-
PDF
- Split View
-
Views
-
Cite
Cite
Bhagu R. Bhavnani, Frank Z. Stanczyk, Misconception and Concerns about Bioidentical Hormones Used for Custom-Compounded Hormone Therapy, The Journal of Clinical Endocrinology & Metabolism, Volume 97, Issue 3, 1 March 2012, Pages 756–759, https://doi.org/10.1210/jc.2011-2492
Close - Share Icon Share
The results of the Women's Health Initiative hormone therapy (HT) trials have caused both apprehension and confusion about the overall risks and benefits associated with postmenopausal HT (1). Since the results were published, patient and clinician interest in potential alternatives to conventional HT has grown immensely and continues to grow. This appears to be particularly true for products or regimens that claim to have fewer risks and side effects than commercially available HT preparations.
One commonly used alternative HT involves custom-compounded hormone preparations. The hormones in these preparations include estrogen [17β-estradiol (estradiol), estrone, and/or estriol], progesterone, testosterone, androstenedione, and dehydroepiandrosterone. The products can be prepared in individualized dosages and forms such as creams, gels, lotions, sublingual tablets, troches (lozenges) for buccal administration, and suppositories by compounding pharmacies from a clinician's prescription. They are often touted by advocates as safer than commercially prepared HT products. Proponents also claim that custom-compounded HT is associated with fewer side effects and may provide better symptom relief than conventional HT because the hormones used in the preparations are “bioidentical,” i.e. identical to those made in the body. The majority of these claims are unsubstantiated, and no rigorous randomized control trials have been carried out to support any of the claims. Because the custom-compounding of HT products is not regulated, a particular concern is that some women may be overdosed, or treated with ineffective products, or subject to unidentified risk.
The objectives of the present commentary are: 1) to show that the so-called “bioidentical” hormones in custom-compounded HT preparations may not be identical to those made in the body; and 2) to point out how the lack of regulation of these hormone products can cause adverse effects in postmenopausal women using them.
Classification of Steroids Used for Postmenopausal HT
For simplicity and to document potential pharmacological differences, various steroid hormones used for HT can be divided into four groups: 1) natural (class A); 2) native to the body and synthesized from natural precursors (class B); 3) native to the body and synthesized from nonsteroidal precursors (class C); and 4) synthetic and not native to the body (class D).
Class A steroids
The steroids in class A are found in nature and are formulated into drugs without undergoing any chemical modifications. For example, conjugated equine estrogens are estrogens in the form of sulfate esters, which are simply extracted from pregnant mare's urine and do not undergo chemical modification. Approximately 50% of conjugated equine estrogens consist of estrone sulfate, and the remaining approximate 50% consists of equine estrogens. Equine estrogens are native to the horse but not the human.
Class B steroids
The class B steroids are semisynthetic. They are steroids that exist in nature and are biosynthesized by the human body, but for these to be formulated as therapeutic agents, they need to be chemically synthesized from a natural starting material, most commonly from a plant source such as the Mexican yam and soybean. These plants contain sterols such as diosgenin and stigmasterol, which are used as precursors for the synthesis of a variety of steroids. Essentially, steroid hormones such as estradiol, estrone, estriol, progesterone, dehydroepiandrosterone, androstenedione, testosterone, cortisol, aldosterone, synthetic conjugated estrogens, etc., have been chemically synthesized from these sources for decades. Contrary to many claims, it is important to note that ingestion of the Mexican yam or soybean does not result in the formation of any of the above steroids because the human body lacks the enzymes needed to convert diosgenin or stigmasterol to those steroids. The chemical synthesis of a steroid hormone such as estradiol from diosgenin requires at least 15 reactions. Another misconception pertains to the occurrence of mammalian steroid hormones in plants. It is only recently that some steroid hormones such as progesterone and androstenedione have been positively identified in plants, using rigorous assay methodology. However, their concentrations are very low (2). There are no hormonal preparations on the market that are derived by extraction of a steroid hormone from a plant.
It is important to recognize that on a biomolecular level steroids synthesized from plant precursors such as diosgenin by the semisynthetic process are not actually identical to the corresponding endogenous steroids in the human body. There are two naturally occurring isotopes of carbon, 12C and 13C, in the carbons that comprise the chemical structure of steroids in plants and humans. Studies show that semisynthetic steroids consist of a different 13C/12C ratio, when compared with the corresponding human endogenous compounds (3). This observation is based on the fact that endogenous steroid hormones reflect an average of the 12C and 13C isotopes of carbon from vegetal and animal food eaten by humans, whereas plant sterols such as those found in soy exhibit a fixed 13C/12C ratio. However, from a physiological standpoint, there is no evidence showing that there is any difference between a semisynthetic steroid and its corresponding endogenous form in the human.
Class C steroids
The steroids in the class C group are “synthetic” compounds that exist in nature but are synthesized from simple nonsteroidal starting materials by a process generally called “total synthesis.” This is one of the oldest means by which steroid hormones were first synthesized. Examples include estrone, estradiol, equilenin, progesterone, etc. Without vigorous analytical analysis, it is extremely difficult to differentiate between class B and class C steroids. However, there can be significant differences in both the chemical and biological properties of the steroids made by these two distinct methods.
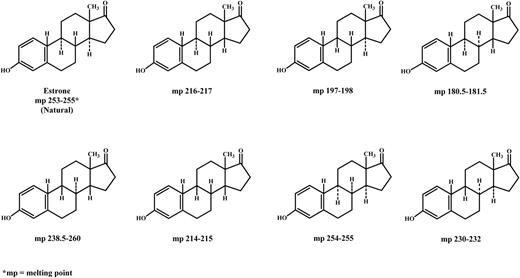
In total synthesis, there is one fundamental requirement and, that is, the steroid synthesized has to have the same precise stereochemistry as that of the naturally occurring hormone. It is only then that the synthesized hormone will have the same biological activity. It is obligatory that the very specific three-dimensional structure be the same for a compound to behave as an agonist because the activity of the compound is expressed by its interaction with its specific endogenous receptor. Unfortunately, total synthesis can give rise to a number of isomers; for example, in the total synthesis of estrone, there are eight possible racemates (16 isomers), as shown in Fig. 1. Only one of these is the natural hormone; others have different physical properties, and some of them are totally inactive (4, 5). Because estrogen preparations compounded by pharmacists do not undergo any vigorous testing or approval by regulatory bodies, it is very possible that some mixtures, depending on the route of synthesis, may not be as potent or can be totally inactive therapeutically, as discussed later. In other words, the so-called bioidentical hormones may not be identical to the natural hormones produced in vivo by the human body.
Class D steroids
The group of class D steroids includes steroids not found in humans, animals, or plants, and includes drugs such as medroxyprogesterone acetate, ethinyl estradiol, norethindrone, norgestrel, etc. These manmade/designed steroidal compounds can be synthesized from the same plant starting materials such as diosgenin or stigmasterol, or alternatively they can be manufactured from nonsteroidal starting material by total synthesis, as described above in Class C steroids.
Regulation of Custom-Compounded Hormone Products
The U.S. Food and Drug Administration (FDA) regards the use of the terminology “bioidentical hormone” as a marketing ploy, implying a benefit for a drug for which there is no medical or scientific basis (6). Although drug compounding is generally subject to FDA oversight, the agency relies on states to regulate the practice as part of their overall regulation of the practice of pharmacy (7). However, the ability of states to oversee the quality and safety of compounded drugs is influenced and limited by the availability of resources for standard inspections and enforcement.
A major concern about the custom-compounded products is that the practice of drug compounding is not subject to the same degree of regulations and oversight by the FDA and by regulatory authorities in other countries, which is required for the commercial production and marketing of prescription medicines. Unlike commercially available drugs, compounded medicines are not tested routinely by any regulatory agency for quality, purity and potency. In addition, there are no product labeling requirements for the custom formulations. This differs from commercially available drugs, which are required to be sold with a package insert that details the product's indications for use, contraindications, pharmacokinetics, and adverse events, using language approved by regulatory authorities.
Concerns about Accuracy of Hormone Doses in Custom-Compounded Hormonal Preparations
Because there is no strict regulation and oversight of compounded medicines, postmenopausal women using custom-prepared hormonal preparations may be subject to underdosing or overdosing of the products. Steroid doses of products prepared by custom-compounding pharmacies are not regulated, and most often little is known about the pharmacokinetics of the products. The doses can be based on a physician's prescription, report(s) from the literature, or information that is not evidence-based. In most instances, serum levels achieved by a particular custom-prepared hormonal preparation are not determined by the physician. In addition, custom-compounded hormonal preparations can be purchased and used by women with no oversight by physicians as to the medical history of the patient, potential presence of risk factors, or the serum levels of active hormones attained with these products.
After becoming aware of product quality control problems associated with custom-compounded hormone preparations, the FDA's Division of Prescription Drug Compliance and Surveillance examined 29 compounded drugs obtained from 12 compounding pharmacies (8). The agency reported that 10 of the products (34%) failed one or more of their standard quality tests, including nine products (one of which was progesterone) that failed because they contained less of the active ingredient than indicated on the label.
Potential underdosing with progesterone has been reported in three women who developed endometrial cancer after using custom-compounded HT to relieve menopausal symptoms (9). The HT preparations included troches containing varying doses of estrone, estradiol, estriol, progesterone, and dehydroepiandrosterone, and were used for 2–4 yr. The final diagnosis of these subjects was stage 1A or 1B, grade 2, endometrial cancer. The authors of the report raised the possibility that the estrogenic component of the troche was significantly absorbed but the progesterone dose was inadequate, thereby causing endometrial hyperplasia.
Although hormones in custom-compounded HT have risks similar to those in regulatory-approved products, e.g. increased risk of a blood clot, heart attack, stroke, breast cancer, gall bladder disease, etc., there is even a higher risk if the hormone dose in a product is greater than expected. In one study, healthy postmenopausal women were treated for 2 wk with a custom-compounded testosterone preparation, either a gel containing 1 mg of testosterone or a lozenge containing 1 mg of testosterone propionate (10). In the women receiving the lozenge, mean maximum testosterone levels occurred 1 h after treatment; the levels rose from a mean baseline level of 16 to 692 ng/dl on d 1 and 836 ng/dl on d 14, and then declined. As for the women receiving the testosterone gel, testosterone levels showed a prolonged rise from a mean baseline value of 20 ng/dl to mean maximal levels of 97 and 100 ng/dl, reached on d 1 and 14, respectively. The testosterone levels observed in these women after treatment are substantially above the upper normal range for women, i.e. 60–70 ng/dl. Such high testosterone levels present for prolonged periods may lead to deleterious effects.
Overdosing can also occur with progesterone creams that are obtained from compounding pharmacies and used by many postmenopausal women because the bioavailability of progesterone after its topical application is poorly understood (11). After the administration of different progesterone doses, a dose response of serum progesterone levels is not observed. The levels do not exceed 3.5 ng/ml, even when relatively high doses of the cream are applied. However, the low progesterone levels do not reflect what is occurring in tissues because levels of progesterone in saliva can increase from baseline levels at least two orders of magnitude after its topical application. Furthermore, the levels in capillary blood are even much higher. In addition, an antiproliferative effect on the endometrium has been reported at serum progesterone levels that were below 3.5 ng/ml. Therefore, it appears that tissue levels of progesterone may be very high, although this is not reflected in its circulating levels. Because progesterone is metabolized to many different metabolites, some of which are active, e.g. neurosteroids such as allopregnanolone, excessive intake of progesterone may cause adverse effects such as a hormonal imbalance and mood swings.
In conclusion, health care providers need to diligently consider the scientific evidence to determine the safety and efficacy of all hormonal preparations used for HT. Use of the misleading term “bioidentical hormone” is inappropriate, and its use should be discouraged.
Acknowledgments
This work was supported by a Medical Research Council of Canada (CIHR) Grant MT-11329 (to B.R.B.).
Disclosure Summary: The authors have no conflicts of interest.



