-
PDF
- Split View
-
Views
-
Cite
Cite
J. T. George, J. D. Veldhuis, A. K. Roseweir, C. L. Newton, E. Faccenda, R. P. Millar, R. A. Anderson, Kisspeptin-10 Is a Potent Stimulator of LH and Increases Pulse Frequency in Men, The Journal of Clinical Endocrinology & Metabolism, Volume 96, Issue 8, 1 August 2011, Pages E1228–E1236, https://doi.org/10.1210/jc.2011-0089
Close - Share Icon Share
Abstract
Kisspeptins stimulate GnRH and thus gonadotropin secretion. Kisspeptin-10 is the minimal kisspeptin sequence with full intrinsic bioactivity, but it has not been studied in man.
We investigated our hypothesis that kisspeptin-10 increases GnRH and thus LH pulse frequency.
The dose response of kisspeptin-10 was investigated by administering iv bolus doses (0.01–3.0 μg/kg) and vehicle to healthy men. Effects on LH pulse frequency and size were determined by deconvolution analysis during infusion of kisspeptin-10 for up to 22.5 h.
Intravenous bolus kisspeptin-10 resulted in a rapid and dose-dependent rise in serum LH concentration, with maximal stimulation at 1 μg/kg (4.1 ± 0.4 to 12.4 ± 1.7 IU/liter at 30 min, P < 0.001, n = 6). Administration of 3 μg/kg elicited a reduced response vs. 1 μg/kg (P < 0.05). Infusion of kisspeptin-10 at 4 μg/kg · h for 22.5 h elicited an increase in LH from a mean of 5.4 ± 0.7 to 20.8 ± 4.9 IU/liter (n = 4; P < 0.05) and serum testosterone increased from 16.6 ± 2.4 to 24.0 ± 2.5 nmol/liter (P < 0.001). LH pulses were obscured at this high rate of secretion, but a lower dose infusion of kisspeptin-10 (1.5 μg/kg · h) increased mean LH from 5.2 ± 0.8 to 14.1 ± 1.7 IU/liter (n = 4; P < 0.01) and increased LH pulse frequency from 0.7 ± 0.1 to 1.0 ± 0.2 pulses/h (P < 0.05) and secretory burst mass from 3.9 ± 0.4 to 12.8 ± 2.6 IU/liter (P < 0.05).
Kisspeptin-10 boluses potently evoke LH secretion in men, and continuous infusion increases testosterone, LH pulse frequency, and pulse size. Kisspeptin analogues have therapeutic potential as regulators of LH and thus testosterone secretion.
The central role of GnRH (LHRH) in regulating reproduction by stimulating the secretion of pituitary gonadotropins (LH and FSH) is well established (1). Kisspeptin, a hypothalamic neuropeptide encoded by the KISS1 gene, has recently emerged as a key central regulator of GnRH secretion. Kisspeptin signaling is obligatory for normal pubertal maturation, as evidenced by absent or advanced pubertal development in individuals with mutations in genes encoding kisspeptin (2) and its receptor [KISS1R; also known as G protein-coupled receptor (GPR) 54] (3–6). Administration of exogenous kisspeptin stimulates LH secretion in both men and women (7–9).
Human studies of kisspeptin have hitherto used the 54-amino acid peptide, kisspeptin-54 (7–10). Kisspeptin-54 is cleaved from the 145-amino acid precursor polypeptide encoded by KISS1 and is further processed to 14, 13, and 10 amino acid (kisspeptin-10) sequences, all sharing the same C-terminal decapeptide RFAmide (arginine-amidated phenylalanine) sequence (11). Although kisspeptin-10 has intrinsic bioactivity similar to the longer kisspeptin fragments (11), it is also characterized by a shorter half-life and more rapid onset of action after iv administration in rodents (12). Kisspeptin-10 also has greater potential for pharmaceutical development because both agonists (13) and antagonists (14, 15) have been developed based on its decapeptide sequence. However, there have been no studies on the activity of kisspeptin-10 in humans.
Alterations in GnRH pulsatility and thus LH secretion are a feature of a number of reproductive disorders. Individuals with some forms of male hypogonadism (16) and hypothalamic amenorrhea (17) show decreased pulse frequency, whereas women with polycystic ovarian syndrome show an increase (18). However, neuroendocrine mechanisms underpinning GnRH pulse generation are yet to be fully delineated (19). Experimental animals exposed to kisspeptin antagonists demonstrate decreased LH pulsatile secretion, suggesting that kisspeptin modulates GnRH pulse frequency (20). Although administration of kisspeptin-54 acutely stimulates LH secretion in men and women (7, 8), it is unclear whether this is mediated by a change in LH pulse frequency, which would indicate an underlying stimulatory effect on GnRH pulse frequency.
In these first-in-human studies of kisspeptin-10, we aimed to establish the dose dependency and time course of stimulation of LH secretion after iv bolus doses of kisspeptin-10 and to compare the magnitude of this stimulated LH secretion with that after a maximally stimulatory dose of GnRH. Having established that kisspeptin-10 is an effective LH secretagogue, we further investigated the effects of infusion of kisspeptin-10 on LH pulsatility. We also examined whether high-dose continuous infusion of kisspeptin-10 induces tachyphylaxis of LH response, as previously suggested in a male primate model (21).
Participants and Methods
Participants
Six healthy male volunteers took part in acute kisspeptin stimulation studies and four healthy males in each of the infusion studies. All volunteers provided informed written consent, and the study was approved by a local research ethics committee (Lothian Research Ethics Committee References 09/S1101/23 and 09/S1101/67). The age of healthy male participants was 35.6 ± 3.4 (mean ± sem) and body mass index was 26.1 ± 0.8 kg/m2, all of whom had a minimum bilateral testicular volume of 40 ml, normal physical examination, and normal secondary sexual characteristics. Baseline full blood count, renal function, liver function, and electrolytes were within normal limits.
Study drugs
Kisspeptin-10 was custom synthesized under GMP standards (Bachem GmBH, Weil am Rhein, Germany). Purity was assessed by HPLC at 97% with a mass balance of 98.8%. For each participant, kisspeptin-10 was made up within the hour before injection by diluting 1 mg of lyophilized kisspeptin-10 in 5 ml sterile normal saline. Stability of kisspeptin-10 in solution was assessed by in vitro receptor binding studies comparing preincubated and freshly constituted 0.2 mg/ml solution (data not shown). A bolus of 0.5 ml sterile normal (0.9%) saline was injected as vehicle. Commercially available GnRH (Relefact; Sanofi Aventis, Frankfurt, Germany) was used for GnRH stimulation.
Protocols
Kisspeptin-10 dose response study
This study was designed to assess the magnitude and dose dependency of gonadotropin secretion in response to six iv bolus doses (0.01, 0.03, 0.1, 0.3, 1.0, and 3.0 μg/kg) of kisspeptin-10 or vehicle (normal saline).
All volunteers attended the study in a fasting state, and all visits commenced between 0800 and 0900 h to avoid diurnal bias. Blood samples were obtained through an indwelling iv cannulae before (60, 45, 30, 20, and 10 min and immediately) and after (10, 20, 30, 45, 60, 75, and 90 min) kisspeptin-10 or vehicle administration. Doses were administered in increasing order for safety reasons and visits were at least 1 wk apart. All volunteers received at least four doses and four volunteers received all doses. Participants were blinded to the dose of kisspeptin-10.
Blood pressure, pulse, and peripheral oxygen saturations were measured every 3 min with standard automated techniques. Full blood count, serum electrolytes, liver function, and renal function were checked at each visit.
High-dose kisspeptin-10 iv infusion
This study was designed to assess LH secretion and potential desensitization of kisspeptin-KISS1R with a continuous high-dose infusion of kisspeptin-10.
After an overnight fast, four volunteers attended our clinical research facility for a 34-h supervised stay. Blood samples were collected at 10-min intervals for two 12-h periods on consecutive days and hourly overnight. After 9 h of baseline sampling, 3 μg/kg of kisspeptin-10 (the highest dose previously investigated) was administered as an iv bolus and a continuous iv infusion of kisspeptin-10 (Crono PCA; Cane, Rivoli, Italy) commenced 90 min later at 4 μg/kg · h. This infusion rate, maintained for 22.5 h, was selected to aim to give a comparable hourly dose to the maximally effective bolus doses, bearing in mind the expected short half-life of kisspeptin-10. A 100-μg/kg iv bolus of GnRH was administered 60 min before the end of the infusion.
Serum LH during the 9-h baseline frequent sampling period was compared with the corresponding 9-h frequent sampling period on the second day of the study during the iv infusion of kisspeptin-10. In addition, we compared the mean LH values observed in the initial 90 min of kisspeptin-10 infusion with the mean LH values observed in the final 90 min of infusion before GnRH administration to identify any potential tachyphylaxis.
Comparisons were also made between mean LH over the 60 min before GnRH administration and the peak LH 30 min afterward. Serum testosterone was analyzed at 30-min intervals during the two 9-h frequent sampling periods before and during the infusion.
All subjects were provided with standardized meals throughout the study and a period of overnight fast maintained between 2200 and 0900 h. Full blood count, electrolytes, liver function, and renal function were checked at the beginning of the study, 24 h later, and at the end of the study.
Pulse frequency study
As there was an apparent increase in LH pulse frequency during the high-dose kisspeptin-10 infusion, we examined this further using a lower dose of kisspeptin-10.
Four healthy men attended our clinical research facility for two visits 5 d apart for 10-min blood sampling. At the first visit, baseline LH pulsatility was assessed over a 9-h period. During the second visit, an iv infusion of kisspeptin-10 was administered for 9 h at 1.5 μg/kg · h after an hour of baseline sampling. Subjects were provided with standardized meals during the study visits.
Hormone assays
Blood samples were centrifuged immediately at 4 C for 10 min at 3000 rpm and serum frozen at −20 C until analysis. LH and FSH were determined by ELISA and testosterone by RIA as previously described (22). Interassay coefficient of variation for all hormonal assays was less than 5% at the concentrations measured. Intraassay coefficient of variation of LH was 2.9%. All samples from each of the study visits were analyzed together in duplicate.
Statistical analysis
For the dose-finding study, 60-min mean LH and FSH concentrations and area under the curve (AUC; by trapezoid integration) before and after the kisspeptin-10 bolus were calculated. Variance in mean LH and ΔAUC values in the dose-response study was analyzed by ANOVA followed by Tukey's simultaneous test. Student's t test was used to analyze changes in mean LH, FSH, and testosterone concentrations in the infusion studies.
LH pulses were identified using a deconvolution algorithm using cluster analysis with 93% sensitivity and specificity on blinded data and basal, pulsatile, and total LH secretion calculated (23). Paired Student's t test was used to assess changes in pulse frequency.
Data are presented as mean ± sem. A two-sided P < 0.05 was regarded as statistically significant for all analyses. The statistical software package Minitab 16 (Minitab Ltd., Coventry, UK) was used.
Results
Kisspeptin-10 dose-response study
Intravenous injections of kisspeptin-10 elicited a rapid increase in LH in all volunteers, with peak concentrations seen by 45 min after injection for all doses studied. The maximum LH stimulation was seen after the 1-μg/kg bolus, achieving peak (12.4 ± 1.7 IU/liter) concentration at 30 min (Fig. 1A).
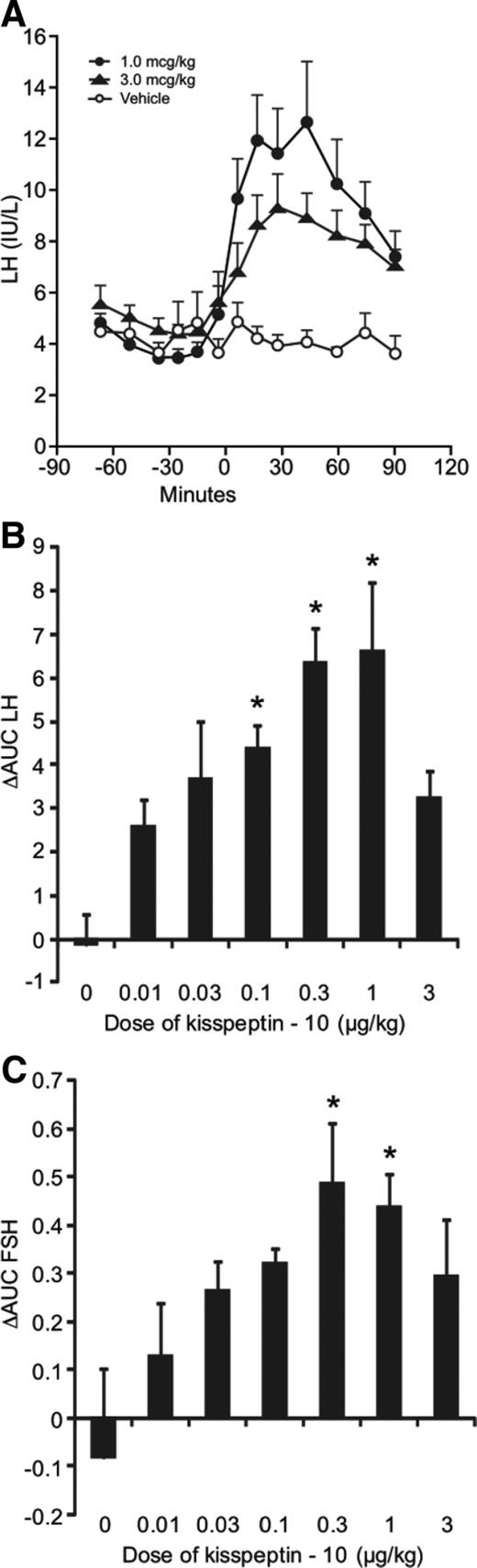
Gonadotropin response to acute administration of kisspeptin-10 to normal men (n = 6). A, Serum LH concentration in response to 1 μg/kg kisspeptin-10 (filled circles), 3 μg/kg kisspeptin-10 (filled triangles), or vehicle (open circles) administered to healthy volunteers at time 0. B, Δ AUC (60 min) of LH after kisspeptin-10 and vehicle administration. C, Δ AUC (60 min) of FSH after kisspeptin-10 and vehicle administration. Mean ± sem. *, P < 0.05 vs. vehicle.
There was a clear dose-dependent increase in LH concentrations in response to kisspeptin-10 (P < 0.0001). Statistically significant increases were observed after the 0.03-, 0.1-, 0.3-, and 1-μg/kg bolus doses (P < 0.01). There was, however, no significant increase in LH concentration after the highest dose administered (3.0 μg/kg), and the mean LH after this dose was significantly less than after the 0.3- and 1-μg/kg doses (both P < 0.05). Calculation of the AUC over 60 min following the kisspeptin-10 administration showed a similar pattern, with the 0.1-, 0.3-, and 1-μg/kg doses achieving statistically significant changes compared with vehicle (Fig. 1B).
FSH secretion also increased dose dependently after kisspeptin-10 administration (P = 0.012). The largest increase was observed with 1 μg/kg in which the mean 60-min FSH rose from 4.6 ± 1.0 to 5.3 ± 1.0 IU/liter. Increases were seen in the AUC of FSH after 0.3 and 1 μg/kg kisspeptin-10, with 3 μg/kg again not resulting in a significant increase in FSH concentration (Fig. 1C). Serum testosterone concentrations showed no statistically significant change with any of the doses studied (data not shown).
Blood pressure, heart rate, peripheral oxygenation, liver function, renal function, hemoglobin, mean corpuscular volume, and electrolytes remained stable in all subjects throughout the study period. No adverse events were reported.
Kisspeptin-10 continuous infusion studies
Infusion of 4 μg/kg · h kisspeptin-10 resulted in sustained increases in LH concentration. The LH profiles observed in the four individual study subjects are represented in Fig. 2. A bolus injection of 3 μg/kg kisspeptin-10 resulted in an increase in LH to 13.6 ± 1.7 IU/liter (P < 0.05 vs. baseline) at 30 min. Continuous kisspeptin-10 infusion (4 μg/kg · h) resulted in a further increase in LH secretion in all subjects, which was sustained throughout the 22.5 h of the infusion. Mean LH during kisspeptin-10 infusion was 20.9 ± 4.9 vs. 5.5 ± 0.8 IU/liter over the 9-h pretreatment period (P < 0.05, Fig. 3A).
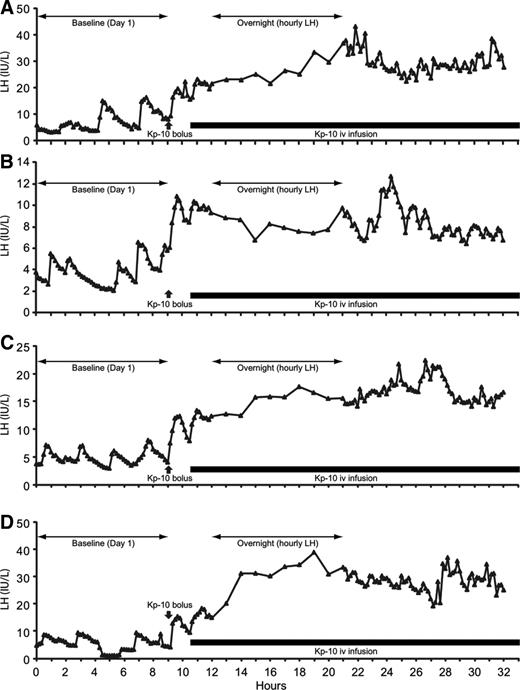
Serum LH profiles of four individual subjects receiving 4 μg/kg · h kisspeptin-10 infusion (black bar) after 9 h baseline sampling and 3 μg/kg iv bolus administered at 9 h (black arrow). Serum samples were obtained at 10 min intervals except overnight when samples were obtained hourly. Note the difference in scale between individual subjects.
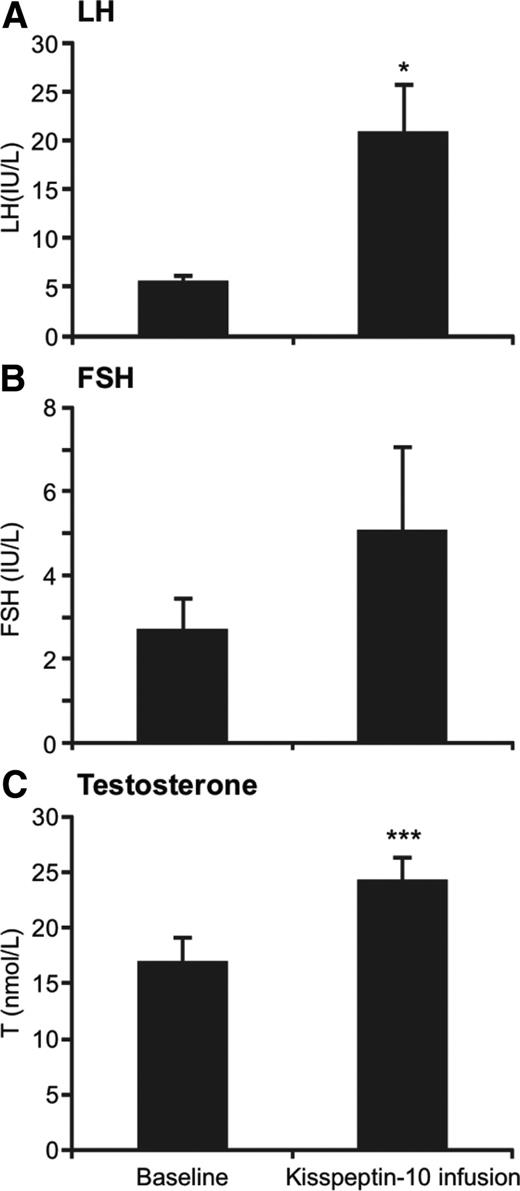
Gonadotropin and testosterone response to infusions of 4 μg/kg of kisspeptin-10 (n = 4). A, Mean serum LH before and during kisspeptin-10 infusion. B, Mean serum FSH before and during kisspeptin-10 infusion. C, Mean serum testosterone before and during kisspeptin-10 infusion. Error bars represent sem. *, P < 0.05; ***, P < 0.001.
Mean LH concentrations in the final 90 min of infusion (23.9 ± 6.8 IU/liter) was comparable with that in the first 90 min of infusion (16.3 ± 2.8 IU/liter, P = 0.34). These data therefore do not indicate tachyphylaxis of the LH response to kisspeptin-10 administration at this dose and duration of exposure.
Intravenous administration of 100 μg bolus of GnRH 60 min before the end of the kisspeptin-10 infusion increased LH further with a peak of 89.3 ± 6.1 IU/liter. This stimulatory effect of GnRH was at least 2.5-fold greater in all subjects than the peak LH observed with the iv bolus of kisspeptin-10 (13.6 ± 1.7 IU/liter, P < 0.001) and the peak LH achieved during kisspeptin-10 infusion (28.7 ± 7.0 IU/liter, P < 0.01).
Analysis of LH pulse frequency during the 9-h baseline sampling and the corresponding 9-h period on the second day of infusion was performed. Characteristic pulsatile LH secretion was observed in all four subjects at baseline but was not well defined during kisspeptin-10 infusion. Nevertheless, deconvolution analysis showed an in increase in pulse frequency in three subjects (from 0.4 to 0.6, 0.6 to 0.8, and 0.6 to 1.2 pulses/h, Fig. 2, A–C, respectively), whereas one subject showed a slight decrease from 0.7 to 0.6 pulse/h (Fig. 2D), giving a mean pulse frequency of 0.6 ± 0.1 pulses/h at baseline and 0.8 ± 0.2 during kisspeptin-10 infusion (P = ns).
Although FSH showed an increase in all subjects during infusion of 4 μg/kg · h kisspeptin-10, this was not statistically significant (2.7 ± 0.8 to 5 ± 2.0 IU/liter, P = 0.17, Fig. 3B), whereas testosterone concentration significantly increased from 16.6 ± 2.4 to 24.0 ± 2.5 nmol/liter (P < 0.001, Fig. 3C).
To investigate further whether continuous kisspeptin-10 exposure increases LH pulse frequency, we infused a lower dose of 1.5 μg/kg · h for 9 h. Mean LH increased significantly from 5.2 ± 0.8 IU/liter at baseline to 14.1 ± 1.7 IU/liter during kisspeptin-10 infusion (P < 0.01, n = 4, Fig. 4). LH showed a steady increase throughout the course of the infusion, increasing from 7.2 ± 2.3 IU/liter in the first 90 min of infusion to 20.4 ± 3.5 IU/liter in the last 90 min (P < 0.05, Fig. 4).
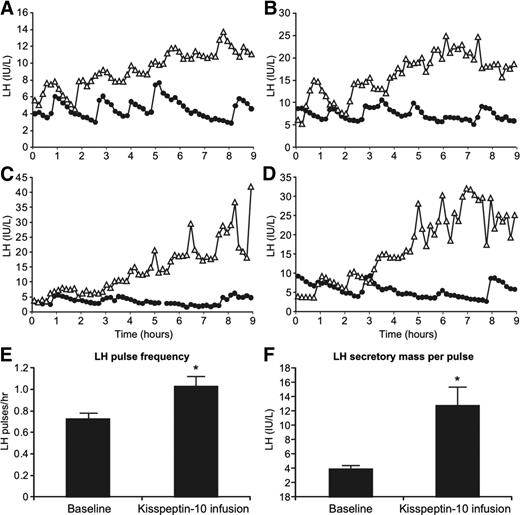
Gonadotrophin response to 9-h iv infusion of 1.5 μg/kg kisspeptin-10 (n = 4). A–D, LH profiles from individual subjects during baseline (closed circles) and kisspeptin-10 infusion (open triangles) visits. Note the difference in scale between individual subjects. E, Mean LH pulse frequency during baseline and kisspeptin-10 infusion visits. F, Secretory mass of LH per pulse during baseline and kisspeptin infusion visits. Error bars represent sem. *, P < 0.05.
Deconvolution analysis demonstrated an increase in LH pulse frequency in all subjects, with a mean increase from 0.7 ± 0.1 to 1.0 ± 0.2 pulses/h (P = 0.01) during kisspeptin-10 infusion at 1.5 μg/kg · h (Fig. 4E). Secretory mass of LH per pulse also increased in all subjects during the infusion, from 3.9 ± 0.4 to 12.8 ± 2.6 IU/liter (P < 0.05, Fig 4F). Pulsatile LH secretion increased as a proportion of total LH secretion in all subjects from 41.3 ± 5.7 to 69.5 ± 5.9% during the infusion.
Serum FSH also increased progressively during the lower-dose kisspeptin-10 infusion. FSH observed during the final 90 min of the infusion (6.6 ± 0.8 IU/liter) showed a significant increase from the first 90 min (3.8 ± 0.8 IU/liter, P < 0.05) of the infusion and baseline (3.9 ± 0.7 IU/liter, P < 0.05).
Discussion
We have demonstrated that iv administration of kisspeptin-10 boluses result in potent and dose-dependent stimulation of LH secretion in healthy men. Doses of kisspeptin-10 as low as 0.03 μg/kg (23 pmol/kg) elicited a significant rise in LH when compared with vehicle, demonstrating the high potency of kisspeptin-10. This is in keeping with data from experimental animals where intracerebroventricular doses of kisspeptin as small as 1 fmol effected robust LH stimulation (24). Central rather than pituitary mediation of the effect of kisspeptin on LH secretion has been demonstrated by abolition of the effect of kisspeptin by pretreatment with a GnRH antagonist (24–26).
The 2.5-fold increase in serum LH from baseline observed after kisspeptin bolus administration in our study is comparable with that observed in men administered equimolar doses of kisspeptin-54 as short infusions (7). Moreover, as in human studies using kisspeptin-54 (7), the stimulatory effect of kisspeptin-10 on LH secretion in our study was also markedly more pronounced than that on FSH secretion. Early animal studies suggested stimulatory effects on LH and testosterone secretion were seen only with the longer kisspeptin peptide, kisspeptin-54, and not shorter forms (27). However, in vivo rodent data have subsequently shown equal potency of kisspeptin-10 and kisspeptin-54 (24, 28), and in in vitro binding assays, both peptides show equal affinity at the KISS1R (11). Our results demonstrate that the 10 amino acid form of kisspeptin is a potent LH secretagogue in vivo in humans.
Although there was a clear dose dependency of LH response to increasing doses of kisspeptin-10 up to 1 μg/kg, the 3-μg/kg (2.3 nmol/kg) bolus dose did not elicit a statistically significant increase when compared with vehicle, and mean and peak LH concentration after this dose was lower than corresponding values after 0.3 and 1 μg/kg. This phenomenon has not been reported before after bolus administration of kisspeptin, but continuous infusion of kisspeptin-10 in primates (21) and repeated sc administration of kisspeptin-54 in human subjects with hypothalamic amenorrhea (9) resulted in tachyphylaxis. The kisspeptin receptor KISS1R is known to desensitize rapidly in vitro (29), raising the possibility of rapid hypothalamic desensitization. Although such desensitization is a parsimonious explanation for the decreased response observed here, desensitization has to be occurring rapidly after kisspeptin-10 administration before maximal stimulation of gonadotrophs is achieved. Because the fast half-life of LH in healthy men is 18 min (30), lower peak LH after the 3 μg/kg would suggest that the gonadotroph stimulation was submaximal.
An alternative explanation for this observation is that at high concentrations, kisspeptin-10 may stimulate another RF-amide receptor with an inhibitory effect on GnRH or LH secretion. Kisspeptin-10, at nanomolar concentrations, has been shown to bind and activate the gonadotropin inhibitory hormone (GnIH) receptor [GPR147, NPFFR1 (neuropeptide FF receptor 1)] (31), which is expressed both in the hypothalamus and on gonadotropes (32). Nanomolar plasma concentrations of kisspeptin were achieved after sc administration of doses of kisspeptin-54 similar to the kisspeptin-10 used in our study (8). However, although peak plasma kisspeptin-10 concentrations after the 3-μg/kg bolus may have been sufficient to activate GnIH receptors, there is little evidence for a functional role for GnIH in humans, and in vivo studies in sheep (33) would suggest that its effects are relatively modest, and it is not possible to exclude an effect of GnIH receptor involvement in the infusion studies. The reduced stimulatory efficacy of kisspeptin-10 at high concentrations demands careful dosing in any potential therapeutic applications.
Importantly, our studies demonstrate the novel phenomenon that continuous infusion of kisspeptin-10 increases LH pulse frequency. Along with increased secretory mass in individual pulses, this increase in pulse frequency resulted in pulsatile secretion contributing to a higher proportion of total LH secreted. Data from other investigators have not shown that GnRH pulse frequency is increased by an acute bolus of kisspeptin (34), indicating the need for continuing kisspeptin stimulation to study its influence on GnRH pulse frequency. The dependency of LH pulsatility on pulsatile GnRH secretion (1, 35) is well established, and kisspeptin has been shown to directly stimulate GnRH secretion (36) with electrophysiological studies showing intense and prolonged activation of rodent GnRH neurons by kisspeptin-10 (37). There is a marked stochastic increase in LH when kisspeptin is applied to the hypothalamic arcuate nucleus in rodents, which might reflect an acceleration of LH pulse frequency (20), and a kisspeptin antagonist decreases LH pulse frequency in this model (20). Systemic administration of kisspeptin-10 in our study prevents us from concluding whether this increase in LH (and by inference GnRH) pulse frequency is mediated by a direct effect on GnRH neurons (19) or through stimulation of the hypothalamic pulse generator (38). However, we have demonstrated that LH pulsatility is dependent on kisspeptin, increasing significantly during continuous administration. However, it is possible that endogenous release of kisspeptin is pulsatile, and pulsatile administration may have a different impact on GnRH pulsatility, modulated by cotransmitters such as neurokinins and dynorphins (39). There may also be species and sex differences in the GnRH pulse regulation because, unlike rodents, a GnRH surge is not obligatory for the preovulatory LH surge in women (40). In addition, the increase in secretory mass in the individual pulses observed during kisspeptin infusion is consistent with increased GnRH secretion.
This effect on LH pulse frequency was most convincingly demonstrated during administration of the lower dose (1.5 μg/kg · h) of kisspeptin-10. Deconvolution analysis (23), like other algorithms to detect LH pulses (41), is less sensitive with high LH secretion rates because the distinct increase in LH concentration achieved by individual pulsatile discharges of LH can be obscured. The high mean LH concentrations during the higher-dose kisspeptin-10 infusion are likely to have prevented reliable analysis of pulse frequency, leading to underestimation.
Another key observation is the relative constancy of serum LH concentrations during kisspeptin-10 infusion, although serum testosterone increased significantly. Androgens have been shown to down-regulate Kiss1 gene expression in the nonhuman primate hypothalamus (42), and kisspeptin-secreting neurones have been proposed to constitute part of the pathway mediating sex steroid-negative feedback (43). Despite significant increases in serum testosterone, although within the normal range, there was no late decline in LH, suggesting that within the physiological range, testosterone has limited ability to modulate the ability of the GnRH neuron to respond to kisspeptin-10 and the pituitary gonadotroph to respond to GnRH.
Tachyphylaxis of the GnRH/LH system during continuous infusion of kisspeptin-10 has also been demonstrated in primate models, with LH returning to baseline a few hours after an initial rise (21, 44). Despite continually infusing kisspeptin-10 for 22.5 h, we found no such desensitization, and indeed LH secretion tended to increase progressively. This is possibly a function of the dose of kisspeptin used in our study being lower than that used in the primate studies (21, 44). Tachyphylaxis of LH response has been seen during the twice-daily administration of kisspeptin-54 to women with hypothalamic amenorrhea, but this occurred over a much longer time period, i.e. 2 wk (9).
Stimulatory effects of kisspeptin-10 on LH, FSH, and testosterone established in this study could inform future studies using kisspeptin as a diagnostic or therapeutic agent. Potent kisspeptin agonists (13) and antagonists (14) currently being tested in animal models are amino acid substitutions of the decapeptide sequence of kisspeptin-10. Dose responsiveness and time course of LH stimulation demonstrated in our study could underpin their translation into clinical studies. Moreover, we have also demonstrated that GnRH responsiveness is preserved in subjects receiving kisspeptin-10 infusion and that maximal LH responses seen with kisspeptin-10 are considerably lower than that achieved with GnRH. Therefore, kisspeptin-10 might provide a more physiological stimulation of the human reproductive axis with potential therapeutic advantages in stimulation of ovulation.
Our study protocols did not incorporate measurement of kisspeptin immunoreactivity for two reasons. With the expected very short plasma half-life of kisspeptin-10 (12), the postinjection blood sampling schedule would need to include samples much earlier and more frequently than at 10 min for accurate analysis. Moreover, immunological assays can potentially give false-positive results in the presence of cleavage products of the peptide because nonspecific binding of kisspeptin antibodies is well established (42). Because the blood samples were not processed appropriately, post hoc immunoassays could not be carried out either. Mass-spectroscopic assays of serum samples for kisspeptin 10 at various time points during the 4 μg/kg · h kisspeptin-10 infusion were therefore attempted, but concentrations of kisspeptin-10 and its cleavage products (kisspeptin-9, -7, or -4) were below the detection limit (1 ng/ml) at almost all time points, most likely due to suboptimal sample processing.
In conclusion, iv kisspeptin-10 boluses evoke rapid and potent LH secretion in men. Continuous infusion of kisspeptin-10 increases mean LH, LH pulse frequency, LH pulse size, and testosterone secretion. These data are consistent with kisspeptin being a key regulator of pulsatile GnRH secretion.
Abbreviations:
- AUC
Area under the curve
- GnIH
gonadotropin inhibitory hormone
- GPR
G protein-coupled receptor
- KISS1R
kisspeptin receptor.
Acknowledgments
We are grateful to Nancy Evans and Robin Sellar for expert technical assistance, Nicholas Malone for coordinating study visits, and the Wellcome Trust and Royal Infirmary of Edinburgh Clinical Research Facilities. We acknowledge the expert advice of Dr. George Merriam on LH pulse detection and thank Dr. Rebecca Reynolds for serving as an independent member of the Study Management Committee. We also thank the men who kindly participated in this study.
This work was supported by Experimental Medicine Grant (G0701682) from the Medical Research Council (United Kingdom).
Disclosure Summary: The authors have nothing to disclose.
References
Author notes
R.P.M. and R.A.A. contributed equally to this work.



