-
PDF
- Split View
-
Views
-
Cite
Cite
Marco Volante, Ida Rapa, Manoj Gandhi, Gianni Bussolati, Daniela Giachino, Mauro Papotti, Yuri E. Nikiforov, RAS Mutations Are the Predominant Molecular Alteration in Poorly Differentiated Thyroid Carcinomas and Bear Prognostic Impact, The Journal of Clinical Endocrinology & Metabolism, Volume 94, Issue 12, 1 December 2009, Pages 4735–4741, https://doi.org/10.1210/jc.2009-1233
Close - Share Icon Share
Context: Poorly differentiated carcinomas represent an aggressive group of thyroid tumors with controversial classification placement and poorly understood pathogenesis. Molecular data in this group of tumors are extremely heterogeneous, possibly reflecting different inclusion criteria. Recently homogeneous diagnostic criteria have been proposed by our group (Turin proposal) that need to be complemented by detailed molecular characterization.
Objective: The objective of the study was to define a comprehensive molecular typing of poorly differentiated thyroid carcinomas classified following homogeneous diagnostic criteria.
Design: Sixty-five cases of poorly differentiated carcinoma selected following the Turin proposal have been screened for N-, K-, H-RAS, BRAF, RET/PTC1 and 3, and PAX8/PPARγ mutations-rearrangements using alternative techniques and in two different laboratories. Molecular data were compared with clinical pathological parameters and survival by univariate and multivariate analysis.
Results: RAS mutations in codon 61 were by far the most common genetic alteration in poorly differentiated carcinomas (23% of cases), with all mutation in NRAS except one in the HRAS gene. A single BRAF mutation was found in a poorly differentiated carcinoma with a residual component of a tall cell variant of papillary carcinoma. No KRAS, RET/PTC, or PAX8/PPARγ genetic alteration was detected. In this series, the presence of RAS mutations was a unique negative prognostic parameter at multivariate analysis.
Conclusions: The present study demonstrates that strictly classified poorly differentiated carcinomas are genetically homogeneous, RAS mutations being the almost exclusive genetic event. Moreover, the detection of RAS mutations might be clinically relevant for the prognostic stratification of these tumors.
Thyroid cancer is the most common type of endocrine malignancy. The vast majority of thyroid tumors originate from thyroid follicular cells and encompass well-differentiated papillary carcinoma and follicular carcinoma. Each of these tumors can dedifferentiate and give rise to undifferentiated (anaplastic) carcinoma. In addition to these well-established tumor entities, poorly differentiated carcinoma has been recently added to the World Health Organization (WHO) classification of thyroid tumors (1). Poorly differentiated thyroid carcinomas comprise a heterogeneous group of tumors that occupy an intermediate position between well-differentiated papillary/follicular carcinomas and anaplastic carcinoma from both a histopathogenetic and a clinical point of view.
The placement of poorly differentiated carcinomas into a separate classification group appears justifiable based on large series of tumors sharing structural and/or other morphological criteria that showed distinct biological and clinical behavior (2–6). However, since the original description (7, 8), different diagnostic criteria have been used to define these tumors (9). Some authors defined poorly differentiated carcinomas primarily based on the presence of the solid, trabecular, and insular growth patterns (10), whereas others considered the presence of high-grade features, such as atypia, high mitotic count, and necrosis, as a specific hallmark of these tumors (4, 11, 12), thus including in some instances the aggressive variants of papillary carcinomas (i.e. tall cell or columnar variants) in the poorly differentiated carcinoma group.
As a result, the molecular genetic data reported to date in the literature are limited by the heterogeneity of the case series analyzed. Generally, irrespective of the selection criteria applied, RAS point mutations appear to be a common molecular alteration in these tumors, although they have found with highly variable frequency and wide variation in specific types of RAS mutations (13–15). The same holds true for the prevalence of BRAF mutations, which have been detected in poorly differentiated carcinomas having residual papillary carcinoma foci (16, 17) but not in cases lacking the morphologic evidence of transition between well-differentiated and poorly differentiated carcinoma (18). Similarly, RET/PTC1 rearrangements have been found in a fraction of poorly differentiated carcinomas having the nuclear features of papillary carcinoma and/or some (residual) foci of papillary carcinoma (19). Controversial findings have been published concerning the presence of β-catenin mutations in poorly differentiated carcinomas because these were detected in 0 (20) and 32% (21) of tumors analyzed in the two studies. PAX8/PPARγ rearrangements have not been reported in poorly differentiated thyroid carcinomas so far to our knowledge.
Taken together, these molecular data indicate that poorly differentiated carcinomas may be related to both well-differentiated follicular and papillary carcinoma lineages, which is also supported by their morphological features, although distinct molecular pathways of progression are still poorly understood (22).
In 2004 poorly differentiated carcinoma has been introduced for the first time as a separate entity in the WHO classification of thyroid tumors (1), providing bases for the use of uniform terminology and diagnostic criteria. An international conference has recently proposed a diagnostic algorithm based on the interpretation of the WHO criteria, offering a uniform, consensus approach for classification of these tumors (23). The relevance of this approach has been recently tested and confirmed by a Japanese group that used the proposed algorithm to identify, among a large series of thyroid malignancies originally diagnosed as papillary carcinomas, a homogeneous group of tumors with unfavorable prognosis (24).
In this study, we report the results of our analysis of molecular alterations in a large series of poorly differentiated carcinomas defined according to criteria of the WHO classification refined by the Turin conference.
Materials and Methods
Case selection
Sixty-five cases of poorly differentiated thyroid carcinoma were selected from the databases of the Divisions of Pathology of the University of Turin (54 cases) and University of Cincinnati (11 cases) and included in the study. All cases have been classified according to the WHO diagnostic criteria refined by the Turin conference (23) and subdivided into poorly differentiated carcinomas of papillary type (PD-PTC; 19 cases) based on the presence of residual papillary carcinoma component (eight of 19) or convoluted nuclei (eight of 19) and poorly differentiated carcinomas not otherwise specified (PD-NOS; 46 cases) in the absence of the former features. xInclusion criteria were the availability of representative hematoxylin and eosin sections for histological review and paraffin blocks for immunohistochemistry and molecular testing. Six additional cases meeting the diagnostic criteria of poorly differentiated carcinoma and having tissue material available were excluded from the study due to unsatisfactory quality of DNA and RNA isolated. The study has been approved by the Institution Review Board of San Luigi Hospital.
Immunohistochemistry
In 44 cases, p53 immunohistochemistry was performed according to standard automated immunohistochemical procedure (Dako Autostainer, Glostrup, Denmark). Monoclonal antibody against p53 (1:200; Neomarkers, Fremont, CA) was used. Immunoreactions were revealed by a biotin-free dextran-chain detection system (Envision; DakoCytomation, Glostrup, Denmark) and developed using diaminobenzidine as the chromogen.
Molecular analysis
Nucleic acid isolation
Genomic DNA was isolated from formalin-fixed, paraffin-embedded tissues using the standard proteinase K-phenol-chloroform extraction method. RNA was isolated from paraffin embedded material using the high pure RNA paraffin kit (Roche, Mannheim, Germany) following the manufacturer’s instructions. The quantity of isolated DNA and RNA was assessed using a Biophotometer (Eppendorf, Hamburg, Germany).
Point mutation analysis
The presence of BRAF V600E and K601E, NRAS codon 61, HRAS codon 61, and KRAS codons 12 and 13 point mutations was analyzed using two different techniques.
All cases were analyzed by pyrosequencing in the laboratory of Turin. PCR and sequencing primers were designed using the PSQ Assay Design Software version 1.0.6 (Biotage AB, Uppsala, Sweden). One primer of each pair has a 5′-biotin label necessary for post-PCR processing. The primer sequences, amplicon sizes and annealing temperatures are shown in Table 1. PCR amplification for the pyrosequencing assay was performed according to standard protocols. The amplicons were mixed with sequencing primers and sequencing was performed using a PyroGold reagent kit (Biotage AB) according to the manufacturer’s protocol. Results were analyzed using the PSQ-96 MA 2.0.2 software (Biotage).
PCR and sequencing primer sequences, annealing temperatures, and amplicon length in pyrosequencing analysis
| Gene . | Codons . | Forward PCR primer . | Reverse PCR primer . | Sequencig primer . | Ann. T . | Product size (bp) . |
|---|---|---|---|---|---|---|
| BRAF NM_004333 | 600–601 | B/CTTCATAATGCTTGCTCTGATAGG | GCATCTCAGGGCCAAAAA | CCACTCCATCGAGATT | 54 C | 235 |
| NRAS NM_002524 | 61 | B/TGAAACCTGTTTGTTGGACATACT | CGCAAATGACTTGCTATTATTGA | CATGGCACTGTACTCTTCT | 55 C | 130 |
| HRAS NM_005343 | 61 | GGTCATTGATGGGGAGACGTG | B/CGCATGGCGCTGTACTCCT | CATCCTGGATACCGC | 57 C | 72 |
| KRAS NM_033360 | 12–13 | B/CTGAATATAAACTTGTGGTAGTTGG | ATATTCGTCCACAAAATGATTCT | AGGCACTCTTGCCTA | 57 C | 92 |
| Gene . | Codons . | Forward PCR primer . | Reverse PCR primer . | Sequencig primer . | Ann. T . | Product size (bp) . |
|---|---|---|---|---|---|---|
| BRAF NM_004333 | 600–601 | B/CTTCATAATGCTTGCTCTGATAGG | GCATCTCAGGGCCAAAAA | CCACTCCATCGAGATT | 54 C | 235 |
| NRAS NM_002524 | 61 | B/TGAAACCTGTTTGTTGGACATACT | CGCAAATGACTTGCTATTATTGA | CATGGCACTGTACTCTTCT | 55 C | 130 |
| HRAS NM_005343 | 61 | GGTCATTGATGGGGAGACGTG | B/CGCATGGCGCTGTACTCCT | CATCCTGGATACCGC | 57 C | 72 |
| KRAS NM_033360 | 12–13 | B/CTGAATATAAACTTGTGGTAGTTGG | ATATTCGTCCACAAAATGATTCT | AGGCACTCTTGCCTA | 57 C | 92 |
B, Biotinylation site; Ann, annealing; T, temperature.
PCR and sequencing primer sequences, annealing temperatures, and amplicon length in pyrosequencing analysis
| Gene . | Codons . | Forward PCR primer . | Reverse PCR primer . | Sequencig primer . | Ann. T . | Product size (bp) . |
|---|---|---|---|---|---|---|
| BRAF NM_004333 | 600–601 | B/CTTCATAATGCTTGCTCTGATAGG | GCATCTCAGGGCCAAAAA | CCACTCCATCGAGATT | 54 C | 235 |
| NRAS NM_002524 | 61 | B/TGAAACCTGTTTGTTGGACATACT | CGCAAATGACTTGCTATTATTGA | CATGGCACTGTACTCTTCT | 55 C | 130 |
| HRAS NM_005343 | 61 | GGTCATTGATGGGGAGACGTG | B/CGCATGGCGCTGTACTCCT | CATCCTGGATACCGC | 57 C | 72 |
| KRAS NM_033360 | 12–13 | B/CTGAATATAAACTTGTGGTAGTTGG | ATATTCGTCCACAAAATGATTCT | AGGCACTCTTGCCTA | 57 C | 92 |
| Gene . | Codons . | Forward PCR primer . | Reverse PCR primer . | Sequencig primer . | Ann. T . | Product size (bp) . |
|---|---|---|---|---|---|---|
| BRAF NM_004333 | 600–601 | B/CTTCATAATGCTTGCTCTGATAGG | GCATCTCAGGGCCAAAAA | CCACTCCATCGAGATT | 54 C | 235 |
| NRAS NM_002524 | 61 | B/TGAAACCTGTTTGTTGGACATACT | CGCAAATGACTTGCTATTATTGA | CATGGCACTGTACTCTTCT | 55 C | 130 |
| HRAS NM_005343 | 61 | GGTCATTGATGGGGAGACGTG | B/CGCATGGCGCTGTACTCCT | CATCCTGGATACCGC | 57 C | 72 |
| KRAS NM_033360 | 12–13 | B/CTGAATATAAACTTGTGGTAGTTGG | ATATTCGTCCACAAAATGATTCT | AGGCACTCTTGCCTA | 57 C | 92 |
B, Biotinylation site; Ann, annealing; T, temperature.
In addition, the same mutations were analyzed in 40 randomly selected tumors using Light Cycler PCR (Roche, Indianapolis, IN) and melting curve analysis as previously described (25) in the laboratory of Cincinnati.
Direct nucleotide (Sanger) sequencing
All samples that tested positive for mutations with one or both techniques were sequenced using BigDye terminator kit on the ABI3100 (Applied Biosystems, Foster City, CA) to confirm the presence and type of mutation.
Detection of rearrangements
RET/PTC1, RET/PTC3, and PAX8/PPARγ rearrangements were detected from RNA by RT-PCR with primers designed to flank the respective fusion point. Quality of RNA in each sample was assessed by amplification of the β-2 microglobulin gene. Primers and PCR conditions used in this study have been previously described in detail (25). In 40 cases, the analysis was performed in parallel in two laboratories (M.P. and Y.E.N.).
Controls
DNA and RNA samples from tumors or cell lines known to carry a specific mutation or rearrangement were used as a positive control.
Statistical analysis
The correlation between genetic alterations and known clinical pathological parameters was assessed by χ2 test. Univariate survival analysis was based on the Kaplan-Meier product limit estimate of overall survival distribution. Unadjusted differences between survival curves were tested using the LogRank test using P = 0.05 as the level of significance. Multivariate analysis of the relative impact on survival of each parameter included in the univariate analysis was estimated using the Cox proportional hazards regression model. Statistical analysis was performed using the Prism 4 (GraphPad, San Diego, CA) and SPSS softwares (Chicago, IL).
Results
Molecular profile
The molecular alterations detected in 65 poorly differentiated carcinomas are summarized in Table 2.
| Poorly differentiated carcinoma type (number of cases analyzed) . | RAS . | BRAF . | RET/PTC 1 and 3 . | PAX8/PPARγ . |
|---|---|---|---|---|
| Overall (65) | 15/65 | 1/65 | 0/65 | 0/65 |
| PD-PTC-type (19) | 7/19 | 1/19 | ||
| Type of mutation | NRAS Q61R (6) | V600E (1) | ||
| H61Q61K (1) | ||||
| PD-NOS (46) | 8/46 | 0/46 | ||
| Type of mutation | NRAS Q61R (7) |
| Poorly differentiated carcinoma type (number of cases analyzed) . | RAS . | BRAF . | RET/PTC 1 and 3 . | PAX8/PPARγ . |
|---|---|---|---|---|
| Overall (65) | 15/65 | 1/65 | 0/65 | 0/65 |
| PD-PTC-type (19) | 7/19 | 1/19 | ||
| Type of mutation | NRAS Q61R (6) | V600E (1) | ||
| H61Q61K (1) | ||||
| PD-NOS (46) | 8/46 | 0/46 | ||
| Type of mutation | NRAS Q61R (7) |
| Poorly differentiated carcinoma type (number of cases analyzed) . | RAS . | BRAF . | RET/PTC 1 and 3 . | PAX8/PPARγ . |
|---|---|---|---|---|
| Overall (65) | 15/65 | 1/65 | 0/65 | 0/65 |
| PD-PTC-type (19) | 7/19 | 1/19 | ||
| Type of mutation | NRAS Q61R (6) | V600E (1) | ||
| H61Q61K (1) | ||||
| PD-NOS (46) | 8/46 | 0/46 | ||
| Type of mutation | NRAS Q61R (7) |
| Poorly differentiated carcinoma type (number of cases analyzed) . | RAS . | BRAF . | RET/PTC 1 and 3 . | PAX8/PPARγ . |
|---|---|---|---|---|
| Overall (65) | 15/65 | 1/65 | 0/65 | 0/65 |
| PD-PTC-type (19) | 7/19 | 1/19 | ||
| Type of mutation | NRAS Q61R (6) | V600E (1) | ||
| H61Q61K (1) | ||||
| PD-NOS (46) | 8/46 | 0/46 | ||
| Type of mutation | NRAS Q61R (7) |
RAS mutations represented by far the most common genetic alteration in poorly differentiated carcinomas, encountered overall in 15 of 65 cases (23%). All but one mutation were in the NRAS gene, codon 61, with the Gln to Arg (Q61R, CAA > CGA) substitution (Fig. 1). The remaining tumor had the HRAS Q61K mutation. No mutation was detected in the KRAS gene. The frequency of RAS mutations was slightly higher in PD-PTC type (seven of 19 cases) than in PD-NOS (eight of 46 cases), although the difference was not statistically significant (χ2 test, P = 0.285). The prevalence of RAS mutations was not significantly different in cases of poorly differentiated carcinoma from Turin compared with those from Cincinnati (13 of 54 and two of 11 cases, respectively; P = 0.761).
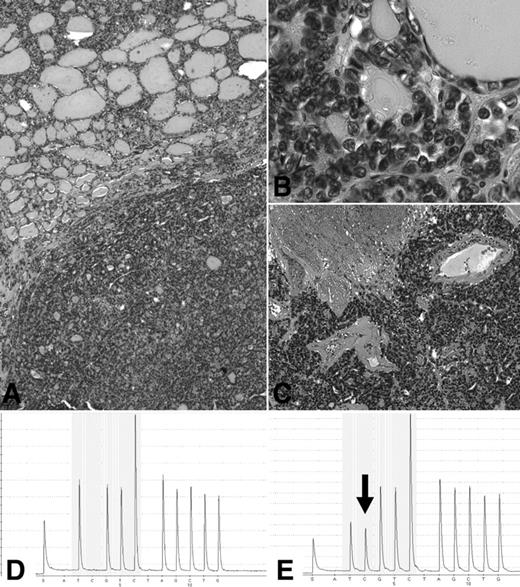
Molecular profile of a poorly differentiated thyroid carcinoma (A) with residual component of follicular variant of papillary carcinoma (A, top, and B) and predominant solid component with extensive necrosis (A, bottom, and C), showing by means of pyrosequencing analysis the presence of wild-type NRAS in the normal peritumoral thyroid (D) and heterozygous mutation at codon 61 in the tumor (E; reverse sequence showing the presence of an additional peak corresponding to the NRAS Q61R-CAA > CGA-substitution).
The common V600E BRAF mutation was encountered in a single case of poorly differentiated carcinoma, which showed a minor component of papillary carcinoma, tall cell variant.
Light Cycler PCR and pyrosequencing methods provided identical results of mutation detection in all cases except one case of NRAS mutation, which was not detected by pyrosequencing but confirmed by direct sequencing.
RET/PTC types 1 and 3 and PAX8/PPARγ translocations were absent in all cases.
Correlation between the presence of RAS mutations and clinical pathological parameters
The status of RAS mutation was compared with the various clinical and pathological characteristics of poorly differentiated carcinomas in the present series. The female to male ratio was 2.2:1, mean age was 62.1 yr (range 23–86), and the mean tumor diameter was 5.6 cm (range 1.5–12). Up to two thirds of the cases presented with high pathological tumor stage (68% stage ≥ pT3) and 38% had extrathyroidal extension. Predominant oncocytic features, defined by the morphological recognition of large cells characterized by abundant deeply eosinophilic and granular cytoplasm in more than 75% of the neoplastic cell population (1) in tumors otherwise fulfilling the Turin proposal for the diagnosis of poorly differentiated carcinoma (23), were observed in 17 cases (26%). Pathological N stage was available in 11 cases only and was not considered in the correlation analysis. Finally, follow-up information were available for 53 cases. More than half of them (53%) had tumor recurrence or were dead of the disease at the time of last follow-up (mean follow-up time: 52 months, range 4–228). None of the above-mentioned characteristics was differentially expressed in the cohort of patients from Italy compared with those from the United States (all P > 0.05).
None of the parameters considered, including gender, age, size, pT, extrathyroidal extension, and predominant oncocytic features, was significantly associated with RAS-positive genotype.
The prevalence of RAS mutations had a tendency to be higher in cases with low/absent p53 protein expression, although without statistical significance (P = 0.196).
Despite the lack of association between RAS mutations and specific disease characteristics, a univariate analysis demonstrated a strong correlation with shorter overall survival in patients harboring RAS mutation (P = 0.004) (Fig. 2). The negative prognostic impact of RAS mutations was confirmed by a multivariate analysis, which identified this mutations as the only independent prognostic indicator in the current series of poorly differentiated carcinomas (Table 3).
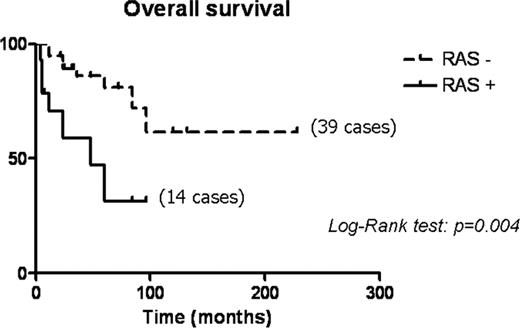
Overall survival analysis of 53 poorly differentiated carcinomas according to RAS mutation status.
Multivariate analysis of overall survival in poorly differentiated thyroid carcinomas according to clinicopathological parameters and RAS mutation status
| Variable . | HR . | 95% CI . | P . |
|---|---|---|---|
| Sex | |||
| Female | |||
| Male | 2.397 | 0.802–7.164 | 0.117 |
| Age (yr) | |||
| ≤45 | |||
| >45 | 0.695 | 0.126–3.825 | 0.676 |
| Size | |||
| ≤4 | |||
| >4 | 0.000 | Undefined | 0.986 |
| Oncocytic features | |||
| Absent | |||
| Present | 0.000 | 0.000–6.174 | 0.967 |
| PD type | |||
| PTC type | |||
| NOS type | 1.301 | 0.433–3.907 | 0.638 |
| pT stage | |||
| pT1–2 | |||
| pT3–4 | 0.000 | Undefined | 0.986 |
| Extrathyroidal extension | |||
| Absent | |||
| Present | 1.077 | 0.266–4.346 | 0.916 |
| RAS mutation | |||
| Absent | |||
| Present | 3.670 | 1.085–12.412 | 0.036 |
| Variable . | HR . | 95% CI . | P . |
|---|---|---|---|
| Sex | |||
| Female | |||
| Male | 2.397 | 0.802–7.164 | 0.117 |
| Age (yr) | |||
| ≤45 | |||
| >45 | 0.695 | 0.126–3.825 | 0.676 |
| Size | |||
| ≤4 | |||
| >4 | 0.000 | Undefined | 0.986 |
| Oncocytic features | |||
| Absent | |||
| Present | 0.000 | 0.000–6.174 | 0.967 |
| PD type | |||
| PTC type | |||
| NOS type | 1.301 | 0.433–3.907 | 0.638 |
| pT stage | |||
| pT1–2 | |||
| pT3–4 | 0.000 | Undefined | 0.986 |
| Extrathyroidal extension | |||
| Absent | |||
| Present | 1.077 | 0.266–4.346 | 0.916 |
| RAS mutation | |||
| Absent | |||
| Present | 3.670 | 1.085–12.412 | 0.036 |
HR, Hazard ratio; CI, confidence intervals.
Multivariate analysis of overall survival in poorly differentiated thyroid carcinomas according to clinicopathological parameters and RAS mutation status
| Variable . | HR . | 95% CI . | P . |
|---|---|---|---|
| Sex | |||
| Female | |||
| Male | 2.397 | 0.802–7.164 | 0.117 |
| Age (yr) | |||
| ≤45 | |||
| >45 | 0.695 | 0.126–3.825 | 0.676 |
| Size | |||
| ≤4 | |||
| >4 | 0.000 | Undefined | 0.986 |
| Oncocytic features | |||
| Absent | |||
| Present | 0.000 | 0.000–6.174 | 0.967 |
| PD type | |||
| PTC type | |||
| NOS type | 1.301 | 0.433–3.907 | 0.638 |
| pT stage | |||
| pT1–2 | |||
| pT3–4 | 0.000 | Undefined | 0.986 |
| Extrathyroidal extension | |||
| Absent | |||
| Present | 1.077 | 0.266–4.346 | 0.916 |
| RAS mutation | |||
| Absent | |||
| Present | 3.670 | 1.085–12.412 | 0.036 |
| Variable . | HR . | 95% CI . | P . |
|---|---|---|---|
| Sex | |||
| Female | |||
| Male | 2.397 | 0.802–7.164 | 0.117 |
| Age (yr) | |||
| ≤45 | |||
| >45 | 0.695 | 0.126–3.825 | 0.676 |
| Size | |||
| ≤4 | |||
| >4 | 0.000 | Undefined | 0.986 |
| Oncocytic features | |||
| Absent | |||
| Present | 0.000 | 0.000–6.174 | 0.967 |
| PD type | |||
| PTC type | |||
| NOS type | 1.301 | 0.433–3.907 | 0.638 |
| pT stage | |||
| pT1–2 | |||
| pT3–4 | 0.000 | Undefined | 0.986 |
| Extrathyroidal extension | |||
| Absent | |||
| Present | 1.077 | 0.266–4.346 | 0.916 |
| RAS mutation | |||
| Absent | |||
| Present | 3.670 | 1.085–12.412 | 0.036 |
HR, Hazard ratio; CI, confidence intervals.
Discussion
Since the original description of poorly differentiated carcinomas as a separate entity among malignant thyroid tumors, several tumor series have been reported, although based on different and sometimes controversial criteria. The variability in diagnostic criteria has relevance and reproducibility of molecular data generated in the literature to date on this type of aggressive thyroid tumors. In fact, it is more than likely that molecular studies performed over the last 2 decades included tumors that do not meet the current WHO definition of poorly differentiated carcinoma. In this study, we performed molecular analysis of a large, homogeneous series of poorly differentiated thyroid carcinomas selected using the strict Turin criteria (23). Our data indicate that RAS mutations constitute the most common genetic alteration in these tumors. With regard to specific mutation hot spot, NRAS mutations at codon 61 were by far the most common. These findings are in agreement with previous data in the literature on the occurrence of NRAS mutations, although the reported frequency ranged from about 18% to more than 60% (13, 14). By contrast, we could not confirm the high prevalence of KRAS mutations observed by another group (15, 17) because no mutations could be detected in our series by two different methods. Interestingly, in our series RAS mutations did not correlate with specific clinical or pathological parameters but were strongly associated with poor prognosis. This suggests that RAS mutation detection might be clinically relevant for the prognostic stratification of these generally more aggressive thyroid tumors because it allows the separation of those poorly differentiated carcinomas that are particularly prone to more aggressive behavior. From a methodological point of view, both the techniques used in the present paper seem to be adequate for RAS mutational screening in poorly differentiated thyroid carcinomas, although Light Cycler PCR methods showed a slightly higher sensitivity.
Concerning papillary carcinoma-related molecular alterations, such as BRAF mutations and RET/PTC translocations, we observed only a single BRAF mutation of the former in a case associated with a residual component of papillary carcinoma, tall cell variant. These data are in partial contrast with previous studies that found a higher occurrence of both alterations in a subset of cases having residual nuclear and/or architectural features of papillary carcinoma (16–19). Although technical and/or geographical differences might act to explain such discrepancy, it should be critically considered that more restrictive inclusion criteria applied in the present study could have negatively selected papillary carcinoma variants (such as solid), formerly included in the poorly differentiated tumor group.
From an histopathogenetic point of view, the absence of papillary carcinoma-related molecular alterations in our series of poorly differentiated carcinomas does not rule out the hypothesis of their possible dedifferentiation from preexisting papillary carcinoma; rather it possibly suggests that progression from papillary to poorly differentiated carcinoma is restricted to those cases harboring RAS mutation (26) and is different from the molecular pathway leading to the progression of papillary to anaplastic carcinoma, in which BRAF mutations have been found in up to 25% of cases (27, 28). Noteworthy, comparable RAS mutation rates were observed in cases with or without predominant oncocytic features, thus suggesting that phenotypical and molecular signatures leading to oncocytic transformation are independent and, possibly, parallel to the molecular alterations involved in poorly differentiated carcinoma onset. Moreover, we could not detect any PAX8/PPARγ translocation in our series of cases, thus suggesting that follicular carcinomas with PAX8/PPARγ rearrangements typically do not undergo dedifferentiation, as also suggested by the absence of this particular alteration in anaplastic carcinomas (29).
Despite a significant prevalence of RAS mutation, more than 70% of cases in our series had no identifiable molecular alterations, indicating that other genes must be involved. Potential candidates are TP53 and PIK3CA. Regarding TP53, there are several reports (30, 31) on the presence of TP53 mutations in a small fraction of poorly differentiated carcinomas. In the present series, up to 30% of cases showed a positive TP53 protein expression, which is expected to correlate with the presence of gene mutation. However, the positive staining was usually restricted to less than 20% of cells, arguing against the importance of TP53 alteration in pathogenesis of this type of cancer. With respect to PIK3CA, its mutations or genomic amplifications have been found in a subset of well-differentiated carcinomas, particularly in follicular carcinomas (32), and its role in the molecular progression of thyroid cancer is suggested by recent studies in anaplastic carcinoma (27, 33). This may warrant further studies of PIK3CA alterations in poorly differentiated carcinomas.
Additional candidate genes may be identified using wide screening genomic and expression profiling technologies, although the few reported studies that included poorly differentiated carcinomas failed to demonstrate additional specific alterations in these tumors (34, 35).
In conclusion, the present study represents the most comprehensive genetic profiling of poorly differentiated thyroid carcinomas diagnosed according to the WHO classification under strict selection criteria, which demonstrates that, among the most common known genetic alterations in thyroid malignancies, the RAS gene mutations represent the most common genetic event in poorly differentiated carcinomas. Moreover, RAS mutations appear to bear prognostic information, identifying a subset of more aggressive tumors within the group.
For editorial see page 4661
Acknowledgements
This work was supported by the Italian Ministry of University, Rome (ex-60% to M.V. and M.P.).
Disclosure Summary: All Authors declare the absence of any conflict of interest.
Abbreviations
- PD-NOS
Poorly differentiated carcinomas not otherwise specified
- PD-PTC
poorly differentiated carcinomas of papillary type



