-
PDF
- Split View
-
Views
-
Cite
Cite
Greisa Vila, Marily Theodoropoulou, Johanna Stalla, Jörg C. Tonn, Marco Losa, Ulrich Renner, Günter K. Stalla, Marcelo Paez-Pereda, Expression and Function of Sonic Hedgehog Pathway Components in Pituitary Adenomas: Evidence for a Direct Role in Hormone Secretion and Cell Proliferation, The Journal of Clinical Endocrinology & Metabolism, Volume 90, Issue 12, 1 December 2005, Pages 6687–6694, https://doi.org/10.1210/jc.2005-1014
Close - Share Icon Share
Context: Sonic hedgehog (Shh) belongs to a family of signaling proteins involved in development and has been recently implicated in cancer. Shh signaling is active in the corticotrophs of the adult pituitary gland, where it cross-talks with the CRH pathway and regulates ACTH secretion. Because developmental pathways are involved in pituitary tumorigenesis, we hypothesized that Shh may be important in pituitary tumors.
Objective: The objective of this study was to examine the expression and function of Shh-pathway components in pituitary adenomas.
Methods: Using immunohistochemistry, we determined the expression of Shh and its receptors Patched 1 (Ptc1) and Patched 2 (Ptc2) in 55 human pituitary adenomas compared with the normal pituitary gland. The AtT-20 and GH3 pituitary tumor cell lines were used as models for studying the role of Shh on cell proliferation and hormone secretion. The effect of Shh on hormone secretion was confirmed in primary cultures of normal rat pituitaries and human pituitary tumors.
Results: Ptc1 and Ptc2 were present, whereas Shh was down-regulated in pituitary adenomas and completely absent in Cushing tumors. Shh inhibited cell proliferation in AtT-20 corticotrophinoma cells and the Shh-specific inhibitor cyclopamine increased proliferation in GH3 mammosomatotrophinoma cells. On the other hand, exogenous administration of Shh increased hormone secretion from normal rat pituitaries, pituitary cell lines, and 10 different pituitary tumors.
Conclusions: Our results suggest that Shh might maintain pituitary cells in a nonproliferative state. We conclude that Shh is a newly described hypophysiotropic cytokine and its down-regulation may be involved in the pathogenesis of pituitary adenomas.
IT IS KNOWN that pituitary hormone production and proliferation of adenohypophysial cells are governed by numerous factors: hormones, growth factors, and cytokines, some of which are locally produced (1–5). The pathways that mediate these responses are responsible for maintaining the normal homeostasis, but their dysregulation can promote tumor development (1–5). Interestingly, pituitary tumorigenesis has different mechanisms from nonendocrine tumors and is classically promoted by hormones and growth factors that are implicated in pituitary development.
Sonic hedgehog (Shh) is a member of the hedgehog family of signaling proteins that acts through the receptors Patched 1 (Ptc1) 1 and Patched 2 (Ptc2) and is a critical regulator of development (6, 7). The signal is transmitted through the Gli transcription factors, among which Gli2 and Gli3 are the primary mediators (8) and activate Gli1, which in turn is responsible for the activation of the downstream target genes (9). Shh is important in pituitary organogenesis, affecting both proliferation and cell-type determination (10). Although Shh is uniformly expressed throughout the oral ectoderm, its expression is down-regulated in the Rathke pouch, as soon as it becomes morphologically visible. This down-regulation of Shh expression allows the Rathke pouch cells to start proliferation and differentiation (11). Studies of the last decade show that alterations of the Shh pathway result in specific types of cancer (12, 13). Growth inhibition or regression was achieved in some of these cases by using cyclopamine (14), a veratrum alkaloid (15) that was shown to inhibit Shh action (16).
We recently described that Shh, its receptors Ptc1 and Ptc2, as well as the transcription factor Gli1 are expressed in the anterior pituitary gland. Shh is colocalized only in the corticotrophs, where it acts through Ptc2 and Gli1. Shh and Gli1 increase proopiomelanocortin transcription and ACTH production through multiple cross-talk with the CRH pathway (17). Being a signaling protein, Shh is also able to act on neighboring cells (18). Prompted by this and the increasing implication of Shh in many forms of cancer, we investigated the Shh pathway in pituitary tumors. The aim of this study was, therefore, to compare the expression of Shh and its receptors in pituitary adenomas vs. the normal pituitary and to examine the possible effects of Shh on pituitary tumor cell proliferation and hormone secretion.
Materials and Methods
Human tissues
Experiments were performed after informed consent and according to the guidelines of the Ethical Committee of the Max-Planck Institute for using autopsy and biopsy material. Human pituitary tissues were obtained from autopsies performed 8–12 h postmortem in healthy subjects after sudden accidental death. The integrity of proteins up to 12 h postmortem was examined by performing immunohistochemistries for all anterior pituitary hormones. Human pituitary adenomas were obtained after their surgical resection from consecutive unselected patients. Clinical diagnosis was determined on the basis of clinical, biochemical, and radiological findings before surgery, as well as morphological and immunohistochemical data during and after surgery. Tissue fragments from both normal pituitaries and pituitary adenomas were shock frozen on dry ice and stored at −80 C. A total of 56 pituitary adenomas were examined, starting with a hormonal analysis by immunohistochemistry. One ACTH-secreting tumor was contaminated with normal pituitary tissue and thus excluded from further analysis. A list of the 55 pituitary adenomas included in the study and their clinical and immunohistochemical diagnosis are given in Table 1. They were classified into acromegaly associated pituitary tumors (ACRO; seven cases), corticotrophinomas (CUSH; 13 cases), prolactinomas (PROL; three cases), TSH-secreting adenomas (TSH; two cases), and clinically nonfunctioning adenomas (NFPA; 30 cases). The latter were divided after immunopathological examination into gonadotrophinomas (17 cases), null cell adenomas (11 cases), one silent ACTH-secreting tumor, and one silent GH/prolactin secreting tumor. Tumors were graded according to a modified Hardy’s classification (19).
| Patient no. . | Age (yr) . | Sex . | Clinical diagnosis . | IHC . | Grade . | Shh ir (%) . | Ptc1 ir (%) . | Ptc2 ir (%) . |
|---|---|---|---|---|---|---|---|---|
| 1 | 31 | M | ACRO | GH | II | 0 | 1 | n.d. |
| 2 | 28 | F | ACRO | GH | II | 0 | 1 | <1 |
| 3 | 51 | F | ACRO | GH | III | 0 | 1 | <1 |
| 4 | 24 | M | ACRO | GH | II | 2 | 50 | 30 |
| 5 | 67 | M | ACRO | GH | III | 0 | 1 | 0 |
| 6 | 50 | M | ACRO | GH/Prl | II | 0 | 0 | 20 |
| 7 | 69 | M | ACRO/PROL | GH/Prl | III | 0 | 0 | 30 |
| 8 | 33 | M | CUSH | ACTH | II | 0 | 0 | n.d. |
| 9 | 32 | F | CUSH | ACTH | II | 0 | 1 | <1 |
| 10 | 18 | F | CUSH | ACTH | II | 0 | 0 | 20 |
| 11 | 36 | F | CUSH | ACTH | III | 0 | 0 | 0 |
| 12 | 45 | F | CUSH | ACTH | III | 0 | 0 | 30 |
| 13 | 14 | M | CUSH | ACTH | III | 0 | 0 | 5 |
| 14 | 40 | F | CUSH | ACTH | III | 0 | 0 | 1 |
| 15 | 31 | M | CUSH | ACTH | II | 0 | 0 | 70 |
| 16 | 52 | F | CUSH | ACTH | II | 0 | 0 | 0 |
| 17 | 59 | M | CUSH | ACTH | III | 0 | 0 | 70 |
| 18 | 63 | F | CUSH | ACTH | III | 0 | 0 | 1 |
| 19 | 20 | F | CUSH | ACTH | II | 0 | 1 | 1 |
| 20 | 47 | F | CUSH | ACTH | II | 0 | 1 | n.d. |
| 21 | 50 | F | NFPA | ACTH | III | 0 | 0 | 1 |
| 22 | 49 | M | NFPA | FSH | III | 0 | 20 | 2 |
| 23 | 29 | F | NFPA | FSH | III | 0 | 0 | 1 |
| 24 | 64 | M | NFPA | FSH | III | 0 | 0 | <1 |
| 25 | 52 | F | NFPA | FSH | III | 0 | 1 | n.d. |
| 26 | 76 | M | NFPA | FSH | III | 0 | 0 | 1 |
| 27 | 34 | M | NFPA | FSH | II | 0 | 0 | n.d. |
| 28 | 61 | F | NFPA | FSH | III | 0 | 0 | 0 |
| 29 | 35 | M | NFPA | FSH | II | 0 | 0 | <1 |
| 30 | 52 | M | NFPA | FSH | III | 0 | 1 | 70 |
| 31 | 64 | M | NFPA | FSH | III | 0 | 0 | n.d. |
| 32 | 36 | F | NFPA | FSH/LH | II | 0 | 70 | <1 |
| 33 | 42 | M | NFPA | FSH/LH | III | 0 | 0 | n.d. |
| 34 | 55 | M | NFPA | FSH/LH | II | 0 | 0 | 0 |
| 35 | 28 | F | NFPA | GH/Prl | II | 1 | 1 | 5 |
| 36 | 28 | M | NFPA | LH | II | 0 | 1 | 30 |
| 37 | 53 | M | NFPA | LH | III | 0 | 20 | n.d. |
| 38 | 59 | M | NFPA | LH | II | 0 | 10 | n.d. |
| 39 | 60 | M | NFPA | LH | III | 0 | 80 | 1 |
| 40 | 51 | F | NFPA | None | III | 0 | 0 | n.d. |
| 41 | 49 | M | NFPA | None | II | 0 | 5 | <1 |
| 42 | 68 | M | NFPA | None | III | 0 | 40 | 30 |
| 43 | 59 | F | NFPA | None | III | 0 | 20 | 1 |
| 44 | 54 | M | NFPA | None | III | 0 | 30 | 0 |
| 45 | 47 | F | NFPA | None | III | 0 | 40 | 10 |
| 46 | 47 | F | NFPA | None | II | 1 | 20 | n.d. |
| 47 | 57 | M | NFPA | None | II | 0 | 50 | 5 |
| 48 | 61 | M | NFPA | None | III | 0 | 0 | 0 |
| 49 | 52 | F | NFPA | None | II | 0 | 80 | 1 |
| 50 | 52 | F | NFPA | None | II | 0 | 0 | 10 |
| 51 | 18 | F | PROL | Prl | II | 0 | 0 | 10 |
| 52 | 26 | F | PROL | Prl | II | 1 | 0 | 5 |
| 53 | 41 | M | PROL | Prl/GH | II | 0 | 0 | 10 |
| 54 | 56 | M | TSH | TSH/α-subunit | II | 2 | 50 | n.d. |
| 55 | 31 | F | TSH/ACRO | TSH/α-subunit | III | 0 | 0 | 20 |
| Patient no. . | Age (yr) . | Sex . | Clinical diagnosis . | IHC . | Grade . | Shh ir (%) . | Ptc1 ir (%) . | Ptc2 ir (%) . |
|---|---|---|---|---|---|---|---|---|
| 1 | 31 | M | ACRO | GH | II | 0 | 1 | n.d. |
| 2 | 28 | F | ACRO | GH | II | 0 | 1 | <1 |
| 3 | 51 | F | ACRO | GH | III | 0 | 1 | <1 |
| 4 | 24 | M | ACRO | GH | II | 2 | 50 | 30 |
| 5 | 67 | M | ACRO | GH | III | 0 | 1 | 0 |
| 6 | 50 | M | ACRO | GH/Prl | II | 0 | 0 | 20 |
| 7 | 69 | M | ACRO/PROL | GH/Prl | III | 0 | 0 | 30 |
| 8 | 33 | M | CUSH | ACTH | II | 0 | 0 | n.d. |
| 9 | 32 | F | CUSH | ACTH | II | 0 | 1 | <1 |
| 10 | 18 | F | CUSH | ACTH | II | 0 | 0 | 20 |
| 11 | 36 | F | CUSH | ACTH | III | 0 | 0 | 0 |
| 12 | 45 | F | CUSH | ACTH | III | 0 | 0 | 30 |
| 13 | 14 | M | CUSH | ACTH | III | 0 | 0 | 5 |
| 14 | 40 | F | CUSH | ACTH | III | 0 | 0 | 1 |
| 15 | 31 | M | CUSH | ACTH | II | 0 | 0 | 70 |
| 16 | 52 | F | CUSH | ACTH | II | 0 | 0 | 0 |
| 17 | 59 | M | CUSH | ACTH | III | 0 | 0 | 70 |
| 18 | 63 | F | CUSH | ACTH | III | 0 | 0 | 1 |
| 19 | 20 | F | CUSH | ACTH | II | 0 | 1 | 1 |
| 20 | 47 | F | CUSH | ACTH | II | 0 | 1 | n.d. |
| 21 | 50 | F | NFPA | ACTH | III | 0 | 0 | 1 |
| 22 | 49 | M | NFPA | FSH | III | 0 | 20 | 2 |
| 23 | 29 | F | NFPA | FSH | III | 0 | 0 | 1 |
| 24 | 64 | M | NFPA | FSH | III | 0 | 0 | <1 |
| 25 | 52 | F | NFPA | FSH | III | 0 | 1 | n.d. |
| 26 | 76 | M | NFPA | FSH | III | 0 | 0 | 1 |
| 27 | 34 | M | NFPA | FSH | II | 0 | 0 | n.d. |
| 28 | 61 | F | NFPA | FSH | III | 0 | 0 | 0 |
| 29 | 35 | M | NFPA | FSH | II | 0 | 0 | <1 |
| 30 | 52 | M | NFPA | FSH | III | 0 | 1 | 70 |
| 31 | 64 | M | NFPA | FSH | III | 0 | 0 | n.d. |
| 32 | 36 | F | NFPA | FSH/LH | II | 0 | 70 | <1 |
| 33 | 42 | M | NFPA | FSH/LH | III | 0 | 0 | n.d. |
| 34 | 55 | M | NFPA | FSH/LH | II | 0 | 0 | 0 |
| 35 | 28 | F | NFPA | GH/Prl | II | 1 | 1 | 5 |
| 36 | 28 | M | NFPA | LH | II | 0 | 1 | 30 |
| 37 | 53 | M | NFPA | LH | III | 0 | 20 | n.d. |
| 38 | 59 | M | NFPA | LH | II | 0 | 10 | n.d. |
| 39 | 60 | M | NFPA | LH | III | 0 | 80 | 1 |
| 40 | 51 | F | NFPA | None | III | 0 | 0 | n.d. |
| 41 | 49 | M | NFPA | None | II | 0 | 5 | <1 |
| 42 | 68 | M | NFPA | None | III | 0 | 40 | 30 |
| 43 | 59 | F | NFPA | None | III | 0 | 20 | 1 |
| 44 | 54 | M | NFPA | None | III | 0 | 30 | 0 |
| 45 | 47 | F | NFPA | None | III | 0 | 40 | 10 |
| 46 | 47 | F | NFPA | None | II | 1 | 20 | n.d. |
| 47 | 57 | M | NFPA | None | II | 0 | 50 | 5 |
| 48 | 61 | M | NFPA | None | III | 0 | 0 | 0 |
| 49 | 52 | F | NFPA | None | II | 0 | 80 | 1 |
| 50 | 52 | F | NFPA | None | II | 0 | 0 | 10 |
| 51 | 18 | F | PROL | Prl | II | 0 | 0 | 10 |
| 52 | 26 | F | PROL | Prl | II | 1 | 0 | 5 |
| 53 | 41 | M | PROL | Prl/GH | II | 0 | 0 | 10 |
| 54 | 56 | M | TSH | TSH/α-subunit | II | 2 | 50 | n.d. |
| 55 | 31 | F | TSH/ACRO | TSH/α-subunit | III | 0 | 0 | 20 |
The numbers represent the percentage of cells that express the indicated gene. M, Male; F, female; IHC, immunohistochemistry of pituitary hormones; ir, immunoreactivity; NFPA, nonfunctioning pituitary adenoma; PROL, prolactinoma; ACRO, acromegaly; CUSH, Cushing’s disease; Prl, prolactin; n.d., not determined.
| Patient no. . | Age (yr) . | Sex . | Clinical diagnosis . | IHC . | Grade . | Shh ir (%) . | Ptc1 ir (%) . | Ptc2 ir (%) . |
|---|---|---|---|---|---|---|---|---|
| 1 | 31 | M | ACRO | GH | II | 0 | 1 | n.d. |
| 2 | 28 | F | ACRO | GH | II | 0 | 1 | <1 |
| 3 | 51 | F | ACRO | GH | III | 0 | 1 | <1 |
| 4 | 24 | M | ACRO | GH | II | 2 | 50 | 30 |
| 5 | 67 | M | ACRO | GH | III | 0 | 1 | 0 |
| 6 | 50 | M | ACRO | GH/Prl | II | 0 | 0 | 20 |
| 7 | 69 | M | ACRO/PROL | GH/Prl | III | 0 | 0 | 30 |
| 8 | 33 | M | CUSH | ACTH | II | 0 | 0 | n.d. |
| 9 | 32 | F | CUSH | ACTH | II | 0 | 1 | <1 |
| 10 | 18 | F | CUSH | ACTH | II | 0 | 0 | 20 |
| 11 | 36 | F | CUSH | ACTH | III | 0 | 0 | 0 |
| 12 | 45 | F | CUSH | ACTH | III | 0 | 0 | 30 |
| 13 | 14 | M | CUSH | ACTH | III | 0 | 0 | 5 |
| 14 | 40 | F | CUSH | ACTH | III | 0 | 0 | 1 |
| 15 | 31 | M | CUSH | ACTH | II | 0 | 0 | 70 |
| 16 | 52 | F | CUSH | ACTH | II | 0 | 0 | 0 |
| 17 | 59 | M | CUSH | ACTH | III | 0 | 0 | 70 |
| 18 | 63 | F | CUSH | ACTH | III | 0 | 0 | 1 |
| 19 | 20 | F | CUSH | ACTH | II | 0 | 1 | 1 |
| 20 | 47 | F | CUSH | ACTH | II | 0 | 1 | n.d. |
| 21 | 50 | F | NFPA | ACTH | III | 0 | 0 | 1 |
| 22 | 49 | M | NFPA | FSH | III | 0 | 20 | 2 |
| 23 | 29 | F | NFPA | FSH | III | 0 | 0 | 1 |
| 24 | 64 | M | NFPA | FSH | III | 0 | 0 | <1 |
| 25 | 52 | F | NFPA | FSH | III | 0 | 1 | n.d. |
| 26 | 76 | M | NFPA | FSH | III | 0 | 0 | 1 |
| 27 | 34 | M | NFPA | FSH | II | 0 | 0 | n.d. |
| 28 | 61 | F | NFPA | FSH | III | 0 | 0 | 0 |
| 29 | 35 | M | NFPA | FSH | II | 0 | 0 | <1 |
| 30 | 52 | M | NFPA | FSH | III | 0 | 1 | 70 |
| 31 | 64 | M | NFPA | FSH | III | 0 | 0 | n.d. |
| 32 | 36 | F | NFPA | FSH/LH | II | 0 | 70 | <1 |
| 33 | 42 | M | NFPA | FSH/LH | III | 0 | 0 | n.d. |
| 34 | 55 | M | NFPA | FSH/LH | II | 0 | 0 | 0 |
| 35 | 28 | F | NFPA | GH/Prl | II | 1 | 1 | 5 |
| 36 | 28 | M | NFPA | LH | II | 0 | 1 | 30 |
| 37 | 53 | M | NFPA | LH | III | 0 | 20 | n.d. |
| 38 | 59 | M | NFPA | LH | II | 0 | 10 | n.d. |
| 39 | 60 | M | NFPA | LH | III | 0 | 80 | 1 |
| 40 | 51 | F | NFPA | None | III | 0 | 0 | n.d. |
| 41 | 49 | M | NFPA | None | II | 0 | 5 | <1 |
| 42 | 68 | M | NFPA | None | III | 0 | 40 | 30 |
| 43 | 59 | F | NFPA | None | III | 0 | 20 | 1 |
| 44 | 54 | M | NFPA | None | III | 0 | 30 | 0 |
| 45 | 47 | F | NFPA | None | III | 0 | 40 | 10 |
| 46 | 47 | F | NFPA | None | II | 1 | 20 | n.d. |
| 47 | 57 | M | NFPA | None | II | 0 | 50 | 5 |
| 48 | 61 | M | NFPA | None | III | 0 | 0 | 0 |
| 49 | 52 | F | NFPA | None | II | 0 | 80 | 1 |
| 50 | 52 | F | NFPA | None | II | 0 | 0 | 10 |
| 51 | 18 | F | PROL | Prl | II | 0 | 0 | 10 |
| 52 | 26 | F | PROL | Prl | II | 1 | 0 | 5 |
| 53 | 41 | M | PROL | Prl/GH | II | 0 | 0 | 10 |
| 54 | 56 | M | TSH | TSH/α-subunit | II | 2 | 50 | n.d. |
| 55 | 31 | F | TSH/ACRO | TSH/α-subunit | III | 0 | 0 | 20 |
| Patient no. . | Age (yr) . | Sex . | Clinical diagnosis . | IHC . | Grade . | Shh ir (%) . | Ptc1 ir (%) . | Ptc2 ir (%) . |
|---|---|---|---|---|---|---|---|---|
| 1 | 31 | M | ACRO | GH | II | 0 | 1 | n.d. |
| 2 | 28 | F | ACRO | GH | II | 0 | 1 | <1 |
| 3 | 51 | F | ACRO | GH | III | 0 | 1 | <1 |
| 4 | 24 | M | ACRO | GH | II | 2 | 50 | 30 |
| 5 | 67 | M | ACRO | GH | III | 0 | 1 | 0 |
| 6 | 50 | M | ACRO | GH/Prl | II | 0 | 0 | 20 |
| 7 | 69 | M | ACRO/PROL | GH/Prl | III | 0 | 0 | 30 |
| 8 | 33 | M | CUSH | ACTH | II | 0 | 0 | n.d. |
| 9 | 32 | F | CUSH | ACTH | II | 0 | 1 | <1 |
| 10 | 18 | F | CUSH | ACTH | II | 0 | 0 | 20 |
| 11 | 36 | F | CUSH | ACTH | III | 0 | 0 | 0 |
| 12 | 45 | F | CUSH | ACTH | III | 0 | 0 | 30 |
| 13 | 14 | M | CUSH | ACTH | III | 0 | 0 | 5 |
| 14 | 40 | F | CUSH | ACTH | III | 0 | 0 | 1 |
| 15 | 31 | M | CUSH | ACTH | II | 0 | 0 | 70 |
| 16 | 52 | F | CUSH | ACTH | II | 0 | 0 | 0 |
| 17 | 59 | M | CUSH | ACTH | III | 0 | 0 | 70 |
| 18 | 63 | F | CUSH | ACTH | III | 0 | 0 | 1 |
| 19 | 20 | F | CUSH | ACTH | II | 0 | 1 | 1 |
| 20 | 47 | F | CUSH | ACTH | II | 0 | 1 | n.d. |
| 21 | 50 | F | NFPA | ACTH | III | 0 | 0 | 1 |
| 22 | 49 | M | NFPA | FSH | III | 0 | 20 | 2 |
| 23 | 29 | F | NFPA | FSH | III | 0 | 0 | 1 |
| 24 | 64 | M | NFPA | FSH | III | 0 | 0 | <1 |
| 25 | 52 | F | NFPA | FSH | III | 0 | 1 | n.d. |
| 26 | 76 | M | NFPA | FSH | III | 0 | 0 | 1 |
| 27 | 34 | M | NFPA | FSH | II | 0 | 0 | n.d. |
| 28 | 61 | F | NFPA | FSH | III | 0 | 0 | 0 |
| 29 | 35 | M | NFPA | FSH | II | 0 | 0 | <1 |
| 30 | 52 | M | NFPA | FSH | III | 0 | 1 | 70 |
| 31 | 64 | M | NFPA | FSH | III | 0 | 0 | n.d. |
| 32 | 36 | F | NFPA | FSH/LH | II | 0 | 70 | <1 |
| 33 | 42 | M | NFPA | FSH/LH | III | 0 | 0 | n.d. |
| 34 | 55 | M | NFPA | FSH/LH | II | 0 | 0 | 0 |
| 35 | 28 | F | NFPA | GH/Prl | II | 1 | 1 | 5 |
| 36 | 28 | M | NFPA | LH | II | 0 | 1 | 30 |
| 37 | 53 | M | NFPA | LH | III | 0 | 20 | n.d. |
| 38 | 59 | M | NFPA | LH | II | 0 | 10 | n.d. |
| 39 | 60 | M | NFPA | LH | III | 0 | 80 | 1 |
| 40 | 51 | F | NFPA | None | III | 0 | 0 | n.d. |
| 41 | 49 | M | NFPA | None | II | 0 | 5 | <1 |
| 42 | 68 | M | NFPA | None | III | 0 | 40 | 30 |
| 43 | 59 | F | NFPA | None | III | 0 | 20 | 1 |
| 44 | 54 | M | NFPA | None | III | 0 | 30 | 0 |
| 45 | 47 | F | NFPA | None | III | 0 | 40 | 10 |
| 46 | 47 | F | NFPA | None | II | 1 | 20 | n.d. |
| 47 | 57 | M | NFPA | None | II | 0 | 50 | 5 |
| 48 | 61 | M | NFPA | None | III | 0 | 0 | 0 |
| 49 | 52 | F | NFPA | None | II | 0 | 80 | 1 |
| 50 | 52 | F | NFPA | None | II | 0 | 0 | 10 |
| 51 | 18 | F | PROL | Prl | II | 0 | 0 | 10 |
| 52 | 26 | F | PROL | Prl | II | 1 | 0 | 5 |
| 53 | 41 | M | PROL | Prl/GH | II | 0 | 0 | 10 |
| 54 | 56 | M | TSH | TSH/α-subunit | II | 2 | 50 | n.d. |
| 55 | 31 | F | TSH/ACRO | TSH/α-subunit | III | 0 | 0 | 20 |
The numbers represent the percentage of cells that express the indicated gene. M, Male; F, female; IHC, immunohistochemistry of pituitary hormones; ir, immunoreactivity; NFPA, nonfunctioning pituitary adenoma; PROL, prolactinoma; ACRO, acromegaly; CUSH, Cushing’s disease; Prl, prolactin; n.d., not determined.
Immunohistochemistry
Frozen tissues were cut in 8-μm sections, thaw mounted onto SuperFrost Plus slides (Menzel-Glaser, Hamburg, Germany), fixed in cold 4% phosphate-buffered paraformaldehyde (Sigma, St. Louis, MO), and stored in ethanol at 4 C until use. The hormone content was assessed using the following mouse monoclonal antibodies and dilutions: antihuman ACTH, 1:1000 (Dako Diagnostika, Hamburg, Germany); antihuman β-FSH, 1:800; antihuman β-LH, 1:1000; antihuman β-TSH, 1:800; antihuman prolactin 1:400 (Immunotech, Karlsruhe, Germany); and antihuman GH, 1:800 (gift from Dr. C. J. Strasburger, Charite Campus Mitte, Berlin, Germany). The expression of Shh and its receptors was studied using goat polyclonal antibodies: antihuman Shh-N, 1:200; antihuman Ptc1, 1:150; and antihuman Ptc2, 1:100 (all from Santa Cruz Biotechnology, Santa Cruz, CA). All other products were from Vector Laboratories (Burlingame, CA). Secondary antibodies were biotinylated antimouse IgG and biotinylated antirabbit IgG, both 1:300. Immunohistochemistry was made as described in Ref.20 using the avidin-biotin-peroxidase complex (ABC). Detection of protein was visualized using 1 mg/ml diaminobenzidine (DAB) (Sigma) as chromogen and 0.01% hydrogen peroxide as substrate. Slides were counterstained with toluidine blue. Negative controls were performed in all experiments by omitting the primary antibody. The specificities of Shh, Ptc1, and Ptc2 were examined by preincubation with the specific blocking peptides (Santa Cruz Biotechnology) according to the protocol of the manufacturer. The double immunohistochemistry was used for colocalization studies and carried out performing, first, the ABC and DAB for detecting Shh, Gli1, Ptc1, and Ptc2 and then the ABC-activator protein complex and the Vector-Red Alkaline Phosphatase Substrate Kit I (Vector Laboratories, Burlingame, CA) for detecting pituitary hormones. Controls were performed by omitting one of the two or both primary antibodies.
Western blot
GH3 and AtT-20 cells were treated for 24 h with Shh or CRH. Then the cell lysates were collected and analyzed by Western blot as described previously (21). The primary antibodies used were as follows: anti-Shh, made in rabbit (Santa Cruz Biotechnology); anti-Ptc, made in rabbit (Dianova, Hamburg, Germany); and anti-Ptc2, made in rabbit (Dianova). The secondary antibody was antirabbit horseradish peroxidase, made in donkey (Amersham Biosciences, Buckinghamshire, UK).
Cell culture and stimulations
Unless stated otherwise, materials were obtained from Invitrogen (Carlsbad, CA). The murine corticotrophinoma AtT-20 cell line and the rat lactosomatotroph GH3 cell line were obtained from the American Type Culture Collection (Manassas, VA). They were grown in DMEM supplemented with 10% fetal calf serum (FCS), l-glutamine, amphotericin, and penicillin/streptomycin. To test for Shh and cyclopamine responsiveness, cells were put in 96-well plates and serum deprived for 24 h before the stimulations. Shh was purchased from R&D Systems (Minneapolis, MN) and diluted in PBS. Cyclopamine was obtained from Toronto Research Chemicals (Ontario, Canada) and dissolved in DMSO. The respective control was tomatidine, a veratrum alkaloid with no effects on the Shh pathway, which was purchased from Sigma and also dissolved in DMSO. For cell proliferation experiments, the incubation lasted 48 h, and the stimulation medium contained 2% FCS. For hormone-secretion experiments, the incubation was 24 h, and the medium contained no FCS. Stimulations with Shh (up to 5 μg/ml) or cyclopamine (up to 5 μm) produced no changes in cell viability compared with the respective controls, as determined by staining with acridine orange and ethidium bromide (data not shown).
Primary cultures of rat pituitary cells and pituitary adenomas were prepared as previously described (20). Tissues were dispersed mechanically and enzymatically, and the obtained cells were centrifuged and then resuspended in DMEM supplemented with 10% FCS, 2 mm essential vitamins, 5 mg/liter insulin, 20 mg/liter selenium, 5 mg/liter transferrin, 30 pm triiodothyronine (Henning, Berlin, Germany), and 10,000 U/ml penicillin-streptomycin. After 48 h in culture, the cells were stimulated with Shh for 24 h in medium containing 0.5% FCS. The amount of hormone secreted into the supernatant was determined by RIA.
Cell proliferation
Cell proliferation and viability were measured as described (21), using the WST-1 reagent (Roche, Mannheim, Germany) according to the instructions of the manufacturer. The reaction product was measured in an ELISA plate reader at 450 nm and validated by total cell count. Acridine orange-ethidium bromide staining was used to rule out toxic effects.
Hormone determination
Hormones were measured by RIA as previously described (22). For recombinant GH and prolactin, reagents were kindly provided by Dr. A. F. Parlow from the National Hormone and Pituitary Program (Torrance, CA).
Statistics
Cell culture experiments were performed in quadruplicate wells. Results were analyzed by ANOVA in combination with the Scheffé’s test. Data are shown as mean ± sd.
Results
Expression of Shh and its receptors in the normal human pituitary
Immunohistochemistry on four different human pituitaries revealed Shh, Ptc1, and Ptc2 expression in endocrine cells of the anterior lobe. Shh immunoreactivity was confirmed in corticotroph cells (Fig. 1A). Double immunohistochemistry showed Ptc1 expression in gonadotrophs and in thyrotrophs (data not shown). Ptc2 was present in corticotrophs, as previously described (17) (see also Fig. 1B), but also in somatotrophs (Fig. 1C) and in a small percentage of lactotrophs (Fig. 1D). The expression of the Patched gene at detectable levels occurs only after the activation of the Shh signal transduction pathway and Patched expression is, therefore, a marker for Shh activation (23, 24). Therefore, its presence in different types of pituitary cells indicates the existence of active Shh signaling in these cells.
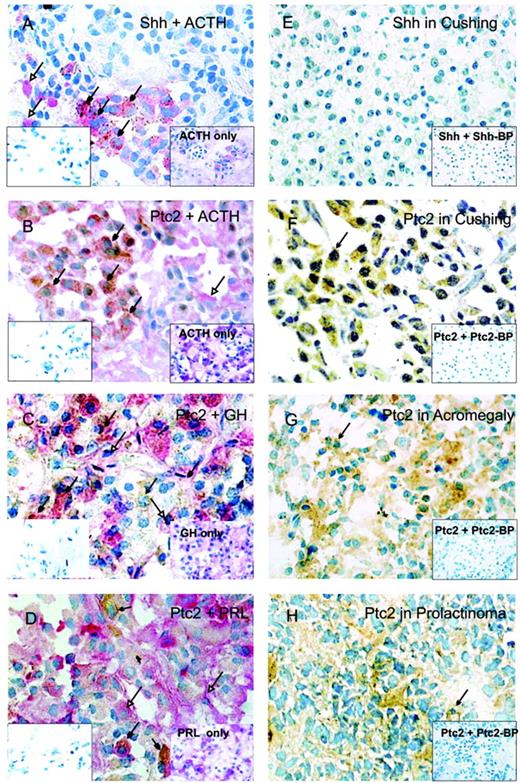
Expression of Shh, Ptc1, and Ptc2 in pituitary adenomas. Colocalization of Shh and ACTH (A), Ptc2 and ACTH (B), Ptc2 and GH (C), and Ptc2 and prolactin (D) in the normal human pituitary gland. In the insets on the left, a parallel section with the negative control is shown. In the insets on the right, a parallel section with the immunohistochemistry of pituitary hormones only is shown. Shh and Ptc2 staining is visualized using DAB (brown color, closed arrowhead). Hormone staining is visualized using Vector Red (red color, open arrowhead). Colocalization of both is indicated by filled arrowheads. Data of A–D are representative of at least three different human adult pituitary samples and at least three independent experiments. E, Shh expression in Cushing’s tumors. Ptc2 expression in Cushing’s tumors (F), sommatotrophinomas (G), and prolactinomas (H). In the insets, a parallel section in the presence of the specific blocking peptide (BP) is shown. Nuclei were counterstained with toluidine blue.
Shh, Ptc1, and Ptc2 expression in pituitary adenomas
Fifty-five pituitary adenomas were analyzed for Shh, Ptc1, and Ptc2 protein expression by immunohistochemistry. Shh immunoreactivity was low or absent in all pituitary adenomas (Table 1). It is interesting that all 13 Cushing tumors included in the study were negative for Shh (Fig. 1E), which contrasts with the high number of Shh immunopositive cells found in the corticotroph population of the normal anterior pituitary (Fig. 1A). Ptc1 (data not shown) and Ptc2 (Fig. 1, F–H) staining was present in variable levels in the tumors studied (Table 1). Therefore, we conclude that Shh expression is reduced, whereas its receptors, Ptc1 and Ptc2, are present in pituitary adenomas.
Shh, Ptc1, and Ptc2 expression in pituitary cell lines
The protein levels of Shh and its receptors, Ptc1 and Ptc2, were studied by Western blot in the mouse corticotrophinoma AtT-20 cell line and in the rat mammosomatotrophinoma GH3 cell line. As shown in Fig. 2A, Shh was present in both cell lines, but higher in GH3 cells. Expression of Ptc1 and Ptc2 protein is also shown in Fig. 2A. The presence of Shh receptors provides the grounds for a functional role of Shh when exogenously administered in cell lines.
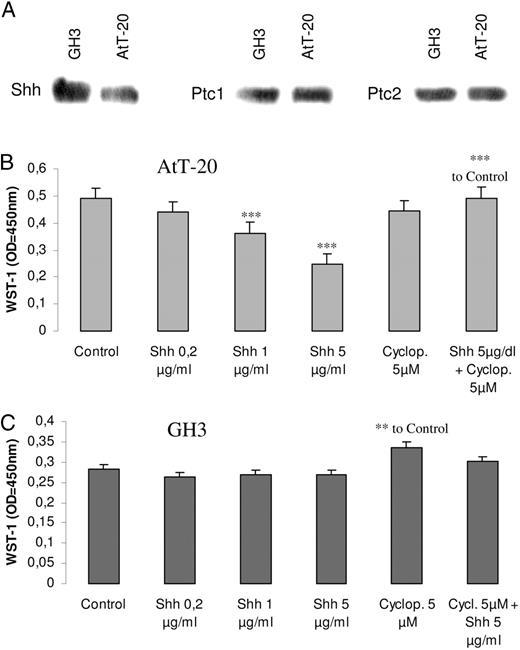
Effects of Shh in pituitary tumor cell proliferation. A, Detection of Shh, Ptc1, and Ptc2 protein in extracts of GH3 and AtT-20 cells. Western blot was performed from three different protein samples, with similar results. AtT-20 cells (B) and GH3 cells (C) were seeded in 96-well plates, serum deprived for 24 h, and then treated for 48 h with Shh 0.2–5 μg/ml, cyclopamine 5 μm, or the combination of both. Proliferation was measured by the WST-1 assay. Results present the mean ± se (n = 4) of one representative of three independent experiments. *, P < 0.01; **, P < 0.005; ***, P < 0.001 with respect to control values.
Shh effects on the proliferation of pituitary cells
Prompted by the differences in Shh expression between normal pituitary and pituitary adenomas, we studied the effect of Shh on pituitary-cell proliferation. AtT-20 and GH3 cells were treated with progressively increasing doses of Shh or its inhibitor, cyclopamine, for 48 h. Shh significantly inhibited AtT-20 cell growth, as determined by the WST-1 assay. Cyclopamine had no effect on its own but abolished the inhibitory effect of Shh (Fig. 2B). In the GH3 cell line, Shh had no effect, whereas cyclopamine caused a small but statistically significant increase in cell proliferation (Fig. 2C). The different effect of cyclopamine in the cell lines could be due to the higher intrinsic expression of Shh in GH3 cells. Therefore, Shh signaling has antiproliferative effects in pituitary tumor cell lines.
Shh effects on hormone secretion by rat normal and tumor pituitary cells
Shh is produced by corticotrophs, where it regulates ACTH secretion (17), but its receptors are also present in other anterior pituitary cells (Fig. 1, B–D). Therefore, we hypothesized that paracrine Shh signaling may also be involved in the regulation of GH and prolactin secretion. To investigate this, we treated rat anterior pituitaries in primary cell culture with Shh or cyclopamine for 24 h. We found that 5 μg/ml Shh increased GH and prolactin secretion almost 3 times (Fig. 3, A–B). The effect of Shh on ACTH release (17) was used as a positive control (basal ACTH values, 633 pg/ml; ACTH after 24-h stimulation with Shh, 1533 pg/ml). Cyclopamine had no significant effect on hormone levels (data not shown). Shh increased GH and prolactin secretion also in the GH3 cell line (Fig. 3, C and D). These results demonstrate that Shh increases GH and prolactin release from normal anterior pituitary cells.
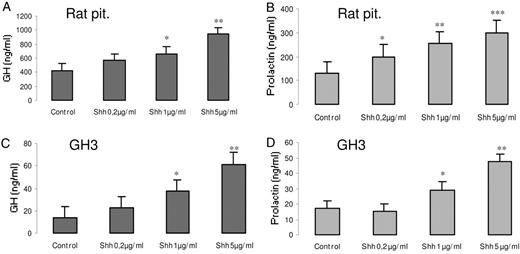
Shh increases GH and prolactin secretion from pituitary normal and tumor cells. Rat pituitary cells in primary culture or the GH3 mamosommatotrophinoma cell line were treated for 24 h with 0.2 μg/ml, 1 μg/ml, or 5 μg/ml Shh, and GH (A and C) and prolactin (B and D) were measured in the supernatants by RIA as described in Materials and Methods. Results present the mean ± se (n = 4) of one representative of three independent experiments. *, P < 0.01; **, P < 0.005; ***, P < 0.001 with respect to control values.
Effects of exogenous administration of Shh on hormone secretion in primary cell cultures of pituitary adenomas
To evaluate whether Shh plays a role in hormone production by pituitary adenomas, we established primary cell cultures from 10 pituitary adenomas (five corticotrophinomas, four acromegaly associated tumors, and one prolactinoma). As shown in Fig. 4, all tumors responded to exogenous Shh by increasing hormone secretion. Therefore, Shh does not only enhance hormone production in the normal pituitary gland but also in human pituitary adenomas in primary culture.
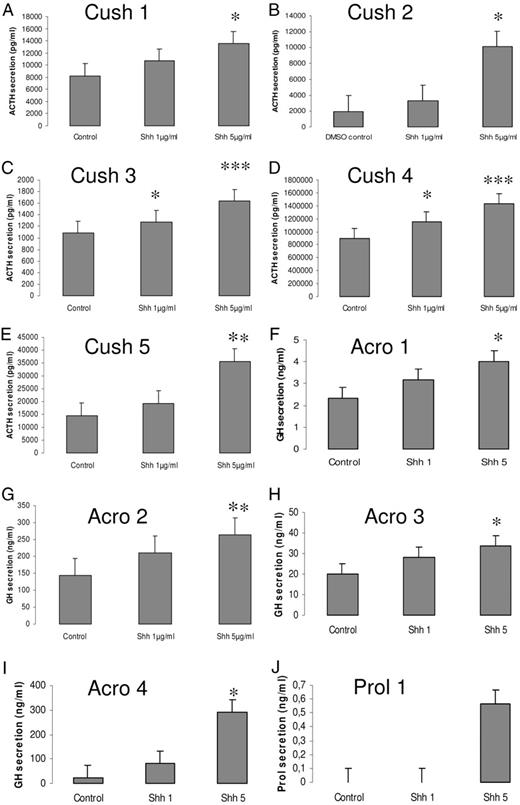
Shh increases hormone secretion from pituitary tumors in primary culture. Human pituitary adenoma cells from five patients with Cushing’s disease, four patients with acromegaly, and one patient with prolactinoma were treated with 1 μg/ml or 5 μg/ml Shh for 24 h; hormone secretion, respectively ACTH (A–E), GH (F–I), and prolactin (J), was measured by RIA in the supernatants. Results present the mean ± se of quadruplicate experiments. *, P < 0.01; **, P < 0.005; ***, P < 0.001 with respect to control values.
Discussion
In the present study, we show that the Shh receptors Ptc1 and Ptc2, are expressed in the human normal anterior pituitary. Ptc1 is present in gonadotrophs and in TSH-producing cells, whereas Ptc2 is expressed not only in corticotrophs but also in somatotrophs and in a small percentage of lactotrophs, providing the molecular basis for active Shh signaling in anterior pituitary endocrine cells. In contrast, the Shh-signaling pathway is down-regulated in all pituitary tumors. Although Shh is produced in normal corticotrophs, it is absent from corticotroph tumors.
A similar expression pattern was observed in pituitary development: Shh is expressed in the early oral ectoderm, but it is excluded from the Rathke pouch as soon as it becomes morphologically visible (10, 11). This down-regulation of Shh allows the proliferation of the anterior pituitary cells. Ptc is expressed throughout the pituitary gland, indicating that the nascent pituitary cells may respond to Shh signaling. We observe the same pattern in pituitary tumors: Shh is absent or down-regulated in tumor endocrine cells, but Ptc is expressed, indicating the existence of an activate Shh pathway. The expression of the receptors provides the molecular basis for the antiproliferative effects of Shh.
In vitro experiments in the AtT-20 and GH3 pituitary tumor cell lines further support an antiproliferative effect of the Shh pathway. Both of these cell lines express Ptc1 and Ptc2 protein. Shh remarkably reduced the proliferation of corticotrophs, whereas its specific antagonist, cyclopamine, slightly but significantly increased the proliferation of lactosomatotrophs. This may be explained by the fact that GH3 cells express higher levels of endogenous Shh compared with AtT-20 cells. These in vitro effects on pituitary-cell proliferation correspond with the expression pattern in pituitary adenomas and suggest that Shh might play a role in pituitary tumorigenesis. Shh expression could hypothetically maintain anterior pituitary cells in a nonproliferative state, and cell proliferation and tumor formation may result from a down-regulation of the Shh signaling. Recent studies show an activation of the Shh pathway in different malignant cancers (25–28). Abnormal expression of Shh has also been found in pancreatic adenocarcinoma deriving from nonendocrine cells (29), but there are no data about the involvement of the Shh pathway in endocrine cancers. This work is, to our knowledge, the first study of the Shh pathway in endocrine tumors and supports the concept that the pathogenesis of pituitary tumors is not controlled by mechanisms involved in highly proliferating and metastasizing cancers.
Shh administration increased ACTH, GH, and prolactin secretion from normal and tumor anterior pituitary cells. The effect on ACTH secretion is achieved through a multiple cross-talk mechanism with the CRH pathway (17). Shh is expressed in corticotrophs but, being a signaling protein, is also able to act in the surrounding cells. The presence of Ptc receptors in pituitary cells other than corticotrophs shows that these cells also receive Shh signaling. Therefore, Shh is a hypophysiotropic cytokine, secreted in the anterior pituitary, which acts through autocrine/paracrine mechanisms to control pituitary hormone release.
In this study, we show that Shh reduces pituitary-cell proliferation and increases hormone production. Also, other cytokines were shown to have opposite effects on hormone release and cell proliferation (5, 30). Our results are another example demonstrating that pituitary adenoma hormone secretion and cell proliferation are not controlled by the same mechanisms.
In summary, here we identify Shh as a locally produced cytokine that acts as a hypophysiotropic factor in regulating pituitary hormone release in normal and tumor cells. Shh signaling has antiproliferative effects in pituitary tumor cell lines and is down-regulated in pituitary adenomas, and exogenous Shh treatment increases hormone secretion from pituitary tumor cells in primary culture. We put forward the hypothesis that the Shh pathway plays a role in pituitary tumorigenesis and could provide a basis for therapeutic interventions.
Acknowledgements
We thank Dr. A. F. Parlow from the National Hormone and Pituitary Program (Baltimore, MD) for kindly providing the reagent kits for GH and prolactin.
First Published Online September 13, 2005
Abbreviations:
- ABC,
Avidin-biotin-peroxidase complex;
- DAB,
diaminobenzidine;
- FCS,
fetal calf serum;
- Ptc,
Patched;
- Shh,
sonic hedgehog.
References
van den



