-
PDF
- Split View
-
Views
-
Cite
Cite
Mangu Srinivas Bharadwaj, Sanjana Ballal, Chandrasekhar Bal, Optimal Cumulative I-131 Activity in Metastatic Differentiated Thyroid Cancer: Balancing Efficacy and Adverse Events, The Journal of Clinical Endocrinology & Metabolism, Volume 109, Issue 11, November 2024, Pages e2120–e2130, https://doi.org/10.1210/clinem/dgae024
Close - Share Icon Share
Abstract
The optimal cumulative activity (CA) of I-131 therapy for patients with metastatic differentiated thyroid cancer (mDTC) remains contentious. This study aimed to determine the maximum CA of I-131 that could be administered without a significant increase in adverse events (AEs) by analyzing a long-term cohort of patients.
Data from patients with mDTC treated with I-131 therapy and followed for at least 2 years from 1967 to 2019 were reviewed. Patients were categorized into 3 groups based on the received CA: group A (≤600 mCi), group B (>600-1000 mCi), and group C (>1000 mCi). The study assessed long-term AEs and survival outcomes.
The study included 671 adult patients with mDTC (mean age, 48 years; range, 19-81) with a median follow-up of 122 months (interquartile range: 82-180). Group A, group B, and group C comprised 269 (40.0%), 212 (31.6%), and 190 (28.4%) patients, respectively. Ten-year survival rates were 72%, 42.7%, and 29% in groups A, B, and C, respectively. A total of 40/671 (6%) AEs were observed in 38 patients: 3 (1.1%), 12 (5.7%), and 25 (13.2%) in groups A, B, and C, respectively. Five patients developed second primary malignancy: 3 in group A and 1 each in group B and C. However, CA >1000 mCi of I-131 was associated with significant increase in bone marrow suppression, decreased pulmonary function, and xerostomia (P < .001).
The study suggests that a maximum CA of up to 1000 mCi strikes a favorable balance between keeping AEs low and benefiting a subset of patients with extensive metastases showing intense I-131 concentration.
The incidence of distant metastasis in differentiated thyroid cancer (DTC) is influenced by economic development and health care accessibility (1). Distant metastasis occurs in 2.3% to 33% of cases (2-6) and is a leading cause of death in DTC (7). Common sites for metastases are the lungs and bones (8). Survival in metastatic DTC (mDTC) varies based on factors such as age, aggressive histological features, radioiodine refractoriness, and the extent of metastases (9-12). Radioactive iodine therapy (I-131) is the primary treatment after thyroidectomy (13-15). However, when metastatic lesions do not take up I-131, managing mDTC becomes challenging. Chemotherapy has shown limited success, and external beam radiotherapy (EBRT) is often used for palliative care (16, 17). In recent years, tyrosine kinase inhibitors (TKIs) have been used for patients with radioiodine-refractory DTC (RR-DTC), with partial responses in 18% to 65% of cases but frequent adverse events (AEs) or severe adverse events (SAEs) leading to dose adjustments or discontinuation of TKIs (18-20).
The American Thyroid Association (ATA) 2015 guidelines introduced the concept of RR-DTC and set a cumulative activity (CA) limit of 600 mCi for defining RR-DTC (21). However, this limit has generated significant debate, especially when metastatic lesions show strong I-131 uptake (22). In real-world scenarios, repeated I-131 treatment cycles are administered until metastatic lesions disappear or patients can no longer tolerate I-131, even if radioiodine CA exceeds 600 mCi. Despite the high CA administered, complete remissions are not achieved in a significant proportion of cases. The higher CA of I-131 therapy for mDTC might lead to better outcomes in some but also increase treatment-emergent AEs. Thus, beyond 600 mCi I-131-administered CA has pros and cons. Moreover, the literature lacks reliable evidence-based data regarding the appropriate CA, associated AEs, and patient clinical outcomes.
In a critical review article, the task force chair, Bryan Haugen, noted that most ATA 2015 Guidelines for managing advanced metastatic disease and RR-DTC recommendations are based on weak evidence (23). This underscores the need for further research in this specific area. This study’s objective was to determine the optimal CA that can be administered without increasing AEs while improving survival outcomes. The analysis is based on a long-term follow-up of a patient cohort from a tertiary care academic institution.
Material and Methods
Study Design
This retrospective cohort study was conducted at the Thyroid Clinic of the All India Institute of Medical Sciences in New Delhi. Approval from the Institute’s ethics committee was obtained (Ref. No. IECPG597/24.10.2019, RT-07/28.-11.2019), and patient records from 1967 to 2019 were reviewed. Data were extracted from patients with a minimum follow-up of 2 years. Patients with de novo RR-DTC were excluded (RR-DTC is defined per the ATA 2015 guidelines (21)). The study included patients who showed tracer uptake on diagnostic I-131 whole-body scans (DxWBS) and confirmed uptake on the posttherapy whole-body scan, with a maximum interval of 3 days between DxWBS and I-131 treatment. TNM staging was done according to the AJCC 8th edition (24), and the primary broad histological groups were papillary, follicular, and Hurtle cell DTC.
Treatment Protocol
The primary treatment consisted of total thyroidectomy ± central and lateral neck dissection, based on preoperative/on-table evaluation, followed by I-131 therapy. Some patients with less than total thyroidectomy also received I-131 therapy. After ruling out stable iodine contamination, patients were scheduled for diagnostic and high-activity I-131 therapy. Thyroid hormone withdrawal was used for preparation, with documented TSH levels >30 µIU/mL for DxWBS and therapy, typically taking 3 to 4 weeks after total thyroidectomy. Empirical I-131 therapy doses were administered, with 100 to 150 mCi for lung metastases and 150 to 250 mCi for bone metastases. The extent of disease was determined based on DxWBS and posttherapy whole-body scan and confirmed by conventional radiologic imaging. Routine baseline blood tests were conducted, including serum thyroglobulin (Tg) and anti-thyroglobulin antibody (ATG), available from 1990 on. Patients received additional cycles of I-131 therapy with a minimum time gap of 6 to 12 months.
Follow-up
Patients achieving complete remission (excellent response) were followed up at 6-month intervals for the first 5 years and annually after that. Levothyroxine dosage was adjusted to maintain serum TSH values ≤0.1 µIU/mL. Major AEs, likely second primary malignancies (SPMs), bone marrow suppression, pulmonary toxicity, salivary toxicity, and gonadal toxicity, which were recorded in the patient's charts, were assessed. AEs were classified according to CTCAE v.5.0 (25). SPMs were confirmed with histopathology reports. Complete blood counts and pulmonary function tests were routinely performed before each I-131 therapy cycle if the patient exceeded a CA of 600 mCi. Salivary gland toxicity was assessed based on symptoms of xerostomia. Patients with recurrences during follow-up were rechallenged with additional I-131 therapy if they showed I-131 avidity. For patients with RR-DTC, redifferentiating agents were used in earlier decades, and more recently, TKIs such as sorafenib or lenvatinib were used, either singly or in combination, if a patient developed severe toxicity to one.
Study Grouping
All patients with mDTC were categorized based on CA:
Group A: Up to 600 mCi (control group as recommended by ATA 2015)
Group B: 601-1000 mCi
Group C: >1000 mCi
Definitions of Bone and Lung Metastases
Bone metastases were classified into solitary and multiple lesions (≥2). Lung metastases were similarly classified into diffuse pulmonary metastasis (X-ray-negative, computed tomography [CT]-negative, and WBS-positive metastases), micronodular metastases (X-ray negative but CT positive, <1 cm in diameter), and macronodular metastases (both X-ray and CT positive).
Criteria for Response
The response was evaluated every 6 months until WBS was negative, followed by Tg and ATG values. The best response category for each patient group included:
Complete response (CR) or excellent response: No clinical or radiological evidence of disease, negative I-131 WBS, stimulated Tg ≤ 10, unstimulated Tg ≤ 1 ng/mL, and ATG negative.
Partial response or structural incomplete response: Clinical and radiological improvement, a reduction in the number of lesions on I-131 WBS, and a serial decrease in Tg not equal to CR.
Biochemical incomplete response: Persistently high Tg/ATG without structural correlation.
Progressive disease: Detection of new metastatic foci on I-131 WBS or any cross-sectional imaging with/without increasing consecutive Tg and/or ATG values.
Statistical Analysis
Categorical variables were expressed as numbers and percentages. Continuous variables were presented as median values with interquartile ranges (IQR). Nonparametric comparisons were assessed using the χ2 test and Kruskal-Wallis test. Overall survival (OS) was estimated as the time from diagnosis to death from any cause. Progression-free survival was defined as the time from treatment initiation to progressive disease or death. Disease-free survival was defined as the amount of time following successful treatment that no evidence of structural disease (recurrent or persistent disease) was found. In the case of persistent disease, the disease-free survival was considered zero. The study used Kaplan-Meier analysis with the log-rank test and univariate and multivariate Cox proportional hazards regression models to examine the impact of various risk factors on survival (26). Receiver operator curve with the area under the curve analysis was performed to find the optimal CA without comprising safety and minimizing AEs. Statistical analysis was conducted using SPSS v.21.0 (IBM Corp., Armonk, NY, USA) and Medcalc (MedCalc Software Ltd, Ostend, Belgium).
Results
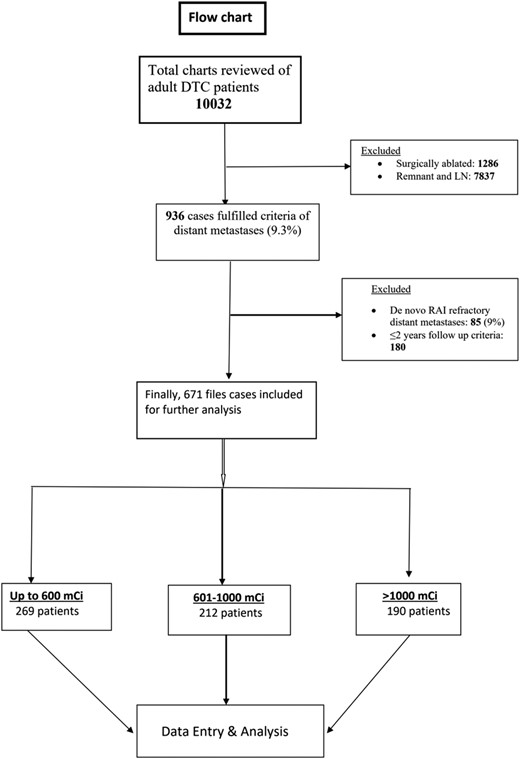
A total of 671 adult patients with mDTC who were I-131 avid and met the inclusion criteria were included in the study (Fig. 1). The baseline characteristics of the entire cohort and 3 subgroups based on CA are summarized in Tables 1 and 2, respectively. The median CA of I-131 administered was 795 mCi (IQR: 450-1095). The median follow-up duration was 122 months (IQR: 82-180), ranging from 2 to 53 years. Notably, follicular carcinoma was the most prevalent histological type in this patient series (49.6%), followed by papillary carcinoma (46.2%). In contrast to a previous publication by Durante et al (5), skeletal metastases were predominant in 43.8% of patients, followed by pulmonary metastases in 30.1%. EBRT was required for 25.2% of patients, and 10% received TKIs. At the time of analysis, 418 (62.3%) patients were alive and 253 (37.7%) had passed away. Among the 418 living patients, 169 (25.2%) achieved complete remission (CR/ER), 228 (34%) had partial remission (structural incomplete response), and 21 (3.1%) had progressive disease. Interestingly, 26 patients (15.4%) experienced recurrences despite initially achieving CR. Multivariate analysis identified several independent poor prognostic factors, including age at presentation ≥55 years, skeletal and multiorgan metastases, and persistent I-131 WBS positivity (Table 3). Gender, histopathology, type of primary surgery, EBRT, and I-131 CA did not independently affect prognosis.
| Characteristic . | Value . |
|---|---|
| Mean age (y) | 48 (range, 19-81) < 55 years: 410 (61%) ≥ 55 years: 261 (39%) |
| Gender (%) (M:F ratio) | Male: 193 (28.8) (1:2.5) Female: 478 (71.2) |
| Initial presentation (%) | STN/MNG ± neck node: 343 (51.1) Metastases: 316 (47.1) (bone 311 and others 5) Locoregional recurrence: 12 (1.8) |
| Primary surgical treatment (%) | Total thyroidectomy: 436 (65.0) Less than total: 235 (35.0) |
| Nodal dissection (%) | No: 454 (67.7) Yes: 217 (32.3) |
| Median tumor size (cm) | 4.3 (IQR: 2.7-5.2) |
| Histopathology (%) | PTC: 310 (46.2) FTC: 333 (49.6) HTC: 28 (4.2) |
| T staging (%) | TX: 34 (5.1)/T1-T2: 333 (49.6) T3-T4: 304(45.3) |
| N staging (%) | NX/N0: 21 (3.1)/426 (63.5) N1: 224 (33.4) |
| Stage (%) | Stage II: 410 (61) Stage IVb: 261 (39) |
| Bone metastases (%) Bone metastases only: 294 (43.8%) | 462 (68.8) Single: 70 (10.4) Multiple (≥2): 392 (58.4) |
| Lung metastases (%) Lung metastases only—202 (30.1%) | 367 (54.7) X-ray (−), CT (−) and WBS +: 40 Micronodular: 187; macronodular: 140 |
| Bone and lungs with other rare sites of distant metastases—175 (26.1%) | Only bone and lung: 140 Brain: 17; liver: 15; renal: 4 Subcutaneous tissue: 1; muscle: 1 |
| Median follow-up in months (IQR) | 122 (82-180) |
| Median cumulative activity (mCi) (IQR), range | 795 (450-1095) 50 −2100 |
| EBRT (%) | 169 (25.2) |
| Tyrosine kinase inhibitors (%) | 67 (10) (sorafenib: 32; lenvatinib: 22; sorafenib f/b lenvatinib: 13) |
| Adverse events (%) | 40 (6) (n = 38; 2 patients have dual adverse events) |
| Patients alive with disease status (n = 418; 62.3%) | Complete response: 169 (25.2%) Partial response: 228 (34%) Progressive disease: 21 (3.1%) |
| Total deaths at the time of reporting | 253 (37.7%) |
| Characteristic . | Value . |
|---|---|
| Mean age (y) | 48 (range, 19-81) < 55 years: 410 (61%) ≥ 55 years: 261 (39%) |
| Gender (%) (M:F ratio) | Male: 193 (28.8) (1:2.5) Female: 478 (71.2) |
| Initial presentation (%) | STN/MNG ± neck node: 343 (51.1) Metastases: 316 (47.1) (bone 311 and others 5) Locoregional recurrence: 12 (1.8) |
| Primary surgical treatment (%) | Total thyroidectomy: 436 (65.0) Less than total: 235 (35.0) |
| Nodal dissection (%) | No: 454 (67.7) Yes: 217 (32.3) |
| Median tumor size (cm) | 4.3 (IQR: 2.7-5.2) |
| Histopathology (%) | PTC: 310 (46.2) FTC: 333 (49.6) HTC: 28 (4.2) |
| T staging (%) | TX: 34 (5.1)/T1-T2: 333 (49.6) T3-T4: 304(45.3) |
| N staging (%) | NX/N0: 21 (3.1)/426 (63.5) N1: 224 (33.4) |
| Stage (%) | Stage II: 410 (61) Stage IVb: 261 (39) |
| Bone metastases (%) Bone metastases only: 294 (43.8%) | 462 (68.8) Single: 70 (10.4) Multiple (≥2): 392 (58.4) |
| Lung metastases (%) Lung metastases only—202 (30.1%) | 367 (54.7) X-ray (−), CT (−) and WBS +: 40 Micronodular: 187; macronodular: 140 |
| Bone and lungs with other rare sites of distant metastases—175 (26.1%) | Only bone and lung: 140 Brain: 17; liver: 15; renal: 4 Subcutaneous tissue: 1; muscle: 1 |
| Median follow-up in months (IQR) | 122 (82-180) |
| Median cumulative activity (mCi) (IQR), range | 795 (450-1095) 50 −2100 |
| EBRT (%) | 169 (25.2) |
| Tyrosine kinase inhibitors (%) | 67 (10) (sorafenib: 32; lenvatinib: 22; sorafenib f/b lenvatinib: 13) |
| Adverse events (%) | 40 (6) (n = 38; 2 patients have dual adverse events) |
| Patients alive with disease status (n = 418; 62.3%) | Complete response: 169 (25.2%) Partial response: 228 (34%) Progressive disease: 21 (3.1%) |
| Total deaths at the time of reporting | 253 (37.7%) |
Abbreviations: EBRT, external beam radiotherapy; FTC, follicular thyroid cancer; HTC, Hurtle cell thyroid carcinoma; IQR, interquartile range; MNG, multinodular goiter; PTC, papillary thyroid cancer; STN, solitary thyroid nodule; WBS, whole body iodine scan.
| Characteristic . | Value . |
|---|---|
| Mean age (y) | 48 (range, 19-81) < 55 years: 410 (61%) ≥ 55 years: 261 (39%) |
| Gender (%) (M:F ratio) | Male: 193 (28.8) (1:2.5) Female: 478 (71.2) |
| Initial presentation (%) | STN/MNG ± neck node: 343 (51.1) Metastases: 316 (47.1) (bone 311 and others 5) Locoregional recurrence: 12 (1.8) |
| Primary surgical treatment (%) | Total thyroidectomy: 436 (65.0) Less than total: 235 (35.0) |
| Nodal dissection (%) | No: 454 (67.7) Yes: 217 (32.3) |
| Median tumor size (cm) | 4.3 (IQR: 2.7-5.2) |
| Histopathology (%) | PTC: 310 (46.2) FTC: 333 (49.6) HTC: 28 (4.2) |
| T staging (%) | TX: 34 (5.1)/T1-T2: 333 (49.6) T3-T4: 304(45.3) |
| N staging (%) | NX/N0: 21 (3.1)/426 (63.5) N1: 224 (33.4) |
| Stage (%) | Stage II: 410 (61) Stage IVb: 261 (39) |
| Bone metastases (%) Bone metastases only: 294 (43.8%) | 462 (68.8) Single: 70 (10.4) Multiple (≥2): 392 (58.4) |
| Lung metastases (%) Lung metastases only—202 (30.1%) | 367 (54.7) X-ray (−), CT (−) and WBS +: 40 Micronodular: 187; macronodular: 140 |
| Bone and lungs with other rare sites of distant metastases—175 (26.1%) | Only bone and lung: 140 Brain: 17; liver: 15; renal: 4 Subcutaneous tissue: 1; muscle: 1 |
| Median follow-up in months (IQR) | 122 (82-180) |
| Median cumulative activity (mCi) (IQR), range | 795 (450-1095) 50 −2100 |
| EBRT (%) | 169 (25.2) |
| Tyrosine kinase inhibitors (%) | 67 (10) (sorafenib: 32; lenvatinib: 22; sorafenib f/b lenvatinib: 13) |
| Adverse events (%) | 40 (6) (n = 38; 2 patients have dual adverse events) |
| Patients alive with disease status (n = 418; 62.3%) | Complete response: 169 (25.2%) Partial response: 228 (34%) Progressive disease: 21 (3.1%) |
| Total deaths at the time of reporting | 253 (37.7%) |
| Characteristic . | Value . |
|---|---|
| Mean age (y) | 48 (range, 19-81) < 55 years: 410 (61%) ≥ 55 years: 261 (39%) |
| Gender (%) (M:F ratio) | Male: 193 (28.8) (1:2.5) Female: 478 (71.2) |
| Initial presentation (%) | STN/MNG ± neck node: 343 (51.1) Metastases: 316 (47.1) (bone 311 and others 5) Locoregional recurrence: 12 (1.8) |
| Primary surgical treatment (%) | Total thyroidectomy: 436 (65.0) Less than total: 235 (35.0) |
| Nodal dissection (%) | No: 454 (67.7) Yes: 217 (32.3) |
| Median tumor size (cm) | 4.3 (IQR: 2.7-5.2) |
| Histopathology (%) | PTC: 310 (46.2) FTC: 333 (49.6) HTC: 28 (4.2) |
| T staging (%) | TX: 34 (5.1)/T1-T2: 333 (49.6) T3-T4: 304(45.3) |
| N staging (%) | NX/N0: 21 (3.1)/426 (63.5) N1: 224 (33.4) |
| Stage (%) | Stage II: 410 (61) Stage IVb: 261 (39) |
| Bone metastases (%) Bone metastases only: 294 (43.8%) | 462 (68.8) Single: 70 (10.4) Multiple (≥2): 392 (58.4) |
| Lung metastases (%) Lung metastases only—202 (30.1%) | 367 (54.7) X-ray (−), CT (−) and WBS +: 40 Micronodular: 187; macronodular: 140 |
| Bone and lungs with other rare sites of distant metastases—175 (26.1%) | Only bone and lung: 140 Brain: 17; liver: 15; renal: 4 Subcutaneous tissue: 1; muscle: 1 |
| Median follow-up in months (IQR) | 122 (82-180) |
| Median cumulative activity (mCi) (IQR), range | 795 (450-1095) 50 −2100 |
| EBRT (%) | 169 (25.2) |
| Tyrosine kinase inhibitors (%) | 67 (10) (sorafenib: 32; lenvatinib: 22; sorafenib f/b lenvatinib: 13) |
| Adverse events (%) | 40 (6) (n = 38; 2 patients have dual adverse events) |
| Patients alive with disease status (n = 418; 62.3%) | Complete response: 169 (25.2%) Partial response: 228 (34%) Progressive disease: 21 (3.1%) |
| Total deaths at the time of reporting | 253 (37.7%) |
Abbreviations: EBRT, external beam radiotherapy; FTC, follicular thyroid cancer; HTC, Hurtle cell thyroid carcinoma; IQR, interquartile range; MNG, multinodular goiter; PTC, papillary thyroid cancer; STN, solitary thyroid nodule; WBS, whole body iodine scan.
| Characteristic . | Group A (n = 269) . | Group B (n = 212) . | Group C (n = 190) . | P . |
|---|---|---|---|---|
| Age (y), median (IQR) | 45 (30-59) | 51.5 (40.5-60) | 50 (41-58) | <.0001 |
| <55 y (%) | 181 (67.3) | 117 (55.2) | 112 (58.9) | .02 |
| ≥55 y (%) | 88 (32.7) | 95 (45.8) | 78 (41.1) | |
| Sex (%) | Male: 88 (32.7) | Male: 50 (23.6) | Male: 55 (28.9) | .90 |
| Female: 181 (67.3) | Female: 162 (73.4) | Female: 135 (71.1) | ||
| Preoperative metabolic equivalent of task (%) | 81 (30.1) | 109 (44.3) | 126 (50.0) | <.0001 |
| Tumor size (cm) Median (IQR) | 4 (2.5-5.0) | 3.8 (2.5-5.0) | 4.0 (3.0-6.0) | .043 |
| Histopathology (%) | FTC: 99 (36.8) | FTC: 111 (52.4) | FTC: 123 (64.7) | <.0001 |
| PTC: 157 (58.4) | PTC:94 (44.3) | PTC: 59 (31.1) | ||
| HTC: 13 (4.8) | HTC: 38 (17) | HTC: 8 (4.2) | ||
| Primary surgery (%) | ||||
| Total thyroidectomy | 171 (63.6) | 136 (64.2) | 129 (67.9) | .622 |
| Less than total | 98 (36.4) | 76 (35.8) | 61(32.1) | |
| T staging (%) | .016 | |||
| TX/T1/T2 | 138 (51.3) | 131 (61.7) | 98 (51.5) | |
| T3/T4 | 131 (48.7) | 81 (38.3) | 92 (48.5) | |
| N staging (%) | <.0001 | |||
| NX/N0 | 148 (55.0) | 149 (70.3) | 150 (79.0) | |
| N1 | 121 (45.0) | 63 (29.7) | 40 (21.0) | |
| Lung metastases (%) | 190 (70.6) | 95 (44.8) | 82 (43.2) | <.0001 |
| Diffuse pulmonary | 31 (11.5) | 6 (2.8) | 3 (1.6) | |
| Micronodular | 105 (39.0) | 49 (23.1) | 33 (17.4) | |
| Macronodular | 54 (20.1) | 40 (18.9) | 46 (24.2) | |
| Bone metastases (%) | 137 (50.9) | 159 (75.0) | 166 (87.4) | <.0001 |
| Single | 27 (10.0) | 23 (10.8) | 20 (10.5) | |
| Multiple(≥2) | 110 (40.9) | 136 (64.2) | 146 (76.9) | |
| Bone and lungs with other rare sites of distant metastases (%) | 61 (22.6) | 51 (24.0) | 63 (33.1) | .025 |
| Median cumulative activity (mCi) IQR | 405 (300-550) | 800 (750-950) | 1350(1220-1551) | <.0001 |
| Median follow-up period (mo) IQR | 57 (24.0-113.0) | 70.0 (41.0-130.0) | 122 (82.0-180.0) | <.0001 |
| Last 131I-WBS (%) | <.0001 | |||
| Negative/positive | 132 (49.1)/137 (50.9) | 55 (25.9)/157 (74.1) | 23 (12.1)/167 (87.9) | |
| EBRT (%) | 33 (12.3) | 49 (23.1) | 87 (45.8) | <.0001 |
| TKI (%) | 15 (5.6) | 10 (4.7) | 42 (21.6) | <.0001 |
| Adverse events (%) | 3 (1.1) | 12 (5.7) | 25 (12.1) | <.0001 |
| Patients alive with disease status (%) | CR: 107 (47.8) | CR: 41 (32.5) | CR: 21 (30.9) | <.0001 |
| PR: 106 (47.3) | PR: 79(62.7) | PR: 43 (63.2) | ||
| PD: 11 (4.9) | PD: 6 (4.8) | PD: 4 (5.9) | ||
| Total deaths at the time of reporting (%) | 45 (16.7) | 86 (40.6) | 122 (64.2) | <.0001 |
| Characteristic . | Group A (n = 269) . | Group B (n = 212) . | Group C (n = 190) . | P . |
|---|---|---|---|---|
| Age (y), median (IQR) | 45 (30-59) | 51.5 (40.5-60) | 50 (41-58) | <.0001 |
| <55 y (%) | 181 (67.3) | 117 (55.2) | 112 (58.9) | .02 |
| ≥55 y (%) | 88 (32.7) | 95 (45.8) | 78 (41.1) | |
| Sex (%) | Male: 88 (32.7) | Male: 50 (23.6) | Male: 55 (28.9) | .90 |
| Female: 181 (67.3) | Female: 162 (73.4) | Female: 135 (71.1) | ||
| Preoperative metabolic equivalent of task (%) | 81 (30.1) | 109 (44.3) | 126 (50.0) | <.0001 |
| Tumor size (cm) Median (IQR) | 4 (2.5-5.0) | 3.8 (2.5-5.0) | 4.0 (3.0-6.0) | .043 |
| Histopathology (%) | FTC: 99 (36.8) | FTC: 111 (52.4) | FTC: 123 (64.7) | <.0001 |
| PTC: 157 (58.4) | PTC:94 (44.3) | PTC: 59 (31.1) | ||
| HTC: 13 (4.8) | HTC: 38 (17) | HTC: 8 (4.2) | ||
| Primary surgery (%) | ||||
| Total thyroidectomy | 171 (63.6) | 136 (64.2) | 129 (67.9) | .622 |
| Less than total | 98 (36.4) | 76 (35.8) | 61(32.1) | |
| T staging (%) | .016 | |||
| TX/T1/T2 | 138 (51.3) | 131 (61.7) | 98 (51.5) | |
| T3/T4 | 131 (48.7) | 81 (38.3) | 92 (48.5) | |
| N staging (%) | <.0001 | |||
| NX/N0 | 148 (55.0) | 149 (70.3) | 150 (79.0) | |
| N1 | 121 (45.0) | 63 (29.7) | 40 (21.0) | |
| Lung metastases (%) | 190 (70.6) | 95 (44.8) | 82 (43.2) | <.0001 |
| Diffuse pulmonary | 31 (11.5) | 6 (2.8) | 3 (1.6) | |
| Micronodular | 105 (39.0) | 49 (23.1) | 33 (17.4) | |
| Macronodular | 54 (20.1) | 40 (18.9) | 46 (24.2) | |
| Bone metastases (%) | 137 (50.9) | 159 (75.0) | 166 (87.4) | <.0001 |
| Single | 27 (10.0) | 23 (10.8) | 20 (10.5) | |
| Multiple(≥2) | 110 (40.9) | 136 (64.2) | 146 (76.9) | |
| Bone and lungs with other rare sites of distant metastases (%) | 61 (22.6) | 51 (24.0) | 63 (33.1) | .025 |
| Median cumulative activity (mCi) IQR | 405 (300-550) | 800 (750-950) | 1350(1220-1551) | <.0001 |
| Median follow-up period (mo) IQR | 57 (24.0-113.0) | 70.0 (41.0-130.0) | 122 (82.0-180.0) | <.0001 |
| Last 131I-WBS (%) | <.0001 | |||
| Negative/positive | 132 (49.1)/137 (50.9) | 55 (25.9)/157 (74.1) | 23 (12.1)/167 (87.9) | |
| EBRT (%) | 33 (12.3) | 49 (23.1) | 87 (45.8) | <.0001 |
| TKI (%) | 15 (5.6) | 10 (4.7) | 42 (21.6) | <.0001 |
| Adverse events (%) | 3 (1.1) | 12 (5.7) | 25 (12.1) | <.0001 |
| Patients alive with disease status (%) | CR: 107 (47.8) | CR: 41 (32.5) | CR: 21 (30.9) | <.0001 |
| PR: 106 (47.3) | PR: 79(62.7) | PR: 43 (63.2) | ||
| PD: 11 (4.9) | PD: 6 (4.8) | PD: 4 (5.9) | ||
| Total deaths at the time of reporting (%) | 45 (16.7) | 86 (40.6) | 122 (64.2) | <.0001 |
Total numbers are highlighted as bold.
Abbreviations: CR, complete response; EBRT, external beam radiotherapy; FTC, follicular thyroid cancer; HTC, Hurtle cell thyroid cancer; IQR, interquartile range; PD, progressive disease; PR, partial response; PTC, papillary thyroid cancer; TKI, tyrosine kinase inhibitors; WBS, whole body scintigraphy.
| Characteristic . | Group A (n = 269) . | Group B (n = 212) . | Group C (n = 190) . | P . |
|---|---|---|---|---|
| Age (y), median (IQR) | 45 (30-59) | 51.5 (40.5-60) | 50 (41-58) | <.0001 |
| <55 y (%) | 181 (67.3) | 117 (55.2) | 112 (58.9) | .02 |
| ≥55 y (%) | 88 (32.7) | 95 (45.8) | 78 (41.1) | |
| Sex (%) | Male: 88 (32.7) | Male: 50 (23.6) | Male: 55 (28.9) | .90 |
| Female: 181 (67.3) | Female: 162 (73.4) | Female: 135 (71.1) | ||
| Preoperative metabolic equivalent of task (%) | 81 (30.1) | 109 (44.3) | 126 (50.0) | <.0001 |
| Tumor size (cm) Median (IQR) | 4 (2.5-5.0) | 3.8 (2.5-5.0) | 4.0 (3.0-6.0) | .043 |
| Histopathology (%) | FTC: 99 (36.8) | FTC: 111 (52.4) | FTC: 123 (64.7) | <.0001 |
| PTC: 157 (58.4) | PTC:94 (44.3) | PTC: 59 (31.1) | ||
| HTC: 13 (4.8) | HTC: 38 (17) | HTC: 8 (4.2) | ||
| Primary surgery (%) | ||||
| Total thyroidectomy | 171 (63.6) | 136 (64.2) | 129 (67.9) | .622 |
| Less than total | 98 (36.4) | 76 (35.8) | 61(32.1) | |
| T staging (%) | .016 | |||
| TX/T1/T2 | 138 (51.3) | 131 (61.7) | 98 (51.5) | |
| T3/T4 | 131 (48.7) | 81 (38.3) | 92 (48.5) | |
| N staging (%) | <.0001 | |||
| NX/N0 | 148 (55.0) | 149 (70.3) | 150 (79.0) | |
| N1 | 121 (45.0) | 63 (29.7) | 40 (21.0) | |
| Lung metastases (%) | 190 (70.6) | 95 (44.8) | 82 (43.2) | <.0001 |
| Diffuse pulmonary | 31 (11.5) | 6 (2.8) | 3 (1.6) | |
| Micronodular | 105 (39.0) | 49 (23.1) | 33 (17.4) | |
| Macronodular | 54 (20.1) | 40 (18.9) | 46 (24.2) | |
| Bone metastases (%) | 137 (50.9) | 159 (75.0) | 166 (87.4) | <.0001 |
| Single | 27 (10.0) | 23 (10.8) | 20 (10.5) | |
| Multiple(≥2) | 110 (40.9) | 136 (64.2) | 146 (76.9) | |
| Bone and lungs with other rare sites of distant metastases (%) | 61 (22.6) | 51 (24.0) | 63 (33.1) | .025 |
| Median cumulative activity (mCi) IQR | 405 (300-550) | 800 (750-950) | 1350(1220-1551) | <.0001 |
| Median follow-up period (mo) IQR | 57 (24.0-113.0) | 70.0 (41.0-130.0) | 122 (82.0-180.0) | <.0001 |
| Last 131I-WBS (%) | <.0001 | |||
| Negative/positive | 132 (49.1)/137 (50.9) | 55 (25.9)/157 (74.1) | 23 (12.1)/167 (87.9) | |
| EBRT (%) | 33 (12.3) | 49 (23.1) | 87 (45.8) | <.0001 |
| TKI (%) | 15 (5.6) | 10 (4.7) | 42 (21.6) | <.0001 |
| Adverse events (%) | 3 (1.1) | 12 (5.7) | 25 (12.1) | <.0001 |
| Patients alive with disease status (%) | CR: 107 (47.8) | CR: 41 (32.5) | CR: 21 (30.9) | <.0001 |
| PR: 106 (47.3) | PR: 79(62.7) | PR: 43 (63.2) | ||
| PD: 11 (4.9) | PD: 6 (4.8) | PD: 4 (5.9) | ||
| Total deaths at the time of reporting (%) | 45 (16.7) | 86 (40.6) | 122 (64.2) | <.0001 |
| Characteristic . | Group A (n = 269) . | Group B (n = 212) . | Group C (n = 190) . | P . |
|---|---|---|---|---|
| Age (y), median (IQR) | 45 (30-59) | 51.5 (40.5-60) | 50 (41-58) | <.0001 |
| <55 y (%) | 181 (67.3) | 117 (55.2) | 112 (58.9) | .02 |
| ≥55 y (%) | 88 (32.7) | 95 (45.8) | 78 (41.1) | |
| Sex (%) | Male: 88 (32.7) | Male: 50 (23.6) | Male: 55 (28.9) | .90 |
| Female: 181 (67.3) | Female: 162 (73.4) | Female: 135 (71.1) | ||
| Preoperative metabolic equivalent of task (%) | 81 (30.1) | 109 (44.3) | 126 (50.0) | <.0001 |
| Tumor size (cm) Median (IQR) | 4 (2.5-5.0) | 3.8 (2.5-5.0) | 4.0 (3.0-6.0) | .043 |
| Histopathology (%) | FTC: 99 (36.8) | FTC: 111 (52.4) | FTC: 123 (64.7) | <.0001 |
| PTC: 157 (58.4) | PTC:94 (44.3) | PTC: 59 (31.1) | ||
| HTC: 13 (4.8) | HTC: 38 (17) | HTC: 8 (4.2) | ||
| Primary surgery (%) | ||||
| Total thyroidectomy | 171 (63.6) | 136 (64.2) | 129 (67.9) | .622 |
| Less than total | 98 (36.4) | 76 (35.8) | 61(32.1) | |
| T staging (%) | .016 | |||
| TX/T1/T2 | 138 (51.3) | 131 (61.7) | 98 (51.5) | |
| T3/T4 | 131 (48.7) | 81 (38.3) | 92 (48.5) | |
| N staging (%) | <.0001 | |||
| NX/N0 | 148 (55.0) | 149 (70.3) | 150 (79.0) | |
| N1 | 121 (45.0) | 63 (29.7) | 40 (21.0) | |
| Lung metastases (%) | 190 (70.6) | 95 (44.8) | 82 (43.2) | <.0001 |
| Diffuse pulmonary | 31 (11.5) | 6 (2.8) | 3 (1.6) | |
| Micronodular | 105 (39.0) | 49 (23.1) | 33 (17.4) | |
| Macronodular | 54 (20.1) | 40 (18.9) | 46 (24.2) | |
| Bone metastases (%) | 137 (50.9) | 159 (75.0) | 166 (87.4) | <.0001 |
| Single | 27 (10.0) | 23 (10.8) | 20 (10.5) | |
| Multiple(≥2) | 110 (40.9) | 136 (64.2) | 146 (76.9) | |
| Bone and lungs with other rare sites of distant metastases (%) | 61 (22.6) | 51 (24.0) | 63 (33.1) | .025 |
| Median cumulative activity (mCi) IQR | 405 (300-550) | 800 (750-950) | 1350(1220-1551) | <.0001 |
| Median follow-up period (mo) IQR | 57 (24.0-113.0) | 70.0 (41.0-130.0) | 122 (82.0-180.0) | <.0001 |
| Last 131I-WBS (%) | <.0001 | |||
| Negative/positive | 132 (49.1)/137 (50.9) | 55 (25.9)/157 (74.1) | 23 (12.1)/167 (87.9) | |
| EBRT (%) | 33 (12.3) | 49 (23.1) | 87 (45.8) | <.0001 |
| TKI (%) | 15 (5.6) | 10 (4.7) | 42 (21.6) | <.0001 |
| Adverse events (%) | 3 (1.1) | 12 (5.7) | 25 (12.1) | <.0001 |
| Patients alive with disease status (%) | CR: 107 (47.8) | CR: 41 (32.5) | CR: 21 (30.9) | <.0001 |
| PR: 106 (47.3) | PR: 79(62.7) | PR: 43 (63.2) | ||
| PD: 11 (4.9) | PD: 6 (4.8) | PD: 4 (5.9) | ||
| Total deaths at the time of reporting (%) | 45 (16.7) | 86 (40.6) | 122 (64.2) | <.0001 |
Total numbers are highlighted as bold.
Abbreviations: CR, complete response; EBRT, external beam radiotherapy; FTC, follicular thyroid cancer; HTC, Hurtle cell thyroid cancer; IQR, interquartile range; PD, progressive disease; PR, partial response; PTC, papillary thyroid cancer; TKI, tyrosine kinase inhibitors; WBS, whole body scintigraphy.
| Characteristic . | AHR . | 95% CI . | Univariate P . | AHR . | 95% CI . | Multivariate P . |
|---|---|---|---|---|---|---|
| Age | ||||||
| <55 y | 1 | <.0001 | 1 | |||
| ≥55 y | 2.576 | 1.997-3.323 | 1.644 | 1.268-2.133 | <.0001 | |
| Sex | — | — | — | |||
| Male | 1 | |||||
| Female | 1.141 | 0.861-1.510 | .359 | |||
| Histopathology | ||||||
| PTC | 1 | 1 | ||||
| FTC | 2.049 | 1.570-2.675 | <.0001 | 0.753 | 0.595-1.068 | .091 |
| HTC | 3.223 | 1.750-5.934 | 1.395 | 0.753-2.587 | ||
| Surgery | — | — | — | |||
| Total thyroidectomy | 1 | |||||
| Less than total thyroidectomy | 1.172 | 0.907-1.515 | .224 | |||
| Metastases | ||||||
| Lung only | 1 | 1 | ||||
| Bone only | 4.852 | 3.257-7.229 | <.0001 | 2.485 | 1.590-3.882 | <.0001 |
| Multiple organs | 5.531 | 3.657-8.365 | 2.526 | 1.618-3.943 | ||
| Groups | ||||||
| A | 1 | 1 | ||||
| B | 2.371 | 1.662-3.384 | <.001 | 1.150 | 0.795-1.665 | .059 |
| C | 2.558 | 1.826-3.584 | 0.816 | 0.567-1.174 | ||
| Radiotherapy | — | — | — | |||
| No | 1 | |||||
| Yes | 1.215 | 0.932-1.582 | .150 | |||
| Last 131I WBS | ||||||
| Negative | 1 | 1 | ||||
| Positive | 12.098 | 7.381-19.831 | <.0001 | 8.907 | 5.249-15.112 | <.0001 |
| Characteristic . | AHR . | 95% CI . | Univariate P . | AHR . | 95% CI . | Multivariate P . |
|---|---|---|---|---|---|---|
| Age | ||||||
| <55 y | 1 | <.0001 | 1 | |||
| ≥55 y | 2.576 | 1.997-3.323 | 1.644 | 1.268-2.133 | <.0001 | |
| Sex | — | — | — | |||
| Male | 1 | |||||
| Female | 1.141 | 0.861-1.510 | .359 | |||
| Histopathology | ||||||
| PTC | 1 | 1 | ||||
| FTC | 2.049 | 1.570-2.675 | <.0001 | 0.753 | 0.595-1.068 | .091 |
| HTC | 3.223 | 1.750-5.934 | 1.395 | 0.753-2.587 | ||
| Surgery | — | — | — | |||
| Total thyroidectomy | 1 | |||||
| Less than total thyroidectomy | 1.172 | 0.907-1.515 | .224 | |||
| Metastases | ||||||
| Lung only | 1 | 1 | ||||
| Bone only | 4.852 | 3.257-7.229 | <.0001 | 2.485 | 1.590-3.882 | <.0001 |
| Multiple organs | 5.531 | 3.657-8.365 | 2.526 | 1.618-3.943 | ||
| Groups | ||||||
| A | 1 | 1 | ||||
| B | 2.371 | 1.662-3.384 | <.001 | 1.150 | 0.795-1.665 | .059 |
| C | 2.558 | 1.826-3.584 | 0.816 | 0.567-1.174 | ||
| Radiotherapy | — | — | — | |||
| No | 1 | |||||
| Yes | 1.215 | 0.932-1.582 | .150 | |||
| Last 131I WBS | ||||||
| Negative | 1 | 1 | ||||
| Positive | 12.098 | 7.381-19.831 | <.0001 | 8.907 | 5.249-15.112 | <.0001 |
Only those variables with P < .05 in univariate analysis were included in the multivariable analysis. Total numbers are highlighted as bold.
Abbreviations: AHR, adjusted hazard ratio; FTC, follicular thyroid cancer; HTC, Hurtle cell thyroid cancer; PTC, papillary thyroid cancer; WBS, whole body scan.
| Characteristic . | AHR . | 95% CI . | Univariate P . | AHR . | 95% CI . | Multivariate P . |
|---|---|---|---|---|---|---|
| Age | ||||||
| <55 y | 1 | <.0001 | 1 | |||
| ≥55 y | 2.576 | 1.997-3.323 | 1.644 | 1.268-2.133 | <.0001 | |
| Sex | — | — | — | |||
| Male | 1 | |||||
| Female | 1.141 | 0.861-1.510 | .359 | |||
| Histopathology | ||||||
| PTC | 1 | 1 | ||||
| FTC | 2.049 | 1.570-2.675 | <.0001 | 0.753 | 0.595-1.068 | .091 |
| HTC | 3.223 | 1.750-5.934 | 1.395 | 0.753-2.587 | ||
| Surgery | — | — | — | |||
| Total thyroidectomy | 1 | |||||
| Less than total thyroidectomy | 1.172 | 0.907-1.515 | .224 | |||
| Metastases | ||||||
| Lung only | 1 | 1 | ||||
| Bone only | 4.852 | 3.257-7.229 | <.0001 | 2.485 | 1.590-3.882 | <.0001 |
| Multiple organs | 5.531 | 3.657-8.365 | 2.526 | 1.618-3.943 | ||
| Groups | ||||||
| A | 1 | 1 | ||||
| B | 2.371 | 1.662-3.384 | <.001 | 1.150 | 0.795-1.665 | .059 |
| C | 2.558 | 1.826-3.584 | 0.816 | 0.567-1.174 | ||
| Radiotherapy | — | — | — | |||
| No | 1 | |||||
| Yes | 1.215 | 0.932-1.582 | .150 | |||
| Last 131I WBS | ||||||
| Negative | 1 | 1 | ||||
| Positive | 12.098 | 7.381-19.831 | <.0001 | 8.907 | 5.249-15.112 | <.0001 |
| Characteristic . | AHR . | 95% CI . | Univariate P . | AHR . | 95% CI . | Multivariate P . |
|---|---|---|---|---|---|---|
| Age | ||||||
| <55 y | 1 | <.0001 | 1 | |||
| ≥55 y | 2.576 | 1.997-3.323 | 1.644 | 1.268-2.133 | <.0001 | |
| Sex | — | — | — | |||
| Male | 1 | |||||
| Female | 1.141 | 0.861-1.510 | .359 | |||
| Histopathology | ||||||
| PTC | 1 | 1 | ||||
| FTC | 2.049 | 1.570-2.675 | <.0001 | 0.753 | 0.595-1.068 | .091 |
| HTC | 3.223 | 1.750-5.934 | 1.395 | 0.753-2.587 | ||
| Surgery | — | — | — | |||
| Total thyroidectomy | 1 | |||||
| Less than total thyroidectomy | 1.172 | 0.907-1.515 | .224 | |||
| Metastases | ||||||
| Lung only | 1 | 1 | ||||
| Bone only | 4.852 | 3.257-7.229 | <.0001 | 2.485 | 1.590-3.882 | <.0001 |
| Multiple organs | 5.531 | 3.657-8.365 | 2.526 | 1.618-3.943 | ||
| Groups | ||||||
| A | 1 | 1 | ||||
| B | 2.371 | 1.662-3.384 | <.001 | 1.150 | 0.795-1.665 | .059 |
| C | 2.558 | 1.826-3.584 | 0.816 | 0.567-1.174 | ||
| Radiotherapy | — | — | — | |||
| No | 1 | |||||
| Yes | 1.215 | 0.932-1.582 | .150 | |||
| Last 131I WBS | ||||||
| Negative | 1 | 1 | ||||
| Positive | 12.098 | 7.381-19.831 | <.0001 | 8.907 | 5.249-15.112 | <.0001 |
Only those variables with P < .05 in univariate analysis were included in the multivariable analysis. Total numbers are highlighted as bold.
Abbreviations: AHR, adjusted hazard ratio; FTC, follicular thyroid cancer; HTC, Hurtle cell thyroid cancer; PTC, papillary thyroid cancer; WBS, whole body scan.
Outcomes According to the Site and Extent of Metastasis
Survival outcomes based on the site and extent of metastasis are presented in Table 4. The 10-year survival rate for patients with lung metastases only was 85.3%; for those with bone metastases only, it was 31.9%; and for patients with multiorgan metastases, it was 26.8%. The overall survival for the entire cohort was 45.1% at 10 years.
| . | Patients (n) . | 5-y survival (%) . | 10-y survival (%) . |
|---|---|---|---|
| Entire cohort overall survival (OS) | 671 | 71 | 45.1 |
| Lung metastases only | 202 | 91.4 | 85.3 |
| Normal X-ray and computed tomography | 35 | 93.8 | 93.8 |
| Micronodular | 130 | 93.3 | 85.7 |
| Macronodular | 37 | 67.9 | 28 |
| Bone metastases only | 294 | 63 | 31.9 |
| Single | 38 | 75.6 | 50.4 |
| Multiple | 256 | 61.2 | 28.4 |
| Multiorgan | 175 | 60.6 | 26.8 |
| . | Patients (n) . | 5-y survival (%) . | 10-y survival (%) . |
|---|---|---|---|
| Entire cohort overall survival (OS) | 671 | 71 | 45.1 |
| Lung metastases only | 202 | 91.4 | 85.3 |
| Normal X-ray and computed tomography | 35 | 93.8 | 93.8 |
| Micronodular | 130 | 93.3 | 85.7 |
| Macronodular | 37 | 67.9 | 28 |
| Bone metastases only | 294 | 63 | 31.9 |
| Single | 38 | 75.6 | 50.4 |
| Multiple | 256 | 61.2 | 28.4 |
| Multiorgan | 175 | 60.6 | 26.8 |
Total numbers are highlighted as bold.
| . | Patients (n) . | 5-y survival (%) . | 10-y survival (%) . |
|---|---|---|---|
| Entire cohort overall survival (OS) | 671 | 71 | 45.1 |
| Lung metastases only | 202 | 91.4 | 85.3 |
| Normal X-ray and computed tomography | 35 | 93.8 | 93.8 |
| Micronodular | 130 | 93.3 | 85.7 |
| Macronodular | 37 | 67.9 | 28 |
| Bone metastases only | 294 | 63 | 31.9 |
| Single | 38 | 75.6 | 50.4 |
| Multiple | 256 | 61.2 | 28.4 |
| Multiorgan | 175 | 60.6 | 26.8 |
| . | Patients (n) . | 5-y survival (%) . | 10-y survival (%) . |
|---|---|---|---|
| Entire cohort overall survival (OS) | 671 | 71 | 45.1 |
| Lung metastases only | 202 | 91.4 | 85.3 |
| Normal X-ray and computed tomography | 35 | 93.8 | 93.8 |
| Micronodular | 130 | 93.3 | 85.7 |
| Macronodular | 37 | 67.9 | 28 |
| Bone metastases only | 294 | 63 | 31.9 |
| Single | 38 | 75.6 | 50.4 |
| Multiple | 256 | 61.2 | 28.4 |
| Multiorgan | 175 | 60.6 | 26.8 |
Total numbers are highlighted as bold.
Outcomes According to Subgroups
Detailed outcomes for each subgroup are presented in Table 2. Group A comprised 269 patients with a median CA of 405 mCi (IQR: 300-550). In this subgroup, 16.7% of patients died during follow-up, whereas 224 patients were alive at the time of reporting. Among these, CR was achieved in 47.8% of patients, whereas 47.3% had a partial response. Progressive disease was observed in 4.9% of surviving patients.
Group B included 212 patients with a median CA of 800 mCi (IQR: 750-950). In this subgroup, 40.6% of patients died during follow-up, whereas 126 were alive. Among them, 32.5% achieved CR, 62.7% had a partial response, and 4.8% had progressive disease among surviving patients.
Group C consisted of 190 patients with a median CA of 1350 mCi (IQR: 1220-1551). Within this subgroup, 64.2% of patients died during follow-up and 35.8% were alive. CR was noted in 31% of patients, partial response in 63%, and progressive disease in 6% among those who survived. Notably, CR was achieved after a CA of 1 curie in 21 patients.
Kaplan-Meier Survival Analysis
The median overall survival for the entire cohort was 110 months (95% CI, 98-122), with a progression-free survival of 95 months (95% CI, 88-105) (Figs. 2 and 3). In group A, group B, and group C, the median overall survival was 261 months (95% CI, 180-342), 106 months (95% CI, 88-124), and 88 months (95% CI, 79-97), respectively (Fig. 4). The median disease-free survival was not reached for the 169 patients who achieved CR (Fig. 5). The 10-year survival rates are 72% in group A, 42.7% in group B, and 29% in group C.
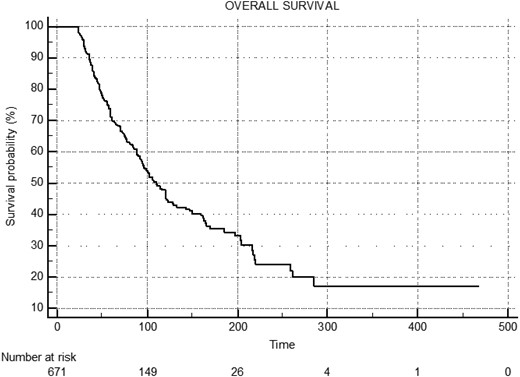
Median overall survival (OS) of entire cohort: 110 months (95% CI, 98.0-122.0).
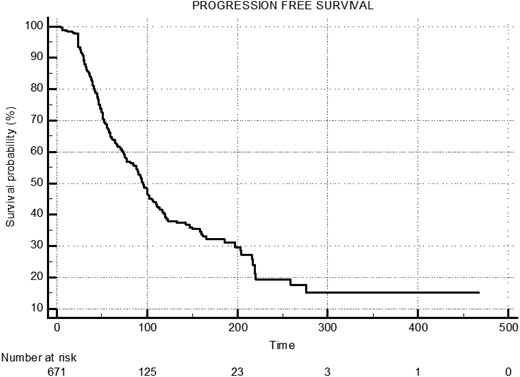
Median-progressive free survival (PFS) of entire cohort: 95.0 months (95% CI, 88.0-105.0).
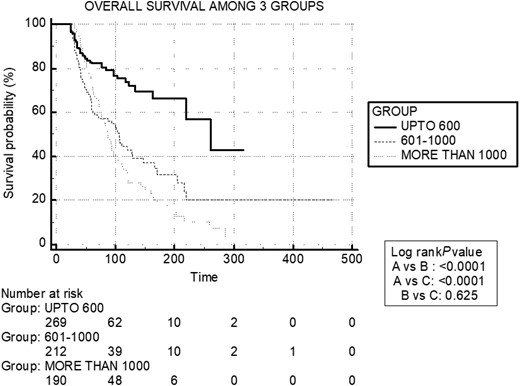
Kaplan-Meier survival curves of 3 groups: median overall survival (OS) of group A: 261.0 months (95% CI, 180.0-342.0), group B: 106.0 months (95% CI, 88.0-124.0), and group C: 88.0 months (95% CI, 79.0-97.0).
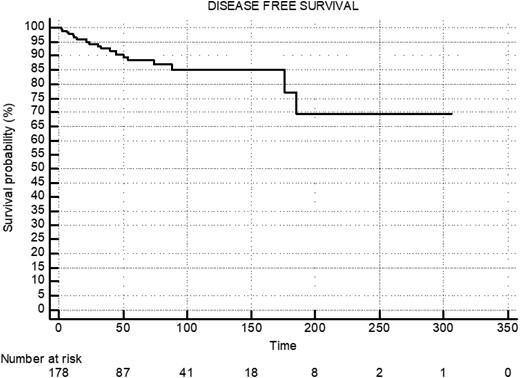
Median disease-free survival (DFS) of patients who achieved complete remission: not reached.
Adverse Events
Details of AEs in different groups are provided in Table 5. A total of 40 (6%) AEs were recorded in 38 patients, with 3 (1.1%) in group A, 12 (5.7%) in group B, and 25 (13.2%) in group C, showing a significant difference with a P value <.001. Five patients developed SPM, with 3 in group A and 1 each in groups B and C. The most common adverse event was bone marrow suppression in 19 patients, followed by pulmonary toxicity (reduced pulmonary function tests) in 9 patients, salivary gland toxicity (xerostomia) in 6 patients, and gonadal toxicity (azoospermia) in 1 patient. Receiver operator curve analysis showed optimal CA to be 840 mCi without comprising safety and without increasing AEs (sensitivity, 82%; specificity,60%; area under the curve, 0.73). A total of 25.2% of patients received radiation therapy; however, there was no significant difference between radiation therapy and AEs (P = .058).
| Group . | Patient number . | Age . | Gender . | Diagnosis (Mets) . | Total cumulative activity (mCi) . | Adverse event . | Grading of adverse event . | Time to the event (mo) . |
|---|---|---|---|---|---|---|---|---|
| A | 1 | 24 | M | CVPTC (lung) | 300 | CML | Grade 3 | 26 |
| A | 2 | 32 | F | CVPTC (lung) | 375 | Breast cancer | Grade 3 | 115 |
| A | 3 | 50 | F | FTC (lung + bone) | 375 | Colon cancer | Grade 3 | 40 |
| B | 4 | 40 | F | CVPTC (lung) | 650 | Pulmonary toxicity | Grade 4 | 38 |
| B | 5 | 26 | F | CVPTC (lung + brain) | 700 | Pulmonary toxicity | Grade 2 | 13 |
| B | 6a | 58 | F | FTC (bone) | 800 | Breast cancer and BMS | Grade 3 Grade 2 | 32 |
| B | 7 | 78 | F | FTC (bone) | 800 | Salivary toxicity | Grade 1 | 26 |
| B | 8 | 55 | F | FVPTC (bone) | 850 | Salivary toxicity | Grade 2 | 24 |
| B | 9 | 51 | F | FA-FTC (bone) | 875 | BMS | Grade 2 | 28 |
| B | 10 | 65 | F | FVPTC (lung + bone) | 880 | BMS | Grade 3 | 105 |
| B | 11 | 31 | M | CVPTC (lung) | 900 | Pulmonary toxicity | Grade 2 | 107 |
| B | 12 | 28 | F | CVPTC (lung) | 970 | Pulmonary toxicity | Grade 3 | 155 |
| B | 13 | 50 | F | FTC (lung + bone) | 1000 | BMS | Grade 1 | 29 |
| B | 14 | 36 | M | FVPTC (lung + bone) | 1000 | Pulmonary toxicity | Grade 1 | 38 |
| C | 15 | 50 | F | FVPTC (bone) | 1005 | BMS | Grade 3 | 59 |
| C | 16 | 36 | F | FVPTC (bone + lung) | 1030 | t-MDS | Grade 4 | 145 |
| C | 17 | 21 | M | CVPTC (lung) | 1043 | Gonadal toxicity | Grade 2 | 80 |
| C | 18 | 21 | F | CVPTC (lung) | 1050 | Pulmonary toxicity | Grade 1 | 60 |
| C | 19 | 30 | F | CVPTC (lung) | 1050 | Salivary toxicity | Grade 1 | 25 |
| C | 20a | 42 | F | CVPTC (lung) | 1075 | Salivary gland tumor and pulmonary toxicity | Grade 1 Grade 3 | 70 288 |
| C | 21 | 62 | M | FTC (lung + bone) | 1100 | BMS | Grade 3 | 113 |
| C | 22 | 58 | F | PDTC (bone) | 1200 | Salivary toxicity | Grade 1 | 36 |
| C | 23 | 42 | F | FVPTC (bone) | 1300 | BMS | Grade 1 | 52 |
| C | 24 | 40 | F | CVPTC (lung) | 1300 | Pulmonary toxicity | Grade 3 | 50 |
| C | 25 | 20 | F | CVPTC (lung) | 1350 | Pulmonary toxicity | Grade 4 | 70 |
| C | 26 | 52 | M | FVPTC (lung + bone) | 1365 | BMS | Grade 1 | 38 |
| C | 27 | 67 | M | FTC (bone) | 1400 | BMS | Grade 1 | 60 |
| C | 28 | 48 | F | FVPTC (bone) | 1400 | BMS | Grade 3 | 65 |
| C | 29 | 60 | F | FTC (lung + bone) | 1400 | BMS | Grade 3 | 56 |
| C | 30 | 45 | F | FTC (bone) | 1450 | BMS | Grade 1 | 128 |
| C | 31 | 52 | M | FTC (bone) | 1470 | Salivary toxicity | Grade 1 | 12 |
| C | 32 | 57 | F | FTC (bone) | 1500 | BMS | Grade 2 | 60 |
| C | 33 | 52 | F | FA-FTC (bone) | 1550 | BMS | Grade 2 | 72 |
| C | 34 | 62 | M | FVPTC (lung + bone) | 1600 | BMS | Grade 1 | 60 |
| C | 35 | 58 | M | FVPTC (bone) | 1600 | Salivary toxicity | Grade 1 | 30 |
| C | 36 | 55 | F | FTC (bone) | 1600 | BMS | Grade 3 | 97 |
| C | 37 | 41 | F | FTC (bone) | 1600 | BMS | Grade 3 | 56 |
| C | 38 | 47 | M | FTC (bone) | 1800 | BMS | Grade 1 | 85 |
| Group . | Patient number . | Age . | Gender . | Diagnosis (Mets) . | Total cumulative activity (mCi) . | Adverse event . | Grading of adverse event . | Time to the event (mo) . |
|---|---|---|---|---|---|---|---|---|
| A | 1 | 24 | M | CVPTC (lung) | 300 | CML | Grade 3 | 26 |
| A | 2 | 32 | F | CVPTC (lung) | 375 | Breast cancer | Grade 3 | 115 |
| A | 3 | 50 | F | FTC (lung + bone) | 375 | Colon cancer | Grade 3 | 40 |
| B | 4 | 40 | F | CVPTC (lung) | 650 | Pulmonary toxicity | Grade 4 | 38 |
| B | 5 | 26 | F | CVPTC (lung + brain) | 700 | Pulmonary toxicity | Grade 2 | 13 |
| B | 6a | 58 | F | FTC (bone) | 800 | Breast cancer and BMS | Grade 3 Grade 2 | 32 |
| B | 7 | 78 | F | FTC (bone) | 800 | Salivary toxicity | Grade 1 | 26 |
| B | 8 | 55 | F | FVPTC (bone) | 850 | Salivary toxicity | Grade 2 | 24 |
| B | 9 | 51 | F | FA-FTC (bone) | 875 | BMS | Grade 2 | 28 |
| B | 10 | 65 | F | FVPTC (lung + bone) | 880 | BMS | Grade 3 | 105 |
| B | 11 | 31 | M | CVPTC (lung) | 900 | Pulmonary toxicity | Grade 2 | 107 |
| B | 12 | 28 | F | CVPTC (lung) | 970 | Pulmonary toxicity | Grade 3 | 155 |
| B | 13 | 50 | F | FTC (lung + bone) | 1000 | BMS | Grade 1 | 29 |
| B | 14 | 36 | M | FVPTC (lung + bone) | 1000 | Pulmonary toxicity | Grade 1 | 38 |
| C | 15 | 50 | F | FVPTC (bone) | 1005 | BMS | Grade 3 | 59 |
| C | 16 | 36 | F | FVPTC (bone + lung) | 1030 | t-MDS | Grade 4 | 145 |
| C | 17 | 21 | M | CVPTC (lung) | 1043 | Gonadal toxicity | Grade 2 | 80 |
| C | 18 | 21 | F | CVPTC (lung) | 1050 | Pulmonary toxicity | Grade 1 | 60 |
| C | 19 | 30 | F | CVPTC (lung) | 1050 | Salivary toxicity | Grade 1 | 25 |
| C | 20a | 42 | F | CVPTC (lung) | 1075 | Salivary gland tumor and pulmonary toxicity | Grade 1 Grade 3 | 70 288 |
| C | 21 | 62 | M | FTC (lung + bone) | 1100 | BMS | Grade 3 | 113 |
| C | 22 | 58 | F | PDTC (bone) | 1200 | Salivary toxicity | Grade 1 | 36 |
| C | 23 | 42 | F | FVPTC (bone) | 1300 | BMS | Grade 1 | 52 |
| C | 24 | 40 | F | CVPTC (lung) | 1300 | Pulmonary toxicity | Grade 3 | 50 |
| C | 25 | 20 | F | CVPTC (lung) | 1350 | Pulmonary toxicity | Grade 4 | 70 |
| C | 26 | 52 | M | FVPTC (lung + bone) | 1365 | BMS | Grade 1 | 38 |
| C | 27 | 67 | M | FTC (bone) | 1400 | BMS | Grade 1 | 60 |
| C | 28 | 48 | F | FVPTC (bone) | 1400 | BMS | Grade 3 | 65 |
| C | 29 | 60 | F | FTC (lung + bone) | 1400 | BMS | Grade 3 | 56 |
| C | 30 | 45 | F | FTC (bone) | 1450 | BMS | Grade 1 | 128 |
| C | 31 | 52 | M | FTC (bone) | 1470 | Salivary toxicity | Grade 1 | 12 |
| C | 32 | 57 | F | FTC (bone) | 1500 | BMS | Grade 2 | 60 |
| C | 33 | 52 | F | FA-FTC (bone) | 1550 | BMS | Grade 2 | 72 |
| C | 34 | 62 | M | FVPTC (lung + bone) | 1600 | BMS | Grade 1 | 60 |
| C | 35 | 58 | M | FVPTC (bone) | 1600 | Salivary toxicity | Grade 1 | 30 |
| C | 36 | 55 | F | FTC (bone) | 1600 | BMS | Grade 3 | 97 |
| C | 37 | 41 | F | FTC (bone) | 1600 | BMS | Grade 3 | 56 |
| C | 38 | 47 | M | FTC (bone) | 1800 | BMS | Grade 1 | 85 |
Pulmonary toxicity: Significant drop in pulmonary function tests; salivary toxicity: xerostomia; gonadal toxicity: azoospermia.
Abbreviations: BMS, bone marrow suppression; CML, chronic myeloid leukemia; CVPTC, classical variant of papillary thyroid cancer; FTC, follicular thyroid cancer; FVPTC, follicular variant of papillary thyroid cancer; Mets, metabolic equivalent of tasks; t-MDS, treatment-related myelodysplastic syndrome.
aPatients with dual toxicity.
| Group . | Patient number . | Age . | Gender . | Diagnosis (Mets) . | Total cumulative activity (mCi) . | Adverse event . | Grading of adverse event . | Time to the event (mo) . |
|---|---|---|---|---|---|---|---|---|
| A | 1 | 24 | M | CVPTC (lung) | 300 | CML | Grade 3 | 26 |
| A | 2 | 32 | F | CVPTC (lung) | 375 | Breast cancer | Grade 3 | 115 |
| A | 3 | 50 | F | FTC (lung + bone) | 375 | Colon cancer | Grade 3 | 40 |
| B | 4 | 40 | F | CVPTC (lung) | 650 | Pulmonary toxicity | Grade 4 | 38 |
| B | 5 | 26 | F | CVPTC (lung + brain) | 700 | Pulmonary toxicity | Grade 2 | 13 |
| B | 6a | 58 | F | FTC (bone) | 800 | Breast cancer and BMS | Grade 3 Grade 2 | 32 |
| B | 7 | 78 | F | FTC (bone) | 800 | Salivary toxicity | Grade 1 | 26 |
| B | 8 | 55 | F | FVPTC (bone) | 850 | Salivary toxicity | Grade 2 | 24 |
| B | 9 | 51 | F | FA-FTC (bone) | 875 | BMS | Grade 2 | 28 |
| B | 10 | 65 | F | FVPTC (lung + bone) | 880 | BMS | Grade 3 | 105 |
| B | 11 | 31 | M | CVPTC (lung) | 900 | Pulmonary toxicity | Grade 2 | 107 |
| B | 12 | 28 | F | CVPTC (lung) | 970 | Pulmonary toxicity | Grade 3 | 155 |
| B | 13 | 50 | F | FTC (lung + bone) | 1000 | BMS | Grade 1 | 29 |
| B | 14 | 36 | M | FVPTC (lung + bone) | 1000 | Pulmonary toxicity | Grade 1 | 38 |
| C | 15 | 50 | F | FVPTC (bone) | 1005 | BMS | Grade 3 | 59 |
| C | 16 | 36 | F | FVPTC (bone + lung) | 1030 | t-MDS | Grade 4 | 145 |
| C | 17 | 21 | M | CVPTC (lung) | 1043 | Gonadal toxicity | Grade 2 | 80 |
| C | 18 | 21 | F | CVPTC (lung) | 1050 | Pulmonary toxicity | Grade 1 | 60 |
| C | 19 | 30 | F | CVPTC (lung) | 1050 | Salivary toxicity | Grade 1 | 25 |
| C | 20a | 42 | F | CVPTC (lung) | 1075 | Salivary gland tumor and pulmonary toxicity | Grade 1 Grade 3 | 70 288 |
| C | 21 | 62 | M | FTC (lung + bone) | 1100 | BMS | Grade 3 | 113 |
| C | 22 | 58 | F | PDTC (bone) | 1200 | Salivary toxicity | Grade 1 | 36 |
| C | 23 | 42 | F | FVPTC (bone) | 1300 | BMS | Grade 1 | 52 |
| C | 24 | 40 | F | CVPTC (lung) | 1300 | Pulmonary toxicity | Grade 3 | 50 |
| C | 25 | 20 | F | CVPTC (lung) | 1350 | Pulmonary toxicity | Grade 4 | 70 |
| C | 26 | 52 | M | FVPTC (lung + bone) | 1365 | BMS | Grade 1 | 38 |
| C | 27 | 67 | M | FTC (bone) | 1400 | BMS | Grade 1 | 60 |
| C | 28 | 48 | F | FVPTC (bone) | 1400 | BMS | Grade 3 | 65 |
| C | 29 | 60 | F | FTC (lung + bone) | 1400 | BMS | Grade 3 | 56 |
| C | 30 | 45 | F | FTC (bone) | 1450 | BMS | Grade 1 | 128 |
| C | 31 | 52 | M | FTC (bone) | 1470 | Salivary toxicity | Grade 1 | 12 |
| C | 32 | 57 | F | FTC (bone) | 1500 | BMS | Grade 2 | 60 |
| C | 33 | 52 | F | FA-FTC (bone) | 1550 | BMS | Grade 2 | 72 |
| C | 34 | 62 | M | FVPTC (lung + bone) | 1600 | BMS | Grade 1 | 60 |
| C | 35 | 58 | M | FVPTC (bone) | 1600 | Salivary toxicity | Grade 1 | 30 |
| C | 36 | 55 | F | FTC (bone) | 1600 | BMS | Grade 3 | 97 |
| C | 37 | 41 | F | FTC (bone) | 1600 | BMS | Grade 3 | 56 |
| C | 38 | 47 | M | FTC (bone) | 1800 | BMS | Grade 1 | 85 |
| Group . | Patient number . | Age . | Gender . | Diagnosis (Mets) . | Total cumulative activity (mCi) . | Adverse event . | Grading of adverse event . | Time to the event (mo) . |
|---|---|---|---|---|---|---|---|---|
| A | 1 | 24 | M | CVPTC (lung) | 300 | CML | Grade 3 | 26 |
| A | 2 | 32 | F | CVPTC (lung) | 375 | Breast cancer | Grade 3 | 115 |
| A | 3 | 50 | F | FTC (lung + bone) | 375 | Colon cancer | Grade 3 | 40 |
| B | 4 | 40 | F | CVPTC (lung) | 650 | Pulmonary toxicity | Grade 4 | 38 |
| B | 5 | 26 | F | CVPTC (lung + brain) | 700 | Pulmonary toxicity | Grade 2 | 13 |
| B | 6a | 58 | F | FTC (bone) | 800 | Breast cancer and BMS | Grade 3 Grade 2 | 32 |
| B | 7 | 78 | F | FTC (bone) | 800 | Salivary toxicity | Grade 1 | 26 |
| B | 8 | 55 | F | FVPTC (bone) | 850 | Salivary toxicity | Grade 2 | 24 |
| B | 9 | 51 | F | FA-FTC (bone) | 875 | BMS | Grade 2 | 28 |
| B | 10 | 65 | F | FVPTC (lung + bone) | 880 | BMS | Grade 3 | 105 |
| B | 11 | 31 | M | CVPTC (lung) | 900 | Pulmonary toxicity | Grade 2 | 107 |
| B | 12 | 28 | F | CVPTC (lung) | 970 | Pulmonary toxicity | Grade 3 | 155 |
| B | 13 | 50 | F | FTC (lung + bone) | 1000 | BMS | Grade 1 | 29 |
| B | 14 | 36 | M | FVPTC (lung + bone) | 1000 | Pulmonary toxicity | Grade 1 | 38 |
| C | 15 | 50 | F | FVPTC (bone) | 1005 | BMS | Grade 3 | 59 |
| C | 16 | 36 | F | FVPTC (bone + lung) | 1030 | t-MDS | Grade 4 | 145 |
| C | 17 | 21 | M | CVPTC (lung) | 1043 | Gonadal toxicity | Grade 2 | 80 |
| C | 18 | 21 | F | CVPTC (lung) | 1050 | Pulmonary toxicity | Grade 1 | 60 |
| C | 19 | 30 | F | CVPTC (lung) | 1050 | Salivary toxicity | Grade 1 | 25 |
| C | 20a | 42 | F | CVPTC (lung) | 1075 | Salivary gland tumor and pulmonary toxicity | Grade 1 Grade 3 | 70 288 |
| C | 21 | 62 | M | FTC (lung + bone) | 1100 | BMS | Grade 3 | 113 |
| C | 22 | 58 | F | PDTC (bone) | 1200 | Salivary toxicity | Grade 1 | 36 |
| C | 23 | 42 | F | FVPTC (bone) | 1300 | BMS | Grade 1 | 52 |
| C | 24 | 40 | F | CVPTC (lung) | 1300 | Pulmonary toxicity | Grade 3 | 50 |
| C | 25 | 20 | F | CVPTC (lung) | 1350 | Pulmonary toxicity | Grade 4 | 70 |
| C | 26 | 52 | M | FVPTC (lung + bone) | 1365 | BMS | Grade 1 | 38 |
| C | 27 | 67 | M | FTC (bone) | 1400 | BMS | Grade 1 | 60 |
| C | 28 | 48 | F | FVPTC (bone) | 1400 | BMS | Grade 3 | 65 |
| C | 29 | 60 | F | FTC (lung + bone) | 1400 | BMS | Grade 3 | 56 |
| C | 30 | 45 | F | FTC (bone) | 1450 | BMS | Grade 1 | 128 |
| C | 31 | 52 | M | FTC (bone) | 1470 | Salivary toxicity | Grade 1 | 12 |
| C | 32 | 57 | F | FTC (bone) | 1500 | BMS | Grade 2 | 60 |
| C | 33 | 52 | F | FA-FTC (bone) | 1550 | BMS | Grade 2 | 72 |
| C | 34 | 62 | M | FVPTC (lung + bone) | 1600 | BMS | Grade 1 | 60 |
| C | 35 | 58 | M | FVPTC (bone) | 1600 | Salivary toxicity | Grade 1 | 30 |
| C | 36 | 55 | F | FTC (bone) | 1600 | BMS | Grade 3 | 97 |
| C | 37 | 41 | F | FTC (bone) | 1600 | BMS | Grade 3 | 56 |
| C | 38 | 47 | M | FTC (bone) | 1800 | BMS | Grade 1 | 85 |
Pulmonary toxicity: Significant drop in pulmonary function tests; salivary toxicity: xerostomia; gonadal toxicity: azoospermia.
Abbreviations: BMS, bone marrow suppression; CML, chronic myeloid leukemia; CVPTC, classical variant of papillary thyroid cancer; FTC, follicular thyroid cancer; FVPTC, follicular variant of papillary thyroid cancer; Mets, metabolic equivalent of tasks; t-MDS, treatment-related myelodysplastic syndrome.
aPatients with dual toxicity.
Discussion
It has been previously reported that most metastases are present either at the time of diagnosis or within a few years of diagnosis (9, 27). Bone metastases have typically been associated with a worse prognosis compared with lung metastases (28-30). Although I-131 therapy has been shown to improve disease-free survival (31) and overall survival (2, 10) in these patients, setting a maximum CA limit when the disease continues to concentrate radioiodine remains a topic of debate. The controversy primarily revolves around whether the recommended limit of 600 mCi in ATA guidelines 2015 is scientifically justified (21) because the evidence supporting this threshold is limited. Therefore, the current study’s primary focus lies in examining the equilibrium between administering the maximum CA while minimizing AEs. In our hospital, our protocol involves treating metastatic patients with DTC every 6 to 12 months, especially when the disease demonstrates a concentration of I-131. This real-world approach is particularly relevant because a considerable number of referred patients present with a substantial metastatic tumor burden. The evidence generated in this study is therefore based on the analysis of a substantial dataset of patients (936 of 10 032) with mDTC treated at a single center over 5 decades. Notably, this is the largest published series of such patients, surpassing the 444 patients analyzed by Durante et al in 2013 (10).
One concern associated with CA exceeding 600 mCi of I-131 is the development of SPMs. Previous studies, such as the trination study by Rubino et al (32) and a series from Taiwan by Lin et al (33), reported SPMs in a significant percentage of patients who received CA >600 mCi. However, this study observed only 5 cases (0.75%) of SPM, including breast cancers, chronic myeloid leukemia, adenocarcinoma of the colon, and a salivary gland tumor. Notably, the incidence of SPM did not increase with higher CA, suggesting that the risk of SPM may not be associated with increasing CA. This finding is consistent with other Asian studies (34, 35) and may be attributed to factors such as the relatively younger age of patients at presentation and different ethnicities. Also, the malignancies reported, especially breast and colon, might be related to other etiologies.
The study also found a higher incidence of AEs in group C (CA >1000 mCi) compared with group A (CA up to 600 mCi) and group B (CA 601-1000 mCi). Bone marrow suppression was more pronounced in group C, with 7.4% experiencing this AE, compared with 2.4% in group B and none in group A. It is consistent with the observation that severe bone marrow suppression is rare when the blood radiation absorbed dose is below 2 Gy (36). Most cases of bone marrow suppression in this study were mild (grade I/II anemia), with only a few cases of prolonged anemia lasting at least 6 months. Interestingly, the majority of anemic patients were women. Leukopenia and thrombocytopenia were also observed in group C, in line with previous studies associating these AEs with high CA of I-131 (37). One case of treatment-related MDS was detected, emphasizing the importance of monitoring for hematologic AEs, particularly in patients receiving high CA. There was no significant association between radiotherapy and AEs (P = .058).
Radiation pneumonitis and pulmonary fibrosis are known complications of large doses of I-131 (38). However, this study observed a relatively low incidence of pulmonary toxicity, likely because of a proper time gap of 6 to 12 months between therapy cycles. Pulmonary function tests were performed in all patients with pulmonary metastases once CA exceeded 600 mCi. Salivary gland toxicity was also minimal, with only a small number of patients experiencing grade 1/2 toxicity. Additionally, transient impairment of testicular function was observed in 1 patient, highlighting the importance of monitoring gonadal function in male patients receiving multiple administrations of I-131. Interestingly, no cases of female infertility were seen, which was in line with the observations of a previously published study from our center (39).
Despite the higher AEs observed in group C, there was no significant difference in Kaplan-Meier survival outcomes between group B and group C (Fig. 3). This paradox can be explained by the group’s nonuniform disease burden, leading to more treatment cycles and higher CA in group C. The debate surrounding CA limits revolves around whether higher CA benefits patients with a high disease burden or leads to increased AEs. In our study, approximately 32.5% of the patients in group B (600-1000 mCi) achieved CR, which starkly contrasts with the results of Durante et al (10), which showed only 4% of patients benefiting beyond 600 mCi. This finding supports that the ceiling of CA of I-131 can be raised to 1000 mCi, limiting AEs and achieving favorable responses. A randomized controlled trial comparing CA limits of 600 mCi to >1000 mCi in high disease-burden patients could potentially provide further insights into this question.
Strengths and Limitations
The study’s strengths include data from a single center with a large cohort of patients with mDTC, a consistent treatment policy, standardized follow-up procedures, and meticulous data collection over a 5-decade period. The relatively long follow-up duration, ranging from 2 to 53 years, provides valuable insights.
However, the study’s retrospective design introduces potential bias, and the long follow-up duration may impact the detection of SPM, given the latency period for radiation-induced malignancies. Furthermore, the study’s single-center nature may limit the generalizability of the findings. With the passing of time, several diagnostic tools of cross-sectional imaging/functional imaging and, most importantly, Thyroglobulin estimations have improved drastically to detect even up to 0.2 ng/mL. In our study, which included patients from 1967, many variable methods in analyzing Thyroglobulin have been used, thereby making it difficult to recategorize into excellent response and indeterminate response separately according to ATA 2015 guidelines.
Nonetheless, this study contributes necessary evidence to the ongoing debate regarding CA limits in the treatment of mDTC. It suggests that increasing CA up to 1000 mCi is feasible with acceptable AEs, particularly in patients with a high disease burden and good uptake in metastatic sites. The study’s observation of no significant increase in the incidence of SPM with CA exceeding 1000 mCi is reassuring.
In conclusion, our study challenges the 2015 ATA Guidelines’ 600 mCi CA limit for I-131 treatment in mDTC. It suggests that increasing CA up to 1000 mCi is possible without a significant increase in SPM and with acceptable AEs. The incidence of SPM in this study was lower than in previous reports, possibly because of a younger patient population and spacing I-131 treatment cycles at longer intervals, thus using more conservative treatment regimes. Although increasing CA may benefit patients with a high disease burden and good uptake in metastatic sites, further research, including randomized controlled trials, is needed to validate these findings.
Acknowledgments
We gratefully acknowledge the contributions of Dr. Avinash Tupalli, Dr. Chandra Teja Reddy, and Dr. Swayamjeet Satapathy for helping in data compilation.
Funding
There were no extramural funding for this work.
Author Contributions
C.S.B. conceived the idea and planned the study. M.S.B. and S.B. compiled and analyzed the data. C.S.B. and M.S.B. wrote the manuscript. All authors have read and approved the manuscript.
Disclosures
The authors have nothing to disclose.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Abbreviations
- AE
adverse event
- ATA
American Thyroid Association
- ATG
anti-thyroglobulin antibody
- CA
cumulative activity
- CR
complete response
- CT
computed tomography
- DTC
differentiated thyroid cancer
- DxWBS
diagnostic I-131 whole-body scan
- EBRT
external beam radiotherapy
- IQR
interquartile range
- mDTC
metastatic differentiated thyroid cancer
- RR-DTC
radioiodine-refractory differentiated thyroid cancer
- SAE
severe adverse event
- SPM
second primary malignancy
- Tg
thyroglobulin
- TKI
tyrosine kinase inhibitor



