-
PDF
- Split View
-
Views
-
Cite
Cite
Youn Hee Jee, Sanjay Jumani, Veronica Mericq, The Association of Accelerated Early Growth, Timing of Puberty, and Metabolic Consequences in Children, The Journal of Clinical Endocrinology & Metabolism, Volume 108, Issue 9, September 2023, Pages e663–e670, https://doi.org/10.1210/clinem/dgad202
Close - Share Icon Share
Abstract
Accelerated early growth and early timing of puberty or pubertal variant have been noticed as risk factors for metabolic syndrome, more frequently observed in children born small for gestational age (SGA) or children with premature adrenarche (PA). Children with SGA, especially if they make an accelerated catch-up growth in early life, carry a higher risk for long-term metabolic consequences, such as type 2 diabetes, insulin resistance, and cardiovascular diseases. Furthermore, multiple studies support that these children, either born SGA or with a history of PA, may have earlier pubertal timing, which is also associated with various metabolic risks. This review aims to summarize the recent studies investigating the association between early infantile growth, the timing of puberty, and metabolic risks to expand our knowledge and gain more insight into the underlying pathophysiology.
Children who are born SGA and experience catch-up growth may have an increased propensity for cardiometabolic risk factors, particularly those who experience robust growth early in the neonatal period.
The finding that children born SGA are at increased risk of early puberty and pubertal variants may be associated with early maturation of the zona reticularis associated with early catch-up growth and elevated DHEA-S.
Premature adrenarche (PA) may be independently associated with increased cardiometabolic risk, possibly mediated through DHEA-S and other adrenal androgens.
There are few biomarkers measured infants born SGA that predict risk of metabolic dysfunction later in life.
Human growth comprises 2 phases: (1) intrauterine or prenatal growth and (2) postnatal growth. Abnormal growth during each phase is linked to certain disorders or disease traits. Specifically, suboptimal weight and/or length at birth—which is termed as small for gestational age (SGA)—has been noted to increase risks of metabolic disorders, such as obesity, type 2 diabetes (T2DM), or cardiovascular diseases (CVDs) (1). However, the mechanism by which suboptimal intrauterine growth resulting in low birth weight for gestational age may lead to postnatal metabolic risks has not been well established, although changes in metabolic programming by leptin signaling, epigenetic reprogramming of mesenchymal stem cells, or alteration of DNA methylation have been proposed (2-5). In addition, several studies have suggested that genetic etiologies (6) or maternal factors (7, 8) are associated with SGA, which may increase metabolic risks in later life for these children. Ninety percent of SGA-born children experience catch-up growth, which has traditionally been thought to be beneficial for children. However, studies have consistently found that the early compensatory rapid growth phase may contribute to adverse health conditions (1), which brings up a need for new approaches to care for these children during early childhood.
Over the years, accumulated evidence has shown that catch-up growth (recovery of weight and/or length to be consistent with genetic potential) during early infancy in children born SGA is associated with metabolic risks (9-11), suggesting that either what triggers the rapid catch-up growth or the rapid restoration of growth itself may increase the risk of the metabolic morbidity. Other studies of SGA children with catch-up growth showed a greater propensity to develop insulin resistance than children born appropriate for gestational age (AGA) during early childhood. In these studies, the markers for insulin resistance were related to catch-up growth in the first year of life (12-14), indicating a clinical clue for the underlying pathogenesis of metabolic risks in these children. The observation of the association may be further complicated later in life because children born SGA may develop earlier timing of puberty or a variant of puberty, such as premature adrenarche (PA), when compared with children born AGA. There has been an overwhelming number of studies that link the timing of puberty to metabolic risks in adulthood. Therefore, the sequence of poor growth prenatally, catch-up growth after birth, and early timing of puberty appear to enhance metabolic consequences in later life (15) (Fig. 1).
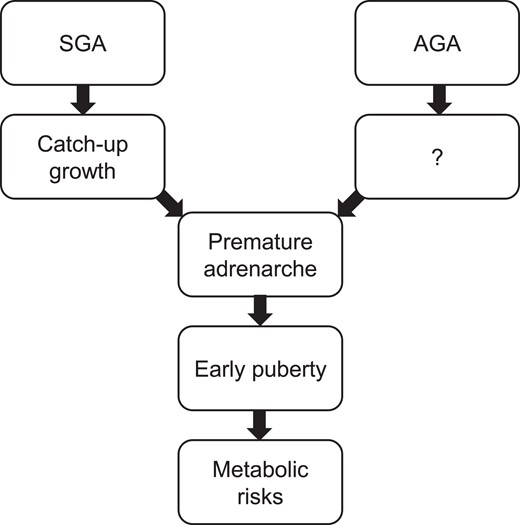
The sequence of the associations between birth weight, early growth, pubertal characteristics, and metabolic risks. “?” reflects a lack of data on early growth in children born AGA who develop PA later in childhood.
Although many are not born SGA, some children with PA show similar sequences to those observed in children born SGA. Adrenarche is biochemically defined by increased production of adrenal androgens (in the range of early puberty) but also clinically by the symptoms, such as adult body odor, oily skin, acne, and pubic and axillary hair growth in the areas sensitive to androgens. Some children with PA also tend to have mild to significantly advanced bone age and early timing of puberty during childhood and carry metabolic risks later in life, similar to children born SGA (16, 17). The common findings observed in children born SGA and those with PA suggest a strong link between accelerated early infantile growth, earlier adrenarche, early puberty, and later metabolic risk. Studies recognize that the underlying common mechanism may primarily involve differences in body adipocyte distribution/composition. Given the high prevalence of metabolic morbidities in the general population, it is critical that we understand the characteristics of early childhood growth and the maturational steps associated with its metabolic consequences not only to expand our knowledge and investigate the underlying pathophysiology, but also to create better approaches to prevent these risks in the future.
In this review, we aim to summarize childhood growth and pubertal conditions that show accelerated early infantile growth and altered timing of puberty or pubertal variant and speculate the underlying biology.
Early Growth and Metabolic Risks in Children Born SGA
It has been well established that birth weight is associated with metabolic risks. Rapid growth during early infancy was shown to be associated with higher fasting insulin and a higher Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) based on a meta-analysis of 22 relevant studies (18). Furthermore, a recent observational study by Leunissen et al explored (1) an association between the degree of weight gain during early life and metabolic disorders (T2DM and CVD) in early adulthood, and (2) the patterns of risk factors in the first year of life among children born SGA that were associated with the risk of metabolic disease in early adulthood (19). The study followed 217 healthy subjects aged 18-24 years longitudinally in the Programming Factors for Growth and Metabolism (PROGRAM) study. The authors showed a direct positive association between weight gain relative to height growth in the first 3 months of life and multiple determinants of metabolic risks in adulthood (reduced insulin sensitivity, serum high-density lipoprotein cholesterol level, increased waist circumference, acute insulin response, ratio of total cholesterol to high-density lipoprotein cholesterol, and serum level of triglycerides). In addition, rapid weight restoration during the first year of life led to a significantly higher percentage of body fat content and a reduced insulin sensitivity in early adulthood compared with slow growth after adjustment for gestational age, sex, age, socioeconomic status, and height growth in the first year of life (19). This was the first study to confirm that postnatal weight gain in the first 3 months is critical for the metabolic risks observed in children born SGA, although many previous studies had proposed the importance of infantile weight gain for later metabolic risks. For example, the importance of weight catch-up in children born SGA was raised by Marcovecchio et al (20). Compared with those without weight catch-up, children with catch-up growth had a higher fat mass index, trunk to limb fat mass ratio, systolic blood pressure SDS, fasting glucose, and C-peptide (20). Prospectively, infants born SGA with catch-up growth developed a thicker carotid intima–media thickness and more preperitoneal fat than AGA controls without a difference in cardiac function (21). Birth weight or early growth may also be associated with changes in reproductive parameters. Girls born SGA who experienced catch-up growth carried increased risks of biochemical hyperandrogenism and have reduced uterine and ovarian volumes in adolescence, which might affect future reproductive function (22). However, whether low birth weight has a direct link to persistent hyperandrogenism, such as polycystic ovarian syndrome, is not known (15). Bone density, in contrast, does not seem to be affected by SGA (23).
Children born SGA may have an increased risk of obesity given the accelerated body mass index (BMI) increase during infancy (24, 25), which may be the main reason for metabolic risks or an independent contributing factor to the risks. Among children with overweight or obesity, the children with a history of SGA showed a higher frequency of prediabetes and abnormal low-density lipoprotein cholesterol compared with those born non-SGA, independent of age, sex, and BMI (26), indicating that even with the same degree of adiposity, being born SGA still carries an independent risk of metabolic syndrome. Obese children born SGA develop a thicker carotid intima–media thickness compared with obese children born AGA of similar age, sex, and BMI (27), indicating an early change in organs affected by metabolic risks.
It is important to dissect out whether SGA with or without catch-up growth would have different metabolic comorbidities. However, these studies may be complicated by the fact that children with SGA but without catch-up growth are often treated with growth hormone (GH), which may influence their metabolic profiles in later life. Studies generally do not observe diabetes (28, 29) in children who received GH for SGA without catch-up growth. Moreover, when compared with untreated short SGA adults, body composition, blood pressure, and lipid levels are similar to treated children at 6.5 years after stopping GH treatment (30). Although there are still limited data, during the course of GH therapy there may be a modest beneficial effect on the adverse metabolic and cardiovascular risk observed in short children born SGA without catch-up growth (31).
Given the strong association between SGA and metabolic risks, investigators explored interventional approaches that could relieve the adverse impact of SGA on metabolic syndrome in adolescents, such as early macronutrient supplementation for infants born SGA (32) or maintaining healthy body weight (33). In an observational study, SGA infants who received an exclusive breast milk–based diet exhibited catch-up growth with increased adiposity measured with dual-energy X-ray absorptiometry but significantly lower insulin level at 2 years of age compared with those born AGA (34). However, the study was limited by the inclusion of only infants with very low birth weight. In addition, because authors did not include a control group of SGA infants who received only formula-based milk, the effect of exclusive breast milk over formula was not explored. In another observational study of infants who were born preterm, continuing a preterm formula enriched with docosahexaenoic acid after discharge from the Neonatal Intensive Care Unit showed a better body composition profile (35). However, this study lacked a separate analysis in only infants born SGA.
The underlying mechanism of SGA being a risk of metabolic syndrome may be partly explained by “thrifty phenotype hypothesis” (36). According to Hales and Barker, the combination of malnutrition in prenatal life and subsequent abundant nutrition after birth may induce metabolic programming of the fetal organs that make them prone to insulin resistance and metabolic syndrome. These findings are repeatedly observed in many clinical studies comparing children with a history of being born SGA and control subjects (36). How to prevent the metabolic programming favoring metabolic risks during nutritional replenish phase still needs to be established.
Interestingly, a genome-wide association study (GWAS) demonstrated that fetal genetic factors may be the major contributor for the association between lower birth weight and higher metabolic risks, likely involving insulin signaling, glucose homeostasis, glycogen biosynthesis and chromatin remodeling (37).
Early Growth and Metabolic Risks in Children With Idiopathic PA
Children Born SGA and Idiopathic PA
Adrenarche is a maturational process of zona reticularis of the adrenal cortex, which begins after the first few years of life (38, 39). The biochemical evaluation of adrenarche is performed by measuring 1 of the adrenal androgens, sulfated dehydroepiandrosterone (DHEA-S), in which the sulfation primarily occurs in the adrenal glands (40) and is the most sensitive to determine this condition (41). DHEA-S serves as a reservoir for androgen so that it is desulfated to DHEA and converted to active androgens and estrogens in peripheral tissues (42). DHEA-S is produced in the fetal adrenal gland, and the concentration is high at birth, then the concentrations rapidly decline during infancy but start rising again gradually with the onset of adrenarche, reaching peak levels in the second decade of life (16, 43).
PA is clinically defined by the appearance of symptoms, such as adult body odor, oily skin, acne, and pubic and axillary hair due to elevated adrenal androgens (typically measured with DHEA-S) (44), at an earlier than the expected age. It has been known that children born SGA would have elevated DHEA-S concentrations between 6 and 8 years of age and, consequently, are more prone to develop PA (45-50), especially when they have had catch-up growth. Children born SGA without catch-up growth do not show an association with elevated DHEA-S concentrations (51), indicating that early adrenarche may also be associated with early growth acceleration or vice versa. How adrenal maturation is affected in children born SGA with catch-up growth causing early DHEA-S elevation is not yet established (52), but it has been proposed that an impaired excretion of adrenal androgen and cortisol metabolites (48) or increased insulin and higher insulin-like growth factor (IGF) 1 concentrations seen in children born SGA with catch-up growth may play a role (53-54).
How is SGA with catch-up growth and adrenal maturation (elevated DHEA-S) linked together? Does early infantile growth induce an early awakening of adrenal gland? What is the role of DHEA-S in metabolic risks? Unfortunately, there is a lack of studies investigating the underlying molecular or hormonal pathophysiology to understand the causes and roles of high DHEA-S in children born SGA. Campbell suggested an interesting hypothetical pathway that links meat consumption, IGF-1 increase in adrenal gland, and zona reticularis maturation and thickening (55). According to this hypothetical sequence, it is plausible that poor nutrition during intrauterine life or the nutritional compensation after birth may hasten the maturation of zona reticularis. Interestingly, IGF-I levels increased rapidly after birth only in children born SGA (56) which support a potential hypothesis that early nutritional compensation increases IGF-1 in the adrenal glands and subsequently triggers early maturation of zona reticularis (16). Well-designed animal studies will be required to explore adrenal maturation and the role of DHEA-S for the metabolic risks in the setting of poor intrauterine growth and nutritional compensation after birth. Other androgens, such as 11β-hydroxy- and 11-ketotestosterone have emerged as the markers of adrenal gland maturation. Further studies require to understand their role in the development of PA and the metabolic risks (16).
Children Born AGA and Idiopathic PA
Adrenal androgens have been often recognized to be associated with metabolic risks. Using stratified random saliva samples in children, Goddings et al found that higher androgen concentrations were positively associated with greater height, weight, BMI, and waist circumference after adjusting for sex and socioeconomic status (57). DHEA-S is a specific adrenal androgen that is frequently studied. In a large longitudinal study of 1052 Chilean children with normal birth weight (58), those with a higher DHEA-S were taller and heavier and presented with a higher Homeostatic Model Assessment for Insulin Resistance and IGF-I than children with lower DHEA-S concentrations. Additionally, girls with high DHEA-S at 7 years of age exhibited a higher BMI, more central fat, and a greater prevalence of hyperglycemia during puberty (59). The findings indicate a strong association between DHEA-S concentrations, early accelerated growth, and increased metabolic risk factors in children with PA (60). Children born large for gestational age had lower serum DHEA-S levels adjusted for BMI and age than the rest of the children (50). Moreover, lower birth weight SDS and higher weight gain during the first 2 years of life predicted higher serum DHEA-S levels (50), again suggesting a strong association between SGA with catch-up growth and adrenal maturation causing high DHEA-S concentrations. Elevated DHEA-S in prepubertal normal birth weight girls was associated with earlier thelarche, pubarche, and menarche and a mild elevation of other androgens throughout puberty (61) confirming the associations.
Whether being overweight is the leading cause of metabolic syndrome in children with PA is debatable (62). In children of normal birth weight, all adiposity markers were positively associated with an elevated DHEA-S at 7 years of age (63). In a large study of adult Latinos, significant associations were observed between several CVD risk factors (total cholesterol and glycemia) and DHEA-S, independent of BMI, suggesting that DHEA-S might have an independent effect on metabolic risk in children with PA (64). However, not all studies identify this association. Mäntyselkä et al observed that higher DHEA-S is not associated with an increased cardiometabolic risk in prepubertal children but a rather protective profile of lower low-density lipoprotein cholesterol and low-density lipoprotein/high-density lipoprotein cholesterol ratio (65). Brahimaj et al observed that both serum DHEA and DHEA-S levels were inversely associated with the risk of T2DM (66). In young Finnish adult women, a history of PA did not show an association with metabolic syndrome or low-grade inflammation, although in those with increased central adiposity continued an increased risk of unfavorable glucose metabolism (67). In Brazil, children with premature pubarche, developed an unfavorable risk of cardiovascular disease (68-69) and showed an increased prevalence of polycystic ovarian syndrome (70). Hence, the associated future risks may be influenced by ethnicity and body composition.
Underlying genetic variants may explain the association between adrenal androgens and metabolic risks. A polymorphism in SULT2A1 encoding sulfotransferase that adds a sulfate to DHEA may be associated with lower circulating DHEA-S (71); however, patients with pathogenic variants in SULT2A1 have not yet been reported. Pathogenic variants in PAPSS2 encoding 3′-phosphoadenosine 5′-phosphosulfate synthase 2 that assists SULT2A1 function have been reported. Patients with pathogenic variants in PAPSS2 develop PA, obesity, hirsutism, and oligomenorrhea (72). However, due to a low number of identified subjects, their metabolic profiles have not been fully investigated.
A meta-analysis of GWASs explored loci associated with serum DHEA-S concentrations in 14 846 Caucasian men and women (73). This study identified several loci associated with serum DHEA-S concentrations: ZKSCAN5, SULT2A1, ARPC1A, TRIM4, BMF, HHEX, BCL2L11, and CYP2C9 (73). Interestingly, a locus nearby the HHEX gene is also identified by a GWAS that links birth weight and T2DM (37) or average distribution of insulin and glycemic trait trajectory between 5- and 16-year-old children (74). However, adult GWAS cannot precisely reflect the dynamic changes of DHEA-S concentrations that occur during childhood. Therefore, a recent GWAS was conducted in prepubertal boys and girls. The study identified loci near GARL1 and PRLR, which encode galanin and prolactin receptors, respectively (75). The role of GARL1 in the adrenal gland is unclear and requires further investigation to understand the cross junction between the galanin pathway and DHEA-S. However, it is experimentally shown that prolactin administration stimulates the maturation and growth of zona reticularis in animal studies suggesting an important association between prolactin signaling and zona reticularis maturation (76, 77).
Timing of Puberty and Metabolic Risks
The evidence that timing of puberty is associated with metabolic risk has been overwhelming and was observed across different ethnic backgrounds. Earlier pubertal timing is observed as a predictor for higher adult BMI and greater risk of obesity (78). In a study of Afro-Caribbean children, earlier menarche and earlier breast development were associated with higher fasting glucose even after adjusting for BMI or prior growth in girls (79). In a Korean study, girls who matured early were taller and heavier in early adolescence than those who matured later (80) but earlier maturation at puberty was found to be an independent factor that influenced metabolic risks (81, 82). This observation is also supported by GWAS, which identify shared etiologies between puberty timing and markers for metabolic syndrome (83, 84). However, these studies do not take early growth into account in the investigation of the association; therefore, what portion of early timing of puberty is affected by early growth pattern remains unclear. Another measure of the timing of puberty in girls is the age of menarche. Earlier age at menarche increases the risk of diabetes in women and this association is mediated by increased adiposity (85, 86).
It should be considered that a greater childhood BMI can contribute to earlier age at menarche because excess adiposity could be the sites of peripheral aromatization of sex steroids. Therefore, it is important to distinguish between whether having obesity causes earlier puberty and metabolic risks independently and whether there is an independent effect of early menarche on metabolic risks during adulthood (87-91). In 2 large prospective cohort studies, the association between age of menarche and the risk of T2DM was largely mediated through excess adult adiposity (92). However, other studies found an independent association. A study of girls with early menarche showed a development of elevated blood pressure and glucose intolerance compared with later maturing girls, independent of body composition (93). Despite many clinical studies in this matter, 1 Mendelian randomization found that puberty timing has only a small influence on adiposity and cardiometabolic traits with adjustment for BMI at age 8 years (94).
The inconsistent results among studies may be due to differences in the characteristics of the study population, measurement of pubertal progression (thelarche, menarche, voice break, gonadarche, spermarche, etc.), determinants of adiposity, and age of determining metabolic risks.
One interesting observation regarding the timing of puberty in children born AGA is that higher prepubertal IGF-1 levels were associated with earlier ages of thelarche and menarche in girls and gonadarche in boys (95), suggesting a role of increased IGF-1 in the initiation of puberty. Animal studies support the clinical observation that central administration of IGF-1 markedly increased luteinizing hormone release and reduced age at vaginal openings in female rats (96).
Potential Biomarkers
Recent investigations using liquid chromatography-tandem mass spectroscopy (LC-MS/MS) have revealed that the androgen repertoire in the adrenal gland is broader than traditional DHEA/DHEAS, such as 11-OH-δ4 androstenedione, 11-OH-testosterone, 11-ketoandrostene-dione, and 11-ketotestosterone, which are clinically more important androgens considering their potency to bind the androgen receptor (97-99). 11-Ketotestosterone is thought to be the dominant circulating bioactive androgen during normal and PA (41). The role of 11-letotestosterone in metabolic risks require investigation.
A small number of studies propose early biomarkers that predict high metabolic risks in children born SGA. In a prospective longitudinal study, the authors found a significant positive correlation between chemerin (1 of the new adipokines) concentrations at 3 months and insulin values at 3 months and also with triglyceride levels at 24 months after adjusting the data with anthropometric parameters. They propose that circulating chemerin concentration, measured at an early age, might be an indicator of future metabolic alterations in SGA children (100). Another proposed marker is an umbilical cord miRNA profile to identify catch-up growth in infants born SGA (101) or a differential profile of circulating miRNAs found in SGA and AGA children with obesity compared with SGA and AGA children with normal weight (102). These circulating miRNAs may represent specific biomarkers for early detection of increased risk of developing metabolic dysfunction in SGA and AGA children with obesity if the findings are reproducible in future studies and if the approach can be standardized and used in clinical settings. Unfortunately, biomarkers for PA are lacking.
Conclusion
The evidence is solid that early compensatory infantile growth in children born SGA or early accelerated growth in children with PA have an increased risk of metabolic syndrome in later life. Both conditions may involve early adrenal maturation. However, the causes of these growth and maturational conditions are largely unknown, and the underlying mechanisms linked to metabolic risks still remain to be elucidated. Future studies, including birth history, early infantile growth, various adrenal androgens, and timing of puberty are required to assess metabolic disease risks in addition to suitable animal models to study the sequence of SGA, adrenal maturation and metabolic disorders.
Disclosures
Authors have no conflict of interest.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Abbreviations
- AGA
appropriate for gestational age
- BMI
body mass index
- CVD
cardiovascular disease
- DHEA-S
dehydroepiandrosterone sulfate
- GH
growth hormone
- GWAS
genome-wide association study
- IGF
insulin-like growth factor
- PA
premature adrenarche
- SGA
small for gestational age
- T2DM
type 2 diabetes



