-
PDF
- Split View
-
Views
-
Cite
Cite
Yan Lin, Zhao-rong Wu, Yao-ping Shi, Min Ding, Xiao-yin Tang, Yi He, Bo Zhai, Ping Li, Radiofrequency Ablation of Unifocal Papillary Thyroid Microcarcinoma With BRAF V600E Mutation, The Journal of Clinical Endocrinology & Metabolism, Volume 108, Issue 11, November 2023, Pages e1298–e1305, https://doi.org/10.1210/clinem/dgad269
Close - Share Icon Share
Abstract
To date there is no study on the feasibility of radiofrequency ablation (RFA) for papillary thyroid microcarcinomas (PTMCs) with BRAF V600E mutation.
This study was designed to evaluate the efficiency, safety, and prognosis of ultrasound (US)-guided percutaneous RFA for unifocal PTMCs with BRAF V600E mutation.
Sixty patients with 60 unifocal BRAF V600E mutation–positive PTMCs who received US-guided RFA between January 2020 and December 2021 were retrospectively analyzed. The mean maximum PTMC tumor diameter was 5.8 ± 1.7 mm (range, 2.5-10.0 mm). All PTMCs were pathologically confirmed by fine needle aspiration or core needle biopsy, and BRAF V600E mutation was confirmed to be positive by real-time fluorescent quantitative polymerase chain reaction. Contrast-enhanced ultrasound (CEUS) was performed immediately after RFA to evaluate whether PTMCs were extendedly ablated. Ultrasound was performed 1, 3, 6, and 12 months after RFA and every 6 months thereafter to evaluate the changes in the ablation zone, local recurrence, and cervical lymph node metastasis (LNM). The complications were recorded and evaluated.
Extended ablation was achieved in all enrolled patients. The ablation zone sizes increased immediately after RFA compared with those of tumors before treatment. One month later, the ablation zone sizes were smaller than immediately after RFA. At the last follow-up assessment, 42 nodules (70.0%) completely disappeared and the ablation zones of 18 nodules (30.0%) showed fissure-like changes. No local recurrence or cervical LNM was detected. Voice change (1.7%) was the only major complication.
RFA is effective and safe in treating unifocal PTMCs with BRAF V600E mutation, especially when surgery is not feasible or refused by patients who are unwilling to continue active surveillance.
Due to the wide application of high-resolution ultrasound, the detection rate of papillary thyroid microcarcinomas (PTMCs) has increased significantly (1, 2). However, the disease-related mortality rates did not increase (3). Benefiting from particular biological PTMC characteristics, some patients can live a lifetime with PTMCs without any symptoms. Therefore, active surveillance is an alternative to surgery for patients with asymptomatic and nonmetastatic PTMC, enabling them to avoid treatment side effects unless the disease shows signs of significant progression (4). However, active surveillance may be a challenging strategy for some patients because of its long-term and dynamic nature. The differences in patients’ understanding of active surveillance and the anxiety evoked by the cancer diagnosis without active treatment may affect the implementation of this strategy and decrease the quality of life (5). Moreover, there is a small but significant proportion of patients whose PTMC displays more aggressive features, such as early metastasis and lymph node involvement, thereby affecting their survival (6-8). Therefore, there remains a cohort of PTMC patients who require a more nuanced risk stratification and an individualized treatment strategy.
Molecular biological examination is one of the important means for risk stratification of thyroid tumors that enables a more individualized treatment of PTMC patients. Among these molecular markers, BRAF V600E is the most prevalent one. Some studies state that there is no significant association of BRAF mutation with negative prognostic indicators in papillary thyroid carcinomas (PTCs) (9, 10). However, other studies have demonstrated that the BRAF V600E mutation status is associated with lymph node metastasis (LNM), extrathyroidal extension, advanced tumor node metastasis stage, increased risk of recurrence and LNM-associated mortality (11-18). Therefore, active surveillance may only be suitable for patients with wild-type BRAF V600E PTMC (19).
In recent years, with the development of interventional ultrasound (US), US-guided thermal ablation, such as radiofrequency ablation (RFA), laser ablation, and microwave ablation have been applied in treating malignant thyroid nodules. These techniques have achieved promising results and have advantages such as fewer complications and less negative impact on patients’ lives compared with surgery (20-22), which is why thermal ablation may be an alternative to active surveillance and surgery. Previous research suggests that the increased recurrence risk and other adverse effects make active surveillance unsuitable for BRAF mutation–positive PTMC (19, 23), and that patients suffering from BRAF V600E-mutated PTMC may require earlier management, such as minimally invasive ablative therapy (19). To date, there is no study on the feasibility of RFA for PTMCs with BRAF V600E mutation; the purpose of this study was to evaluate the efficacy, safety, and prognosis of this treatment modality.
Methods
Patients
This retrospective study was approved by the Institutional Review Board of Renji Hospital. In our department, all PTMC patients (with or without BRAF V600E mutation) have been informed about the possible treatment options, such as active surveillance, RFA, and surgery; the advantages and disadvantages of the different treatment strategies; and that extended ablation would be achieved in all patients. Those PTMC patients with BRAF V600E mutation have been informed about the characteristics of PTMCs with BRAF V600E mutations. The patients enrolled in the present retrospective study were those who declined active surveillance or surgical resection but consented to receive RFA. Moreover, all patients who received RFA were selected based on suitability for our treatment. The suitability required the feasibility of the hydrodissection technique and that the patients displayed no cervical LNM or invasion of the trachea or esophagus. Written informed consent for RFA as well as related clinical research was obtained from each patient prior to RFA. All patients enrolled in this study consented to the publication of their anonymized examination results and radiological images. The inclusion criteria of the present study were as follows: (i) unifocal PTMC confirmed by fine needle aspiration (FNA) or core needle biopsy (CNB); (ii) PTMC nodules with BRAF V600E mutation confirmed by quantitative polymerase chain reaction; (iii) no cervical LNM; (iv) no distant metastasis shown on images; and (v) complete follow-up data.
From January 2020 to December 2021, a total of 60 PTMC patients (20 men and 40 women) with BRAF V600E mutation treated with US-guided RFA, were enrolled in the present study according to the selection flowchart as shown in Fig. 1. The mean age was 43.6 ± 11.6 years (range, 23-67 years).
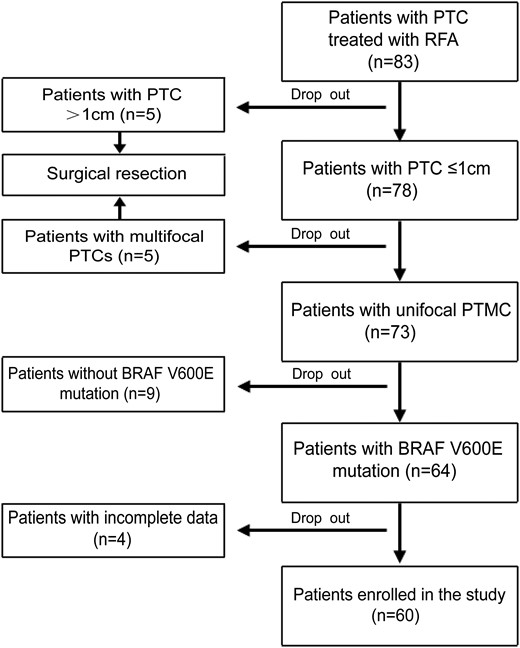
Pretreatment Assessments
All patients underwent laboratory examinations, enhanced CT of the neck, x-ray or computed tomography (CT) examination of the chest, US examination, and US-guided FNA or CNB before RFA. Laboratory examinations mainly included blood routine examination, coagulation, and thyroid function tests. The enhanced CT of the neck and x-ray or CT examination of the chest were conducted to detect possible LNM or distant metastasis. Ultrasound examinations and guidance were performed using ultrasonography (Italy, Esaote) equipped with a linear, high-frequency probe (5 to 14 MHz). A cytological or histopathological analysis, as well as a BRAF V600E mutation test, were conducted prior to RFA. Three orthogonal diameters and locations of all PTMC nodules were presented on the US image. The volume of each nodule was calculated by the following equation: volume = πabc/6, where a is the largest diameter, and b and c are the other mutually perpendicular diameters (24).
BRAF V600E Mutation Examination
A portion of the sample taken from FNA or CNB was used for pathological diagnosis and the remainder of the samples were stored in a nucleic acid preservation bottle at 2 to 8 °C. After DNA extraction, BRAF V600E gene mutations were analyzed by quantitative polymerase chain reaction (25, 26).
Continuous Hydrodissection Technique
Before RFA, cool sterile water was injected into the interstitial space of the thyroid and surrounding tissues such as the skin, carotid artery, trachea, and esophagus until they were completely separated (about 2 mm between nodules and the vitals) as shown in Fig. 2. To avoid thermal injury and to ensure extended ablation completion, during the RFA process cool sterile water was continuously injected into the interstitial space to keep the thyroid tissue separated from the surrounding important tissues through the water needle retained in the interstitial space.
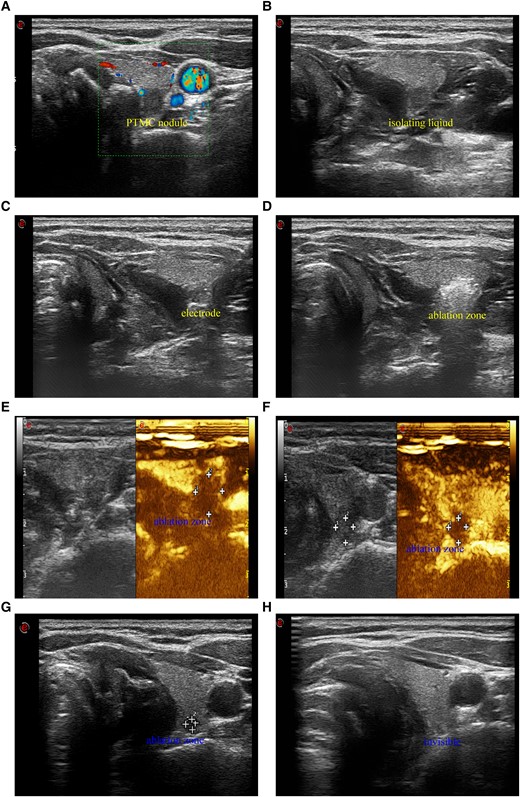
A 39-year-old woman with PTMC nodule and BRAF V600E mutation received ultrasound-guided RFA. A, Color Doppler flow imaging (CDFI) shows a PTMC nodule (5.4 × 5.1 × 4.1 mm) with little signals of blood stream and superior thyroid artery. B, Ultrasound image in transverse view shows that cool sterile water was injected between thyroid tissue, trachea, esophagus, and carotid artery to make the PTMC nodule an isolated island. C, Ultrasound image in transverse view shows that under the protection of isolation fluids, the RF electrode was inserted into the lowest pole of the PTMC nodule through the lateral cervical approach to avoid of damage to superior thyroid artery and the trachea. D, Ultrasound image in transverse view shows that the hyperechoic area covers and exceeds the original PTMC nodule, and extended ablation was achieved successfully. E, Contrast-enhanced ultrasound (CEUS) shows that immediately after RFA the area without enhancement was about 7.9 × 7.8 × 7.1 mm and it exceeded the original size of the PTMC nodule before RFA. F, CEUS shows that 1 month after RFA the area without enhancement was about 5.9 × 5.6 × 5.3 mm and it was smaller than that immediately after RFA. G, Ultrasound image in transverse view shows that 3 months after RFA the ablation zone was significantly reduced (3.0 × 2.1 × 2.1 mm). H, Ultrasound image in transverse view shows that 6 months after RFA the ablation zone was completely invisible.
RFA Procedure
RFA treatment was performed by interventional radiologists with more than 3 years of experience in RFA treatment of thyroid nodules. For RFA treatment, an RF Generator S-500 was applied, with a 19-gauge, 7 cm shaft length, 0.5 cm or 1 cm internally cooled electrode with an active tip (Medsphere, Shanghai, China) that is specifically designed for thyroid lesion applications and minimizes the risk of healthy tissue injury because of its easier control. RFA power was adjusted to 20W or 40W using the impedance mode.
When treating PTMC nodules that are close to the trachea, discomfort may be caused by continuously injecting cool sterilized water into the tracheoesophageal space. Therefore, general anesthesia with tracheal intubation was applied to avoid affecting the RFA procedure performed in patients who tended to be nervous or had problems controlling their swallowing. The heart rate, blood pressure, respiration rate, as well as peripheral oxygenation were monitored throughout the treatment.
Before treatment, all patients lay down in supine position to keep their neck extended. Two interventional radiologists worked together to choose the appropriate route for electrode insertion with careful observation of the blood vessel location to prevent hemorrhage. For most PTMCs located in the isthmus or the lateral thyroid lobe, we usually adopted the trans-isthmus approach. However, for PTMCs located in the medial region of the upper pole of either thyroid lobe, sometimes the lateral cervical approach was used to avoid damage to superior thyroid artery and the trachea (Fig. 2A-2D). Under real-time US guidance, percutaneous RFA was performed using the fixed-applicator technique or the moving shot technique from a single puncture site to reduce the possibility of needle track implantation metastasis. The puncture needle path from the tumor to the subcutis was ablated to avoid bleeding or implantation metastasis. Contrast-enhanced US was performed immediately after RFA to determine whether complete and extended ablation had been achieved, that is, whether the ablation zone completely covered the original tumor with the outer edge of the ablation zone exceeding the tumor size by at least 2 mm. The technical success was defined as a complete absence of enhancement on CEUS immediately after RFA (27).
Postablation Assessment and Follow-ups
One day and 1 month after RFA, thyroid function indexes including free triiodothyronine (FT3), free thyroxine (FT4), and thyrotropin (TSH, thyroid-stimulating hormone) levels were evaluated to assess how RFA influenced thyroid function. A CEUS examination was performed 1 month after RFA, whereas US was performed after 1, 3, 6, and 12 months, and every 6 months thereafter for all patients. Three orthogonal diameters of the ablation zone were evaluated by US and CEUS examinations from which the RFA efficacy was evaluated by assessing the volume changes of the inactive ablation zones. Due to extended ablation, the sizes of the inactive ablation zones became generally larger than those of the original tumors immediately after RFA.
After extended ablation confirmed by CEUS immediately after RFA as well as 1 month later, we reconfirmed that there were no local residual tumors. Local recurrence during follow-up was defined as the appearance of vascular structures inside the ablation zone even if the overall volume of the ablation zone decreased. In addition, CEUS and biopsy would be performed to determine whether there was recurrence. In the absence of local recurrence, the ablation zones without blood supply became necrotic and were gradually shrinking with fissure-like changes and eventually disappeared.
During follow-up, we also detected and evaluated any suspicious cervical lymph nodes via US. If necessary, US-guided FNA or CNB was performed to determine whether the lymph nodes were metastatic. Computed tomography examination was performed annually to rule out distant metastases.
Thyroid-stimulating hormone has a potential role in the initiation or progression of differentiated thyroid carcinoma (28, 29). Therefore, TSH suppressive therapy has been widely used in patients with differentiated thyroid cancer to prevent disease progression (30). Previous studies have illustrated that TSH suppressive therapy was performed after RFA to reduce tumor recurrence and metastasis of PTMC (31) and T1bN0M0 PTC (32). In our department, after RFA all PTMC patients were advised to take levothyroxine to maintain TSH levels between 0.1 and 0.5 mU/L until the ablation zones disappeared completely, then levothyroxine was withdrawn in a tapered manner (32), except for those patients with a previous diagnosis of hypothyroidism who were maintained on the previous replacement dose.
Complications were classified as major and minor in accordance with the Society of Interventional Radiology (33). Complications during and after the RFA procedure were checked for proper management and were recorded (34).
Statistical Analysis
All statistical analyses were performed using the SPSS statistical software package (version 19.0 for Microsoft Windows, SPSS). The outcomes, such as changes in the largest diameter, volume, and thyroid function were compared by a Wilcoxon signed-rank test. Continuous variables are reported as the means ± SD. The significance level was defined as P < .05.
Results
Clinical Characteristics
The clinical characteristics of the enrolled patients are summarized in Table 1. Among the 60 cases enrolled in this study, 56 patients refused surgery due to cosmetic concerns and worries about surgery-related complications, including 2 patients with hypothyroidism. Two patients had a history of hyperthyroidism and preferred RFA because they worried about the bleeding risk during surgical resection. One patient already underwent a thyroid surgery and due to the possible local tissue adhesion, the head and neck surgeon judged this patient as unsuitable for surgical resection. One patient with hypertrophic cardiomyopathy with cardiac insufficiency received RFA under local anesthesia because of the high risk of general anesthesia. RFA was successfully completed in 53 patients under local anesthesia. General anesthesia with tracheal intubation was applied in 7 patients who tended to be nervous or displayed swallowing problems (Table 1).
The clinicopathological and therapeutic characteristics of enrolled PTMC patients
| Variables . | Data (n = 60) . |
|---|---|
| Patients | |
| Age (years), mean ± SD (range) | 43.6 ± 11.6 years (23–67 years) |
| Sex, male/female | 20/40 |
| PTMCs | |
| Location of PTMCs | |
| Right lobe | 39 (65%) |
| Left lobe | 13 (21.7%) |
| isthmus | 8 (13.3%) |
| PTMCs adjacent to important structure | |
| Trachea | 27 (45%) |
| Esophagus | 9 (15%) |
| Internal carotid artery | 12 (20%) |
| Tracheoesophageal groove | 5 (8.3%) |
| Diameter (mm), mean ± SD (range) | 6.0 ± 2.0 mm (2.5-15.0 mm) |
| Volume (mL), mean ± SD (range) | 0.10 ± 0.09 mL (0.01-0.43 mL) |
| Reasons for refusal of surgery | |
| Cosmetic concerns | 43 (71.7%) |
| Worries about surgery-related complications | 13 (21.7%) |
| High risk of general anesthesia | 1 (1.7%) |
| Combined with hyperthyroidism | 2 (3.3%) |
| History of thyroid surgery | 1 (1.7%) |
| Type of anesthesia | |
| General anesthesia | 7 (11.7%) |
| Local anesthesia | 53 (88.3%) |
| Ablation time (seconds), mean ± SD (range) | 96.9 ± 55.8 seconds (20–256 seconds) |
| Mean follow-up time (months) mean ± SD (range) | 23.2 ± 6.2 months (11.3–36.3 months) |
| Ablation zones at the last follow-up | |
| Invisible | 42 (70%) |
| Fissure-like changes | 18 (30%) |
| Variables . | Data (n = 60) . |
|---|---|
| Patients | |
| Age (years), mean ± SD (range) | 43.6 ± 11.6 years (23–67 years) |
| Sex, male/female | 20/40 |
| PTMCs | |
| Location of PTMCs | |
| Right lobe | 39 (65%) |
| Left lobe | 13 (21.7%) |
| isthmus | 8 (13.3%) |
| PTMCs adjacent to important structure | |
| Trachea | 27 (45%) |
| Esophagus | 9 (15%) |
| Internal carotid artery | 12 (20%) |
| Tracheoesophageal groove | 5 (8.3%) |
| Diameter (mm), mean ± SD (range) | 6.0 ± 2.0 mm (2.5-15.0 mm) |
| Volume (mL), mean ± SD (range) | 0.10 ± 0.09 mL (0.01-0.43 mL) |
| Reasons for refusal of surgery | |
| Cosmetic concerns | 43 (71.7%) |
| Worries about surgery-related complications | 13 (21.7%) |
| High risk of general anesthesia | 1 (1.7%) |
| Combined with hyperthyroidism | 2 (3.3%) |
| History of thyroid surgery | 1 (1.7%) |
| Type of anesthesia | |
| General anesthesia | 7 (11.7%) |
| Local anesthesia | 53 (88.3%) |
| Ablation time (seconds), mean ± SD (range) | 96.9 ± 55.8 seconds (20–256 seconds) |
| Mean follow-up time (months) mean ± SD (range) | 23.2 ± 6.2 months (11.3–36.3 months) |
| Ablation zones at the last follow-up | |
| Invisible | 42 (70%) |
| Fissure-like changes | 18 (30%) |
The clinicopathological and therapeutic characteristics of enrolled PTMC patients
| Variables . | Data (n = 60) . |
|---|---|
| Patients | |
| Age (years), mean ± SD (range) | 43.6 ± 11.6 years (23–67 years) |
| Sex, male/female | 20/40 |
| PTMCs | |
| Location of PTMCs | |
| Right lobe | 39 (65%) |
| Left lobe | 13 (21.7%) |
| isthmus | 8 (13.3%) |
| PTMCs adjacent to important structure | |
| Trachea | 27 (45%) |
| Esophagus | 9 (15%) |
| Internal carotid artery | 12 (20%) |
| Tracheoesophageal groove | 5 (8.3%) |
| Diameter (mm), mean ± SD (range) | 6.0 ± 2.0 mm (2.5-15.0 mm) |
| Volume (mL), mean ± SD (range) | 0.10 ± 0.09 mL (0.01-0.43 mL) |
| Reasons for refusal of surgery | |
| Cosmetic concerns | 43 (71.7%) |
| Worries about surgery-related complications | 13 (21.7%) |
| High risk of general anesthesia | 1 (1.7%) |
| Combined with hyperthyroidism | 2 (3.3%) |
| History of thyroid surgery | 1 (1.7%) |
| Type of anesthesia | |
| General anesthesia | 7 (11.7%) |
| Local anesthesia | 53 (88.3%) |
| Ablation time (seconds), mean ± SD (range) | 96.9 ± 55.8 seconds (20–256 seconds) |
| Mean follow-up time (months) mean ± SD (range) | 23.2 ± 6.2 months (11.3–36.3 months) |
| Ablation zones at the last follow-up | |
| Invisible | 42 (70%) |
| Fissure-like changes | 18 (30%) |
| Variables . | Data (n = 60) . |
|---|---|
| Patients | |
| Age (years), mean ± SD (range) | 43.6 ± 11.6 years (23–67 years) |
| Sex, male/female | 20/40 |
| PTMCs | |
| Location of PTMCs | |
| Right lobe | 39 (65%) |
| Left lobe | 13 (21.7%) |
| isthmus | 8 (13.3%) |
| PTMCs adjacent to important structure | |
| Trachea | 27 (45%) |
| Esophagus | 9 (15%) |
| Internal carotid artery | 12 (20%) |
| Tracheoesophageal groove | 5 (8.3%) |
| Diameter (mm), mean ± SD (range) | 6.0 ± 2.0 mm (2.5-15.0 mm) |
| Volume (mL), mean ± SD (range) | 0.10 ± 0.09 mL (0.01-0.43 mL) |
| Reasons for refusal of surgery | |
| Cosmetic concerns | 43 (71.7%) |
| Worries about surgery-related complications | 13 (21.7%) |
| High risk of general anesthesia | 1 (1.7%) |
| Combined with hyperthyroidism | 2 (3.3%) |
| History of thyroid surgery | 1 (1.7%) |
| Type of anesthesia | |
| General anesthesia | 7 (11.7%) |
| Local anesthesia | 53 (88.3%) |
| Ablation time (seconds), mean ± SD (range) | 96.9 ± 55.8 seconds (20–256 seconds) |
| Mean follow-up time (months) mean ± SD (range) | 23.2 ± 6.2 months (11.3–36.3 months) |
| Ablation zones at the last follow-up | |
| Invisible | 42 (70%) |
| Fissure-like changes | 18 (30%) |
The mean maximum PTMC tumor diameter was 5.8 ± 1.7 mm (range, 2.5-10.0 mm), and the mean target lesion volume was 0.09 ± 0.08 mL (range, 0.01-0.43 mL). All (100%) cases were tested positive for BRAF V600E mutation. The median follow-up time was 23.2 months. The mean follow-up time after RFA was 23.1 ± 6.2 months (range, 11.3-36.3 months), and 59, 28, and 10 patients were followed for more than 12, 24, and 30 months, respectively.
Technical Success of RFA
All PTMC nodules have been extendedly ablated in one RFA session. According to the CEUS results immediately after RFA, extended ablation was achieved in all cases with a technical success rate of 100%. The mean ablation time was 96.8 ± 56.2 seconds (20-256 seconds).
Changes of Thyroid Function Indexes
In the light of the extended ablation strategy, the possible influence of RFA treatment on thyroid function was also assessed. One day after RFA, the thyroxine level was significantly higher than that before treatment, and 1 month after RFA, the concentrations of FT3, FT4, and TSH did not return to the preoperative level as shown in Fig. 3.
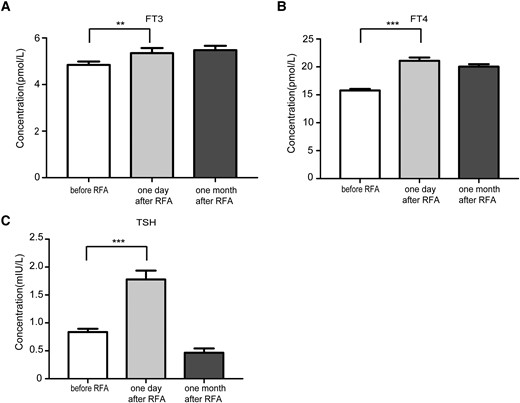
The influence of RFA on thyroid function. A, The serum FT3 concentration before RFA, 1 day and 1 month after RFA. B, The serum FT4 concentration before RFA, 1 day and 1 month after RFA. C, The serum TSH concentration before RFA, 1 day and 1 month after RFA.
Changes in Size of Ablation Zone After RFA
Due to the extended ablation, the sizes of the inactive ablation zones increased immediately after RFA compared to the original tumors prior to treatment (P < .05). One month after RFA, the sizes of the inactive ablation zones were smaller than those immediately after RFA (P < .05) as shown in Table 2. At the last follow-up, the ablation zones in 42 cases (70.0%) could not be seen completely and the ablation zones of 18 nodules showed fissure-like changes without vascular structures appearing inside these fissures.
| Variable . | Initial . | Immediately after RFA . | 1 month after RFA . | P value . |
|---|---|---|---|---|
| Volume (ml) | 0.09 ± 0.08(0.01–0.43) | 0.44 ± 0.38(0.07–2.26) | 0.36 ± 0.35(0.04–1.50) | <.0001a/< .01b |
| Variable . | Initial . | Immediately after RFA . | 1 month after RFA . | P value . |
|---|---|---|---|---|
| Volume (ml) | 0.09 ± 0.08(0.01–0.43) | 0.44 ± 0.38(0.07–2.26) | 0.36 ± 0.35(0.04–1.50) | <.0001a/< .01b |
Values are means ± SD (range).
Initial vs immediately after RFA.
Immediately after RFA vs 1 month after RFA.
| Variable . | Initial . | Immediately after RFA . | 1 month after RFA . | P value . |
|---|---|---|---|---|
| Volume (ml) | 0.09 ± 0.08(0.01–0.43) | 0.44 ± 0.38(0.07–2.26) | 0.36 ± 0.35(0.04–1.50) | <.0001a/< .01b |
| Variable . | Initial . | Immediately after RFA . | 1 month after RFA . | P value . |
|---|---|---|---|---|
| Volume (ml) | 0.09 ± 0.08(0.01–0.43) | 0.44 ± 0.38(0.07–2.26) | 0.36 ± 0.35(0.04–1.50) | <.0001a/< .01b |
Values are means ± SD (range).
Initial vs immediately after RFA.
Immediately after RFA vs 1 month after RFA.
Disease Progression After RFA
At the last follow-up, no local recurrence or progression was detected by US and CEUS. Moreover, there was no cervical LNM or distant organ metastasis so far.
Complications
In the present study, a temporary voice change (1.7%) was the only major complication and the patient recovered after oral Mecobalamin treatment 2 months after RFA. No serious bleeding or implantation metastasis in the needle path occurred after the ablation of the puncture needle path from tumor to subcutis. By employing the hydrodissection technique, no skin burns were detected during RFA. Six (10.0%) patients who received RFA with local anesthesia complained of mild pain during RFA which subsided after RFA. Four (6.7%) patients complained of local swelling after RFA, which may be caused by injection of isolation fluid during ablation. After treatment with a +15° head-up tilt position, the swelling symptoms were significantly relieved the next day after RFA.
Discussion
Recently, the application of thermal ablation has achieved promising results in PTC treatment, not only for the low-risk primary PTMCs (27, 35-38), but moreover, even for those T1N0M0 PTCs characterized by capsular invasion (39). At present, there is still a controversial debate about the optimal clinical treatment for PTMCs with BRAF V600E mutation.
Some studies suggest that the presence or absence of the BRAF mutation does not significantly alter the therapy result (40). The 2015 American Thyroid Association (ATA) guidelines indicated that the impact of the BRAF status on the recurrence risk in very low-risk patients (intrathyroidal unifocal papillary microcarcinomas <1 cm) appears to be weak (30). However, other studies reported that BRAF mutations can increase the risk of PTC recurrence (13) and that this is associated with LNM and LNM-associated mortality (16, 41). In addition, BRAF V600E mutations may make the feasibility of long-term conservative surveillance uncertain in BRAF V600E-mutated PTMC patients (23).
We started testing for BRAF V600E mutations in all patients with suspicious nodules who received FNA or CNB in January 2020. By December 2021, 64 patients diagnosed as unifocal PTMC with BRAF V600E mutation received radical and extended RFA in our department. Four patients with incomplete data were excluded from this study. In this study, the technical success rate was 100%. Previous meta-analysis regarding RFA in PTMC showed that the complete disappearance rate at the end of the follow-up period ranged between 29.3% and 100% (38). In our study, during a mean follow-up period of 23.2 months, 42 ablation zones (70.0%) completely disappeared. The ablation zones of 18 nodules showed fissure-like changes with color Doppler US showing that there was no blood flow inside these fissures. According to our observation of inactive ablation zones by US during the follow-up, fissure-like changes can be regarded as an omen for a complete disappearance of the ablation zone.
According to regular US and CEUS examinations, there was no local recurrence or cervical LNM during the follow-up period. Also, no distant metastasis was observed with annual CT examinations, which is comparable to the rate of 0.2% after initial surgery (42). In the present study, the lack of local recurrence and relatively low complication rate in the postablation period may be attributed to the successful application of the continuous hydrodissection technique. This seems to be a key factor to effectively and safely ensure extended ablation of the PTMC nodules, especially for the nodules close to the important structures, such as trachea, esophagus, internal carotid artery, and the posterior capsule of the thyroid. In the present study, the incidence of temporary voice changes was only 1.7%, which is lower than the reported overall complication rate of 7.1% after surgery and 6.0% after microwave ablation (35, 43). A wide enough liquid barrier in the paratracheal parenchyma can reduce the incidence of recurrent laryngeal nerve injury. Figure 2A-2D shows that with the help of the continuous hydrodissection technique, extended RFA was achieved in the PTMC nodule close to the trachea through the lateral cervical approach, thereby avoiding damage to superior thyroid artery and the trachea.
In a previous study on RFA of large benign thyroid nodules, the concentrations of FT3 and FT4 increased slightly 1 day after RFA and returned to normal 1 month after RFA, with the TSH concentration decreasing slightly 1 day after RFA and also returning to normal 1 month thereafter (44). In this study, 1 day after RFA, the thyroxine level was significantly higher than that before treatment (Fig. 3A-3C), which may be due to an increased thyroxine release caused by RFA. One month after RFA, the concentrations of FT3, FT4, and TSH did not return to the preoperative level (Fig. 3A-3C), which may be caused by the TSH suppressive therapy. Therefore, we believe that although the extended ablation strategy was applied to ensure complete ablation of the PTMC nodules, the treatment strategy did not influence the thyroid function significantly.
Our study has several limitations. First, the number of patients enrolled in the present study is limited and the follow-up time is relatively short. In addition, consistent with previous research that East Asian PTC patients have the highest rate of BRAF V600E mutations (76.4%) (45), there were more BRAF mutation–positive than –negative PTMC patients in our department. Only 9 patients with 9 PTMC nodules who were confirmed to be negative in BRAF V600E mutations received RFA in our department, and at the last follow-up the ablation zones in 6 patients could not be seen completely and the ablation zones of 3 nodules showed fissure-like changes. The number of these BRAF mutation–negative PTMC patients is too small, thus there were not enough cases for the control group in this study. Further prospective multicenter comparative studies involving more patients with long-term follow-ups would definitely contribute to further clarification of the efficacy and safety of RFA for PTMCs with BRAF V600E mutation.
Conclusion
The present results indicate that with the help of a continuous hydrodissection technique, extended RFA is an effective and safe modality for unifocal PTMCs displaying BRAF V600E mutation, especially when surgery is not feasible or is refused for some reason by those patients who decline an active surveillance.
Disclosures
None.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
References
Abbreviations
- CEUS
contrast-enhanced ultrasound
- CNB
core needle biopsy
- CT
computed tomography
- FNA
fine needle aspiration
- LNM
lymph node metastasis
- PTC
papillary thyroid carcinoma
- PTMC
papillary thyroid microcarcinoma
- RFA
radiofrequency ablation
- FT3
free triiodothyronine
- FT4
free thyroxine
- TSH
thyrotropin (thyroid-stimulating hormone)
- US
ultrasound
Author notes
Co-first authors: Yan Lin, Zhao-rong Wu.



