-
PDF
- Split View
-
Views
-
Cite
Cite
Nanna Maria Uldall Torp, Niels Henrik Bruun, Peter Astrup Christensen, Aase Handberg, Stig Andersen, Stine Linding Andersen, Thyrotropin Receptor Antibodies in Early Pregnancy, The Journal of Clinical Endocrinology & Metabolism, Volume 107, Issue 9, September 2022, Pages e3705–e3713, https://doi.org/10.1210/clinem/dgac383
Close - Share Icon Share
Abstract
Thyrotropin (TSH) receptor antibodies (TRAb) are important when distinguishing between Graves’ and gestational hyperthyroidism, but sparse evidence exists on the recommended cutoff during pregnancy.
This work aimed to establish a method- and pregnancy-specific cutoff for TRAb, to describe the frequency of TRAb positivity in early pregnancy, and to follow up the women in the years after pregnancy.
This cohort study used the North Denmark Region Pregnancy Cohort and Danish nationwide registers of women in the North Denmark Region who had a blood sample drawn in early pregnancy, 2011 to 2015, that was stored in a biobank for assessment of thyroid function and thyroid autoantibodies. A cutoff value for TRAb was established in a reference cohort (n = 524) and used to identify TRAb-positive and TRAb-negative hyperthyroidism in early pregnancy for evaluation of frequency and follow-up.
The method- and cohort-specific cutoff for TRAb in early pregnancy was 0.98 IU/L (95% CI, 0.96-0.99 IU/L). Among women with low TSH in early pregnancy and no known thyroid disease (n = 414), 21 women (5.1%) were TRAb positive and 393 (94.9%) were TRAb negative. Follow-up in the years following the pregnancy (median 8.1 years) revealed that 52.4% of women with TRAb-positive hyperthyroidism and 8.4% of the women with TRAb-negative hyperthyroidism were diagnosed with hyperthyroidism.
This is the first study to measure TRAb in a large group of women in early pregnancy and to establish a pregnancy-specific cutoff. Results reveal that TRAb-negative hyperthyroidism is predominant in early pregnancy and rarely associated with later development of hyperthyroidism.
The measurement of thyrotropin (TSH) receptor antibodies (TRAb) is a key determinant in the diagnosis of Graves’ disease (GD). Thus, the assessment of TRAb is commonly used to identify the cause of hyperthyroidism in newly diagnosed patients (1). Particularly in pregnant patients, TRAb is important to distinguish between hyperthyroidism caused by GD and gestational hyperthyroidism in the early pregnancy. TRAb are autoantibodies that bind to the TSH receptor that can cause the excessive production of thyroid hormone (2). TRAb can be stimulatory, inhibitory, or neutral, and the methods used for assessment of TRAb in clinical laboratories are immunoassays. In contrast to bioassays, these routinely used methods do not distinguish between stimulatory and blocking TRAb (3). TRAb immunoassays are not standardized, and levels obtained with different assays are not necessarily similar. Therefore, assay-specific cutoffs are important for correct diagnosis, and for monitoring of disease activity it is preferable that the same assay is used in each patient. Besides assay type, several other determinants may possibly influence the level of TRAb observed in individual patients. The pregnancy period is characterized by immune suppression, to allow for the development of the fetus, which is followed by a strong immune rebound after birth of the child (4, 5). Consequently, TRAb levels may differ between pregnant and nonpregnant individuals, and the levels may also vary during different parts of a pregnancy. How the levels of TRAb deviate during and after a pregnancy in individual patients has previously been considered and described (6-9). On the other hand, sparse evidence is available on the recommended cutoff for TRAb in early pregnancy. Such a cutoff is of clinical importance as part of the evaluation of newly diagnosed hyperthyroidism, which could be caused by GD or be part of gestational hyperthyroidism. The clinical distinction between these types of hyperthyroidism in pregnant women is crucial because the management and treatment in pregnancy differ substantially (10). To inform clinical practice, more evidence on the course and outcome of each type of hyperthyroidism in and after the pregnancy is warranted.
The North Denmark Region Pregnancy Cohort (NDRPC) is a large cohort including all pregnant women in the North Denmark Region from 2011 to 2015 (11). Each woman had thyroid function assessed retrospectively in a stored blood sample from the early pregnancy. In all women with suppressed TSH and in a random subgroup of women, we measured TRAb for the present study. Such measurements were performed with the aim of establishing an early pregnancy cutoff for TRAb. Furthermore, we aimed to describe the frequency of GD as opposed to gestational hyperthyroidism in the cohort and to follow the thyroid status in each group of women during the years after the pregnancy using Danish nationwide health registers.
Materials and Methods
Study Cohorts
This study was a retrospective cohort study within the NDRPC (11). The NDRPC was established by collection of the residues of blood samples drawn from pregnant women as part of the Danish nationwide prenatal screening program for fetal chromosomal anomalies (12). As previously described in detail (11), the cohort holds information on thyroid function parameters in 17 669 blood samples drawn in early pregnancy from 14 323 unique women (Fig. 1). For the present study, 2 cohorts were selected among women in the NDRPC for measurement of TRAb (see Fig. 1). If a woman had more than one blood sample during the inclusion period, the first blood sample in the first pregnancy was assessed. All blood samples in NDRPC with TSH below 0.1 mIU/L were selected as a “low TSH cohort.” The TSH cutoff applied was the previously established lower refence limit in the first trimester of pregnancy within the same cohort (11). Furthermore, all blood samples drawn from November 1, 2012, to December 31, 2012, were included as a “random cohort.” A small group of women (n = 25) fulfilled the criteria for inclusion in both cohorts (see Fig. 1). From the random cohort, a reference cohort for establishment of a cutoff for TRAb in early pregnancy was selected after the exclusion of women with multiple pregnancies, late timing of blood sampling, and/or markers of thyroid disease (see Fig. 1). The selection of women with TSH in the range from 0.1 to 2.9 mIU/L for the reference cohort was made from the previously established first trimester cohort- and method-specific reference ranges (11). The study was approved by the North Denmark Region Committee on Health Research Ethics (No. N-20150015) and registered according to the General Data Protection Regulation in the North Denmark Region.
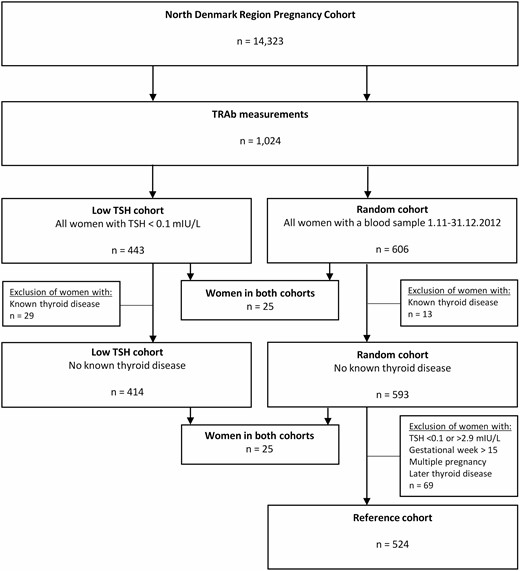
Flowchart of the study population illustrating the selection of the “low TSH cohort,” the random cohort, and the reference cohort.
Biochemical Measurements
As previously described, the serum residues were stored at –80 °C until the biochemical measurements (11). In short, TSH, free thyroxine (fT4), thyroid-peroxidase antibodies (TPO-Ab), and thyroglobulin antibodies (Tg-Ab) were measured during 2015 to 2016 by an automated immunoassay (ADVIA Centaur XPT, Siemens Healthineers). The measurement range for TSH was 0.008 to 150 mIU/L, and values below the functional sensitivity were replaced by half the functional sensitivity (0.004 mIU/L). To determine whether women were TPO-Ab and/or Tg-Ab positive in the present study, we used a cutoff of 60 IU/L as provided by the manufacturer. From September 2020 to November 2020, TRAb was measured in the established cohorts for the present study. The measurements were conducted by an automated immunoassay (BRAHMS TRAK Human, Kryptor Compact, Thermo Fisher Diagnostics Aps) in the Department of Clinical Biochemistry, Aalborg University Hospital. The detection limit was 0.27 IU/l, and the cutoff value provided by the manufacturer for nonpregnant adults was 1.8 IU/L. The measurement of β-human chorionic gonadotropin (β-hCG) is part of the Danish nationwide prenatal screening program (12) and was assessed by BRAHMS free βhCG, Kryptor Compact, Thermo Fisher Diagnostics Aps.
Diagnoses and Treatment
Every citizen in Denmark is provided a unique personal identification number (13), which enabled the linkage of the biochemical measurements to information in the Danish nationwide registers (the Medical Birth Register (14), the Danish National Hospital Register [DNHR] (15), and the Danish National Prescription Register [DNPR]) (16). The Medical Birth Register holds information on maternal age, parity, origin, smoking in pregnancy, and prepregnancy body mass index (BMI) as well as information on fetal sex, gestational age at birth, whether the pregnancy was a singleton or multiple pregnancy, and whether the outcome was a live birth. From the DNHR, we collected information on inpatient and outpatient hospital diagnoses during the period 1994 to 2018, coded according to the 10th International Classification of Diseases (ICD-10). Medical treatment was identified by redeemed prescriptions in the period from 1995 to 2020, coded according to the Anatomic Therapeutic Chemical classification system in the DNPR. The date of blood sampling for each pregnant woman was within a 5-year period (2011-2015) and follow-up was fixed until 2018 in the DNHR and 2020 in the DNPR. The combined information of diagnoses and prescriptions was used to assess subtypes of thyroid disease and medication use in the cohorts, as previously described in detail (17). The present study focused on maternal hyperthyroidism, which was defined by a hyperthyroid diagnosis (ICD-10: DE05) in the DNHR and/or a prescription of antithyroid drugs (ATD) in the DNPR (Anatomic Therapeutic Chemical: H03B). The date of diagnosis was defined as the date of first prescription or first diagnosis, whichever came first. Use of ATD in pregnancy was defined by a redeemed prescription of ATD within the time window from 1 month before the pregnancy start and throughout the pregnancy.
Statistical Analyses
Maternal characteristics were presented nonparametrically by median and interquartile range (IQR) or 95% CI (for continuous variables) or as relative frequency (RF) and 95% CI (for categorical variables). For RFs, CIs were calculated using the binomial exact model. The distribution of TRAb levels in the reference cohort showed a high degree of left-censoring with 79.6% of the values below the detection limit. Consequently, we established a cutoff (95-percentile) by regression on order statistics after log-transformation, also known as log-probit regression, which is a recommended method for such distributions (18, 19). Statistical analyses were performed using STATA version 16 (Stata Corp).
Results
Study Population
In the NDRPC, 443 women had a TSH less than 0.1 mIU/L in early pregnancy and were included in the “low TSH cohort,” while 606 women were part of the random cohort (see Fig. 1). A small group of women were overlapping between the cohorts, thus, TRAb was measured in a total of 1024 unique women. Overall, the random cohort was comparable with the full NDRPC (Table 1). In contrast, the women in the “low TSH cohort” had a higher median β-hCG value and were more often carrying a female fetus and a multiple pregnancy. Furthermore, the women in the “low TSH cohort” were generally older, more often multiparous and of non-Danish origin, and less often overweight (see Table 1).
| . | Full cohort . | . | Random cohort . | . | Low TSH cohorta . | . |
|---|---|---|---|---|---|---|
| . | n = 14 323 . | . | n = 606 . | . | n = 443 . | . |
| . | Median . | IQR . | Median . | IQR . | Median . | IQR . |
| TSH, mIU/L | 1.109 | 0.661-1.689 | 1.068 | 0.597-1.564 | 0.033 | 0.014-0.064 |
| fT4, pmol/L | 16.01 | 14.76-17.35 | 15.89 | 14.61-17.20 | 20.22 | 18.24-22.71 |
| β-hCG, IU/L | 61 | 39-92 | 64 | 40-96 | 97 | 60-138 |
| Gestational wk | 10 | 9-11 | 10 | 9-11 | 10 | 10-11 |
| . | Full cohort . | . | Random cohort . | . | Low TSH cohorta . | . |
|---|---|---|---|---|---|---|
| . | n = 14 323 . | . | n = 606 . | . | n = 443 . | . |
| . | Median . | IQR . | Median . | IQR . | Median . | IQR . |
| TSH, mIU/L | 1.109 | 0.661-1.689 | 1.068 | 0.597-1.564 | 0.033 | 0.014-0.064 |
| fT4, pmol/L | 16.01 | 14.76-17.35 | 15.89 | 14.61-17.20 | 20.22 | 18.24-22.71 |
| β-hCG, IU/L | 61 | 39-92 | 64 | 40-96 | 97 | 60-138 |
| Gestational wk | 10 | 9-11 | 10 | 9-11 | 10 | 10-11 |
| . | n . | % . | n . | % . | n . | % . |
|---|---|---|---|---|---|---|
| Age ≥ 30 y | 6935 | 48.4 | 305 | 50.3 | 257 | 58.0 |
| Origin other than Danishb | 1670 | 11.7 | 77 | 12.7 | 84 | 19.0 |
| Multiple pregnancy | 293 | 2.0 | 8 | 1.3 | 42 | 9.5 |
| Live birth | 13 160 | 91.9 | 567 | 93.6 | 419 | 94.6 |
| Male fetal sexc | 6592 | 51.0 | 299 | 53.3 | 150 | 39.3 |
| Multiparityd | 6511 | 49.5 | 296 | 52.3 | 259 | 62.0 |
| Smokerd | 1564 | 11.9 | 62 | 10.9 | 51 | 12.2 |
| BMId ≥ 25.0 | 5178 | 39.4 | 217 | 38.3 | 130 | 31.2 |
| . | n . | % . | n . | % . | n . | % . |
|---|---|---|---|---|---|---|
| Age ≥ 30 y | 6935 | 48.4 | 305 | 50.3 | 257 | 58.0 |
| Origin other than Danishb | 1670 | 11.7 | 77 | 12.7 | 84 | 19.0 |
| Multiple pregnancy | 293 | 2.0 | 8 | 1.3 | 42 | 9.5 |
| Live birth | 13 160 | 91.9 | 567 | 93.6 | 419 | 94.6 |
| Male fetal sexc | 6592 | 51.0 | 299 | 53.3 | 150 | 39.3 |
| Multiparityd | 6511 | 49.5 | 296 | 52.3 | 259 | 62.0 |
| Smokerd | 1564 | 11.9 | 62 | 10.9 | 51 | 12.2 |
| BMId ≥ 25.0 | 5178 | 39.4 | 217 | 38.3 | 130 | 31.2 |
Abbreviations: β-hCG, β-human chorionic gonadotropin; BMI, body mass index; fT4, free thyroxine; IQR, interquartile range, TSH, thyrotropin.
aPregnant women in the full cohort with TSH less than 0.1 mIU/L.
bIndividuals with missing values not included (n = 37).
cInformation available only for singleton live and still births, missing values not included (n = 1126).
dInformation available only for live and still births, missing values not included: parity (n = 1180), smoking (n = 1176), and BMI (n = 1187).
| . | Full cohort . | . | Random cohort . | . | Low TSH cohorta . | . |
|---|---|---|---|---|---|---|
| . | n = 14 323 . | . | n = 606 . | . | n = 443 . | . |
| . | Median . | IQR . | Median . | IQR . | Median . | IQR . |
| TSH, mIU/L | 1.109 | 0.661-1.689 | 1.068 | 0.597-1.564 | 0.033 | 0.014-0.064 |
| fT4, pmol/L | 16.01 | 14.76-17.35 | 15.89 | 14.61-17.20 | 20.22 | 18.24-22.71 |
| β-hCG, IU/L | 61 | 39-92 | 64 | 40-96 | 97 | 60-138 |
| Gestational wk | 10 | 9-11 | 10 | 9-11 | 10 | 10-11 |
| . | Full cohort . | . | Random cohort . | . | Low TSH cohorta . | . |
|---|---|---|---|---|---|---|
| . | n = 14 323 . | . | n = 606 . | . | n = 443 . | . |
| . | Median . | IQR . | Median . | IQR . | Median . | IQR . |
| TSH, mIU/L | 1.109 | 0.661-1.689 | 1.068 | 0.597-1.564 | 0.033 | 0.014-0.064 |
| fT4, pmol/L | 16.01 | 14.76-17.35 | 15.89 | 14.61-17.20 | 20.22 | 18.24-22.71 |
| β-hCG, IU/L | 61 | 39-92 | 64 | 40-96 | 97 | 60-138 |
| Gestational wk | 10 | 9-11 | 10 | 9-11 | 10 | 10-11 |
| . | n . | % . | n . | % . | n . | % . |
|---|---|---|---|---|---|---|
| Age ≥ 30 y | 6935 | 48.4 | 305 | 50.3 | 257 | 58.0 |
| Origin other than Danishb | 1670 | 11.7 | 77 | 12.7 | 84 | 19.0 |
| Multiple pregnancy | 293 | 2.0 | 8 | 1.3 | 42 | 9.5 |
| Live birth | 13 160 | 91.9 | 567 | 93.6 | 419 | 94.6 |
| Male fetal sexc | 6592 | 51.0 | 299 | 53.3 | 150 | 39.3 |
| Multiparityd | 6511 | 49.5 | 296 | 52.3 | 259 | 62.0 |
| Smokerd | 1564 | 11.9 | 62 | 10.9 | 51 | 12.2 |
| BMId ≥ 25.0 | 5178 | 39.4 | 217 | 38.3 | 130 | 31.2 |
| . | n . | % . | n . | % . | n . | % . |
|---|---|---|---|---|---|---|
| Age ≥ 30 y | 6935 | 48.4 | 305 | 50.3 | 257 | 58.0 |
| Origin other than Danishb | 1670 | 11.7 | 77 | 12.7 | 84 | 19.0 |
| Multiple pregnancy | 293 | 2.0 | 8 | 1.3 | 42 | 9.5 |
| Live birth | 13 160 | 91.9 | 567 | 93.6 | 419 | 94.6 |
| Male fetal sexc | 6592 | 51.0 | 299 | 53.3 | 150 | 39.3 |
| Multiparityd | 6511 | 49.5 | 296 | 52.3 | 259 | 62.0 |
| Smokerd | 1564 | 11.9 | 62 | 10.9 | 51 | 12.2 |
| BMId ≥ 25.0 | 5178 | 39.4 | 217 | 38.3 | 130 | 31.2 |
Abbreviations: β-hCG, β-human chorionic gonadotropin; BMI, body mass index; fT4, free thyroxine; IQR, interquartile range, TSH, thyrotropin.
aPregnant women in the full cohort with TSH less than 0.1 mIU/L.
bIndividuals with missing values not included (n = 37).
cInformation available only for singleton live and still births, missing values not included (n = 1126).
dInformation available only for live and still births, missing values not included: parity (n = 1180), smoking (n = 1176), and BMI (n = 1187).
TRAb Cutoff in Early Pregnancy
The “reference cohort” for the establishment of the TRAb cutoff included 524 women (see Fig. 1) with a median age of 29.9 years (IQR: 26.9-33.0), and a median gestational week at blood sampling of 10 (IQR: 9-11). With the use of regression on order statistics, a cutoff of 0.98 IU/L (95% CI, 0.96-0.99 IU/L) for TRAb positivity in early pregnancy was found. The cutoff was robust when analyses were restricted to live-born pregnancies (n = 493; cutoff: 0.98 IU/L [95% CI, 0.96-1.00 IU/L]) and when no selection was made according to maternal TSH in early pregnancy (n = 565; cutoff: 0.95 IU/L [95% CI, 0.93-0.96 IU/L]).
Considering maternal thyroid function tests in relation to the established cutoff (1.0 IU/L) and the cutoff recommended by the manufacturer (1.8 IU/L) among women with low TSH in early pregnancy, median maternal TSH was lower and fT4 higher also among women with TRAb in the range from 1.0 to 1.8 IU/L as compared to the large group of women with TRAb less than or equal to 1.0 IU/L (Table 2). Furthermore, median β-hCG was higher and the RF of prior or later hyperthyroid diagnoses was much lower among the women with TRAb less than or equal to 1.0 IU/L compared to the TRAb-positive groups (see Table 2).
Characteristics of pregnant women with thyrotropin (TSH) less than 0.1 mIU/L (n = 443) stratified by level of thyrotropin receptor antibodies (TRAb) according to the established cohort-specific cutoff (1.0 IU/L) and the cutoff provided by the manufacturer (1.8 IU/L)
| . | TRAb ≤ 1.0 IU/L . | . | TRAb > 1.0 and ≤ 1.8 IU/L . | . | TRAb > 1.8 IU/L . | . |
|---|---|---|---|---|---|---|
| . | n = 414 . | . | n = 8 . | . | n = 21 . | . |
| . | Median . | 95% CI . | Median . | 95% CI . | Median . | 95% CI . |
| TSH, mIU/L | 0.037 | 0.032-0.041 | 0.017 | 0.004-0.061 | 0.004 | 0.004-0.009 |
| fT4, pmol/L | 20.18 | 19.87-20.54 | 21.17 | 16.02-24.05 | 22.66 | 20.18-25.92 |
| β-hCG, IU/L | 100.5 | 93.5-106.5 | 62.0 | 27.6-114.3 | 57.0 | 45.4-78.0 |
| Gestational wk | 10 | 10-10 | 10 | 9-12 | 9 | 9-10 |
| Age, y | 30.9 | 30.3-31.3 | 32.2 | 27.0-38.4 | 29.4 | 27.2-33.7 |
| BMIa | 23.1 | 22.7-23.7 | 22.8 | 20.5-25.6 | 25.0 | 24.2-25.9 |
| . | TRAb ≤ 1.0 IU/L . | . | TRAb > 1.0 and ≤ 1.8 IU/L . | . | TRAb > 1.8 IU/L . | . |
|---|---|---|---|---|---|---|
| . | n = 414 . | . | n = 8 . | . | n = 21 . | . |
| . | Median . | 95% CI . | Median . | 95% CI . | Median . | 95% CI . |
| TSH, mIU/L | 0.037 | 0.032-0.041 | 0.017 | 0.004-0.061 | 0.004 | 0.004-0.009 |
| fT4, pmol/L | 20.18 | 19.87-20.54 | 21.17 | 16.02-24.05 | 22.66 | 20.18-25.92 |
| β-hCG, IU/L | 100.5 | 93.5-106.5 | 62.0 | 27.6-114.3 | 57.0 | 45.4-78.0 |
| Gestational wk | 10 | 10-10 | 10 | 9-12 | 9 | 9-10 |
| Age, y | 30.9 | 30.3-31.3 | 32.2 | 27.0-38.4 | 29.4 | 27.2-33.7 |
| BMIa | 23.1 | 22.7-23.7 | 22.8 | 20.5-25.6 | 25.0 | 24.2-25.9 |
| . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . |
|---|---|---|---|---|---|---|---|---|---|
| Known or later hyperthyroid diagnosis | 45 | 10.9 | 8.0-14.3 | 5 | 62.5 | 24.5-91.5 | 14 | 66.7 | 43.0-85.4 |
| . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . |
|---|---|---|---|---|---|---|---|---|---|
| Known or later hyperthyroid diagnosis | 45 | 10.9 | 8.0-14.3 | 5 | 62.5 | 24.5-91.5 | 14 | 66.7 | 43.0-85.4 |
Abbreviations: β-hCG, β-human chorionic gonadotropin; BMI, body mass index; fT4, free thyroxine; RF, relative frequency; TRAb, thyrotropin receptor antibodies; TSH, thyrotropin.
aInformation available only for live and still births, missing values not included: BMI (n = 1187).
Characteristics of pregnant women with thyrotropin (TSH) less than 0.1 mIU/L (n = 443) stratified by level of thyrotropin receptor antibodies (TRAb) according to the established cohort-specific cutoff (1.0 IU/L) and the cutoff provided by the manufacturer (1.8 IU/L)
| . | TRAb ≤ 1.0 IU/L . | . | TRAb > 1.0 and ≤ 1.8 IU/L . | . | TRAb > 1.8 IU/L . | . |
|---|---|---|---|---|---|---|
| . | n = 414 . | . | n = 8 . | . | n = 21 . | . |
| . | Median . | 95% CI . | Median . | 95% CI . | Median . | 95% CI . |
| TSH, mIU/L | 0.037 | 0.032-0.041 | 0.017 | 0.004-0.061 | 0.004 | 0.004-0.009 |
| fT4, pmol/L | 20.18 | 19.87-20.54 | 21.17 | 16.02-24.05 | 22.66 | 20.18-25.92 |
| β-hCG, IU/L | 100.5 | 93.5-106.5 | 62.0 | 27.6-114.3 | 57.0 | 45.4-78.0 |
| Gestational wk | 10 | 10-10 | 10 | 9-12 | 9 | 9-10 |
| Age, y | 30.9 | 30.3-31.3 | 32.2 | 27.0-38.4 | 29.4 | 27.2-33.7 |
| BMIa | 23.1 | 22.7-23.7 | 22.8 | 20.5-25.6 | 25.0 | 24.2-25.9 |
| . | TRAb ≤ 1.0 IU/L . | . | TRAb > 1.0 and ≤ 1.8 IU/L . | . | TRAb > 1.8 IU/L . | . |
|---|---|---|---|---|---|---|
| . | n = 414 . | . | n = 8 . | . | n = 21 . | . |
| . | Median . | 95% CI . | Median . | 95% CI . | Median . | 95% CI . |
| TSH, mIU/L | 0.037 | 0.032-0.041 | 0.017 | 0.004-0.061 | 0.004 | 0.004-0.009 |
| fT4, pmol/L | 20.18 | 19.87-20.54 | 21.17 | 16.02-24.05 | 22.66 | 20.18-25.92 |
| β-hCG, IU/L | 100.5 | 93.5-106.5 | 62.0 | 27.6-114.3 | 57.0 | 45.4-78.0 |
| Gestational wk | 10 | 10-10 | 10 | 9-12 | 9 | 9-10 |
| Age, y | 30.9 | 30.3-31.3 | 32.2 | 27.0-38.4 | 29.4 | 27.2-33.7 |
| BMIa | 23.1 | 22.7-23.7 | 22.8 | 20.5-25.6 | 25.0 | 24.2-25.9 |
| . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . |
|---|---|---|---|---|---|---|---|---|---|
| Known or later hyperthyroid diagnosis | 45 | 10.9 | 8.0-14.3 | 5 | 62.5 | 24.5-91.5 | 14 | 66.7 | 43.0-85.4 |
| . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . |
|---|---|---|---|---|---|---|---|---|---|
| Known or later hyperthyroid diagnosis | 45 | 10.9 | 8.0-14.3 | 5 | 62.5 | 24.5-91.5 | 14 | 66.7 | 43.0-85.4 |
Abbreviations: β-hCG, β-human chorionic gonadotropin; BMI, body mass index; fT4, free thyroxine; RF, relative frequency; TRAb, thyrotropin receptor antibodies; TSH, thyrotropin.
aInformation available only for live and still births, missing values not included: BMI (n = 1187).
TRAb Positivity in Early Pregnancy
After the establishment of a cohort- and method-specific cutoff for TRAb, the RF of TRAb positivity was evaluated. As expected, the RF was higher in the “low TSH cohort” compared with the random cohort (Table 3). The RF of TPO-Ab and Tg-Ab positivity was not higher in the “low TSH cohort” compared with the random cohort; however, the combined positivity of TRAb and TPO-Ab and/or Tg-Ab was more frequent in the “low TSH cohort” (see Table 3). We subsequently considered all women with a measurement of TRAb (n = 1024) and stratified the women by TRAb positivity and by level of TSH (Table 4). Among women with low TSH in early pregnancy, TRAb positive women had lower median TSH, higher fT4, and lower β-hCG levels. Furthermore, the TRAb-positive women were younger, more often smoking, and TPO-Ab/Tg-Ab positive, less often carried a multiple pregnancy or a female fetus, and they less often terminated the pregnancy with live birth (see Table 4). Finally, they more often had known thyroid disease at the time of blood sampling. When the same variables were considered among women who did not have a low TSH in early pregnancy (see Table 4), the aforementioned differences in TRAb positivity were not seen. On the other hand, TRAb-positive women with TSH greater than or equal to 0.1 mIU/L were older with higher parity and a higher BMI and were more often TPO-Ab/Tg-Ab positive than TRAb-negative women (see Table 4).
Frequency of thyroid autoantibodies among pregnant women in the random cohort and in the low thyrotropin (TSH) cohort (TSH < 0.1 mIU/L)
| . | All women . | . | . | . | . | . | No known thyroid diseasea . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Random cohort . | . | . | Low TSH cohort . | . | . | Random cohort . | . | . | Low TSH cohort . | . | . |
| . | n = 606 . | . | . | n = 443 . | . | . | n = 593 . | . | . | n = 414 . | . | . |
| . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . |
| TRAb > 1.0 IU/L | 28 | 4.6 | 3.1-6.6 | 29 | 6.5 | 4.4-9.3 | 26 | 4.4 | 2.9-6.4 | 21 | 5.1 | 3.2-7.6 |
| TPO-Ab positiveb | 62 | 10.2 | 7.9-12.9 | 45 | 10.2 | 7.5-13.4 | 52 | 8.8 | 6.6-11.3 | 34 | 8.2 | 5.8-11.3 |
| Tg-Ab positiveb | 66 | 10.9 | 8.5-13.6 | 35 | 7.9 | 5.6-10.8 | 59 | 9.9 | 7.7-12.6 | 24 | 5.8 | 3.7-8.5 |
| TRAb > 1.0 IU/L and TPO- and/or Tg-Ab positiveb | 8 | 1.3 | 0.6-2.6 | 18 | 4.1 | 2.4-6.3 | 6 | 1.0 | 0.4-2.2 | 12 | 2.9 | 1.5-5.0 |
| . | All women . | . | . | . | . | . | No known thyroid diseasea . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Random cohort . | . | . | Low TSH cohort . | . | . | Random cohort . | . | . | Low TSH cohort . | . | . |
| . | n = 606 . | . | . | n = 443 . | . | . | n = 593 . | . | . | n = 414 . | . | . |
| . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . |
| TRAb > 1.0 IU/L | 28 | 4.6 | 3.1-6.6 | 29 | 6.5 | 4.4-9.3 | 26 | 4.4 | 2.9-6.4 | 21 | 5.1 | 3.2-7.6 |
| TPO-Ab positiveb | 62 | 10.2 | 7.9-12.9 | 45 | 10.2 | 7.5-13.4 | 52 | 8.8 | 6.6-11.3 | 34 | 8.2 | 5.8-11.3 |
| Tg-Ab positiveb | 66 | 10.9 | 8.5-13.6 | 35 | 7.9 | 5.6-10.8 | 59 | 9.9 | 7.7-12.6 | 24 | 5.8 | 3.7-8.5 |
| TRAb > 1.0 IU/L and TPO- and/or Tg-Ab positiveb | 8 | 1.3 | 0.6-2.6 | 18 | 4.1 | 2.4-6.3 | 6 | 1.0 | 0.4-2.2 | 12 | 2.9 | 1.5-5.0 |
Abbreviations: RF, relative frequency; Tg-Ab, thyroglobulin antibodies; TPO-Ab, thyroid peroxidase antibodies; TRAb, thyrotropin receptor antibodies; TSH, thyrotropin.
aNo hospital diagnosis or redeemed prescription of drug before date of blood sampling in early pregnancy.
bTPO-Ab and/or Tg-Ab greater than 60 IU/L.
Frequency of thyroid autoantibodies among pregnant women in the random cohort and in the low thyrotropin (TSH) cohort (TSH < 0.1 mIU/L)
| . | All women . | . | . | . | . | . | No known thyroid diseasea . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Random cohort . | . | . | Low TSH cohort . | . | . | Random cohort . | . | . | Low TSH cohort . | . | . |
| . | n = 606 . | . | . | n = 443 . | . | . | n = 593 . | . | . | n = 414 . | . | . |
| . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . |
| TRAb > 1.0 IU/L | 28 | 4.6 | 3.1-6.6 | 29 | 6.5 | 4.4-9.3 | 26 | 4.4 | 2.9-6.4 | 21 | 5.1 | 3.2-7.6 |
| TPO-Ab positiveb | 62 | 10.2 | 7.9-12.9 | 45 | 10.2 | 7.5-13.4 | 52 | 8.8 | 6.6-11.3 | 34 | 8.2 | 5.8-11.3 |
| Tg-Ab positiveb | 66 | 10.9 | 8.5-13.6 | 35 | 7.9 | 5.6-10.8 | 59 | 9.9 | 7.7-12.6 | 24 | 5.8 | 3.7-8.5 |
| TRAb > 1.0 IU/L and TPO- and/or Tg-Ab positiveb | 8 | 1.3 | 0.6-2.6 | 18 | 4.1 | 2.4-6.3 | 6 | 1.0 | 0.4-2.2 | 12 | 2.9 | 1.5-5.0 |
| . | All women . | . | . | . | . | . | No known thyroid diseasea . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Random cohort . | . | . | Low TSH cohort . | . | . | Random cohort . | . | . | Low TSH cohort . | . | . |
| . | n = 606 . | . | . | n = 443 . | . | . | n = 593 . | . | . | n = 414 . | . | . |
| . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . |
| TRAb > 1.0 IU/L | 28 | 4.6 | 3.1-6.6 | 29 | 6.5 | 4.4-9.3 | 26 | 4.4 | 2.9-6.4 | 21 | 5.1 | 3.2-7.6 |
| TPO-Ab positiveb | 62 | 10.2 | 7.9-12.9 | 45 | 10.2 | 7.5-13.4 | 52 | 8.8 | 6.6-11.3 | 34 | 8.2 | 5.8-11.3 |
| Tg-Ab positiveb | 66 | 10.9 | 8.5-13.6 | 35 | 7.9 | 5.6-10.8 | 59 | 9.9 | 7.7-12.6 | 24 | 5.8 | 3.7-8.5 |
| TRAb > 1.0 IU/L and TPO- and/or Tg-Ab positiveb | 8 | 1.3 | 0.6-2.6 | 18 | 4.1 | 2.4-6.3 | 6 | 1.0 | 0.4-2.2 | 12 | 2.9 | 1.5-5.0 |
Abbreviations: RF, relative frequency; Tg-Ab, thyroglobulin antibodies; TPO-Ab, thyroid peroxidase antibodies; TRAb, thyrotropin receptor antibodies; TSH, thyrotropin.
aNo hospital diagnosis or redeemed prescription of drug before date of blood sampling in early pregnancy.
bTPO-Ab and/or Tg-Ab greater than 60 IU/L.
Characteristics of all women with early pregnancy measurement of thyrotropin receptor antibodies (TRAb) when stratified by level of TRAb and thyrotropin (TSH)
| . | TSH < 0.1 mIU/L . | . | . | . | TSH ≥ 0.1 mIU/L . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| . | TRAb > 1.0 IU/L . | . | TRAb ≤ 1.0 IU/L . | . | TRAb > 1.0 IU/L . | . | TRAb ≤ 1.0 IU/L . | . |
| . | n = 29 . | . | n = 414 . | . | n = 28 . | . | n = 553 . | . |
| . | Median . | 95% CI . | Median . | 95% CI . | Median . | 95% CI . | Median . | 95% CI . |
| TSH, mIU/L | 0.004 | 0.004-0.012 | 0.037 | 0.032-0.041 | 1.151 | 0.721-1.411 | 1.101 | 1.021-1.176 |
| fT4, pmol/L | 22.66 | 20.13-25.16 | 20.18 | 19.87-20.54 | 15.30 | 14.69-16.11 | 15.82 | 15.64-16.00 |
| β-hCG, IU/L | 57.0 | 49.1-78.0 | 100.5 | 93.5-106.5 | 59.0 | 38.6-69.4 | 64 | 59.0-69.0 |
| Gestational wk | 10 | 9-10 | 10 | 10-10 | 10 | 9-11 | 10 | 10-10 |
| . | TSH < 0.1 mIU/L . | . | . | . | TSH ≥ 0.1 mIU/L . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| . | TRAb > 1.0 IU/L . | . | TRAb ≤ 1.0 IU/L . | . | TRAb > 1.0 IU/L . | . | TRAb ≤ 1.0 IU/L . | . |
| . | n = 29 . | . | n = 414 . | . | n = 28 . | . | n = 553 . | . |
| . | Median . | 95% CI . | Median . | 95% CI . | Median . | 95% CI . | Median . | 95% CI . |
| TSH, mIU/L | 0.004 | 0.004-0.012 | 0.037 | 0.032-0.041 | 1.151 | 0.721-1.411 | 1.101 | 1.021-1.176 |
| fT4, pmol/L | 22.66 | 20.13-25.16 | 20.18 | 19.87-20.54 | 15.30 | 14.69-16.11 | 15.82 | 15.64-16.00 |
| β-hCG, IU/L | 57.0 | 49.1-78.0 | 100.5 | 93.5-106.5 | 59.0 | 38.6-69.4 | 64 | 59.0-69.0 |
| Gestational wk | 10 | 9-10 | 10 | 10-10 | 10 | 9-11 | 10 | 10-10 |
| . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPO-Ab positivea | 15 | 51.7 | 32.5-70.6 | 30 | 7.2 | 4.9-10.2 | 7 | 25.0 | 10.7-44.9 | 52 | 9.5 | 7.1-12.1 |
| Tg-Ab positivea | 12 | 41.4 | 23.5-61.1 | 23 | 5.6 | 3.6-8.2 | 6 | 21.4 | 8.3-41.0 | 57 | 10.3 | 7.9-13.1 |
| Age ≥ 30 y | 15 | 51.7 | 32.5-70.6 | 242 | 58.5 | 53.5-63.2 | 17 | 60.7 | 40.6-78.5 | 271 | 49.0 | 44.8-53.3 |
| Origin other than Danishb | < 5 | N/A | N/A | 81 | 19.6 | 15.9-23.8 | < 5 | N/A | N/A | 69 | 12.5 | 9.9-15.6 |
| Multiple pregnancies | < 5 | N/A | N/A | 41 | 9.9 | 7.2-13.2 | < 5 | N/A | N/A | 6 | 1.1 | 0.4-2.3 |
| Live birth | 24 | 82.8 | 64.2-94.2 | 395 | 95.4 | 92.9-97.2 | N/A | N/A | N/A | 518 | 93.7 | 91.3-95.6 |
| Male fetusc | 11 | 47.8 | 26.8-69.4 | 139 | 38.7 | 33.7-44.0 | 14 | 53.8 | 33.4-73.4 | 278 | 54.2 | 49.8-58.6 |
| Multiparousd | 14 | 58.3 | 36.6-77.9 | 245 | 62.2 | 57.2-67.0 | 15 | 57.7 | 36.9-76.6 | 265 | 51.3 | 46.9-55.6 |
| Smokersd | 5 | 20.8 | 7.1-42.2 | 46 | 11.7 | 8.7-15.3 | < 5 | N/A | N/A | 59 | 11.4 | 8.8-14.4 |
| BMId ≥ 25 | 9 | 37.5 | 18.8-59.4 | 121 | 30.8 | 26.3-35.6 | 14 | 53.8 | 33.4-73.4 | 197 | 38.1 | 33.9-42.4 |
| Thyroid disease at blood sampling | 8 | 27.6 | 12.7-47.2 | 21 | 5.1 | 3.2-7.6 | < 5 | N/A | N/A | 11 | 2.0 | 1.0-3.5 |
| Hyperthyroidism at blood sampling | 8 | 27.6 | 12.7-47.2 | 12 | 2.9 | 1.5-5.0 | < 5 | N/A | N/A | 5 | 0.9 | 0.3-2.1 |
| . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPO-Ab positivea | 15 | 51.7 | 32.5-70.6 | 30 | 7.2 | 4.9-10.2 | 7 | 25.0 | 10.7-44.9 | 52 | 9.5 | 7.1-12.1 |
| Tg-Ab positivea | 12 | 41.4 | 23.5-61.1 | 23 | 5.6 | 3.6-8.2 | 6 | 21.4 | 8.3-41.0 | 57 | 10.3 | 7.9-13.1 |
| Age ≥ 30 y | 15 | 51.7 | 32.5-70.6 | 242 | 58.5 | 53.5-63.2 | 17 | 60.7 | 40.6-78.5 | 271 | 49.0 | 44.8-53.3 |
| Origin other than Danishb | < 5 | N/A | N/A | 81 | 19.6 | 15.9-23.8 | < 5 | N/A | N/A | 69 | 12.5 | 9.9-15.6 |
| Multiple pregnancies | < 5 | N/A | N/A | 41 | 9.9 | 7.2-13.2 | < 5 | N/A | N/A | 6 | 1.1 | 0.4-2.3 |
| Live birth | 24 | 82.8 | 64.2-94.2 | 395 | 95.4 | 92.9-97.2 | N/A | N/A | N/A | 518 | 93.7 | 91.3-95.6 |
| Male fetusc | 11 | 47.8 | 26.8-69.4 | 139 | 38.7 | 33.7-44.0 | 14 | 53.8 | 33.4-73.4 | 278 | 54.2 | 49.8-58.6 |
| Multiparousd | 14 | 58.3 | 36.6-77.9 | 245 | 62.2 | 57.2-67.0 | 15 | 57.7 | 36.9-76.6 | 265 | 51.3 | 46.9-55.6 |
| Smokersd | 5 | 20.8 | 7.1-42.2 | 46 | 11.7 | 8.7-15.3 | < 5 | N/A | N/A | 59 | 11.4 | 8.8-14.4 |
| BMId ≥ 25 | 9 | 37.5 | 18.8-59.4 | 121 | 30.8 | 26.3-35.6 | 14 | 53.8 | 33.4-73.4 | 197 | 38.1 | 33.9-42.4 |
| Thyroid disease at blood sampling | 8 | 27.6 | 12.7-47.2 | 21 | 5.1 | 3.2-7.6 | < 5 | N/A | N/A | 11 | 2.0 | 1.0-3.5 |
| Hyperthyroidism at blood sampling | 8 | 27.6 | 12.7-47.2 | 12 | 2.9 | 1.5-5.0 | < 5 | N/A | N/A | 5 | 0.9 | 0.3-2.1 |
Abbreviations: β-hCG, β-human chorionic gonadotropin; ATD, antithyroid drugs; BMI, body mass index; fT4, free thyroxine; N/A, not available; RF, relative frequency; TRAb, thyrotropin receptor antibodies; TSH, thyrotropin.
aTPOAb- or Tg-Ab greater than 60 IU/L.
bIndividuals with missing values not included (n = 37).
cInformation available only for singleton live and still births, missing values not included (n = 1126).
dInformation available only for live and still births, missing values not included: parity (n = 1180), smoking (n = 1167), BMI (n = 1187).
Characteristics of all women with early pregnancy measurement of thyrotropin receptor antibodies (TRAb) when stratified by level of TRAb and thyrotropin (TSH)
| . | TSH < 0.1 mIU/L . | . | . | . | TSH ≥ 0.1 mIU/L . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| . | TRAb > 1.0 IU/L . | . | TRAb ≤ 1.0 IU/L . | . | TRAb > 1.0 IU/L . | . | TRAb ≤ 1.0 IU/L . | . |
| . | n = 29 . | . | n = 414 . | . | n = 28 . | . | n = 553 . | . |
| . | Median . | 95% CI . | Median . | 95% CI . | Median . | 95% CI . | Median . | 95% CI . |
| TSH, mIU/L | 0.004 | 0.004-0.012 | 0.037 | 0.032-0.041 | 1.151 | 0.721-1.411 | 1.101 | 1.021-1.176 |
| fT4, pmol/L | 22.66 | 20.13-25.16 | 20.18 | 19.87-20.54 | 15.30 | 14.69-16.11 | 15.82 | 15.64-16.00 |
| β-hCG, IU/L | 57.0 | 49.1-78.0 | 100.5 | 93.5-106.5 | 59.0 | 38.6-69.4 | 64 | 59.0-69.0 |
| Gestational wk | 10 | 9-10 | 10 | 10-10 | 10 | 9-11 | 10 | 10-10 |
| . | TSH < 0.1 mIU/L . | . | . | . | TSH ≥ 0.1 mIU/L . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| . | TRAb > 1.0 IU/L . | . | TRAb ≤ 1.0 IU/L . | . | TRAb > 1.0 IU/L . | . | TRAb ≤ 1.0 IU/L . | . |
| . | n = 29 . | . | n = 414 . | . | n = 28 . | . | n = 553 . | . |
| . | Median . | 95% CI . | Median . | 95% CI . | Median . | 95% CI . | Median . | 95% CI . |
| TSH, mIU/L | 0.004 | 0.004-0.012 | 0.037 | 0.032-0.041 | 1.151 | 0.721-1.411 | 1.101 | 1.021-1.176 |
| fT4, pmol/L | 22.66 | 20.13-25.16 | 20.18 | 19.87-20.54 | 15.30 | 14.69-16.11 | 15.82 | 15.64-16.00 |
| β-hCG, IU/L | 57.0 | 49.1-78.0 | 100.5 | 93.5-106.5 | 59.0 | 38.6-69.4 | 64 | 59.0-69.0 |
| Gestational wk | 10 | 9-10 | 10 | 10-10 | 10 | 9-11 | 10 | 10-10 |
| . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPO-Ab positivea | 15 | 51.7 | 32.5-70.6 | 30 | 7.2 | 4.9-10.2 | 7 | 25.0 | 10.7-44.9 | 52 | 9.5 | 7.1-12.1 |
| Tg-Ab positivea | 12 | 41.4 | 23.5-61.1 | 23 | 5.6 | 3.6-8.2 | 6 | 21.4 | 8.3-41.0 | 57 | 10.3 | 7.9-13.1 |
| Age ≥ 30 y | 15 | 51.7 | 32.5-70.6 | 242 | 58.5 | 53.5-63.2 | 17 | 60.7 | 40.6-78.5 | 271 | 49.0 | 44.8-53.3 |
| Origin other than Danishb | < 5 | N/A | N/A | 81 | 19.6 | 15.9-23.8 | < 5 | N/A | N/A | 69 | 12.5 | 9.9-15.6 |
| Multiple pregnancies | < 5 | N/A | N/A | 41 | 9.9 | 7.2-13.2 | < 5 | N/A | N/A | 6 | 1.1 | 0.4-2.3 |
| Live birth | 24 | 82.8 | 64.2-94.2 | 395 | 95.4 | 92.9-97.2 | N/A | N/A | N/A | 518 | 93.7 | 91.3-95.6 |
| Male fetusc | 11 | 47.8 | 26.8-69.4 | 139 | 38.7 | 33.7-44.0 | 14 | 53.8 | 33.4-73.4 | 278 | 54.2 | 49.8-58.6 |
| Multiparousd | 14 | 58.3 | 36.6-77.9 | 245 | 62.2 | 57.2-67.0 | 15 | 57.7 | 36.9-76.6 | 265 | 51.3 | 46.9-55.6 |
| Smokersd | 5 | 20.8 | 7.1-42.2 | 46 | 11.7 | 8.7-15.3 | < 5 | N/A | N/A | 59 | 11.4 | 8.8-14.4 |
| BMId ≥ 25 | 9 | 37.5 | 18.8-59.4 | 121 | 30.8 | 26.3-35.6 | 14 | 53.8 | 33.4-73.4 | 197 | 38.1 | 33.9-42.4 |
| Thyroid disease at blood sampling | 8 | 27.6 | 12.7-47.2 | 21 | 5.1 | 3.2-7.6 | < 5 | N/A | N/A | 11 | 2.0 | 1.0-3.5 |
| Hyperthyroidism at blood sampling | 8 | 27.6 | 12.7-47.2 | 12 | 2.9 | 1.5-5.0 | < 5 | N/A | N/A | 5 | 0.9 | 0.3-2.1 |
| . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPO-Ab positivea | 15 | 51.7 | 32.5-70.6 | 30 | 7.2 | 4.9-10.2 | 7 | 25.0 | 10.7-44.9 | 52 | 9.5 | 7.1-12.1 |
| Tg-Ab positivea | 12 | 41.4 | 23.5-61.1 | 23 | 5.6 | 3.6-8.2 | 6 | 21.4 | 8.3-41.0 | 57 | 10.3 | 7.9-13.1 |
| Age ≥ 30 y | 15 | 51.7 | 32.5-70.6 | 242 | 58.5 | 53.5-63.2 | 17 | 60.7 | 40.6-78.5 | 271 | 49.0 | 44.8-53.3 |
| Origin other than Danishb | < 5 | N/A | N/A | 81 | 19.6 | 15.9-23.8 | < 5 | N/A | N/A | 69 | 12.5 | 9.9-15.6 |
| Multiple pregnancies | < 5 | N/A | N/A | 41 | 9.9 | 7.2-13.2 | < 5 | N/A | N/A | 6 | 1.1 | 0.4-2.3 |
| Live birth | 24 | 82.8 | 64.2-94.2 | 395 | 95.4 | 92.9-97.2 | N/A | N/A | N/A | 518 | 93.7 | 91.3-95.6 |
| Male fetusc | 11 | 47.8 | 26.8-69.4 | 139 | 38.7 | 33.7-44.0 | 14 | 53.8 | 33.4-73.4 | 278 | 54.2 | 49.8-58.6 |
| Multiparousd | 14 | 58.3 | 36.6-77.9 | 245 | 62.2 | 57.2-67.0 | 15 | 57.7 | 36.9-76.6 | 265 | 51.3 | 46.9-55.6 |
| Smokersd | 5 | 20.8 | 7.1-42.2 | 46 | 11.7 | 8.7-15.3 | < 5 | N/A | N/A | 59 | 11.4 | 8.8-14.4 |
| BMId ≥ 25 | 9 | 37.5 | 18.8-59.4 | 121 | 30.8 | 26.3-35.6 | 14 | 53.8 | 33.4-73.4 | 197 | 38.1 | 33.9-42.4 |
| Thyroid disease at blood sampling | 8 | 27.6 | 12.7-47.2 | 21 | 5.1 | 3.2-7.6 | < 5 | N/A | N/A | 11 | 2.0 | 1.0-3.5 |
| Hyperthyroidism at blood sampling | 8 | 27.6 | 12.7-47.2 | 12 | 2.9 | 1.5-5.0 | < 5 | N/A | N/A | 5 | 0.9 | 0.3-2.1 |
Abbreviations: β-hCG, β-human chorionic gonadotropin; ATD, antithyroid drugs; BMI, body mass index; fT4, free thyroxine; N/A, not available; RF, relative frequency; TRAb, thyrotropin receptor antibodies; TSH, thyrotropin.
aTPOAb- or Tg-Ab greater than 60 IU/L.
bIndividuals with missing values not included (n = 37).
cInformation available only for singleton live and still births, missing values not included (n = 1126).
dInformation available only for live and still births, missing values not included: parity (n = 1180), smoking (n = 1167), BMI (n = 1187).
Later Follow-up
Finally, we performed follow-up on each woman after the pregnancy according to level of TRAb in early pregnancy (Table 5). For this assessment, women with known thyroid disease before blood sampling in the pregnancy were excluded, leaving 47 TRAb-positive and 935 TRAb-negative women for follow-up. The median follow-up time was 8.1 years (range, 4-10 years). Among women with low TSH in pregnancy, it was consistently found that women who were TRAb positive were much more likely to be diagnosed and treated for hyperthyroidism in the years following the pregnancy and in a later pregnancy. This finding was also reflected a more frequent use of ATD assessed from the number of redeemed prescriptions (see Table 5). Notably, ATD was the main treatment used, whereas thyroid surgery and radioiodine treatment rarely occurred and only among women who had low TSH and were TRAb negative. Among women with TSH greater than or equal to 0.1 mIU/L, the frequency of later diagnosis and treatment for hyperthyroidism was low irrespective of the TRAb positivity in the pregnancy (see Table 5).
Follow-up of all women with no prior thyroid diagnosis when stratified by levels of thyrotropin receptor antibodies and thyrotropin in early pregnancy
| . | TSH < 0.1 mIU/L . | . | . | . | . | . | TSH ≥ 0.1 mIU/L . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | TRAb > 1.0 IU/L . | . | . | TRAb ≤ 1.0 IU/L . | . | . | TRAb > 1.0 IU/L . | . | . | TRAb ≤ 1.0 IU/L . | . | . |
| . | n = 21 . | . | . | n = 393 . | . | . | n = 26 . | . | . | n = 542 . | . | . |
| . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . |
| Later hyperthyroid diagnosis | 11 | 52.4 | 29.8-74.3 | 33 | 8.4 | 5.9-11.6 | < 5 | N/A | N/A | < 5 | N/A | N/A |
| Later ATD treatment | 10 | 47.6 | 25.7-70.2 | 17 | 4.3 | 2.5-6.8 | < 5 | N/A | N/A | < 5 | N/A | N/A |
| Later thyroid surgery or radioiodine treatment | < 5 | N/A | N/A | 5 | 1.3 | 0.4-2.9 | < 5 | N/A | N/A | < 5 | N/A | N/A |
| Later pregnancy | 9 | 42.9 | 21.8-66.0 | 154 | 39.2 | 34.3-44.2 | 11 | 42.3 | 23.4-63.1 | 271 | 50.0 | 45.7-54.3 |
| Hyperthyroidism in pregnancy or 2 y post partuma | 5 | 23.8 | 8.2-47.2 | 10 | 2.5 | 1.2-4.6 | < 5 | N/A | N/A | < 5 | N/A | N/A |
| ATD in pregnancy or 2 y post partumb | 5 | 23.8 | 8.2-47.2 | 5 | 1.3 | 0.4-2.9 | < 5 | N/A | N/A | < 5 | N/A | N/A |
| . | TSH < 0.1 mIU/L . | . | . | . | . | . | TSH ≥ 0.1 mIU/L . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | TRAb > 1.0 IU/L . | . | . | TRAb ≤ 1.0 IU/L . | . | . | TRAb > 1.0 IU/L . | . | . | TRAb ≤ 1.0 IU/L . | . | . |
| . | n = 21 . | . | . | n = 393 . | . | . | n = 26 . | . | . | n = 542 . | . | . |
| . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . |
| Later hyperthyroid diagnosis | 11 | 52.4 | 29.8-74.3 | 33 | 8.4 | 5.9-11.6 | < 5 | N/A | N/A | < 5 | N/A | N/A |
| Later ATD treatment | 10 | 47.6 | 25.7-70.2 | 17 | 4.3 | 2.5-6.8 | < 5 | N/A | N/A | < 5 | N/A | N/A |
| Later thyroid surgery or radioiodine treatment | < 5 | N/A | N/A | 5 | 1.3 | 0.4-2.9 | < 5 | N/A | N/A | < 5 | N/A | N/A |
| Later pregnancy | 9 | 42.9 | 21.8-66.0 | 154 | 39.2 | 34.3-44.2 | 11 | 42.3 | 23.4-63.1 | 271 | 50.0 | 45.7-54.3 |
| Hyperthyroidism in pregnancy or 2 y post partuma | 5 | 23.8 | 8.2-47.2 | 10 | 2.5 | 1.2-4.6 | < 5 | N/A | N/A | < 5 | N/A | N/A |
| ATD in pregnancy or 2 y post partumb | 5 | 23.8 | 8.2-47.2 | 5 | 1.3 | 0.4-2.9 | < 5 | N/A | N/A | < 5 | N/A | N/A |
| . | Median . | 95% CI . | Median . | 95% CI . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| No. of later ATD prescriptionsc | 13 | 4-25 | 3 | 3-7 |
| . | Median . | 95% CI . | Median . | 95% CI . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| No. of later ATD prescriptionsc | 13 | 4-25 | 3 | 3-7 |
Abbreviations: β-hCG, β-human chorionic gonadotropin; ATD, antithyroid drugs; BMI, body mass index; fT4, free thyroxine; N/A, not available; RF, relative frequency; TRAb, thyrotropin receptor antibodies; TSH, thyrotropin.
aHospital diagnosis of hyperthyroidism while pregnant or 2 years post partum.
bPrescription of ATD in the period from 1 month before pregnancy until 2 years post partum.
cNumber of prescriptions from the first prescription until December 31, 2020.
Follow-up of all women with no prior thyroid diagnosis when stratified by levels of thyrotropin receptor antibodies and thyrotropin in early pregnancy
| . | TSH < 0.1 mIU/L . | . | . | . | . | . | TSH ≥ 0.1 mIU/L . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | TRAb > 1.0 IU/L . | . | . | TRAb ≤ 1.0 IU/L . | . | . | TRAb > 1.0 IU/L . | . | . | TRAb ≤ 1.0 IU/L . | . | . |
| . | n = 21 . | . | . | n = 393 . | . | . | n = 26 . | . | . | n = 542 . | . | . |
| . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . |
| Later hyperthyroid diagnosis | 11 | 52.4 | 29.8-74.3 | 33 | 8.4 | 5.9-11.6 | < 5 | N/A | N/A | < 5 | N/A | N/A |
| Later ATD treatment | 10 | 47.6 | 25.7-70.2 | 17 | 4.3 | 2.5-6.8 | < 5 | N/A | N/A | < 5 | N/A | N/A |
| Later thyroid surgery or radioiodine treatment | < 5 | N/A | N/A | 5 | 1.3 | 0.4-2.9 | < 5 | N/A | N/A | < 5 | N/A | N/A |
| Later pregnancy | 9 | 42.9 | 21.8-66.0 | 154 | 39.2 | 34.3-44.2 | 11 | 42.3 | 23.4-63.1 | 271 | 50.0 | 45.7-54.3 |
| Hyperthyroidism in pregnancy or 2 y post partuma | 5 | 23.8 | 8.2-47.2 | 10 | 2.5 | 1.2-4.6 | < 5 | N/A | N/A | < 5 | N/A | N/A |
| ATD in pregnancy or 2 y post partumb | 5 | 23.8 | 8.2-47.2 | 5 | 1.3 | 0.4-2.9 | < 5 | N/A | N/A | < 5 | N/A | N/A |
| . | TSH < 0.1 mIU/L . | . | . | . | . | . | TSH ≥ 0.1 mIU/L . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | TRAb > 1.0 IU/L . | . | . | TRAb ≤ 1.0 IU/L . | . | . | TRAb > 1.0 IU/L . | . | . | TRAb ≤ 1.0 IU/L . | . | . |
| . | n = 21 . | . | . | n = 393 . | . | . | n = 26 . | . | . | n = 542 . | . | . |
| . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . | n . | RF (%) . | 95% CI . |
| Later hyperthyroid diagnosis | 11 | 52.4 | 29.8-74.3 | 33 | 8.4 | 5.9-11.6 | < 5 | N/A | N/A | < 5 | N/A | N/A |
| Later ATD treatment | 10 | 47.6 | 25.7-70.2 | 17 | 4.3 | 2.5-6.8 | < 5 | N/A | N/A | < 5 | N/A | N/A |
| Later thyroid surgery or radioiodine treatment | < 5 | N/A | N/A | 5 | 1.3 | 0.4-2.9 | < 5 | N/A | N/A | < 5 | N/A | N/A |
| Later pregnancy | 9 | 42.9 | 21.8-66.0 | 154 | 39.2 | 34.3-44.2 | 11 | 42.3 | 23.4-63.1 | 271 | 50.0 | 45.7-54.3 |
| Hyperthyroidism in pregnancy or 2 y post partuma | 5 | 23.8 | 8.2-47.2 | 10 | 2.5 | 1.2-4.6 | < 5 | N/A | N/A | < 5 | N/A | N/A |
| ATD in pregnancy or 2 y post partumb | 5 | 23.8 | 8.2-47.2 | 5 | 1.3 | 0.4-2.9 | < 5 | N/A | N/A | < 5 | N/A | N/A |
| . | Median . | 95% CI . | Median . | 95% CI . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| No. of later ATD prescriptionsc | 13 | 4-25 | 3 | 3-7 |
| . | Median . | 95% CI . | Median . | 95% CI . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| No. of later ATD prescriptionsc | 13 | 4-25 | 3 | 3-7 |
Abbreviations: β-hCG, β-human chorionic gonadotropin; ATD, antithyroid drugs; BMI, body mass index; fT4, free thyroxine; N/A, not available; RF, relative frequency; TRAb, thyrotropin receptor antibodies; TSH, thyrotropin.
aHospital diagnosis of hyperthyroidism while pregnant or 2 years post partum.
bPrescription of ATD in the period from 1 month before pregnancy until 2 years post partum.
cNumber of prescriptions from the first prescription until December 31, 2020.
Discussion
Principal Findings
This study reports results of early pregnancy TRAb measurements in a large cohort of Danish pregnant women. The study design and selection of study participants allowed for establishment of a method- and pregnancy-specific cutoff for TRAb, for assessment of TRAb positivity, and for later follow-up. The established pregnancy-specific cutoff revealed a frequency of TRAb-positive hyperthyroidism in early pregnancy of 0.2%, whereas the frequency of TRAb-negative hyperthyroidism was 2.9%. Other maternal characteristics as well as follow-up on maternal later development of hyperthyroidism substantiated the different etiologies of maternal hyperthyroidism identified using TRAb. On the other hand, TRAb was not a predictor of maternal hyperthyroidism in pregnancy or during later follow-up in the absence of early pregnancy hyperthyroidism.
Interpretation
A normal pregnancy is characterized by immune suppression. This is evident by the low TRAb values reached in the later part of pregnancy followed by an increase postpartum in GD (6-9) and healthy women (8). Similarly, TPO-Ab and Tg-Ab gradually decrease over the course of a pregnancy (20). Prior studies aiming to assess cutoffs for TRAb were performed in nonpregnant individuals using various biochemical and statistical methods (21-23). Among the methods used in clinical laboratories in Denmark, Pedersen et al reported a cutoff of 1.0 IU/L for the second-generation radioimmunoassay DYNOtest TRAK human by BRAHMS Diagnostica (21, 24), whereas for the third-generation automatic immunoassay by Roche Diagnostics, the recommended cutoff is 1.75 IU/L (25). The disparity between the cutoff established in pregnant women in our study and the nonpregnant cutoff recommended by the manufacturer may reflect immune suppression in pregnancy. However, the cutoff was established in the early pregnancy at a time when alterations in the immune system are not fully introduced. Alternatively, our findings may indicate that the optimal cutoff for this specific method is lower than the recommended 1.8 IU/L, also in nonpregnant individuals. The high degree of left-censoring of the TRAb results in our study necessitated a certain statistical approach when establishing the cutoff. We used a recommended method (18, 19) and the established cutoff was robust in sensitivity analyses.
In our cohort, only 5.1% of the women identified with low TSH were TRAb positive, corresponding to 0.2% of the full cohort. This estimate is in line with the general figures on the prevalence of GD in pregnant women (10). Solitary adenomas or toxic multinodular goiter as the cause of hyperthyroidism rarely occurs in women of fertile age (26, 27), thus, it is most likely that the TRAb-negative women in our study had low TSH as part of gestational hyperthyroidism. We observed that more than 90% of women with low TSH had TRAb-negative hyperthyroidism, corresponding to 2.9% of the full cohort. Previous reports have stated that gestational hyperthyroidism is 10 times more frequent than GD (28). In our study, thyroid function was measured retrospectively in early pregnancy blood samples drawn as part of the Danish prenatal screening program for chromosomal anomalies (12). As a result, the frequency that we report includes cases of undetected thyroid function abnormalities.
Our study design did not include a “gold standard” for evaluation of the established cutoff because the blood samples were drawn for nonthyroidal reasons and some women would have unidentified thyroid disease and because assessment of maternal thyroid disease was performed indirectly from hospital diagnosis and prescriptions of drugs, and not from the review of medical records. However, we had information on maternal thyroid function and other characteristics as well as the unique opportunity to perform later follow-up, which could be used in the evaluation of the cutoff. Our results indicate that a cutoff of 1.0 IU/L for the TRAb method used could distinguish between the 2 main types of hyperthyroidism in early pregnancy (GD and gestational hyperthyroidism). This was evident from the fact that women identified with TRAb-positive hyperthyroidism had more pronounced hyperthyroidism and were also more often positive for other thyroid autoantibodies. Furthermore, they were more often smoking, which is known to be associated with GD (29). On the other hand, women identified with TRAb-negative hyperthyroidism had higher β-hCG levels, more often carried a multiple pregnancy and a female fetus, which are all characteristics of gestational hyperthyroidism (30, 31). Finally, the follow-up on women with unidentified early pregnancy hyperthyroidism clearly showed that later hyperthyroidism predominantly developed in women with early-pregnancy TRAb-positive hyperthyroidism. This is compatible with the fact that gestational hyperthyroidism is a transient pregnancy phenomenon (2), whereas GD may persist or may relapse after pregnancy (32).
To further evaluate the established cutoff, we specifically considered the group of women with TRAb in the “grey zone” between 1.0 and 1.8 IU/L. Although the group was small, results supported a cutoff of 1.0 IU/L since these women were comparable to women with TRAb above 1.8 IU/L on the frequency of known hyperthyroidism as well as thyroid function and β-hCG levels. Our study also included the measurement of TRAb among pregnant women who did not have low TSH. In this group, the later development of hyperthyroidism was rare irrespective of TRAb status. This finding corroborates the clinical recommendation of using TRAb only when hyperthyroidism is detected. Considering underlying mechanisms of autoimmune thyroid disease, it was a notable finding that we identified a group of women who did not have low TSH but were TRAb positive in the early pregnancy. It appeared that these women were more often also positive for TPO-Ab and Tg-Ab. Thyroid autoantibodies are often overlapping, and TRAb may also be positive in patients with autoimmune hypothyroidism (25). We speculate if this group of women suffered from underlying thyroid autoimmunity which was not (yet) reflected by thyroid dysfunction and whether they were predisposed to develop autoimmune hypothyroidism. This hypothesis is compatible with the older age, higher parity, and higher BMI in this group, which are predictors of hypothyroidism (29).
Methodological Comments
Study participants were selected from the NDRPC, which is large cohort of nearly 15 000 pregnant women. Nevertheless, the number of women was limited in some of the stratified analyses and results should be interpreted cautiously. It is the first study to measure TRAb in a large cohort of early pregnant women, and further studies in different populations and using different TRAb assays are warranted to corroborate and extend our findings. Our study was register-based, and in general, the Danish registries are considered to have a high validity (15, 16). Specifically, thyroid diagnoses in the DNHR have previously been validated, with less than 2% of 900 patients with a thyroid diagnosis misclassified (33). The combined use of hospital diagnoses and redeemed prescriptions of drugs ensured the inclusion of patient contacts in general practice. The length of follow-up for each woman varied slightly depending on the year of blood sampling. However, we find it unlikely that length of follow-up would be influenced by our study exposure.
For the selection of women with low TSH, we used the lower first-trimester reference limit for TSH. TSH is dynamic within the early pregnancy (11); however, the number of women identified differed only slightly and results did not change when week-specific reference ranges were applied. We measured TRAb after storage of the blood samples at –80 °C for 5 to 9 years. A prior study has shown that TRAb was stable for after 6 to 7 years of storage at –20 °C (24). Likewise, previous studies have found measurements of thyroid function parameters and autoantibodies stable after storage for more than 20 years at –25 °C (34). As we did not conduct bioassay measurements of TRAb, we were unable to discriminate between stimulating and inhibitory antibodies. A recent study by de Morais et al (35) compared the use of immunoassays and bioassays and found a similar performance in discriminating GD from other causes of hyperthyroidism in adult patients with active thyrotoxicosis. However, we measured TRAb in biobank samples and not in individuals with active thyrotoxicosis.
Conclusion
This study included the combined measurement of thyroid function and TRAb in early pregnant women. When a pregnancy-specific cutoff was established and used, TRAb-negative hyperthyroidism was predominant and differed from TRAb-positive hyperthyroidism regarding thyroid function tests, β-hCG levels, and later development of hyperthyroidism. On the other hand, TRAb was not a predictor of known or later hyperthyroidism among early pregnant women with nonsuppressed TSH. Results emphasize that a pregnancy-specific cutoff for TRAb can be established and used as part of the evaluation of early-pregnancy maternal hyperthyroidism. On the other hand, TRAb should be used and interpretated with caution in pregnant women without biochemical hyperthyroidism. Further large studies with the measurement of TRAb in early pregnant women using different assays are warranted to extend the findings.
Abbreviations
- β-hCG
β-human chorionic gonadotropin
- ATD
antithyroid drugs
- BMI
body mass index
- DNHR
Danish National Hospital Register
- DNPR
Danish National Prescription Register
- fT4
free thyroxine
- GD
Graves’ disease
- ICD-10
10th International Classification of Diseases
- IQR
interquartile range
- NDRPC
North Denmark Region Pregnancy Cohort
- RF
relative frequency
- Tg-Ab
thyroglobulin antibodies
- TPO-Ab
thyroid peroxidase antibodies
- TRAb
thyrotropin receptor antibodies
- TSH
thyrotropin
Financial Support
This work was supported by The Novo Nordisk Foundation (grant No. NNF20OC0059465) and Beckett-Fonden. Beckett- Fonden. Thermo Fisher Diagnostics Aps supported the biochemical measurements of TRAb.
Disclosures
The authors have nothing to disclose.
Data Availability
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license according to the EU General Data Protection Regulation. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.



