-
PDF
- Split View
-
Views
-
Cite
Cite
Solene Rerat, Jessica Amsellem-Jager, Marie Clemence L’hour, Natacha Bouhours-Nouet, Aurelie Donzeau, Stephanie Rouleau, Lucie Levaillant, Fabienne Emeriau, Valerie Moal, Florence Boux de Casson, Najiba Lahlou, Regis Coutant, Lower Circulating Sertoli and Leydig Cell Hormone Levels During Puberty in Obese Boys: A Cross-sectional Study, The Journal of Clinical Endocrinology & Metabolism, Volume 107, Issue 4, April 2022, Pages e1568–e1576, https://doi.org/10.1210/clinem/dgab845
Close - Share Icon Share
Abstract
Alterations in semen characteristics and circulating Sertoli and Leydig cell hormones have been described in obese male adults. Whether hormonal alterations occur before adulthood has not been fully evaluated.
We describe circulating Sertoli and Leydig cell hormone levels in overweight–obese (ow/ob) boys through childhood and adolescence in a cross-sectional study.
Monocentric study in the Pediatric Endocrinology Unit of Angers University Hospital. Three hundred and fifty-one obese and overweight boys aged 5-19 years underwent physical examination, dual-energy X-ray absorptiometry for body composition, oral glucose tolerance test on insulin and glucose, and measurements of follicle-stimulating hormone, luteinizing hormone, anti-Müllerian hormone (AMH), inhibin B, testosterone, and estradiol. Hormonal levels were compared with normative data obtained from 652 healthy nonoverweight nonobese boys of similar age or Tanner stage.
Median inhibin B and testosterone levels during puberty were significantly lower in ow/ob than in healthy boys (1) from age >12 years and thereafter for inhibin B, and (2) from age >14 years and thereafter for testosterone. At Tanner stages 4 and 5, 26%, 31%, and 18% of inhibin B, testosterone, and AMH values were below the 5th percentile in ow/ob subjects (P < .01). In multiple regression analyses, estradiol and total bone mineral density Z-score were negative predictors of inhibin B, fat mass percentage was a negative predictor of testosterone, and insulin was a negative predictor of AMH.
Lower Sertoli and Leydig cell hormone levels during puberty were observed in the ow/ob boys.
The prevalence of obesity has nearly tripled worldwide in the last 4 decades. The number of obesity cases among children has risen dramatically. Indeed, the obesity prevalence among European adolescents reached 14.5% in 2017, while among US adolescents it reached 20.6% in 2016 (1-3). In France, 17% of children and adolescents aged 6-17 years were overweight and 4% were obese in 2015 (4).
One of the consequences of obesity, although often silent, may be the impairment of reproductive function. There are conflicting data concerning the impact of male overweight and obesity on sperm quality. Some clinical and experimental data have shown that obesity was negatively correlated with normal reproductive function (5, 6). A meta‐analysis by Sermondade et al. (7) found that in a sample of 13 077 men, most of them attending fertility clinics, those with overweight or obesity displayed an increased prevalence of azoospermia or oligospermia. It should be noted that most of the studies regarding fertility and obesity were conducted in fertility clinics, which represents an inherent recruitment bias (6). Regarding reproductive hormones levels in adult men, some studies found that body mass index (BMI) was inversely related to circulating reproductive hormones concentrations (3, 8-11). In cross-sectional studies, decreases in testosterone and inhibin B levels were shown in obese in comparison with normal weight adult men (9-11). Lower levels of anti-Müllerian hormone (AMH) with increased BMI have also been reported (12). The pathophysiological relevance of these abnormalities is still a matter of discussion (13).
It is not known whether the lower levels of testicular hormones observed in obese adults can be observed in prepubertal or pubertal obese boys. Obesity has been associated with an alteration in testicular growth during puberty in some (14) but not all studies (15). Regarding testosterone levels, lower mean testosterone values were observed in obese pubertal boys aged >12 years than references from normal weight, normal stature boys (15). In a small study comparing 15 prepubertal (age 5-9 years) overweight and obese boys to nonobese prepubertal boys, inhibin B levels were unaffected by obesity, suggesting that the alteration in Sertoli cell hormone level might occur later (16).
The primary objective of this cross-sectional study was to describe the circulating testicular and gonadotrophins hormones (8 am follicle-stimulating hormone [FSH], luteinizing hormone [LH], testosterone, inhibin B, and AMH) of 351 overweight and obese (ow/ob) children and adolescents compared with those of 652 healthy nonoverweight nonobese boys before and during each stage of puberty. The secondary objective was to determine whether BMI and/or body composition (as assessed by dual-energy X-ray absorptiometry [DXA]) and/or metabolic factors (lipids, oral glucose tolerance test on insulin, and blood glucose) are related to these hormonal values.
Subjects and Methods
Study Design
From 2010 to 2018, this cross-sectional study included 351 overweight and obese male children and adolescents aged 5-19 years who had been referred to the Pediatric Endocrinology Unit of Angers University Hospital for overweight or obesity. Exclusion criteria were obesity of syndromic, genetic, hypothalamic, or endocrine origin, diabetes, and/or the use of any medication. During the recruitment period, 523 boys aged 4-19 were consecutively referred to our center and eligible: 7 were excluded because of syndromic obesity or obesity of hypothalamic origin, 40 were excluded because of the use of medications, 125 declined to participate in the program, and 351 were finally included.
The study received 3 different regulatory approvals. The first approval was for the program of care of obese subjects by our regional health agency (ARS des Pays de la Loire) on behalf of the national health system: within our specialized center for childhood obesity, we offered to the referred obese children to participate in a 2-year obesity outpatient program. The program included an initial day hospitalization for clinical and paraclinical evaluation, lifestyle intervention, group sessions, and physicians’ nurses’, dieticians,’ and psychologists’ appointments, with meetings every 2 months for 2 years. The second approval was for the collection and storage of blood for research purpose (in the Center for biological research at the University Hospital) from the ethics committee of Angers University Hospital. The third approval was for the collection of medical data from the patients’ medical records, by the ethics committee of Angers University Hospital.
All parents of children signed a first written consent (all the children gave their assent and those who were old enough to sign the consent form did so) for their child to participate in the program, and a second written consent for collection and storage of blood samples. The collection of personal data from the medical records for research purposes requested only the nonopposition from the children and their families, in agreement with French regulation (“loi Jardé”).
Six hundred and fifty-two healthy nonoverweight nonobese boys were recruited from 2008 to 2018 for establishing normative data for gonadotrophins and testicular hormones according to age and pubertal stage. Most of the boys were selected during a systematic clinical and biological examination in a medical center dedicated to the annual examination of school children (Vandoeuvre-lès-Nancy, France). The others were brothers of children with an allergic disease, clinically investigated in the medical center at St Vincent de Paul Hospital (Paris, France), and found free of any disease. Height and weight were in the normal range (between –2 and +2 SD scores) of French growth charts according to age and gender (17), and overweight and obesity were exclusion criteria (see below for definition of overweight and obesity).
Study Procedure
Anthropometric measurements included weight (kg), height (cm), and waist and hip circumferences (cm). The physical examination comprised pubertal staging according to Tanner (18) and blood pressure measurement (mmHg). BMI Z-scores were calculated based on the CDC references (19, 20). Overweight was defined as a BMI >+ 1 (corresponding to the 85th percentile) and <+ 2 Z-score, and obesity as a BMI >+ 2 Z-score. Pubertal assessment (Tanner genital and pubic hair staging) of ow/ob boys was performed by 2 pediatric endocrinologists (R.C. and J.A.J.) with the use of an orchidometer. In the healthy nonoverweight nonobese boys, age and Tanner genital and pubic hair stages were collected. The involved physicians received hands-on training for the appropriate use of an orchidometer and the appropriate staging of pubic hair.
After a 12-hour overnight fast, a peripheral venous catheter was placed for blood sampling. Blood samples were obtained for each patient and included 8 am fasting levels of FSH, LH, testosterone, estradiol, inhibin B, and AMH, as well as levels of total cholesterol, high-density lipoprotein (HDL) cholesterol, total triglycerides, blood glucose and insulin. Intravenous blood samples for measurements of FSH, LH, testosterone, estradiol, inhibin B, and AMH were immediately clotted, centrifuged, and refrigerated (4°C). Serum samples were divided into small volumes in several tubes, stoppered with plastic caps, and sealed with a layer of parafilm, prior to being frozen (–40°C) for storage until assay. Each of these aliquots underwent a single freeze/thaw cycle. The freeze/thaw cycle is similar to the experiment to assess interassay reproducibility. In our experience, and that of others, inhibin B, AMH, testosterone, estradiol, FSH, and LH are sufficiently stable under these conditions to give reasonably reproducible results (21-23). The maximum storage time was 10 years in freezers equipped with automated continuous temperature monitoring.
An oral glucose tolerance test was performed with the administration of 1.75 g glucose/kg body wight (maximal dose 75 g). Blood samples were drawn at −30, 0, 30, 60, 90, and 120 minutes for measurements of glucose and insulin.
Body composition was investigated by DXA using a QDR 4500A densitometer (Hologic, Waltham, MA). Whole-body scans were performed, and body compartments were analyzed using software from Hologic (version V8.24a:3) (24). Whole-body and posteroanterior lumbar spine (L1-L4, fast array) scans were also acquired for each study participant for whole body bone mineral content and whole body and lumbar bone mineral density measurements (24).
Analytical Determinations
Plasma glucose was measured with a Hitachi 917 analyzer (Roche-Diagnostics, Meylan, France). Plasma insulin was measured by radioimmunoassay (RIA) (Schering AG Cis Bio International, Gif sur Yvette, France) (CISBIO cat. no. 62IN1PEG, RRID:AB_2890910; https://antibodyregistry.org/search.php?q=AB_2890910). The intra- and interassay coefficients of variation (CVs) were 1.9 and 2.7%, respectively.
Inhibin B levels were measured by means of a solid-phase sandwich assay using Ansh Labs reagents (Webster, TX) (Ansh Labs cat. no. AL-107, RRID:AB_2783661; https://antibodyregistry.org/search.php?q=AB_2783661). Inhibin A exhibited 1% cross-reactivity in the inhibin B assay. The intra- and interassay CVs were 6.8% and 21.5%, respectively, at the level of 39 pg/mL, and 5.7% and 12%, respectively, at the level of 112 pg/mL. The sensitivity was 6 pg/mL. When values were <6, they were arbitrarily set at 3 pg/mL.
AMH levels were measured by a solid-phase sandwich assay using Ansh Labs reagents (Webster, TX) (Ansh Labs cat. no AL105, RRID:AB_2783659; https://antibodyregistry.org/search.php?q=AB_2783659). There was no cross-reactivity of related proteins including transforming growth factor β. The intra- and interassay CVs were 2.3% and 3.1%, respectively, at the level of 107 pmol/L, and 1.4 and 2.5%, respectively, at the level of 557 pmol/L. The sensitivity was 0.7 pmol/L.
FSH and LH were measured by a time-resolved fluorometric assay using Delfia reagents (Perkin Elmer Life Sciences, Courtaboeuf, France) (FSH Kit AutoDelfia, PerkinElmer cat. no B017-201, RRID:AB_2783738; https://antibodyregistry.org/search.php?q=AB_2783738) (LH spec Kit AutoDelfia, PerkinElmer cat. no B031-101, RRID:AB_2783737; https://antibodyregistry.org/search.php?q=AB_2783737). In the FSH assay, the intra- and interassay CVs were 1.2% and 3.9%, respectively, at the level of 3.1 IU/L, and 1.5% and 2.8%, respectively, at the level of 16.6 IU/L. In the LH assay, the intra- and interassay CVs were 1.4% and 2.6%, respectively, at the level of 0.3 IU/L, and 1.7% and 2%, respectively, at the level of 6.9 IU/L. The sensitivity was 0.01 IU/L for both assays.
Testosterone was measured by RIA, after a steroid extraction step, using Orion reagents (Cis Bio International, Gif sur Yvette, France) (Abcam cat. no. ab33863, RRID:AB_778304; https://antibodyregistry.org/search?q=RRID:AB_778304). The intra- and interassay CVs were 6.8% and 11.5%, respectively, at the level of 0.81 nmol/L, and 5.9% and 8.2%, respectively, at the level 1.6 nmol/L. The sensitivity was 0.046 nmol/L. Accuracy was not different from that of the tandem mass spectrometric assay developed in the same laboratory (Quattro Premier; Waters, Guyancourt, France), the regression curve being testosterone value measured by RIA = 1.0 × testosterone value measured by mass spectrometry + 0.0049.
Estradiol was measured by Cis Bio International ultrasensitive RIA (Gif sur Yvette, France) (RRID:AB_10578018; https://antibodyregistry.org/search.php?q=AB_10578018) designed for low estradiol levels: sensitivity was 8 pmol/L. At 12 pmol/L, the intra- and interassay CVs were 17% and 18%, respectively. At 90 pmol/L, the intra- and interassay CVs were 2.8% and 5.8%, respectively. At 300 pmol/L, the intra- and interassay CVs were 5% and 10%, respectively. Cross-reaction with estrone was less than 1%.
Total cholesterol, HDL, and low-density lipoprotein cholesterol and triglycerides were measured by commercially available kits.
Statistical Methods
Continuous variables were expressed as mean ± SD or median (5th and 95th percentiles). Discrete variables were expressed as percent. The precision for age was at the day level. Kruskal–Wallis, Wilcoxon rank-sum tests, chi-squared, and Fisher exact tests were used for comparisons. Significance was defined as P < .05.
Reference values (median, 5th and 95th percentiles) for inhibin B, AMH, testosterone, estradiol, LH, and FSH were established in our laboratory from 652 healthy nonoverweight nonobese boys. Reference values including some of the subjects from the present study have been published (25, 26). When frozen samples from healthy boys from these previous studies were still available, they were used for the present study.
Regression analyses were performed to determine the best combination of predictors of inhibin B, AMH, and testosterone levels. First, simple regression analyses were performed, with clinical and biological variables as independent variables. Then, backward stepwise multiple regression analyses were performed using the variables (P < .2) from the simple regression analyses. Interactions between the variables were also included in the analyses. Any nonsignificant interaction was deleted. We used SPSS Statistics v25 (IBM Corp., Armonk, NY) and GraphPad Prism 9 (GraphPad Software Inc.).
Results
Characteristics of the population according to age groups and pubertal stage are shown in Table 1 and Table 2. Sixty-eight (19%) of the 351 patients were overweight and 283 (81%) were obese. One patient had a delayed onset of puberty, and 1 had early sexual maturation.
Clinical characteristics and hormonal values in overweight/obese and healthy boys (reference values) according to age groups
| Age (years) . | 4-< 8 years . | 8-< 10 years . | 10-<12 years . | 12-< 14 years . | 14-<16 years . | 16-19 years . |
|---|---|---|---|---|---|---|
| Obese boys (N) | 17 | 42 | 65 | 105 | 76 | 46 |
| Age (years) | 6.26 ± 1.7 | 9.1 ± 0.5 | 11.1 ± 0.6 | 13 ± 0.6 | 14.9 ± 0.6 | 17.1 ± 0.8 |
| BMI Z score | 2.7 (2.2; 5) | 2.2 (1.8; 2.7) | 2.2 (1.7; 2.7) | 2.2 (1.6; 2.7) | 2.4 (1.7; 2.8) | 2.5 (1.9; 3.1) |
| Percent fat mass (%) | 37 (46; 42) | 42 (36; 50) | 45 (37; 52) | 43 (35; 51) | 41 (32; 50) | 40 (32; 48) |
| BMD Z score | 1.0 ± 1.1 | 1.0 ± 1.3 | 0.9 ± 0.8 | 0.5 ± 1.0 | 0.7 ± 1.3 | 0.9 ± 1.0 |
| Inhibin B (pg/mL) | 77 (20; 163) | 56 (34; 151) | 87 (35; 211) | 139c (36; 260) | 135c (36; 264) | 126c (33; 226) |
| AMH (pmol/L) | 705 (418; 1321) | 610 (248; 1166) | 401 (120;1115) | 138 (20; 811) | 60 (15; 425) | 43 (12; 213) |
| Testosterone (nmol/L) | 0.1 (0.07; 0.30) | 0.38 (0.1; 0.69) | 0.5 (0.20;5) | 3.15 (0.46; 13.8) | 8.8b (0.7; 20.4) | 11.7c (3.8; 20.4) |
| LH (IU/L) | 0.08 (0.05; 0.15) | 0.08 (0.05; 0.50) | 0.1 (0.05;3.1) | 1.6 (0.05; 4.2) | 2.9 (0.7; 6.7) | 3.6 (1.6; 7.0) |
| FSH (IU/L) | 0.4 (0.09; 0.9) | 0.47 (0.09;1.96) | 0.96 (0.19; 2.8) | 2.1 (0.4; 4.5) | 2.52 (0.71; 8.5) | 2.52 (0.77; 10.0) |
| Estradiol (pmol/L) | <8 | 40a (8;55) | 51c (8;70) | 62c (8;136) | 95c (55;176) | 121c (92;209) |
| Healthy boys (N) | 109 | 117 | 124 | 163 | 88 | 51 |
| Age (years) | 6.1 ± 1.1 | 8.8 ± 0.9 | 10.7 ± 0.59 | 12.9 ± 0.61 | 14.7 ± 0.53 | 17.2 ± 0.97 |
| BMI Z score | –0.1 (–1.4; 0.9) | –0.2 (–1.6; 0.9) | –0.2 (–1.6; 0.9) | –0.4 (–1.7; 0.7) | –0.0 (–1.5; 0.7) | –0.3 (–1.5; 0.6) |
| Inhibin B (pg/mL) | 75 (30; 150) | 80 (35; 175) | 125 (60; 220) | 169 (80; 300) | 190 (90; 350) | 200 (110; 400) |
| AMH (pmol/L) | 650 (220; 1560) | 550 (170; 1400) | 500 (120; 1290) | 190 (60; 820) | 65 (28; 310) | 48 (25; 105) |
| Testosterone (nmol/L) | 0.20 (0.15; 0.5) | 0.45 (0.18; 1.0) | 0.70 (0.30; 7.5) | 4.0 (0.35; 21) | 11.1 (3.8; 25) | 16.5 (11; 30) |
| LH (IU/L) | 0.08 (0.06; 0.20) | 0.15 (0.01; 0.38) | 0.25 (0.02; 1.17) | 1.5 (0.25; 2.83) | 2.6 (0.6; 6.1) | 3.4 (0.8; 6.2) |
| FSH (IU/L) | 0.40 (0.04; 1.0) | 0.6 (0.1; 1.7) | 1.08 (0.55; 2.85) | 1.9 (0.75; 3.92) | 2.2 (0.8; 5.2) | 2.5 (1.0; 6.0) |
| Estradiol (pmol/L) | <8 | 22 (8; 38) | 30 (8; 44) | 36 (8; 60) | 46 (8; 86) | 70 (28; 120) |
| Age (years) . | 4-< 8 years . | 8-< 10 years . | 10-<12 years . | 12-< 14 years . | 14-<16 years . | 16-19 years . |
|---|---|---|---|---|---|---|
| Obese boys (N) | 17 | 42 | 65 | 105 | 76 | 46 |
| Age (years) | 6.26 ± 1.7 | 9.1 ± 0.5 | 11.1 ± 0.6 | 13 ± 0.6 | 14.9 ± 0.6 | 17.1 ± 0.8 |
| BMI Z score | 2.7 (2.2; 5) | 2.2 (1.8; 2.7) | 2.2 (1.7; 2.7) | 2.2 (1.6; 2.7) | 2.4 (1.7; 2.8) | 2.5 (1.9; 3.1) |
| Percent fat mass (%) | 37 (46; 42) | 42 (36; 50) | 45 (37; 52) | 43 (35; 51) | 41 (32; 50) | 40 (32; 48) |
| BMD Z score | 1.0 ± 1.1 | 1.0 ± 1.3 | 0.9 ± 0.8 | 0.5 ± 1.0 | 0.7 ± 1.3 | 0.9 ± 1.0 |
| Inhibin B (pg/mL) | 77 (20; 163) | 56 (34; 151) | 87 (35; 211) | 139c (36; 260) | 135c (36; 264) | 126c (33; 226) |
| AMH (pmol/L) | 705 (418; 1321) | 610 (248; 1166) | 401 (120;1115) | 138 (20; 811) | 60 (15; 425) | 43 (12; 213) |
| Testosterone (nmol/L) | 0.1 (0.07; 0.30) | 0.38 (0.1; 0.69) | 0.5 (0.20;5) | 3.15 (0.46; 13.8) | 8.8b (0.7; 20.4) | 11.7c (3.8; 20.4) |
| LH (IU/L) | 0.08 (0.05; 0.15) | 0.08 (0.05; 0.50) | 0.1 (0.05;3.1) | 1.6 (0.05; 4.2) | 2.9 (0.7; 6.7) | 3.6 (1.6; 7.0) |
| FSH (IU/L) | 0.4 (0.09; 0.9) | 0.47 (0.09;1.96) | 0.96 (0.19; 2.8) | 2.1 (0.4; 4.5) | 2.52 (0.71; 8.5) | 2.52 (0.77; 10.0) |
| Estradiol (pmol/L) | <8 | 40a (8;55) | 51c (8;70) | 62c (8;136) | 95c (55;176) | 121c (92;209) |
| Healthy boys (N) | 109 | 117 | 124 | 163 | 88 | 51 |
| Age (years) | 6.1 ± 1.1 | 8.8 ± 0.9 | 10.7 ± 0.59 | 12.9 ± 0.61 | 14.7 ± 0.53 | 17.2 ± 0.97 |
| BMI Z score | –0.1 (–1.4; 0.9) | –0.2 (–1.6; 0.9) | –0.2 (–1.6; 0.9) | –0.4 (–1.7; 0.7) | –0.0 (–1.5; 0.7) | –0.3 (–1.5; 0.6) |
| Inhibin B (pg/mL) | 75 (30; 150) | 80 (35; 175) | 125 (60; 220) | 169 (80; 300) | 190 (90; 350) | 200 (110; 400) |
| AMH (pmol/L) | 650 (220; 1560) | 550 (170; 1400) | 500 (120; 1290) | 190 (60; 820) | 65 (28; 310) | 48 (25; 105) |
| Testosterone (nmol/L) | 0.20 (0.15; 0.5) | 0.45 (0.18; 1.0) | 0.70 (0.30; 7.5) | 4.0 (0.35; 21) | 11.1 (3.8; 25) | 16.5 (11; 30) |
| LH (IU/L) | 0.08 (0.06; 0.20) | 0.15 (0.01; 0.38) | 0.25 (0.02; 1.17) | 1.5 (0.25; 2.83) | 2.6 (0.6; 6.1) | 3.4 (0.8; 6.2) |
| FSH (IU/L) | 0.40 (0.04; 1.0) | 0.6 (0.1; 1.7) | 1.08 (0.55; 2.85) | 1.9 (0.75; 3.92) | 2.2 (0.8; 5.2) | 2.5 (1.0; 6.0) |
| Estradiol (pmol/L) | <8 | 22 (8; 38) | 30 (8; 44) | 36 (8; 60) | 46 (8; 86) | 70 (28; 120) |
Data are presented as mean ± SD or median (5th; 95th). Comparison vs median in healthy boys (Wilcoxon rank sum test, see “Subjects and Methods”). Percent fat mass and BMD by DXA.
Abbreviations: AMH, anti-Müllerian hormone; BMD, bone mineral density; BMI, body mass index; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
aP < .05;
bP < .01;
cP < .001.
Clinical characteristics and hormonal values in overweight/obese and healthy boys (reference values) according to age groups
| Age (years) . | 4-< 8 years . | 8-< 10 years . | 10-<12 years . | 12-< 14 years . | 14-<16 years . | 16-19 years . |
|---|---|---|---|---|---|---|
| Obese boys (N) | 17 | 42 | 65 | 105 | 76 | 46 |
| Age (years) | 6.26 ± 1.7 | 9.1 ± 0.5 | 11.1 ± 0.6 | 13 ± 0.6 | 14.9 ± 0.6 | 17.1 ± 0.8 |
| BMI Z score | 2.7 (2.2; 5) | 2.2 (1.8; 2.7) | 2.2 (1.7; 2.7) | 2.2 (1.6; 2.7) | 2.4 (1.7; 2.8) | 2.5 (1.9; 3.1) |
| Percent fat mass (%) | 37 (46; 42) | 42 (36; 50) | 45 (37; 52) | 43 (35; 51) | 41 (32; 50) | 40 (32; 48) |
| BMD Z score | 1.0 ± 1.1 | 1.0 ± 1.3 | 0.9 ± 0.8 | 0.5 ± 1.0 | 0.7 ± 1.3 | 0.9 ± 1.0 |
| Inhibin B (pg/mL) | 77 (20; 163) | 56 (34; 151) | 87 (35; 211) | 139c (36; 260) | 135c (36; 264) | 126c (33; 226) |
| AMH (pmol/L) | 705 (418; 1321) | 610 (248; 1166) | 401 (120;1115) | 138 (20; 811) | 60 (15; 425) | 43 (12; 213) |
| Testosterone (nmol/L) | 0.1 (0.07; 0.30) | 0.38 (0.1; 0.69) | 0.5 (0.20;5) | 3.15 (0.46; 13.8) | 8.8b (0.7; 20.4) | 11.7c (3.8; 20.4) |
| LH (IU/L) | 0.08 (0.05; 0.15) | 0.08 (0.05; 0.50) | 0.1 (0.05;3.1) | 1.6 (0.05; 4.2) | 2.9 (0.7; 6.7) | 3.6 (1.6; 7.0) |
| FSH (IU/L) | 0.4 (0.09; 0.9) | 0.47 (0.09;1.96) | 0.96 (0.19; 2.8) | 2.1 (0.4; 4.5) | 2.52 (0.71; 8.5) | 2.52 (0.77; 10.0) |
| Estradiol (pmol/L) | <8 | 40a (8;55) | 51c (8;70) | 62c (8;136) | 95c (55;176) | 121c (92;209) |
| Healthy boys (N) | 109 | 117 | 124 | 163 | 88 | 51 |
| Age (years) | 6.1 ± 1.1 | 8.8 ± 0.9 | 10.7 ± 0.59 | 12.9 ± 0.61 | 14.7 ± 0.53 | 17.2 ± 0.97 |
| BMI Z score | –0.1 (–1.4; 0.9) | –0.2 (–1.6; 0.9) | –0.2 (–1.6; 0.9) | –0.4 (–1.7; 0.7) | –0.0 (–1.5; 0.7) | –0.3 (–1.5; 0.6) |
| Inhibin B (pg/mL) | 75 (30; 150) | 80 (35; 175) | 125 (60; 220) | 169 (80; 300) | 190 (90; 350) | 200 (110; 400) |
| AMH (pmol/L) | 650 (220; 1560) | 550 (170; 1400) | 500 (120; 1290) | 190 (60; 820) | 65 (28; 310) | 48 (25; 105) |
| Testosterone (nmol/L) | 0.20 (0.15; 0.5) | 0.45 (0.18; 1.0) | 0.70 (0.30; 7.5) | 4.0 (0.35; 21) | 11.1 (3.8; 25) | 16.5 (11; 30) |
| LH (IU/L) | 0.08 (0.06; 0.20) | 0.15 (0.01; 0.38) | 0.25 (0.02; 1.17) | 1.5 (0.25; 2.83) | 2.6 (0.6; 6.1) | 3.4 (0.8; 6.2) |
| FSH (IU/L) | 0.40 (0.04; 1.0) | 0.6 (0.1; 1.7) | 1.08 (0.55; 2.85) | 1.9 (0.75; 3.92) | 2.2 (0.8; 5.2) | 2.5 (1.0; 6.0) |
| Estradiol (pmol/L) | <8 | 22 (8; 38) | 30 (8; 44) | 36 (8; 60) | 46 (8; 86) | 70 (28; 120) |
| Age (years) . | 4-< 8 years . | 8-< 10 years . | 10-<12 years . | 12-< 14 years . | 14-<16 years . | 16-19 years . |
|---|---|---|---|---|---|---|
| Obese boys (N) | 17 | 42 | 65 | 105 | 76 | 46 |
| Age (years) | 6.26 ± 1.7 | 9.1 ± 0.5 | 11.1 ± 0.6 | 13 ± 0.6 | 14.9 ± 0.6 | 17.1 ± 0.8 |
| BMI Z score | 2.7 (2.2; 5) | 2.2 (1.8; 2.7) | 2.2 (1.7; 2.7) | 2.2 (1.6; 2.7) | 2.4 (1.7; 2.8) | 2.5 (1.9; 3.1) |
| Percent fat mass (%) | 37 (46; 42) | 42 (36; 50) | 45 (37; 52) | 43 (35; 51) | 41 (32; 50) | 40 (32; 48) |
| BMD Z score | 1.0 ± 1.1 | 1.0 ± 1.3 | 0.9 ± 0.8 | 0.5 ± 1.0 | 0.7 ± 1.3 | 0.9 ± 1.0 |
| Inhibin B (pg/mL) | 77 (20; 163) | 56 (34; 151) | 87 (35; 211) | 139c (36; 260) | 135c (36; 264) | 126c (33; 226) |
| AMH (pmol/L) | 705 (418; 1321) | 610 (248; 1166) | 401 (120;1115) | 138 (20; 811) | 60 (15; 425) | 43 (12; 213) |
| Testosterone (nmol/L) | 0.1 (0.07; 0.30) | 0.38 (0.1; 0.69) | 0.5 (0.20;5) | 3.15 (0.46; 13.8) | 8.8b (0.7; 20.4) | 11.7c (3.8; 20.4) |
| LH (IU/L) | 0.08 (0.05; 0.15) | 0.08 (0.05; 0.50) | 0.1 (0.05;3.1) | 1.6 (0.05; 4.2) | 2.9 (0.7; 6.7) | 3.6 (1.6; 7.0) |
| FSH (IU/L) | 0.4 (0.09; 0.9) | 0.47 (0.09;1.96) | 0.96 (0.19; 2.8) | 2.1 (0.4; 4.5) | 2.52 (0.71; 8.5) | 2.52 (0.77; 10.0) |
| Estradiol (pmol/L) | <8 | 40a (8;55) | 51c (8;70) | 62c (8;136) | 95c (55;176) | 121c (92;209) |
| Healthy boys (N) | 109 | 117 | 124 | 163 | 88 | 51 |
| Age (years) | 6.1 ± 1.1 | 8.8 ± 0.9 | 10.7 ± 0.59 | 12.9 ± 0.61 | 14.7 ± 0.53 | 17.2 ± 0.97 |
| BMI Z score | –0.1 (–1.4; 0.9) | –0.2 (–1.6; 0.9) | –0.2 (–1.6; 0.9) | –0.4 (–1.7; 0.7) | –0.0 (–1.5; 0.7) | –0.3 (–1.5; 0.6) |
| Inhibin B (pg/mL) | 75 (30; 150) | 80 (35; 175) | 125 (60; 220) | 169 (80; 300) | 190 (90; 350) | 200 (110; 400) |
| AMH (pmol/L) | 650 (220; 1560) | 550 (170; 1400) | 500 (120; 1290) | 190 (60; 820) | 65 (28; 310) | 48 (25; 105) |
| Testosterone (nmol/L) | 0.20 (0.15; 0.5) | 0.45 (0.18; 1.0) | 0.70 (0.30; 7.5) | 4.0 (0.35; 21) | 11.1 (3.8; 25) | 16.5 (11; 30) |
| LH (IU/L) | 0.08 (0.06; 0.20) | 0.15 (0.01; 0.38) | 0.25 (0.02; 1.17) | 1.5 (0.25; 2.83) | 2.6 (0.6; 6.1) | 3.4 (0.8; 6.2) |
| FSH (IU/L) | 0.40 (0.04; 1.0) | 0.6 (0.1; 1.7) | 1.08 (0.55; 2.85) | 1.9 (0.75; 3.92) | 2.2 (0.8; 5.2) | 2.5 (1.0; 6.0) |
| Estradiol (pmol/L) | <8 | 22 (8; 38) | 30 (8; 44) | 36 (8; 60) | 46 (8; 86) | 70 (28; 120) |
Data are presented as mean ± SD or median (5th; 95th). Comparison vs median in healthy boys (Wilcoxon rank sum test, see “Subjects and Methods”). Percent fat mass and BMD by DXA.
Abbreviations: AMH, anti-Müllerian hormone; BMD, bone mineral density; BMI, body mass index; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
aP < .05;
bP < .01;
cP < .001.
Clinical characteristics and hormonal values in overweight/obese and healthy boys (reference values) according to Tanner stage groups
| . | Tanner 1 . | Tanner 1 . | Tanner 2 . | Tanner 3 . | Tanner 4 . | Tanner 5 . |
|---|---|---|---|---|---|---|
| . | Age <10 years . | Age >10 years . | . | . | . | . |
| Obese boys (N) | 58 | 72 | 66 | 57 | 57 | 41 |
| Age (years) | 8.3 ± 1.6 | 11.7 ± 1.1 | 12.7 ± 1.2 | 13.9 ± 1.2 | 15 ± 1.3 | 16.7 ± 1.3 |
| BMI Z score | 2.3 (1.9; 4.9) | 2.2 (1.7; 2.7) | 2.2 (1.7; 2.6) | 2.3 (1.6; 2.9) | 2.3 (1.5; 3) | 2.4 (1.9; 3) |
| Percent fat mass (%) | 42 (36; 50) | 46 (38; 52) | 43 (36; 50) | 41 (33; 49) | 40 (30; 48) | 40 (33; 49) |
| Total BMD Z score | 1.1 ± 1.2 | 0.7 ± 0.9 | 0.6 ± 0.9 | 0.6 ± 1.2 | 0.7 ± 1.2 | 1.1 ± 1.1 |
| Inhibin B (pg/mL) | 62 (30; 158) | 75c (33; 154) | 149a (24; 266) | 138a (64; 270) | 150c (33; 294) | 138c (57; 229) |
| AMH (pmol/L) | 623 (279;1514) | 436 (186; 1114) | 173 (40; 852) | 57a (10; 198) | 58 (15; 207) | 40 (10; 310) |
| Testosterone(nmol/L) | 0.1 (0.07; 0.59) | 0.4 (0.1; 1.03) | 2.4 (0.37; 12.5) | 7 (0.67; 17.4) | 9.9c (3.0; 21.1) | 11.6c (6.5; 19.5) |
| LH (IU/L) | 0.05 (0.05; 0.35) | 0.1 (0.05; 2.04) | 1.5 (0.1; 4.72) | 2 (0.41; 5.50) | 3 (1.0; 7.8) | 3.2 (1.6; 6.3) |
| FSH (IU/L) | 0.4 (0.09; 2.2) | 0.93 (0.17; 2.9) | 2.1 (0.55; 5.3) | 2.5 (0.7; 7.5) | 2.52 (0.75; 10.7) | 2.24 (0.75; 7.19) |
| Estradiol (pmol/L) | 40b (8; 66) | 40b (8; 92) | 44b (8; 106) | 95c (8; 132) | 99c (8; 209) | 114a (59; 176) |
| Healthy boys (N) | 224 | 137 | 97 | 81 | 54 | 59 |
| Age (years) | 7.5 ± 1.7 | 11.5 ± .1.2 | 13.1 ± 1.0 | 14.2 ± 1.0 | 14.7 ± 0.8 | 16.7 ± 1.5 |
| BMI Z score | –0.1 (–1.6; 0.9) | –0.4 (–1.7; 0.8) | –0.2 (–1.6; 0.9) | –0.3 (–1.8; 0.7) | –0.2 (–1.8; 0.6) | –0.2 (–1.5; 0.6) |
| Inhibin B (pg/mL) | 80 (34; 180) | 110 (45; 220) | 160 (70; 300) | 170 (90; 315) | 195 (100; 350) | 200 (105; 400) |
| AMH (pmol/L) | 642 (185; 1475) | 540 (150; 1400) | 188 (70; 820) | 70 (30; 500) | 53 (25; 310) | 46 (25; 105) |
| Testosterone (nmol/L) | 0.07 (0.03; 0.6) | 0.35 (0.07; 1.25) | 2.1 (0.65; 8.50) | 8.0 (3.81; 15.9) | 13 (7.2; 18) | 20 (11; 35) |
| LH (IU/L) | 0.065 (0.01; 0.32) | 0.1 (0.07; 2.15) | 1.2 (0.30; 2.85) | 1.9 (0.70; 3.8) | 2.5 (0.90; 3.95) | 2.8 (0.5; 5.0) |
| FSH (IU/L) | 0.5 (0.05; 1.2) | 0.7 (0.20; 2.3) | 1.9 (0.30; 3.1) | 2.5 (0.70; 3.8) | 2.5 (0.80; 4.1) | 2.3 (0.8; 4.4) |
| Estradiol (pmol/L) | 14 (8; 24) | 18 (8; 34) | 21 (8; 45) | 36 (8; 85) | 59 (29; 102) | 71 (44; 117) |
| . | Tanner 1 . | Tanner 1 . | Tanner 2 . | Tanner 3 . | Tanner 4 . | Tanner 5 . |
|---|---|---|---|---|---|---|
| . | Age <10 years . | Age >10 years . | . | . | . | . |
| Obese boys (N) | 58 | 72 | 66 | 57 | 57 | 41 |
| Age (years) | 8.3 ± 1.6 | 11.7 ± 1.1 | 12.7 ± 1.2 | 13.9 ± 1.2 | 15 ± 1.3 | 16.7 ± 1.3 |
| BMI Z score | 2.3 (1.9; 4.9) | 2.2 (1.7; 2.7) | 2.2 (1.7; 2.6) | 2.3 (1.6; 2.9) | 2.3 (1.5; 3) | 2.4 (1.9; 3) |
| Percent fat mass (%) | 42 (36; 50) | 46 (38; 52) | 43 (36; 50) | 41 (33; 49) | 40 (30; 48) | 40 (33; 49) |
| Total BMD Z score | 1.1 ± 1.2 | 0.7 ± 0.9 | 0.6 ± 0.9 | 0.6 ± 1.2 | 0.7 ± 1.2 | 1.1 ± 1.1 |
| Inhibin B (pg/mL) | 62 (30; 158) | 75c (33; 154) | 149a (24; 266) | 138a (64; 270) | 150c (33; 294) | 138c (57; 229) |
| AMH (pmol/L) | 623 (279;1514) | 436 (186; 1114) | 173 (40; 852) | 57a (10; 198) | 58 (15; 207) | 40 (10; 310) |
| Testosterone(nmol/L) | 0.1 (0.07; 0.59) | 0.4 (0.1; 1.03) | 2.4 (0.37; 12.5) | 7 (0.67; 17.4) | 9.9c (3.0; 21.1) | 11.6c (6.5; 19.5) |
| LH (IU/L) | 0.05 (0.05; 0.35) | 0.1 (0.05; 2.04) | 1.5 (0.1; 4.72) | 2 (0.41; 5.50) | 3 (1.0; 7.8) | 3.2 (1.6; 6.3) |
| FSH (IU/L) | 0.4 (0.09; 2.2) | 0.93 (0.17; 2.9) | 2.1 (0.55; 5.3) | 2.5 (0.7; 7.5) | 2.52 (0.75; 10.7) | 2.24 (0.75; 7.19) |
| Estradiol (pmol/L) | 40b (8; 66) | 40b (8; 92) | 44b (8; 106) | 95c (8; 132) | 99c (8; 209) | 114a (59; 176) |
| Healthy boys (N) | 224 | 137 | 97 | 81 | 54 | 59 |
| Age (years) | 7.5 ± 1.7 | 11.5 ± .1.2 | 13.1 ± 1.0 | 14.2 ± 1.0 | 14.7 ± 0.8 | 16.7 ± 1.5 |
| BMI Z score | –0.1 (–1.6; 0.9) | –0.4 (–1.7; 0.8) | –0.2 (–1.6; 0.9) | –0.3 (–1.8; 0.7) | –0.2 (–1.8; 0.6) | –0.2 (–1.5; 0.6) |
| Inhibin B (pg/mL) | 80 (34; 180) | 110 (45; 220) | 160 (70; 300) | 170 (90; 315) | 195 (100; 350) | 200 (105; 400) |
| AMH (pmol/L) | 642 (185; 1475) | 540 (150; 1400) | 188 (70; 820) | 70 (30; 500) | 53 (25; 310) | 46 (25; 105) |
| Testosterone (nmol/L) | 0.07 (0.03; 0.6) | 0.35 (0.07; 1.25) | 2.1 (0.65; 8.50) | 8.0 (3.81; 15.9) | 13 (7.2; 18) | 20 (11; 35) |
| LH (IU/L) | 0.065 (0.01; 0.32) | 0.1 (0.07; 2.15) | 1.2 (0.30; 2.85) | 1.9 (0.70; 3.8) | 2.5 (0.90; 3.95) | 2.8 (0.5; 5.0) |
| FSH (IU/L) | 0.5 (0.05; 1.2) | 0.7 (0.20; 2.3) | 1.9 (0.30; 3.1) | 2.5 (0.70; 3.8) | 2.5 (0.80; 4.1) | 2.3 (0.8; 4.4) |
| Estradiol (pmol/L) | 14 (8; 24) | 18 (8; 34) | 21 (8; 45) | 36 (8; 85) | 59 (29; 102) | 71 (44; 117) |
Data are presented as mean ± SD or median (5th; 95th). Comparison vs median in healthy boys (Wilcoxon rank sum test, see “Subjects and Methods”). Percent fat mass and BMD by DXA.
Abbreviations: AMH, anti-Müllerian hormone; BMD, bone mineral density; BMI, body mass index; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
aP < .05;
bP < .01;
cP < .001.
Clinical characteristics and hormonal values in overweight/obese and healthy boys (reference values) according to Tanner stage groups
| . | Tanner 1 . | Tanner 1 . | Tanner 2 . | Tanner 3 . | Tanner 4 . | Tanner 5 . |
|---|---|---|---|---|---|---|
| . | Age <10 years . | Age >10 years . | . | . | . | . |
| Obese boys (N) | 58 | 72 | 66 | 57 | 57 | 41 |
| Age (years) | 8.3 ± 1.6 | 11.7 ± 1.1 | 12.7 ± 1.2 | 13.9 ± 1.2 | 15 ± 1.3 | 16.7 ± 1.3 |
| BMI Z score | 2.3 (1.9; 4.9) | 2.2 (1.7; 2.7) | 2.2 (1.7; 2.6) | 2.3 (1.6; 2.9) | 2.3 (1.5; 3) | 2.4 (1.9; 3) |
| Percent fat mass (%) | 42 (36; 50) | 46 (38; 52) | 43 (36; 50) | 41 (33; 49) | 40 (30; 48) | 40 (33; 49) |
| Total BMD Z score | 1.1 ± 1.2 | 0.7 ± 0.9 | 0.6 ± 0.9 | 0.6 ± 1.2 | 0.7 ± 1.2 | 1.1 ± 1.1 |
| Inhibin B (pg/mL) | 62 (30; 158) | 75c (33; 154) | 149a (24; 266) | 138a (64; 270) | 150c (33; 294) | 138c (57; 229) |
| AMH (pmol/L) | 623 (279;1514) | 436 (186; 1114) | 173 (40; 852) | 57a (10; 198) | 58 (15; 207) | 40 (10; 310) |
| Testosterone(nmol/L) | 0.1 (0.07; 0.59) | 0.4 (0.1; 1.03) | 2.4 (0.37; 12.5) | 7 (0.67; 17.4) | 9.9c (3.0; 21.1) | 11.6c (6.5; 19.5) |
| LH (IU/L) | 0.05 (0.05; 0.35) | 0.1 (0.05; 2.04) | 1.5 (0.1; 4.72) | 2 (0.41; 5.50) | 3 (1.0; 7.8) | 3.2 (1.6; 6.3) |
| FSH (IU/L) | 0.4 (0.09; 2.2) | 0.93 (0.17; 2.9) | 2.1 (0.55; 5.3) | 2.5 (0.7; 7.5) | 2.52 (0.75; 10.7) | 2.24 (0.75; 7.19) |
| Estradiol (pmol/L) | 40b (8; 66) | 40b (8; 92) | 44b (8; 106) | 95c (8; 132) | 99c (8; 209) | 114a (59; 176) |
| Healthy boys (N) | 224 | 137 | 97 | 81 | 54 | 59 |
| Age (years) | 7.5 ± 1.7 | 11.5 ± .1.2 | 13.1 ± 1.0 | 14.2 ± 1.0 | 14.7 ± 0.8 | 16.7 ± 1.5 |
| BMI Z score | –0.1 (–1.6; 0.9) | –0.4 (–1.7; 0.8) | –0.2 (–1.6; 0.9) | –0.3 (–1.8; 0.7) | –0.2 (–1.8; 0.6) | –0.2 (–1.5; 0.6) |
| Inhibin B (pg/mL) | 80 (34; 180) | 110 (45; 220) | 160 (70; 300) | 170 (90; 315) | 195 (100; 350) | 200 (105; 400) |
| AMH (pmol/L) | 642 (185; 1475) | 540 (150; 1400) | 188 (70; 820) | 70 (30; 500) | 53 (25; 310) | 46 (25; 105) |
| Testosterone (nmol/L) | 0.07 (0.03; 0.6) | 0.35 (0.07; 1.25) | 2.1 (0.65; 8.50) | 8.0 (3.81; 15.9) | 13 (7.2; 18) | 20 (11; 35) |
| LH (IU/L) | 0.065 (0.01; 0.32) | 0.1 (0.07; 2.15) | 1.2 (0.30; 2.85) | 1.9 (0.70; 3.8) | 2.5 (0.90; 3.95) | 2.8 (0.5; 5.0) |
| FSH (IU/L) | 0.5 (0.05; 1.2) | 0.7 (0.20; 2.3) | 1.9 (0.30; 3.1) | 2.5 (0.70; 3.8) | 2.5 (0.80; 4.1) | 2.3 (0.8; 4.4) |
| Estradiol (pmol/L) | 14 (8; 24) | 18 (8; 34) | 21 (8; 45) | 36 (8; 85) | 59 (29; 102) | 71 (44; 117) |
| . | Tanner 1 . | Tanner 1 . | Tanner 2 . | Tanner 3 . | Tanner 4 . | Tanner 5 . |
|---|---|---|---|---|---|---|
| . | Age <10 years . | Age >10 years . | . | . | . | . |
| Obese boys (N) | 58 | 72 | 66 | 57 | 57 | 41 |
| Age (years) | 8.3 ± 1.6 | 11.7 ± 1.1 | 12.7 ± 1.2 | 13.9 ± 1.2 | 15 ± 1.3 | 16.7 ± 1.3 |
| BMI Z score | 2.3 (1.9; 4.9) | 2.2 (1.7; 2.7) | 2.2 (1.7; 2.6) | 2.3 (1.6; 2.9) | 2.3 (1.5; 3) | 2.4 (1.9; 3) |
| Percent fat mass (%) | 42 (36; 50) | 46 (38; 52) | 43 (36; 50) | 41 (33; 49) | 40 (30; 48) | 40 (33; 49) |
| Total BMD Z score | 1.1 ± 1.2 | 0.7 ± 0.9 | 0.6 ± 0.9 | 0.6 ± 1.2 | 0.7 ± 1.2 | 1.1 ± 1.1 |
| Inhibin B (pg/mL) | 62 (30; 158) | 75c (33; 154) | 149a (24; 266) | 138a (64; 270) | 150c (33; 294) | 138c (57; 229) |
| AMH (pmol/L) | 623 (279;1514) | 436 (186; 1114) | 173 (40; 852) | 57a (10; 198) | 58 (15; 207) | 40 (10; 310) |
| Testosterone(nmol/L) | 0.1 (0.07; 0.59) | 0.4 (0.1; 1.03) | 2.4 (0.37; 12.5) | 7 (0.67; 17.4) | 9.9c (3.0; 21.1) | 11.6c (6.5; 19.5) |
| LH (IU/L) | 0.05 (0.05; 0.35) | 0.1 (0.05; 2.04) | 1.5 (0.1; 4.72) | 2 (0.41; 5.50) | 3 (1.0; 7.8) | 3.2 (1.6; 6.3) |
| FSH (IU/L) | 0.4 (0.09; 2.2) | 0.93 (0.17; 2.9) | 2.1 (0.55; 5.3) | 2.5 (0.7; 7.5) | 2.52 (0.75; 10.7) | 2.24 (0.75; 7.19) |
| Estradiol (pmol/L) | 40b (8; 66) | 40b (8; 92) | 44b (8; 106) | 95c (8; 132) | 99c (8; 209) | 114a (59; 176) |
| Healthy boys (N) | 224 | 137 | 97 | 81 | 54 | 59 |
| Age (years) | 7.5 ± 1.7 | 11.5 ± .1.2 | 13.1 ± 1.0 | 14.2 ± 1.0 | 14.7 ± 0.8 | 16.7 ± 1.5 |
| BMI Z score | –0.1 (–1.6; 0.9) | –0.4 (–1.7; 0.8) | –0.2 (–1.6; 0.9) | –0.3 (–1.8; 0.7) | –0.2 (–1.8; 0.6) | –0.2 (–1.5; 0.6) |
| Inhibin B (pg/mL) | 80 (34; 180) | 110 (45; 220) | 160 (70; 300) | 170 (90; 315) | 195 (100; 350) | 200 (105; 400) |
| AMH (pmol/L) | 642 (185; 1475) | 540 (150; 1400) | 188 (70; 820) | 70 (30; 500) | 53 (25; 310) | 46 (25; 105) |
| Testosterone (nmol/L) | 0.07 (0.03; 0.6) | 0.35 (0.07; 1.25) | 2.1 (0.65; 8.50) | 8.0 (3.81; 15.9) | 13 (7.2; 18) | 20 (11; 35) |
| LH (IU/L) | 0.065 (0.01; 0.32) | 0.1 (0.07; 2.15) | 1.2 (0.30; 2.85) | 1.9 (0.70; 3.8) | 2.5 (0.90; 3.95) | 2.8 (0.5; 5.0) |
| FSH (IU/L) | 0.5 (0.05; 1.2) | 0.7 (0.20; 2.3) | 1.9 (0.30; 3.1) | 2.5 (0.70; 3.8) | 2.5 (0.80; 4.1) | 2.3 (0.8; 4.4) |
| Estradiol (pmol/L) | 14 (8; 24) | 18 (8; 34) | 21 (8; 45) | 36 (8; 85) | 59 (29; 102) | 71 (44; 117) |
Data are presented as mean ± SD or median (5th; 95th). Comparison vs median in healthy boys (Wilcoxon rank sum test, see “Subjects and Methods”). Percent fat mass and BMD by DXA.
Abbreviations: AMH, anti-Müllerian hormone; BMD, bone mineral density; BMI, body mass index; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
aP < .05;
bP < .01;
cP < .001.
In prepuberty and before the age of 8 years, median LH, FSH, inhibin B, AMH, testosterone, and estradiol levels were similar between ow/ob and healthy nonoverweight nonobese boys.
Inhibin B
Median inhibin B levels during puberty were significantly lower (P < 0.5-.001) in ow/ob compared with healthy nonoverweight nonobese boys from the age of 12 years (Table 1 and Fig. 1) and from Tanner stage 1 and age >10 years and thereafter (Table 2). Accordingly, 22% of the inhibin B values of ow/ob boys were below the 5th percentile of reference of healthy boys from the age of 12 years (P < .05) (Fig. 1). At Tanner stages 4 and 5, 26% of the inhibin B values of ow/ob boys were below the 5th percentile of reference of healthy boys (P < .05).
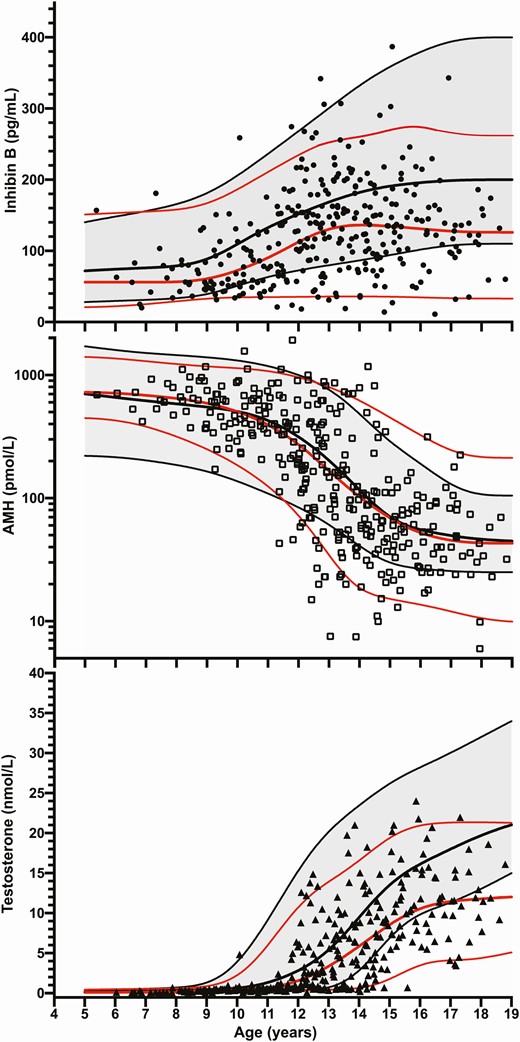
Inhibin B, AMH, and testosterone levels in overweight and obese boys versus healthy boys (expressed as 5th, 50th, and 95th percentiles) according to age. Black lines represent 5th, median, and 95th percentiles of healthy boys. Red lines represent 5th, median, and 95th percentiles of overweight/obese boys.
Testosterone Levels
Median testosterone levels were lower in ow/ob than in healthy nonoverweight nonobese boys from the age of 14 years (Table 1 and Fig. 1) and from Tanner stage 4 and thereafter (P < .01) (Table 2). Accordingly, 28% of the testosterone values of ow/ob boys were below the 5th percentile of reference of healthy boys from the age of 14 years (P < .05) (Fig. 1). At Tanner stages 4 and 5, 31% of the testosterone values of ow/ob boys were below the 5th percentile of reference of healthy boys (P < .05).
AMH Levels
Median AMH values were similar between ow/ob and healthy nonoverweight nonobese boys across ages and Tanner stages (Tables 1 and 2, and Fig. 1). However, a greater dispersion of AMH values was observed in ow/ob than in healthy boys from the age of 12 years: 22% of the AMH values of obese boys were below the 5th percentile of reference of healthy boys from the age of 12 years (P < .05) (Fig. 1). At Tanner stages 4 and 5, 19% of the AMH values of obese boys were below the 5th percentile of reference of healthy boys (P < .05).
Estradiol Levels
Median estradiol levels were significantly higher in ow/ob than in healthy nonoverweight nonobese boys from the age of 8 years and thereafter (P < .05).
LH and FSH Levels
Median LH and FSH levels were similar in ow/ob and healthy nonoverweight nonobese boys regardless of age or Tanner stage (Tables 1 and 2)
Multiple Regression Analyses
The best combination of predictors of inhibin B levels was LH and testosterone levels (both positive predictors), and FSH and estradiol levels and total bone mineral density (BMD) Z-score (all negative predictors) (multiple R = 0.62, P < .0001) (Table 3).
Backward stepwise multiple regression analyses for inhibin B, testosterone, and AMH
| . | Variables . | β ± SD . | P . |
|---|---|---|---|
| Inhibin B (pg/mL) | FSH (IU/L) | –15.35 ± 4.02 | <.0001 |
| Multiple R = 0.62 | Testosterone (nmol/L) | 7.22 ± 1.98 | <.001 |
| P < .0001 | LH (IU/L) | +15.74 ± 6.97 | <.05 |
| Total BMD Z-score | –23.22 ± 7.31 | <.01 | |
| Estradiol (pmol/L) | –8.74 ± 3.19 | <.01 | |
| Testosterone (nmol/L) | Tanner genital stage | 1.96 ± 0.20 | <.0001 |
| Multiple R = 0.82 | LH (IU/L) | 0.94 ± 0.15 | <.0001 |
| P < .0001 | % fat mass | –0.21 ± 0.04 | <.0001 |
| AMHa (pmol/L) | Testosterone (nmol/L) | –0.06 ± 0.00 | <.0001 |
| Multiple R = 0.74 | Insulina (mIU/L) | –0.54 ± 0.09 | <.0001 |
| P < .0001 |
| . | Variables . | β ± SD . | P . |
|---|---|---|---|
| Inhibin B (pg/mL) | FSH (IU/L) | –15.35 ± 4.02 | <.0001 |
| Multiple R = 0.62 | Testosterone (nmol/L) | 7.22 ± 1.98 | <.001 |
| P < .0001 | LH (IU/L) | +15.74 ± 6.97 | <.05 |
| Total BMD Z-score | –23.22 ± 7.31 | <.01 | |
| Estradiol (pmol/L) | –8.74 ± 3.19 | <.01 | |
| Testosterone (nmol/L) | Tanner genital stage | 1.96 ± 0.20 | <.0001 |
| Multiple R = 0.82 | LH (IU/L) | 0.94 ± 0.15 | <.0001 |
| P < .0001 | % fat mass | –0.21 ± 0.04 | <.0001 |
| AMHa (pmol/L) | Testosterone (nmol/L) | –0.06 ± 0.00 | <.0001 |
| Multiple R = 0.74 | Insulina (mIU/L) | –0.54 ± 0.09 | <.0001 |
| P < .0001 |
Abbreviations: AMH, anti-Müllerian hormone; BMD, bone mineral density; BMI, body mass index; FSH, follicle-stimulating hormone; HDL< high-density lipoprotein; LH, luteinizing hormone.
aAMH and insulin were log transformed to normalize their distribution. In simple regression analyses, inhibin B was related to age, Tanner genital stage, BMI Z-score, systolic blood pressure, fat mass percentage, testosterone, estradiol, AMH, FSH, LH, total bone mineral density Z-score (BMD Z score), and lumbar BMD Z-score. In simple regression analyses, testosterone was related to age, Tanner genital stage, systolic blood pressure, fat mass percentage, inhibin B, AMH, estradiol, FSH, LH, insulin, and total and HDL cholesterol. In simple regression analyses, AMH was related to age, Tanner genital stage, systolic and diastolic blood pressure, fat mass percentage, fasting insulin, HDL cholesterol, total BMD Z-score, and inhibin B, testosterone, FSH, LH, and estradiol levels.
Backward stepwise multiple regression analyses for inhibin B, testosterone, and AMH
| . | Variables . | β ± SD . | P . |
|---|---|---|---|
| Inhibin B (pg/mL) | FSH (IU/L) | –15.35 ± 4.02 | <.0001 |
| Multiple R = 0.62 | Testosterone (nmol/L) | 7.22 ± 1.98 | <.001 |
| P < .0001 | LH (IU/L) | +15.74 ± 6.97 | <.05 |
| Total BMD Z-score | –23.22 ± 7.31 | <.01 | |
| Estradiol (pmol/L) | –8.74 ± 3.19 | <.01 | |
| Testosterone (nmol/L) | Tanner genital stage | 1.96 ± 0.20 | <.0001 |
| Multiple R = 0.82 | LH (IU/L) | 0.94 ± 0.15 | <.0001 |
| P < .0001 | % fat mass | –0.21 ± 0.04 | <.0001 |
| AMHa (pmol/L) | Testosterone (nmol/L) | –0.06 ± 0.00 | <.0001 |
| Multiple R = 0.74 | Insulina (mIU/L) | –0.54 ± 0.09 | <.0001 |
| P < .0001 |
| . | Variables . | β ± SD . | P . |
|---|---|---|---|
| Inhibin B (pg/mL) | FSH (IU/L) | –15.35 ± 4.02 | <.0001 |
| Multiple R = 0.62 | Testosterone (nmol/L) | 7.22 ± 1.98 | <.001 |
| P < .0001 | LH (IU/L) | +15.74 ± 6.97 | <.05 |
| Total BMD Z-score | –23.22 ± 7.31 | <.01 | |
| Estradiol (pmol/L) | –8.74 ± 3.19 | <.01 | |
| Testosterone (nmol/L) | Tanner genital stage | 1.96 ± 0.20 | <.0001 |
| Multiple R = 0.82 | LH (IU/L) | 0.94 ± 0.15 | <.0001 |
| P < .0001 | % fat mass | –0.21 ± 0.04 | <.0001 |
| AMHa (pmol/L) | Testosterone (nmol/L) | –0.06 ± 0.00 | <.0001 |
| Multiple R = 0.74 | Insulina (mIU/L) | –0.54 ± 0.09 | <.0001 |
| P < .0001 |
Abbreviations: AMH, anti-Müllerian hormone; BMD, bone mineral density; BMI, body mass index; FSH, follicle-stimulating hormone; HDL< high-density lipoprotein; LH, luteinizing hormone.
aAMH and insulin were log transformed to normalize their distribution. In simple regression analyses, inhibin B was related to age, Tanner genital stage, BMI Z-score, systolic blood pressure, fat mass percentage, testosterone, estradiol, AMH, FSH, LH, total bone mineral density Z-score (BMD Z score), and lumbar BMD Z-score. In simple regression analyses, testosterone was related to age, Tanner genital stage, systolic blood pressure, fat mass percentage, inhibin B, AMH, estradiol, FSH, LH, insulin, and total and HDL cholesterol. In simple regression analyses, AMH was related to age, Tanner genital stage, systolic and diastolic blood pressure, fat mass percentage, fasting insulin, HDL cholesterol, total BMD Z-score, and inhibin B, testosterone, FSH, LH, and estradiol levels.
The best combination of predictors of testosterone levels was Tanner genital stage and LH level (positive predictors), and fat mass percentage (negative predictor) (multiple R = 0.82, P < .0001).
The best combination of predictors of AMH levels was testosterone and insulin levels (negative predictors) (multiple R = 0.74, P < .00001).
Discussion
We observed lower median levels of inhibin B and testosterone levels in overweight and obese adolescents than in healthy nonoverweight nonobese boys during puberty. Accordingly, 22% and 28% of the inhibin B and testosterone values were below the 5th percentile from the age of 12 and 14 years, respectively. Although median AMH was not different between obese and healthy boys across ages, the dispersion of the values was greater in obese boys, and 22% of AMH values were below the 5th percentile from the age of 12 years. The lower Sertoli cell hormone levels in obese boys than in references from healthy boys was observed earlier in puberty than those of Leydig cells. It is not clear from this cross-sectional study whether these alterations correspond to a simple pubertal delay in Sertoli and Leydig cell hormone levels or whether they persist into adulthood. Longitudinal follow-up of obese boys from prepubertal to adult age would be necessary.
A few studies have investigated Sertoli cell hormone levels in obese boys. In overweight and obese prepubertal boys, inhibin B levels were similar to those of nonobese (as defined by the BMI) age-matched boys (16). In 2 other studies of obese pubertal boys, circulating inhibin B concentrations were significantly lower than those in age-matched nonobese subjects, but comparisons across Tanner stages were not performed (27, 28). In 1 large study including 231 nonobese and 208 obese male children and adolescents aged 5.5-18 years, a negative association between boys’ testicular volume and BMI was reported, but inhibin B levels were not measured (14). With regard to AMH levels, which is another peptide produced by Sertoli cells, Buyukinan et al. found AMH to be inversely related to BMI and insulin resistance in obese adolescent boys aged 12-16 years (27). More specifically, AMH values were significantly lower only in the obese insulin-resistant boys compared with lean boys (27). In our study, median AMH was similar in obese compared with healthy boys, but the dispersion was greater. This could point to a complex regulation of circulating AMH levels in obese adolescent boys. On the one hand, a stimulating effect of estradiol on AMH production has been demonstrated in Sertoli cell lines (29): since median estradiol levels were elevated in our obese boys, this could explain the maintenance of elevated AMH levels through puberty in some obese boys. On the other hand, we also observed that 22% of the AMH values were below the 5th percentile in obese boys from the age of 12 years, in agreement with the study by Buyukinan et al. (27). As testosterone levels were also lower in ow/ob boys at the same time, with testosterone being a negative regulator of AMH production (30-32), the lower AMH values could not be accounted for merely by a physiological negative regulation by testosterone. In our study and that of Buyukinan et al., fasting insulin was inversely related to AMH levels, suggesting a link between insulin resistance and AMH levels. In adults, a negative association between BMI and inhibin B and AMH levels has been reported (9-12, 33), suggesting Sertoli cell impairment in some obese adults.
Circulating Leydig cell hormone levels during puberty in obese boys has been more extensively studied. Lower total testosterone levels were found in the obese compared with nonobese subjects, as in our study, but comparisons across Tanner stages were not performed (15, 27, 28, 34). In large adult studies, testosterone levels were generally lower in obese compared with nonobese males (9-12).
Gonadotrophin levels have been investigated in adult and adolescent studies (12, 16, 27, 28) and found to be unaffected by obesity and BMI, in agreement with our study. Since lower levels of testosterone and inhibin B in obese subjects did not elicit higher LH and FSH levels, this impaired response was considered to evidence a dysregulation of the hypothalamic–pituitary–gonadal axis (6).
In our study, median estradiol levels were higher in obese than in healthy boys from the age of 8 years, that is before puberty onset. Aromatization in fat tissue from adrenal androgens may explain this early difference, while the difference in later stages of puberty may be due to the aromatization of androgens both from adrenal and testicular origin. There are not many studies about estradiol levels in obese boys, likely because sensitive assays are needed (16, 34, 35). Higher estradiol values and/or excess aromatization have been demonstrated in relation to fat mass in obese adults (11, 36, 37).
Regarding the factors potentially mediating Sertoli cell hormone levels, the multiple regression analyses revealed a negative association of circulating inhibin B with estradiol, FSH, and total BMD Z-score in the ow/ob subjects. Estradiol and the total BMD Z-score likely indicated a negative effect of excess aromatization on Sertoli cell hormone levels. In adults, the positive association between obesity and BMD is well known (38), and estrogen levels are more robust predictors of BMD than testosterone levels in men (39, 40). Surprisingly, we also found a negative association between inhibin B and FSH in obese boys. This may suggest that the lower Sertoli cell hormone levels not only results from hypothalamic alteration, as usually assumed (6), but also from direct testis involvement. Regarding the factors associated with circulating Leydig cell hormone levels, we found a negative association between testosterone and fat mass percentage. Similarly, Crocker et al. (14) reported a negative association between fat mass and testosterone in male adolescents. Among the potential mediators of this effect, excess leptin levels resulting from increased secretion from adipose tissue are known to have a deleterious effect on the production of androgens by Leydig cells (41).
Our study has several limitations. It was a cross-sectional and not a longitudinal study: whether we observed a simple pubertal delay in physiological circulating Sertoli and Leydig cell hormone levels or an alteration persistent into adulthood could not be known. Tanner stage assessment was based on orchidometer use and not ultrasound, which could have increased the accuracy of the measurements (42). Our study’s strengths were a large set of overweight, obese, and healthy nonoverweight nonobese subjects, assayed by the same techniques for all the hormones studied, and a precise assessment of body composition and metabolic characteristics of the ow/ob boys.
Conclusion
In conclusion, we showed lower Sertoli and Leydig cell hormone levels in ow/ob boys than in healthy nonoverweight nonobese boys during puberty. This was related to excess aromatization, insulin resistance, and excess fat mass. It remains to determine whether this is a simple delay or a persistent alteration in adulthood, and whether this is reversible with weight loss.
Abbreviations
- AMH
anti-Müllerian hormone
- BMD
bone mineral density
- BMI
body mass index
- CV
coefficient of variation
- DXA
dual-energy X-ray absorptiometry
- FSH
follicle-stimulating hormone
- HDL
high-density lipoprotein
- LH
luteinizing hormone
- ow/ob
overweight–obese
- RIA
radioimmunoassay
Acknowledgments
We are indebted to Valerie Cognee, Celine Barbot, Nathalie Barre, Emmanuelle Labarre (Specialized Nurses, Angers). The authors thank Cathy Stott, medical writer, for help with preparing the manuscript.
Funding: None.
Additional Information
Disclosures: The authors declare no conflict of interest.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Zemel B. BMI Z-score calculator. https://zscore.research.chop.edu/[email protected].
Author notes
These authors contributed equally to this work.
These authors contributed equally to this work.



