-
PDF
- Split View
-
Views
-
Cite
Cite
Carrie Riestenberg, Anika Jagasia, Daniela Markovic, Richard P Buyalos, Ricardo Azziz, Health Care-Related Economic Burden of Polycystic Ovary Syndrome in the United States: Pregnancy-Related and Long-Term Health Consequences, The Journal of Clinical Endocrinology & Metabolism, Volume 107, Issue 2, February 2022, Pages 575–585, https://doi.org/10.1210/clinem/dgab613
Close - Share Icon Share
Abstract
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder of reproductive-aged women, affecting approximately 5% to 20% of women of reproductive age. The economic burden of PCOS was previously estimated at approximately $3.7 billion annually in 2020 USD when considering only the costs of the initial diagnosis and of reproductive endocrine morbidities, without considering the costs of pregnancy-related and long-term morbidities.
This study aimed to estimate the excess prevalence and economic burden of pregnancy-related and long-term health morbidities attributable to PCOS.
PubMed, EmBase, and Cochrane Library were searched, and studies were selected in which the diagnosis of PCOS was consistent with the Rotterdam, National Institutes of Health, or Androgen Excess and PCOS Society criteria, or that used electronic medical record diagnosis codes, or diagnosis based on histopathologic sampling. Studies that included an outcome of interest and a control group of non-PCOS patients who were matched or controlled for body mass index were included. Two investigators working independently extracted data on study characteristics and outcomes. Data were pooled using random effects meta-analysis. The I2 statistic was used to assess inter-study heterogeneity. The quality of selected studies was assessed using the Newcastle-Ottawa Scale.
The additional total healthcare-related economic burden of PCOS due to pregnancy-related and long-term morbidities in the United States is estimated to be $4.3 billion annually in 2020 USD.
Together with our prior analysis, the economic burden of PCOS is estimated at $8 billion annually in 2020 USD.
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age, affecting 5% to 20% of reproductive-aged women, depending on the diagnostic criteria used (1). It is also one of the most common causes of subfertility (2) and a common antecedent of type 2 diabetes mellitus (T2DM), endometrial malignancy, and vascular morbidity (3). Despite the prevalence of this syndrome, many physicians are unfamiliar with the PCOS diagnostic criteria, and PCOS patients have a high level of dissatisfaction with their health care (4). Additionally, funding for research on PCOS appears to be deficient relative to the high prevalence and cost of the disorder (5), and the disorder is not included in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) 2020 Strategic Plan (6), the principal funding agency for PCOS research in the United States (5). A clearer estimate of the economic burden of PCOS would be valuable to allow for a more accurate prioritization of the disorder as a public health interest.
In a previous study, excluding the cost of T2DM, which is assessed in the present study using more current data, the cost of the initial diagnosis and treatment of the most common reproductive endocrine disorders (ie, menstrual dysfunction/abnormal uterine bleeding, infertility, and hirsutism) in PCOS was estimated to be $2.6 billion annually in 2004 US dollars (USD) (7), or approximately $3.7 billion annually in 2020 USD. That study did not assess the cost of pregnancy-related complications or of long-term morbidities (except T2DM), providing an incomplete estimate of the disorder’s economic impact. Considering the high prevalence and public health relevance of the disorder, the objective of the present study was to expand upon our previous analysis and estimate the excess prevalence and economic burden of PCOS associated with pregnancy-related complications, including gestational diabetes (GDM), gestational hypertension (gHTN), and preeclampsia, and with select long-term morbidities, including T2DM, myocardial infarction (MI), and stroke.
Methods
A systematic review of the published medical literature was performed adhering to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement and Checklist (8) (Fig. 1).
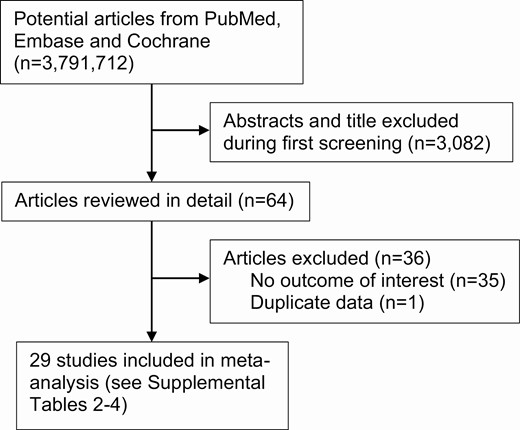
Flow diagram of the literature search and study selection process.
Information Sources and Search Strategy
PubMed, EmBase, and the Cochrane Library were searched to identify English-language articles with no date restriction. The search strategy for the pregnancy-related morbidities was: ((“Polycystic Ovary Syndrome”[Mesh] OR “Hyperandrogenism”[Mesh] OR “Amenorrhea”[Mesh] OR “Oligomenorrhea”[Mesh] OR “Anovulation”[Mesh]) OR (“Polycystic Ovary Syndrome”[tw] OR pcos[tw] OR “Stein Leventhal”[tw] OR “Stein-Leventhal”[tw] OR “Amenorrhea”[tw] OR “Oligomenorrhea”[tw] OR “Irregular Menses”[tw] OR “Irregular Menstruation”[tw] OR “Menstruation Disorder”[tw] OR “Menstrual Irregularity”[tw] OR Anovulation*[tw] OR oligoovulation*[tw] OR “Hyperandrogenism”[tw])) AND ((“Diabetes, Gestational”[Mesh] OR “Hypertension, Pregnancy-Induced”[Mesh] OR “Pre-Eclampsia”[Mesh]) OR (“Diabetes, Gestational”[tw] OR “Hypertension, Pregnancy-Induced”[tw] OR “Pre-Eclampsia”)). The search strategy for the long-term morbidities was: ((“Polycystic Ovary Syndrome”[Mesh] OR “Hyperandrogenism”[Mesh] OR “Amenorrhea”[Mesh] OR “Oligomenorrhea”[Mesh] OR “Anovulation”[Mesh]) OR (“Polycystic Ovary Syndrome”[tw] OR pcos[tw] OR “Stein Leventhal”[tw] OR “Stein-Leventhal”[tw] OR “Amenorrhea”[tw] OR “Oligomenorrhea”[tw] OR “Irregular Menses”[tw] OR “Irregular Menstruation”[tw] OR “Menstruation Disorder”[tw] OR “Menstrual Irregularity”[tw] OR Anovulation*[tw] OR oligoovulation*[tw] OR “Hyperandrogenism”[tw])) AND ((“Stroke”[Mesh] OR “Cardiovascular Diseases”[Mesh] OR “Diabetes Mellitus, Type 2”[Mesh]) OR (“Stroke”[tw] OR “Cardiovascular Diseases”[tw] OR “Diabetes Mellitus, Type 2”[tw])). We also performed a manual search of reference lists from relevant articles and reviews to identify additional eligible studies.
Eligibility Criteria
Observational, controlled studies that examined the risk of adverse pregnancy outcomes (GDM, gHTN, and preeclampsia), or long-term health outcomes (T2DM, MI, or stroke) in women with PCOS compared with a control group were eligible for inclusion. Studies without a control group, or that did not match or control for body mass index (BMI) were excluded. Studies that reported relative risks, odds ratios, or incidence data were eligible for inclusion, while those reporting hazard ratios were excluded.
Study Selection and Data Extraction
Article titles and abstracts were reviewed and those involving case reports, editorials, or reviews/meta-analyses were excluded. Studies in which the diagnosis of PCOS was consistent with the 1990 National Institutes of Health (NIH), the 2003 Rotterdam, or the 2006 Androgen Excess and PCOS (AE-PCOS) Society criteria, or that used electronic medical record diagnosis codes, or diagnosis based on histopathologic sampling were eligible for inclusion. Full-text selected articles were then reviewed in detail and rejected if they did not include an outcome of interest.
Search strategy and study identification was performed by one investigator (C.R.) using a standardized approach. Articles selected for inclusion were then screened by a second author (A.Z.). Two investigators worked in duplicate to independently extract data on study characteristics and outcomes (C.R., D.M.). Disagreements were discussed until consensus was reached.
Quality Assessment
Two investigators worked in duplicate to independently assess the quality of eligible studies using the Newcastle-Ottawa Scale (C.R., R.B.). Details of the Newcastle-Ottawa Scale scores for studies included in the analysis can be found in the supplemental materials (9).
Statistical Analysis
The meta-analysis was performed using the DerSimonian-Laird random effects model (10) to compute weighted effect estimates (relative risk or odds ratio) for a given outcome measure. The study specific and overall effect estimates were graphically presented using forest plots. Between-study heterogeneity was evaluated using the I2 statistic. Since the analyses indicated the presence of significant heterogeneity across studies, additional subgroup analyses were performed to explore the sources of this heterogeneity. The following study characteristics were considered for the subgroup analyses including age as well as study design (prospective or retrospective). Most of the heterogeneity could be explained by differences in age, and separate summary statistics were therefore computed in subgroups by age (young/premenopause, close to menopause, postmenopause). Because of the relatively low frequency of the outcomes of interest, the odds ratios (ORs) generated from the meta-analyses were used to approximate the risk ratios and to calculate the relative risk of each outcome for women with PCOS versus controls.
Definition of PCOS for Prevalence Estimation
Four sets of diagnostic criteria for PCOS have been proposed over the last 30 years (11-13). The first was developed by the NIH in 1990, which required the presence of both hyperandrogenism/hyperandrogenemia and oligo-ovulation (ovulatory disorder), with or without the presence of polycystic ovarian morphology (PCOM) on ultrasound, and excluding other disorders such as Cushing syndrome, hyperprolactinemia, and congenital adrenal hyperplasia (14).
The second set of criteria was based on expert consensus reached at a meeting cosponsored by the European Society for Human Reproduction and Embryology (ESHRE) and the American Society for Reproductive Medicine (ASRM) in Rotterdam, the Netherlands in 2003 (12). These criteria, commonly referred to as the Rotterdam criteria, require 2 of the following 3 features to be present for the diagnosis: (1) oligo- or anovulation, (2) clinical and/or biochemical signs of hyperandrogenism, (3) PCOM on ultrasound, after the exclusion of other etiologies. Institution of the Rotterdam criteria broadened the PCOS diagnosis, significantly increasing the number of patients meeting the diagnosis of PCOS.
In 2006, the AE-PCOS Society published the third set of diagnostic guidelines based on recommendations from an expert task force (13). In contrast to the Rotterdam criteria, the AE-PCOS criteria made androgen excess requisite for the diagnosis, with either ovulatory disorder or PCOM as the second criteria. Prevalence estimates based on these various criteria range from 5% to 20%, with the NIH criteria being the most strict (1).
Finally, in 2018, the International PCOS Network published an international consensus guideline on the assessment and management of PCOS based on an Appraisal of Guidelines for Research and Evaluation (AGREE)-compliant literature review and synthesis (15). This consensus document endorsed the Rotterdam criteria for PCOS diagnosis in adults, heavily discouraged diagnosis in adolescents based on PCOM on ultrasound, and provided more nuanced recommendations for follicular count and menstrual cycle irregularity.
Because the NIH diagnostic criteria yielded the most consistent and conservative prevalence estimates, we chose to use these criteria for the purpose of the economic burden calculations, although we recognize that this may underestimate the true prevalence and therefore economic burden of the disorder. While subfertility affects the majority of women with PCOS, studies that have previously compared fecundity and family size of PCOS and non-PCOS controls have found no differences (16, 17). This is likely a result of the increased availability and effectiveness of fertility treatment in women with PCOS and the decreasing number of children being borne by the average family. We therefore will assume that the proportion of births by women with PCOS approximates their prevalence in the overall population. For the calculations pertaining to the long-term health consequences of PCOS, we will also assume that the prevalence of PCOS is 6.6% across age groups. We feel justified in this assumption because the prevalence of PCOS, as with any genetic disorder, would not be expected to change with age, regardless of whether signs and symptoms of the disorder ameliorate (1-3). Consequently, a prevalence of 6.6% based on the NIH criteria, as was used in our prior analysis, is applied here to all of our cost calculations (7).
Estimating Excess Attributable Costs
Cost data for medical treatment of the health outcomes of interest were obtained from The Medical Expenditure Panel Survey (MEPS) data from the Agency for Healthcare Research and Quality (AHRQ) where possible (18) and otherwise from review of published literature of healthcare expense in the United States.
Adjusting for Inflation
We adjusted for inflation using the consumer price index (19).
Results
A total of 3146 studies were identified during the initial electronic search and bibliography review, of which 3082 were excluded after title and abstract screening. A total of 64 potentially eligible studies were reviewed in detail, of which 35 were excluded because of the lack of an outcome of interest or because they did not match or control for BMI, and 1 study was excluded because it contained duplicate data published in an earlier study. The general characteristics of the 29 included studies are detailed in the supplemental materials (9).
Economic Burden of Pregnancy-Related Morbidities in PCOS
A number of pregnancy-related morbidities have been associated with PCOS (20). For the purpose of this economic burden analysis, we considered those morbidities which have been most consistently and strongly associated with PCOS in pregnancy: GDM, gHTN, and preeclampsia.
Prevalence of PCOS in pregnancy
A total of 3 745 540 births were registered in the United States in 2019 according to the Centers for Disease Prevention and Control (CDC) National Vital Statistics Birth Data (21). Assuming a prevalence of 6.6%, we can estimate that approximately 250 000 PCOS-related births took place in the United States in 2020.
Prevalence of GDM in women with PCOS during pregnancy
GDM is the pregnancy complication most commonly described in women with PCOS. Seventeen studies reported an association between PCOS in pregnancy and gestational diabetes in 1 230 648 women (14 388 with PCOS and 1 216 260 controls). Compared with BMI-matched women without PCOS, women with PCOS had a higher prevalence of GDM (random effects OR: 2.78; 95% CI, 1.97-3.91; I2 78.4%; Fig. 2A). For our calculations of economic burden, we considered PCOS patients as having a 2.78-fold (95% CI, 1.97-3.91) greater risk of GDM compared with unaffected women (see below). Four meta-analyses investigating pregnancy complications in women with PCOS published within the last 10 years reported a similar increased risk of GDM, ranging from a 2.8- to 3.6-fold increase, although not all included studies controlled for BMI (22-25).
Considering that comparably aged women of the general population have a prevalence of GDM of 6% (26), the overall prevalence of GDM in women with PCOS can be estimated to be 2.78 × 6% = 16.7%. The excess prevalence of pregnancies with GDM due to PCOS is therefore 16.7% − 6% = 10.7%. Therefore, the excess number of pregnancies with GDM due to PCOS is 250 000 × 10.7% = 26 700 pregnancies (95% CI, 14 550 to 43 650 pregnancies).
Prevalence of gHTN in women with PCOS during pregnancy
Fifteen studies reported an association between PCOS in pregnancy and the risk of gHTN in 11 052 women (1596 with PCOS and 9456 controls). Compared with BMI-matched women without PCOS, women with PCOS had a higher prevalence of gHTN (random effects OR: 2.04; 95% CI, 1.30-3.22; I2 63.6%; Fig. 2B). For our calculations of economic burden, we will consider PCOS patients as having a 2.04-fold greater risk of gHTN compared with unaffected women (see below). The 4 meta-analyses previously referenced reported a 2.5- to 4.1-fold increase in risk of gHTN among women with PCOS compared to controls, which is commensurate with our findings (22-25).
According to National Health and Nutrition Examination Survey (NHANES) data, gHTN affects 6% to 8% of pregnancies in women 20 to 44 years of age in the United States (27). Based on this and a 2.04-fold increase in risk among women with PCOS, the prevalence of gHTN among women with PCOS can be estimated to be 2.04 × 7% = 14.28%. The excess prevalence of pregnancies with gHTN due to PCOS is therefore 14.28% − 7% = 7.28%. Therefore, the excess number of pregnancies with gHTN due to PCOS is 250 000 × 7.28% = 18 200 pregnancies (95% CI, 5250 to 38 850 pregnancies).
Prevalence of preeclampsia in women with PCOS during pregnancy
Seventeen studies reported an association between PCOS in pregnancy and the risk of preeclampsia in 1 229 016 women (14 136 with PCOS and 1 214 880 controls). Compared with BMI-matched women without PCOS, women with PCOS had a higher prevalence of preeclampsia (random effects OR: 2.03; 95% CI, 1.43-2.87; I2 49.0%; Fig. 2C). For our calculations of economic burden, we will consider PCOS patients as having a 2.03-fold greater risk of preeclampsia compared with unaffected women (see below). A 1.9 to 4.2-fold increased risk of preeclampsia was reported in the 4 meta-analyses, which is in agreement with our findings (22-25).
The Centers for Disease Prevention and Control estimates that preeclampsia affects 1 in 25, or roughly 4% of pregnancies in the United States (28, 29). Given a 2.03-fold increased risk, the prevalence of preeclampsia in women with PCOS would be estimated to be 2.03 × 4% = 8.12%. The excess prevalence of pregnancies with preeclampsia due to PCOS is therefore 8.12% − 4% = 4.12%. Therefore, the excess number of pregnancies complicated by preeclampsia due to PCOS is 250 000 × 4.12% = 10 300 pregnancies (95% CI, 4300 to 18 700 pregnancies).
Economic burden of GDM in women with PCOS during pregnancy
The cost of pregnancy-related care per uncomplicated pregnancy was estimated to be $9705 in 2009 USD (18), equivalent to $11 972 in 2020 USD. The excess cost of pregnancy-related care due to GDM per pregnancy is $1971 in 2012 USD, or $2265 in 2020 USD (30). Therefore, the excess cost of pregnancy-related care due to GDM in PCOS is 26 700 × $2265 = $60 475 500 (95% CI, $32 955 750 to $98 867 250) in 2020 USD (Table 1).
Estimates of the excess prevalence and economic burden associated with pregnancy-related and long-term morbidities of PCOS in 2020 in the United States
| Morbidity . | Excess prevalence of morbidity in PCOS (%) . | Annual costs in millions in 2020 USD (% of total costs in category) . |
|---|---|---|
| Pregnancy-related morbidities | ||
| GDM | 16.7 % | 61 (16) |
| gHTN | 14.3 % | 187 (50) |
| Preeclampsia | 8.1 % | 127 (34) |
| Total cost of pregnancy-related morbidities | 375 (100.0) | |
| Long-term morbidities | ||
| Type 2 diabetes mellitus | 3.93%* | 1500 (38) |
| MI | ___ | ___ |
| Stroke | 4.3%* | 2400 (62) |
| Total cost of long-term morbidities | 3900 (100.0) | |
| Total economic burden due to pregnancy-related or long-term morbidities | 4275 |
| Morbidity . | Excess prevalence of morbidity in PCOS (%) . | Annual costs in millions in 2020 USD (% of total costs in category) . |
|---|---|---|
| Pregnancy-related morbidities | ||
| GDM | 16.7 % | 61 (16) |
| gHTN | 14.3 % | 187 (50) |
| Preeclampsia | 8.1 % | 127 (34) |
| Total cost of pregnancy-related morbidities | 375 (100.0) | |
| Long-term morbidities | ||
| Type 2 diabetes mellitus | 3.93%* | 1500 (38) |
| MI | ___ | ___ |
| Stroke | 4.3%* | 2400 (62) |
| Total cost of long-term morbidities | 3900 (100.0) | |
| Total economic burden due to pregnancy-related or long-term morbidities | 4275 |
Abbreviations: GDM, gestational diabetes mellitus; gHTN, gestational hypertension; MI, myocardial infarction; PCOS, polycystic ovary syndrome; USD, US dollars.
*Weighted average prevalence of T2DM and stroke across age groups.
Estimates of the excess prevalence and economic burden associated with pregnancy-related and long-term morbidities of PCOS in 2020 in the United States
| Morbidity . | Excess prevalence of morbidity in PCOS (%) . | Annual costs in millions in 2020 USD (% of total costs in category) . |
|---|---|---|
| Pregnancy-related morbidities | ||
| GDM | 16.7 % | 61 (16) |
| gHTN | 14.3 % | 187 (50) |
| Preeclampsia | 8.1 % | 127 (34) |
| Total cost of pregnancy-related morbidities | 375 (100.0) | |
| Long-term morbidities | ||
| Type 2 diabetes mellitus | 3.93%* | 1500 (38) |
| MI | ___ | ___ |
| Stroke | 4.3%* | 2400 (62) |
| Total cost of long-term morbidities | 3900 (100.0) | |
| Total economic burden due to pregnancy-related or long-term morbidities | 4275 |
| Morbidity . | Excess prevalence of morbidity in PCOS (%) . | Annual costs in millions in 2020 USD (% of total costs in category) . |
|---|---|---|
| Pregnancy-related morbidities | ||
| GDM | 16.7 % | 61 (16) |
| gHTN | 14.3 % | 187 (50) |
| Preeclampsia | 8.1 % | 127 (34) |
| Total cost of pregnancy-related morbidities | 375 (100.0) | |
| Long-term morbidities | ||
| Type 2 diabetes mellitus | 3.93%* | 1500 (38) |
| MI | ___ | ___ |
| Stroke | 4.3%* | 2400 (62) |
| Total cost of long-term morbidities | 3900 (100.0) | |
| Total economic burden due to pregnancy-related or long-term morbidities | 4275 |
Abbreviations: GDM, gestational diabetes mellitus; gHTN, gestational hypertension; MI, myocardial infarction; PCOS, polycystic ovary syndrome; USD, US dollars.
*Weighted average prevalence of T2DM and stroke across age groups.
Economic burden of gHTN in women with PCOS during pregnancy
The excess cost of pregnancy-related care due to gHTN is estimated to be $9200 in 2013 USD (31), equivalent to $10 254 in 2020 USD. Therefore, the excess cost of pregnancy-related care due to gHTN in PCOS is 18 200 × $10 254 = $186 622 800 (95% CI, $53 833 500 to $398 367 900) in 2020 USD (Table 1).
Economic burden of preeclampsia in women with PCOS during pregnancy
The average cost of care for a pregnancy complicated by preeclampsia in the United States was estimated to be $21 167 in 2012, equivalent to $24 324 in 2020 USD (30). By subtracting the cost of care for an uncomplicated pregnancy of $11 393, we can estimate that the excess cost of care for a pregnancy complicated by preeclampsia to be $12 352 per pregnancy. Therefore, the excess cost of pregnancy-related care due to preeclampsia in PCOS is 10 300 × $12 352 = $127 225 600 (95% CI, $53 113 600 to $230 982 400) in 2020 USD (Table 1).
Economic Burden of Long-Term Morbidities in PCOS
Women with PCOS have higher rates of insulin resistance and secondary hyperinsulinemia than weight-matched controls, and therefore, unsurprisingly a higher lifetime risk of T2DM, metabolic disorders and metabolic syndrome (32). Recent evidence, however, suggests that some of the negative impact of PCOS on long-term health may be attenuated or even abolished after the menopausal transition (33). In light of recent data suggesting that after menopause PCOS may not be a strong risk factor for the development of T2DM, cardiovascular diseases (CVD), or stroke, studies included in the meta-analysis were analyzed by mean age to determine if the effect of PCOS on the risk of developing these long-term outcomes was age-dependent.
In the total US population, the prevalence of women by age group is: 15-19 years, 10.7 million women; 20-34 years, 39.9 million women; 35-44 years, 20.6 million women; 45-49 years, 11.5 million women; 50-54 years, 11.4 million women; 55-64 years, 18.8 million women; 65-74 years, 11.6 million women; and >75 years, 15 million women (34). Assuming a prevalence of 6.6%, we estimate that 0.7 million women 15-19 years of age, 2.6 million women 20-34 years of age, 1.4 million women 35-44 years of age, 0.76 million women 45-49 years of age, 2.0 million women 50-64 years of age, 0.8 million women 65-74 years of age, and 1 million women >75 years of age are living with PCOS. This is the population we will consider to be at risk for the development of T2DM and CVD/stroke in our cost calculations below.
Prevalence of T2DM associated with PCOS
Compared to BMI-matched women without PCOS, premenopausal women with PCOS have a higher prevalence of T2DM, which was reported in 4 studies. Interestingly, this increased risk of T2DM was not seen in the 2 studies whose participants had a mean age in the postmenopausal range (>55 years). Given this finding, we considered only women <55 years of age in our cost analyses for T2DM. The overall OR for T2DM in women with PCOS compared to age- and BMI-matched controls was 2.41 (random effects OR: 2.41; 95% CI, 1.85-3.13; I2 59.3%; Fig. 3A). For our calculations of economic burden, we considered PCOS patients as having a 2.41-fold greater risk of T2DM compared with unaffected women (see below).
The prevalence of T2DM in all women increases with age, from 0.2% among individuals younger than 18 years, to 1.3% from 19 to 34 years, 4.7% from 35 to 44 years, and 8.9% from 45 to 54 years (34). Given a 2.41-fold increased risk, the prevalence of T2DM in women with PCOS would be estimated to be 0.5%, 3.1%, 11.3%, and 21.4% respectively. The excess prevalence of T2DM due to PCOS is therefore 0.3%, 1.8%, 6.6%, and 12.5% among these age groups. Based on the population of women with PCOS in each age category, we can estimate that 1974 (95% CI, 1190-2982) excess cases in women <18 years of age, 47 658 (95% CI, 28 730 to 71 994) excess cases in women 19-34 years of age, 92 778 (95% CI, 55 930 to 140 154) excess cases in women 35-44 years of age, and 95 372 (95% CI, 57 494 to 144 073) excess cases of T2DM due to PCOS in women 45-54 years of age (34).
Prevalence of MI associated with PCOS
Compared to BMI-matched women without PCOS, women with PCOS were reported to have a higher prevalence of MI in 6 studies; however, the difference did not reach statistical significance (random effects OR: 1.46; 95% CI, 0.88-2.42; I2 55.9%; Fig. 3B). Interestingly, the studies reporting the strongest association of PCOS with risk of MI were those whose mean age was in the perimenopausal range (35, 36). Because the association between PCOS and MI was not found to be statistically significant in this meta-analysis, MI was excluded from our calculations of economic burden.
Prevalence of stroke associated with PCOS
Because of the paucity of data on stroke in PCOS, we were unable to subdivide the included studies by mean age as we did for the other long-term outcomes. Interestingly, however, 2 of the included studies had an average patient age older than 55 years (37, 38) and the third had an average age younger than 35 years (39). Collectively, compared to BMI-matched women without PCOS, women with PCOS had a higher prevalence of stroke as reported in the 3 studies (random effects OR: 1.77; 95% CI, 1.28-2.44; I2 7.4%; Fig. 3C). For our calculations of economic burden, we will consider PCOS patients as having a 1.77-fold greater risk of stroke compared with unaffected women at all ages (see below).
The prevalence of stroke in all women increases with age, from 0.6% among individuals from ages 18-44 years, to 3.1% in those 45-64 years of age, 6.9% in those 65-74 years of age, and 11.8% in those older than 75 years (40). Given a 1.77-fold increased risk, the prevalence of stroke in women with PCOS would be estimated to be 1.1%, 5.5%, 12.2%, and 20.9% in these age groups, respectively. The excess prevalence of stroke due to PCOS is therefore 0.5%, 2.4%, 5.3%, and 9.1%, respectively, in these age groups. Based on the population of women with PCOS in each age category, we can estimate that there are 18 480 (95% CI, 6720 to 34 560) excess cases of stroke due to PCOS in women younger than 44 years; 65 881 (95% CI, 23 957 to 123 206) excess cases in women 45 to 64 years; 42 504 (95% CI, 15 456 to 79 488) excess cases in women 65 to 74 years; and 90 860 (95% CI, 33 040 to 169 920) excess cases in women 75 years of age and older. In total, we can estimate that there are 217 725 (95% CI, 79 173 to 407 174) excess cases of stroke due to PCOS annually.
Economic burden of T2DM in women with PCOS
The American Diabetes Association published new data in 2018 that estimates the annual direct medical cost (health resource use and direct medical cost) of T2DM in the United States to be $327 billion USD, which is a 26% increase compared to 5 years prior (34). Costs for affected individuals younger than 54 years, numbering 7.1 million, were estimated to be $44.4 billion or $46.7 billion in 2020 USD. The average cost per person with diabetes in 2020 USD was $7892 in patients <18 years of age, $7083 in 18- to 34-year-olds, $5759 in 35- to 44-year-olds, and $6768 in 45- to 54-year-olds. Based on our prevalence calculations and this cost data, we can estimate that the excess cost of T2DM attributable to PCOS to be $1.5 billion (95% CI, $0.9-$2.3 billion) in 2020 USD annually (Table 1).
Economic burden of stroke in women with PCOS
The American Heart Association published new data in 2020 which estimates that the annual direct medical cost of stroke in the United States in 2015 for individuals 18-44, 45-64, 65-79, and >80 years of age to be $4.55, $23.6, $25.3, and $12.9 billion USD, respectively (41). Taking into account the US female population in each age group (61 million 18-44 years, 42 million 45-64 years, 29 million 65-79 years of age, and 11.2 million older than 80 years) and the prevalence of stroke by age group, we estimate that $12 432, $1813, $12 643, and $9761 in 2020 USD was spent for stroke care per individual by age group, respectively (42). Based on the excess number of stroke cases due to PCOS in each age group, we can estimate that the excess cost of stroke attributable to PCOS is $2.4 billion (95% CI, $0.9 to 4.5 billion) USD annually (Table 1).
Discussion
The economic burden of PCOS due to pregnancy and long-term health complications associated with the disease is estimated to be $4.3 billion annually in 2020 USD, based upon this systematic review, meta-analysis, and economic burden analysis. When combined with our previous estimate of the economic burden of the initial evaluation and treatment of reproductive endocrine disorders (menstrual dysfunction/abnormal uterine bleeding, hirsutism, and infertility) (7), the total economic burden of PCOS can be estimated to be $8 billion annually in 2020 USD.
Our current meta-analysis and economic burden calculations estimates that the excess cost of pregnancy-related comorbidities (gHTN, GDM, and preeclampsia) attributable to PCOS is $375 million in 2020 USD annually. The cost of treatment of gHTN accounts for just under half of this cost, while preeclampsia accounts for one-third, and GDM accounts for 16%. Together, the cost of the pregnancy-related comorbidities account for just over 4% of the total economic burden of PCOS.
The cost of the long-term comorbidities associated with PCOS is estimated to be $3.9 billion in 2020 USD, based on the current meta-analysis and economic burden calculations. The risk of MI was not significantly increased in women with PCOS compared with BMI-matched controls and was therefore not included in our cost analysis. The excess cost of stroke attributable to PCOS accounted for 62% of the total cost of the long-term comorbidities, with T2DM making up the remaining 38%. The economic burden calculations in the present study found the excess cost of T2DM attributable to PCOS in women of reproductive age and in the perimenopause to be estimated at $1.5 billion in 2020 USD annually, based on an OR of 2.41. This is lower than our prior estimation of $2.4 billion in 2020 USD, potentially because there are now more studies estimating the risk for T2DM adjusting for BMI differences between PCOS and controls.
The bulk of the cost of treatment for the long-term health consequences was accounted for in the late reproductive and perimenopausal years, due to the increased baseline prevalence of these outcomes in those age groups along with the increased cost as compared to younger patients, likely due to increased severity of illness and complexity of care. Consistent with a recent large long-term prospective population-based study (33), as well as several cohort studies (38, 43), the results of our meta-analysis suggest that the excess burden of comorbidities associated with PCOS is most prominent in women of reproductive age and in the perimenopausal period, and it diminishes or even disappears in the postmenopausal years. As previously suggested (44), the attenuation of excess disease risk in the postmenopausal years may reflect a protective effect of antecedent PCOS diagnosis or, more likely, the much stronger influence of age on the development of these comorbidities.
It is important to acknowledge that our calculations do not encompass all potential long-term health consequences of PCOS, including increased risks of endometrial, breast, and ovarian neoplasia and mental health disorders (3). While there are data supporting an increased prevalence of neoplasia and mental health issues among women with PCOS, we chose to focus on those long-term health consequences that are easily quantifiable and best established by the published literature.
The total economic burden estimated for PCOS, including our prior assessment (7), is estimated to be $8 billion in 2020 USD. Of this cost, approximately 46% is accounted for by the cost of treating reproductive endocrine morbidities (menstrual dysfunction/abnormal uterine bleeding, hirsutism, and infertility), consistent with the high symptomatology of PCOS during the reproductive years. Metabolic and vascular morbidities account for more than 48% of the economic burden of PCOS, with strokes accounting for 30% and T2DM for 19% of all costs. Pregnancy-related complications (GDM, gHTN, and preeclampsia) account for just 5% of the total costs. Finally, the cost of the initial diagnostic evaluation of PCOS is very low ($139 million annually in 2020 USD), accounting for <2% of the overall economic burden of the disorder, suggesting that ensuring quality diagnosis and evaluation for all patients is a cost-effective approach to ameliorating the complications and costs associated with PCOS.
The present study is only the second report to estimate the economic burden of PCOS, and the first to evaluate the economic impact of pregnancy-related complications and long-term health comorbidities in the disorder. Inclusion of only BMI-matched or controlled studies is a strength of our economic burden and meta-analysis. Alternatively, our analysis is limited by the small number of studies that met inclusion criteria, particularly for MI and stroke. This is largely due to the fact that the majority of studies use markers of CVD, rather than MI and stroke, as their primary outcomes. Our findings are also limited by the high risk of potential bias caused by the design of included studies and inconsistencies in results across these studies.
Our estimate should be considered conservative for several reasons: we used the lower PCOS prevalence based on the stricter NIH diagnostic criteria; we included only direct costs, excluding indirect costs due to lost productivity and time off work due to illness, and other intangible costs related to impacts on quality of life; and we included only those pregnancy and long-term health complications which have been most strongly associated with the disorder. For example, the estimate does not include the cost of evaluation and treatment of general metabolic dysfunction, mental health disorders, and neoplastic disease that women with PCOS are at increased risk for (12).
Overall, our studies, taken together, suggest that even when using the most restrictive diagnostic criteria and prevalence for PCOS, the overall economic burden exceeds for the disorder exceeds $7.9 billion annually in 2020 USD, with almost one-half accounted for by the treatment of reproductive endocrine morbidities (ie, menstrual dysfunction/abnormal uterine bleeding, hirsutism, and infertility) and the other half by the direct costs of stroke and T2DM. The costs of pregnancy-related complications (GDM, gHTN, and preeclampsia) account for less than 5% of the total economic burden. Considering our previous study (7), the cost of the initial diagnostic evaluation of PCOS is very low, accounting for <2% of the overall economic burden of PCOS. Lifestyle and medical intervention have been shown to slow and potentially prevent long-term health complications, including T2DM (45, 46). These data suggest that greater clinician vigilance and public awareness, combined with more liberal screening and early diagnosis, would be a cost-effective approach to ameliorating the economic, health and quality of life impact of PCOS. Additionally, our findings support the need for increased investment in PCOS research and education.
Abbreviations
- AE-PCOS
Androgen Excess and PCOS (Society)
- BMI
body mass index
- GDM
gestational diabetes mellitus
- gHTN
gestational hypertension
- MI
myocardial infarction
- NIH
National Institutes of Health
- OR
odds ratio
- PCOS
polycystic ovary syndrome
- T2DM
type 2 diabetes mellitus
- USD
US dollars
Acknowledgments
This study was supported, in part, by funding from the Foundation for Research and Education Excellence.
Additional Information
Disclosures: R.A. serves as consultant for Spruce Biosciences and was employed by the American Society for Reproductive Medicine. The other authors have no conflicts to report.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.





