-
PDF
- Split View
-
Views
-
Cite
Cite
Mesut Savas, Sonal Mehta, Nidhi Agrawal, Elisabeth F C van Rossum, Richard A Feelders, Approach to the Patient: Diagnosis of Cushing Syndrome, The Journal of Clinical Endocrinology & Metabolism, Volume 107, Issue 11, November 2022, Pages 3162–3174, https://doi.org/10.1210/clinem/dgac492
Close - Share Icon Share
Abstract
Cushing syndrome results from supraphysiological exposure to glucocorticoids and is associated with significant morbidity and mortality. The pathogenesis includes administration of corticosteroids (exogenous Cushing syndrome) or autonomous cortisol overproduction, whether or not ACTH-dependent (endogenous Cushing syndrome). An early diagnosis of Cushing syndrome is warranted; however, in clinical practice, it is very challenging partly because of resemblance with other common conditions (ie, pseudo-Cushing syndrome). Initial workup should start with excluding local and systemic corticosteroid use. First-line screening tests including the 1-mg dexamethasone suppression test, 24-hour urinary free cortisol excretion, and late-night salivary cortisol measurement should be performed to screen for endogenous Cushing syndrome. Scalp-hair cortisol/cortisone analysis helps in the assessment of long-term glucocorticoid exposure as well as in detection of transient periods of hypercortisolism as observed in cyclical Cushing syndrome. Interpretation of results can be difficult because of individual patient characteristics and hence requires awareness of test limitations. Once endogenous Cushing syndrome is established, measurement of plasma ACTH concentrations differentiates between ACTH-dependent (80%-85%) or ACTH-independent (15%-20%) causes. Further assessment with different imaging modalities and dynamic biochemical testing including bilateral inferior petrosal sinus sampling helps further pinpoint the cause of Cushing’s syndrome. In this issue of “Approach to the patient,” the diagnostic workup of Cushing syndrome is discussed with answering the questions when to screen, how to screen, and how to differentiate the different causes. In this respect, the latest developments in biochemical and imaging techniques are discussed as well.
Case 1
A 45-year-old woman was referred to the University Medical Center from another hospital for possible Cushing syndrome. She had a weight gain of 6 kg in 18 months, central obesity, moderate muscle weakness, and insomnia. Hypertension had been diagnosed 3 years ago and was treated with nifedipine 30 mg. She consulted a psychiatrist for 14 months because of depressive complaints and there was suspicion of bipolar disorder. For this, she is treated with carbamazepine 200 mg twice daily. There is no alcohol or drug abuse.
At physical examination, a body mass index of 28 kg/m2 and blood pressure of 150/95 mmHg was measured. The patient had a moderate plethoric facial appearance, supraclavicular fat pads, and some central obesity without striae. There was minimal muscle atrophy of the upper legs, and no ecchymoses, hirsutism, or edema was observed.
In the hospital, an increased urinary free cortisol excretion (UFC) was found of 2 times the upper limit of normal (ULN), a disturbed 1-mg dexamethasone suppression test (DST) with a cortisol value of 5.62 µg/dL (155 nmol/L) and a high-normal ACTH level of 40.9 pg/mL (ULN 50 pg/mL; 9 pmol/L with ULN of 11 pmol/L). Pituitary imaging with magnetic resonance imaging (MRI) showed a small cystic lesion at the left side of the pituitary.
In consultation with the psychiatrist, the carbamazepine was replaced by lithium and psychotherapy was started. Endocrine evaluation at the University Medical Center 6 weeks later revealed a UFC of 1.5 to 2.0 times ULN, a postdexamethasone cortisol dose of 3.05 µg/dL (84 nmol/L), midnight salivary cortisol levels of 0.047 and 0.065 µg/dL (1.3 and 1.8 nmol/L, ULN 0.11 µg/dL or 3.0 nmol/L). In addition, a dexamethasone-corticotropin-releasing hormone (CRH) test was performed showing undetectable cortisol levels after administration of 4 mg dexamethasone for 2 days and no stimulation of cortisol levels after 1 μg/kg CRH IV. A pseudo-Cushing syndrome secondary to her psychiatric disorder and a nonfunctional pituitary lesion was considered the most likely diagnosis. The patient responded well to the psychiatric treatment and 10 months later she felt better with control of depressive symptoms, improvement of her condition, and weight loss of 3 kg. UFC levels were measured and were below the ULN, whereas the DST showed a cortisol level of 1.16 µg/dL (32 nmol/L).
Learning Points
A pseudo-Cushing syndrome should always be considered in patients with endogenous hypercortisolism
Patients with a pseudo-Cushing syndrome can have symptoms associated with endogenous hypercortisolism resembling true Cushing syndrome
The results of first-line screening tests for Cushing syndrome can be influenced by the use of concomitant medication (eg, antiepileptic drugs can cause a false-positive DST)
Midnight salivary cortisol levels and the second-line dexamethasone-CRH test can be useful to differentiate pseudo-Cushing syndrome from ACTH-dependent Cushing syndrome
Case 2
A 52-year-old woman was referred by a hospital elsewhere to the University Medical Center for surgical treatment of adrenal Cushing syndrome. The patient presented with nephrolithiasis and on a computed tomography (CT) scan of the abdomen, an enlarged left adrenal gland was found with radiological features compatible with an adenoma (lipid-rich, low Hounsfield units). Six months before the patient was seen at the emergency room because of deep venous thrombosis of the right leg. Then a high blood pressure was measured (210/110 mmHg) for which treatment was started with valsartan. The patient reported weight gain (8 kg in the past 2 years), increased abdominal circumference, hirsutism, easy bruisability, and proximal muscle weakness. At physical examination, she had a cushingoid phenotype with a moon face with plethora and moderate hirsutism, central obesity, proximal muscle atrophy of the extremities, and skin atrophy with some hematomas. A body mass index of 31 kg/m2 and blood pressure of 170/10 mmHg were measured.
Endocrine evaluation revealed the following results: UFC values of 4.5 to 5.0 times ULN, a DST with a cortisol level of 17.33 µg/dL (478 nmol/L), and an ACTH concentration of 19.07 pg/mL (4.2 pmol/L). After referral, ACTH measurement was repeated and showed values of 12.71 pg/mL (2.8 pmol/L) and 18.16 pg/mL (4.0 pmol/L). Because ACTH levels were not suppressed, an MRI of the sellar region was performed that demonstrated a pituitary adenoma of 7 mm. Additional investigations included measurement of dehydroepiandrosterone-sulfate (DHEAS) and a CRH test. The DHEAS concentration was 2.36 µg/mL or 6.4 µmol/L (reference range, < 2.28 µg/mL or 6.2 µmol/L) and the CRH test showed an ACTH increase of 170% and a cortisol increase of 140% of baseline. Considering these results, the diagnosis pituitary-dependent Cushing syndrome was considered most likely. The patient underwent a transsphenoidal adenomectomy, which resulted in biochemical remission. Pathological examination confirmed a basophilic adenoma with a positive ACTH staining.
Learning points
Cushing syndrome is associated with a high risk of venous thromboembolic events which can be a presenting symptom
Pituitary-dependent Cushing syndrome can be accompanied by unilateral adrenal enlargement
In Cushing syndrome patients with ACTH levels in the low-normal range and uni- or bilateral adrenal enlargement, measurement of DHEAS levels and a CRH test can be helpful to differentiate between a pituitary and an adrenal cause
Cushing syndrome (CS) results from prolonged exposure to excess glucocorticoids, either from exogenous glucocorticoids or an endogenous source of excess cortisol. The most common cause of CS is iatrogenic, resulting from exogenous pharmacologic doses of corticosteroids. Endogenous CS is caused by ACTH-dependent or ACTH-independent excess of cortisol production (1-3). The estimated incidence of endogenous CS is 0.2 to 5.0 per million people per year and the estimated prevalence is 39 to 79 per million in various populations (2). ACTH-dependent CS accounts for 80% to 85% of cases and ACTH-independent accounts for 15% to 20% (2, 4, 5).
CS is a severe disease with often long-lasting effects, yielding a low quality of life (6). Hypercortisolism is associated with an increase in cardiovascular events (myocardial infarction), cerebrovascular events (stroke), sepsis, and thromboembolism with 3.5 to 5 times increased mortality risk compared with the general population (7-11). The risk of myocardial infarction is approximately 4.5 times higher in patients with CS compared with the general population (8, 9). Surgical remission does not eliminate the risk of complications from systemic comorbidities completely (12, 13). The prevalence and pathophysiology of comorbidities in CS (5, 10, 14-28) are shown in Table 1. Even if improvements occur after successful treatment, recovery often does not seem to be complete, and physical and neuropsychological comorbidities may persist (29). Therefore, early diagnosis of CS is important, but in clinical practice it is often challenging because there is substantial overlap in signs and symptoms with other (common) conditions.
| Comorbidity . | Description/pathophysiology in Cushing syndrome . | Prevalence in Cushing syndrome (%) (5, 10, 14-17) . |
|---|---|---|
| Hypertension | • Mineralocorticoid and glucocorticoid effect of cortisol • Activation of renin-angiotensin system • Impaired balance between vasodilators and vasoconstrictors • Increase in sympathetic nervous system (5, 10) | 70-85 |
| Hyperlipidemia | • Cortisol increases (peripheral) lipolysis and free fatty acid production and very-low-density lipoprotein synthesis • Fatty acid accumulation causes increase in total cholesterol and triglyceride levels • Insulin resistance also plays a role in dyslipidemia (18, 19) | 70 |
| Insulin resistance and impaired glucose tolerance | • Stimulation of gluconeogenesis • Development of insulin resistance • Decrease in insulin secretion from the pancreas (20) | 45-70 |
| Obesity | • Promotion of lipogenesis/adipogenesis resulting in visceral fat accumulation, most commonly abdominal (21) • Adipocyte hypertrophy by increasing synthesis and storage of lipids (22) • Adipose tissue hyperplasia by increasing differentiation of preadipocytes to mature adipocytes (22) • Contribution to weight gain by increasing food intake with a preferential choice of high-caloric, high-fat “comfort foods” (23) | 70-95 |
| Hypercoagulability | • Hypercoagulability from increased clotting factors and impaired fibrinolysis • Prothrombotic state causes venous thromboembolisms (18, 21, 24) | 20 |
| Osteoporosis | • Decrease bone collagenous matrix synthesis • Increase degradation of bone matrix (25) | 50 |
| Cardiovascular disease | • Increased cardiovascular risk factors, cardiac remodeling, dysfunction, and vascular atherosclerosis • Left ventricular hypertrophy and remodeling, reduced systolic function, and impaired relaxation seen • Risk factors for myocardial infarction and stroke include vascular damage and increase in atherosclerotic plaques (10, 25) | 29 |
| Neuropsychiatric | • Emotional lability, depression, irritability • Other symptoms include psychosis, mania, anxiety, paranoia • Associated with decrease in brain volume and impairment of memory, visual and spatial information, verbal learning and language (25, 26) | 70-85 |
| Infectious diseases | • Hypercortisolism causes immunosuppression by impairing both cellular and humoral components of the innate immune system and inhibiting steps in the adaptive immune response • Predisposes patients to opportunistic infections: bacterial, fungal, viral, and parasitic • Susceptibility of infection correlates with degree of hypercortisolism (16, 17, 27) | 21-51 |
| Others (nephrolithiasis, hyperandrogenism gonadal dysfunction) | • High prevalence of nephrolithiasis from synergic effects of several lithogenic factors particularly systemic arterial hypertension and excess urinary of uric acid (28) • Adrenal androgens are the main cause of hirsutism, acne, alopecia (17) • Hypercortisolism can inhibit release of GnRH, LH, and FSH, leading to hypogonadotropic hypogonadism (17) | 21-50 20-75 24-80 |
| Comorbidity . | Description/pathophysiology in Cushing syndrome . | Prevalence in Cushing syndrome (%) (5, 10, 14-17) . |
|---|---|---|
| Hypertension | • Mineralocorticoid and glucocorticoid effect of cortisol • Activation of renin-angiotensin system • Impaired balance between vasodilators and vasoconstrictors • Increase in sympathetic nervous system (5, 10) | 70-85 |
| Hyperlipidemia | • Cortisol increases (peripheral) lipolysis and free fatty acid production and very-low-density lipoprotein synthesis • Fatty acid accumulation causes increase in total cholesterol and triglyceride levels • Insulin resistance also plays a role in dyslipidemia (18, 19) | 70 |
| Insulin resistance and impaired glucose tolerance | • Stimulation of gluconeogenesis • Development of insulin resistance • Decrease in insulin secretion from the pancreas (20) | 45-70 |
| Obesity | • Promotion of lipogenesis/adipogenesis resulting in visceral fat accumulation, most commonly abdominal (21) • Adipocyte hypertrophy by increasing synthesis and storage of lipids (22) • Adipose tissue hyperplasia by increasing differentiation of preadipocytes to mature adipocytes (22) • Contribution to weight gain by increasing food intake with a preferential choice of high-caloric, high-fat “comfort foods” (23) | 70-95 |
| Hypercoagulability | • Hypercoagulability from increased clotting factors and impaired fibrinolysis • Prothrombotic state causes venous thromboembolisms (18, 21, 24) | 20 |
| Osteoporosis | • Decrease bone collagenous matrix synthesis • Increase degradation of bone matrix (25) | 50 |
| Cardiovascular disease | • Increased cardiovascular risk factors, cardiac remodeling, dysfunction, and vascular atherosclerosis • Left ventricular hypertrophy and remodeling, reduced systolic function, and impaired relaxation seen • Risk factors for myocardial infarction and stroke include vascular damage and increase in atherosclerotic plaques (10, 25) | 29 |
| Neuropsychiatric | • Emotional lability, depression, irritability • Other symptoms include psychosis, mania, anxiety, paranoia • Associated with decrease in brain volume and impairment of memory, visual and spatial information, verbal learning and language (25, 26) | 70-85 |
| Infectious diseases | • Hypercortisolism causes immunosuppression by impairing both cellular and humoral components of the innate immune system and inhibiting steps in the adaptive immune response • Predisposes patients to opportunistic infections: bacterial, fungal, viral, and parasitic • Susceptibility of infection correlates with degree of hypercortisolism (16, 17, 27) | 21-51 |
| Others (nephrolithiasis, hyperandrogenism gonadal dysfunction) | • High prevalence of nephrolithiasis from synergic effects of several lithogenic factors particularly systemic arterial hypertension and excess urinary of uric acid (28) • Adrenal androgens are the main cause of hirsutism, acne, alopecia (17) • Hypercortisolism can inhibit release of GnRH, LH, and FSH, leading to hypogonadotropic hypogonadism (17) | 21-50 20-75 24-80 |
| Comorbidity . | Description/pathophysiology in Cushing syndrome . | Prevalence in Cushing syndrome (%) (5, 10, 14-17) . |
|---|---|---|
| Hypertension | • Mineralocorticoid and glucocorticoid effect of cortisol • Activation of renin-angiotensin system • Impaired balance between vasodilators and vasoconstrictors • Increase in sympathetic nervous system (5, 10) | 70-85 |
| Hyperlipidemia | • Cortisol increases (peripheral) lipolysis and free fatty acid production and very-low-density lipoprotein synthesis • Fatty acid accumulation causes increase in total cholesterol and triglyceride levels • Insulin resistance also plays a role in dyslipidemia (18, 19) | 70 |
| Insulin resistance and impaired glucose tolerance | • Stimulation of gluconeogenesis • Development of insulin resistance • Decrease in insulin secretion from the pancreas (20) | 45-70 |
| Obesity | • Promotion of lipogenesis/adipogenesis resulting in visceral fat accumulation, most commonly abdominal (21) • Adipocyte hypertrophy by increasing synthesis and storage of lipids (22) • Adipose tissue hyperplasia by increasing differentiation of preadipocytes to mature adipocytes (22) • Contribution to weight gain by increasing food intake with a preferential choice of high-caloric, high-fat “comfort foods” (23) | 70-95 |
| Hypercoagulability | • Hypercoagulability from increased clotting factors and impaired fibrinolysis • Prothrombotic state causes venous thromboembolisms (18, 21, 24) | 20 |
| Osteoporosis | • Decrease bone collagenous matrix synthesis • Increase degradation of bone matrix (25) | 50 |
| Cardiovascular disease | • Increased cardiovascular risk factors, cardiac remodeling, dysfunction, and vascular atherosclerosis • Left ventricular hypertrophy and remodeling, reduced systolic function, and impaired relaxation seen • Risk factors for myocardial infarction and stroke include vascular damage and increase in atherosclerotic plaques (10, 25) | 29 |
| Neuropsychiatric | • Emotional lability, depression, irritability • Other symptoms include psychosis, mania, anxiety, paranoia • Associated with decrease in brain volume and impairment of memory, visual and spatial information, verbal learning and language (25, 26) | 70-85 |
| Infectious diseases | • Hypercortisolism causes immunosuppression by impairing both cellular and humoral components of the innate immune system and inhibiting steps in the adaptive immune response • Predisposes patients to opportunistic infections: bacterial, fungal, viral, and parasitic • Susceptibility of infection correlates with degree of hypercortisolism (16, 17, 27) | 21-51 |
| Others (nephrolithiasis, hyperandrogenism gonadal dysfunction) | • High prevalence of nephrolithiasis from synergic effects of several lithogenic factors particularly systemic arterial hypertension and excess urinary of uric acid (28) • Adrenal androgens are the main cause of hirsutism, acne, alopecia (17) • Hypercortisolism can inhibit release of GnRH, LH, and FSH, leading to hypogonadotropic hypogonadism (17) | 21-50 20-75 24-80 |
| Comorbidity . | Description/pathophysiology in Cushing syndrome . | Prevalence in Cushing syndrome (%) (5, 10, 14-17) . |
|---|---|---|
| Hypertension | • Mineralocorticoid and glucocorticoid effect of cortisol • Activation of renin-angiotensin system • Impaired balance between vasodilators and vasoconstrictors • Increase in sympathetic nervous system (5, 10) | 70-85 |
| Hyperlipidemia | • Cortisol increases (peripheral) lipolysis and free fatty acid production and very-low-density lipoprotein synthesis • Fatty acid accumulation causes increase in total cholesterol and triglyceride levels • Insulin resistance also plays a role in dyslipidemia (18, 19) | 70 |
| Insulin resistance and impaired glucose tolerance | • Stimulation of gluconeogenesis • Development of insulin resistance • Decrease in insulin secretion from the pancreas (20) | 45-70 |
| Obesity | • Promotion of lipogenesis/adipogenesis resulting in visceral fat accumulation, most commonly abdominal (21) • Adipocyte hypertrophy by increasing synthesis and storage of lipids (22) • Adipose tissue hyperplasia by increasing differentiation of preadipocytes to mature adipocytes (22) • Contribution to weight gain by increasing food intake with a preferential choice of high-caloric, high-fat “comfort foods” (23) | 70-95 |
| Hypercoagulability | • Hypercoagulability from increased clotting factors and impaired fibrinolysis • Prothrombotic state causes venous thromboembolisms (18, 21, 24) | 20 |
| Osteoporosis | • Decrease bone collagenous matrix synthesis • Increase degradation of bone matrix (25) | 50 |
| Cardiovascular disease | • Increased cardiovascular risk factors, cardiac remodeling, dysfunction, and vascular atherosclerosis • Left ventricular hypertrophy and remodeling, reduced systolic function, and impaired relaxation seen • Risk factors for myocardial infarction and stroke include vascular damage and increase in atherosclerotic plaques (10, 25) | 29 |
| Neuropsychiatric | • Emotional lability, depression, irritability • Other symptoms include psychosis, mania, anxiety, paranoia • Associated with decrease in brain volume and impairment of memory, visual and spatial information, verbal learning and language (25, 26) | 70-85 |
| Infectious diseases | • Hypercortisolism causes immunosuppression by impairing both cellular and humoral components of the innate immune system and inhibiting steps in the adaptive immune response • Predisposes patients to opportunistic infections: bacterial, fungal, viral, and parasitic • Susceptibility of infection correlates with degree of hypercortisolism (16, 17, 27) | 21-51 |
| Others (nephrolithiasis, hyperandrogenism gonadal dysfunction) | • High prevalence of nephrolithiasis from synergic effects of several lithogenic factors particularly systemic arterial hypertension and excess urinary of uric acid (28) • Adrenal androgens are the main cause of hirsutism, acne, alopecia (17) • Hypercortisolism can inhibit release of GnRH, LH, and FSH, leading to hypogonadotropic hypogonadism (17) | 21-50 20-75 24-80 |
In this article, we focus on the diagnostic approach to the patient suspected of (exogenous, endogenous, or cyclic) CS, and take the latest developments in the field of novel diagnostic measurements and technology into account.
Exogenous Steroids
Synthetic glucocorticoids (ie, corticosteroids) have the potential to induce similar symptoms as seen in endogenous CS. Glucocorticoid use is in fact the most prevalent cause of CS. The profuse prescription and over-the-counter availability in some countries justify the inclusion of drug history in the initial approach to CS. The net systemic effect of glucocorticoids depends on the bioavailability as well as other pharmacokinetic and the pharmacodynamic properties of the applied drug. Also, the duration of use and the route of administration are important for the development of features of CS. Serious adverse events are in general more likely to occur in systemic corticosteroid users and especially with longer duration and higher dosage of use (30). Suspected unreported exogenous glucocorticoid administration can be detected with urinary or blood mass spectrometry assays designed to detect exogenous glucocorticoids (31). Moreover, it is also important to screen for concomitant use of other drugs such as antifungals, protease inhibitors, or estrogens given the potential drug–drug interaction resulting in increased glucocorticoid effect (32, 33).
From the patient’s perspective, weight gain has been reported as the most common adverse event followed by skin problems (bruising/thinning) and sleep disturbances (34). Interestingly, 2 distinct patterns in the occurrence of glucocorticoid-associated adverse events have been described in chronic users. A dose-related pattern was found for clinical features such as cushingoid phenotype, skin thinning, ecchymosis, and sleep disturbances. Although other adverse effects manifested above a certain threshold of daily glucocorticoid dosage (eg, epistaxis and weight gain with daily prednisone equivalent dose of > 5.0-7.5 mg), whereas depression and high blood pressure were especially prevalent with > 7.5 mg/d (35). With regard to exogenous corticosteroid assessment, administration forms other than the oral types should also be taken into consideration. A meta-analysis on the occurrence of adrenal insufficiency in corticosteroid users has found similar percentages in users of intra-articular injection (52.2% absolute risk) as for oral corticosteroids (48.7%) (36). Furthermore, the locally applied corticosteroids such as nasal, dermal, and inhaled types were also significantly associated with adrenal insufficiency (4.2%, 4.7%, and 7.8%, respectively), which implies systemic availability of these types. When drugs of different administration routes were combined, which is not uncommon for asthma, eczema, and hay fever, among others, the absolute risk of adrenal insufficiency even increased to 42.7% in this study. In this light, it is of interest that we recently showed associations between use of local corticosteroids, particularly inhaled types, and higher likelihood of metabolic syndrome, higher body mass index, reduced executive cognitive functioning, and a higher likelihood of mood and anxiety disorders (37, 38). All these features are also (although nonspecific) characteristics of increased glucocorticoid exposure. These relations between corticosteroid use and cardiometabolic sequelae seemed to be related to glucocorticoid receptor gene variants that are associated with increased or normal glucocorticoid sensitivity. Interestingly, the adverse effects were less pronounced in corticosteroid users harboring gene polymorphisms, which are associated with glucocorticoid receptor resistance (39). Despite the lower probability of systemic adverse events in locally administered use, it is still of great importance given the fact that the vast majority of corticosteroid use involves the local types (37). Moreover, in the case of local corticosteroids, also other individual factors that determine glucocorticoid metabolism or can promote absorption and thus systemic adverse events must be considered, such as type of delivery device for inhaled corticosteroids or application of dermal corticosteroids in skin folds or under occlusion.
When to Screen
The clinical presentation of CS can be variable, depending on a patient’s age, sex, severity, and duration of cortisol excess (Table 2) (1, 3, 5). Patients often present with nonspecific features such as (abdominal) obesity and weight gain, rounded (moon) face, menstrual irregularity, and depression (1, 3, 5) as depicted in Figure 1. The diagnosis is even more complicated if signs and symptoms gradually develop over time and emerge sequentially. It is therefore a challenging task to diagnose endogenous CS at an early stage. There is additionally a large overlap of cushingoid-related features with other conditions associated with relatively mild increased cortisol levels. These pseudo-Cushing states, such as with severe obesity, alcoholism, polycystic ovary syndrome, and neuropsychiatric disorders, are beyond compare more prevalent than endogenous CS (40) (see also subparagraph “Pseudo-Cushing’s syndrome”). Testing is however recommended (3) in:
| Signs/symptoms of Cushing syndrome . | Prevalence (%) . |
|---|---|
| (Abdominal) obesity/weight gain | 75-95 |
| Rounded face (moon face) | 81-90 |
| Supraclavicular/dorsocervical fat pad (buffalo hump) | 50 |
| Hirsutism/alopecia | 75 |
| Facial plethora | 70-90 |
| Violaceous striae | 44-50 |
| Acne | 20-35 |
| Easy bruising | 35-65 |
| Menstrual irregularity | 70-80 |
| Decreased libido | 24-80 |
| Neuropsychiatric (emotional lability/depression, psychosis/mania, cognitive dysfunction) | 70-85 |
| Muscle weakness/atrophy | 60-82 |
| Osteopenia/fractures | 40-70 |
| Signs/symptoms of Cushing syndrome . | Prevalence (%) . |
|---|---|
| (Abdominal) obesity/weight gain | 75-95 |
| Rounded face (moon face) | 81-90 |
| Supraclavicular/dorsocervical fat pad (buffalo hump) | 50 |
| Hirsutism/alopecia | 75 |
| Facial plethora | 70-90 |
| Violaceous striae | 44-50 |
| Acne | 20-35 |
| Easy bruising | 35-65 |
| Menstrual irregularity | 70-80 |
| Decreased libido | 24-80 |
| Neuropsychiatric (emotional lability/depression, psychosis/mania, cognitive dysfunction) | 70-85 |
| Muscle weakness/atrophy | 60-82 |
| Osteopenia/fractures | 40-70 |
Features with highest discriminatory value are depicted in bold. Table adapted from Sharma et al (5).
| Signs/symptoms of Cushing syndrome . | Prevalence (%) . |
|---|---|
| (Abdominal) obesity/weight gain | 75-95 |
| Rounded face (moon face) | 81-90 |
| Supraclavicular/dorsocervical fat pad (buffalo hump) | 50 |
| Hirsutism/alopecia | 75 |
| Facial plethora | 70-90 |
| Violaceous striae | 44-50 |
| Acne | 20-35 |
| Easy bruising | 35-65 |
| Menstrual irregularity | 70-80 |
| Decreased libido | 24-80 |
| Neuropsychiatric (emotional lability/depression, psychosis/mania, cognitive dysfunction) | 70-85 |
| Muscle weakness/atrophy | 60-82 |
| Osteopenia/fractures | 40-70 |
| Signs/symptoms of Cushing syndrome . | Prevalence (%) . |
|---|---|
| (Abdominal) obesity/weight gain | 75-95 |
| Rounded face (moon face) | 81-90 |
| Supraclavicular/dorsocervical fat pad (buffalo hump) | 50 |
| Hirsutism/alopecia | 75 |
| Facial plethora | 70-90 |
| Violaceous striae | 44-50 |
| Acne | 20-35 |
| Easy bruising | 35-65 |
| Menstrual irregularity | 70-80 |
| Decreased libido | 24-80 |
| Neuropsychiatric (emotional lability/depression, psychosis/mania, cognitive dysfunction) | 70-85 |
| Muscle weakness/atrophy | 60-82 |
| Osteopenia/fractures | 40-70 |
Features with highest discriminatory value are depicted in bold. Table adapted from Sharma et al (5).
Patients with adrenal incidentalomas (adenoma).
Patients who show Cushingoid-related features which are uncommon for age (such as hypertension, osteoporosis, or female balding).
Patients who have multiple symptoms, which are progressive over time, in particular when specific cushingoid features are present (3). Clinical features such as ecchymoses, proximal myopathy, wide reddish-purple striae, facial plethora, recurrent infections, and osteopenia have been found to be more characteristic of CS (41, 42) and aid in the decision to perform screening tests.
Children with a combination of increasing weight and decreasing height percentile.
In addition, screening can be considered in patients with difficult to treat diabetes or hypertension, although it is generally not recommended to perform large-scale screening for CS in populations with diabetes, hypertension, or obesity. In case of pituitary incidentaloma, routine screening for ACTH-hypersecretion is not recommended (43). It remains a matter of debate whether screening for hypercortisolism in asymptomatic persons is useful for detecting preclinical Cushing disease (44). Individuals with clinical suspicion of Cushing disease should undergo testing, as mentioned in the next section. Finally, patients with active CS have a high risk of venous thromboembolism (VTE) in comparison to the general population (8, 45, 46). The patient in case 2 presented earlier with VTE, but CS was not recognized yet. The high VTE risk is due to glucocorticoid-induced activation of the coagulation cascade, whereas fibrinolysis is impaired (3, 24). Hence, screening for hypercortisolism may be considered in patients with unexplained venous thrombotic events.
How to Screen/Establishment of Hypercortisolism
If a patient is suspected of CS and exogenous glucocorticoid use is excluded, it is recommended to start by performing one of the first-line screening tests (3). Recommended initial tests include:
overnight 1-mg DST;
24-hour UFC; and
late-night salivary cortisol test (LNSC).
The latter 2 tests should be performed at least twice because of significant day-to-day variations in cortisol production. The pooled diagnostic accuracy of the various tests (47) is presented in Figure 2. There is no specific order of screening, but the choice for a specific test can be made based on individual patient characteristics (see also caveats in Figure 2). A promising relatively novel test to detect chronic hypercortisolism is measurement of cortisol in scalp hair (see “New developments: potential of hair cortisol measurement as diagnostic tool”).
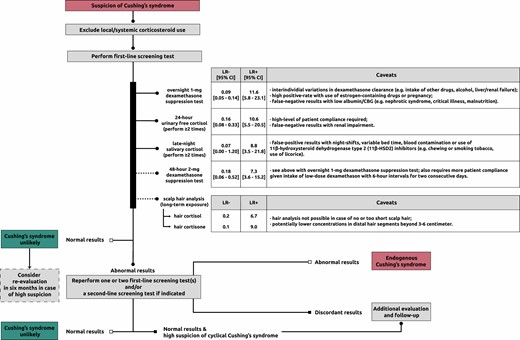
Diagnostic workup of Cushing syndrome. Flowchart is based on Lacroix et al (3). The likelihood ratios (LRs) for the first-line screening tests and scalp hair analysis concern pooled data from (47) and findings from (48), respectively. LRs take sensitivity and specificity into account and determine the posttest probability given a certain pretest probability (higher LR+ = increasing probability of disease with positive test result; lower LR- = decreasing probability of disease with negative test result). Abbreviations: CBG, cortisol-binding globulin; LR-, negative likelihood ratio; LR+, positive likelihood ratio.
CS is unlikely with normal test results; however, referral to an endocrinologist is recommended in patients with a high likelihood. In other cases, a reevaluation in 6 months should be considered if a patient has progressive features. An abnormal test result prompts further evaluation by an endocrinologist. Patients should be subsequently tested again with 1 or 2 first-line screening tests or a second-line screening test (eg, combined dexamethasone-CRH test or midnight serum cortisol) if necessary (3). The diagnosis of CS is established with concordant abnormal results indicating hypercortisolism. Further evaluation should be focused on identifying the underlying cause. Endogenous CS is unlikely with 2 normal test results and requires no further evaluation unless a cyclical CS or a (rare) glucocorticoid hypersensitivity is suspected. In the rare condition of increased glucocorticoid sensitivity, a clinical picture of CS is present, but laboratory tests show (borderline) low plasma and urinary cortisol values while responsiveness to ACTH (Cortrosyn) or metyrapone stimulation test and/or insulin-induced hypoglycemia is normal (31, 49). An ultra-low-dose DST showing suppressed morning serum cortisol levels is also indicative of this rare condition, and functional testing of the glucocorticoid receptor or sequencing of the glucocorticoid receptor gene may be considered in specialized centers (31, 49, 50). In these cases, use of any type of exogenous corticosteroids should be ruled out or only use of low dosages equivalent to hydrocortisone replacement therapy or below with a history of development of cushingoid features after initiation. Primary glucocorticoid resistance, a rare genetic condition resulting mainly from mutations in the glucocorticoid receptor gene, would on the contrary yield abnormal test results (51). These patients present with symptoms of increased mineralocorticoid and/or androgen action, combined with biochemical hypercortisolism from compensatory overdrive of the hypothalamus-pituitary-adrenal (HPA) axis, but lack of specific cushingoid features. This increased HPA-axis activity from the decreased peripheral glucocorticoid receptor sensitivity should be distinguished from pathological hypercortisolism from CS (52). In general, follow-up and further evaluation is recommended in the event of discordant results or high clinical suspicion of cyclical CS (3).
Each of the first-line screening tests has its limitations, the most important factors that can affect the outcome are mentioned in Figure 2. Regarding the 1-mg DST, it is essential to screen for current drug use, which could alter dexamethasone clearance and/or levels of cortisol-binding globulin. This mainly relates to antiepileptic drugs, as in case 1, and use of estrogen-containing medication; a detailed overview is available elsewhere (3). In case of positive DST, measurement of serum dexamethasone concentration could be of value in identifying insufficient levels (53) (eg, from altered dexamethasone metabolism or inadequate test adherence) and in determining in whom a second DST would be useful (54). As to the LNSC, use of substances containing glycyrrhizic acid (ie, 11beta-hydroxysteroid dehydrogenase type 2 [11β-HSD2] inhibitor) should be avoided. This is because cortisol in the salivary glands is naturally inactivated by 11β-HSD2 and inhibition of this can therefore lead to falsely elevated cortisol levels. Glycyrrhizic acid is among others present in licorice candies and some teas. Other less prevalent 11β-HSD2 inhibitors include the glycyrrhizic acid derivative carbenoxolone, gossypol, and various endocrine disruptors such as phthalates (55).
Pseudo-CS
One diagnostic challenge in the evaluation of endogenous hypercortisolism is differentiating neoplastic CS from pseudo-CS. Pseudo-CS, or nonneoplastic physiologic hypercortisolism, is a phenomenon that can occur in many medical disorders such as chronic alcoholism, chronic kidney disease, type 2 diabetes mellitus, and psychiatric conditions. Hypercortisolism in these conditions is mainly mediated by activation of the HPA axis through neural pathways without tumorous hypercortisolemia. There is also decreased sensitivity to glucocorticoid negative feedback in the majority of these states which may lead to mild increases in cortisol. Over time, the effects of small increases in cortisol can lead to significant and longitudinal glucocorticoid exposure and can result in pathologic features of hypercortisolism as is also illustrated in case 1 (1, 56, 57). A detailed history and physical examination are important first steps to take in evaluating patients with hypercortisolism and most patients with pseudo-CS will have mild cortisol excess and not have overt clinical manifestations of glucocorticoid excess. When undergoing biochemical testing in patients, if the first-line tests show normal LNSC measurements (57) and appropriate suppression of cortisol with DST, patients are unlikely to have neoplastic hypercortisolism. However, if there is diagnostic uncertainty, a 48-hour 2 mg/d DST or secondary tests can be performed, including DDAVP stimulation, and dexamethasone-CRH testing (1, 3, 56-58). The latter tests and their interpretation are described in Table 3.
Secondary testing to differentiate pathologic Cushing syndrome from nonneoplastic physiologic hypercortisolism (pseudo-Cushing syndrome)
| Test . | Basis . | Technique . | Interpretation of results . | Sensitivity . | Specificity . |
|---|---|---|---|---|---|
| DDAVP stimulation | Corticotroph adenomas have vasopressin receptors. DDAVP will stimulate ACTH secretion in Cushing disease, but response typically absent in physiologic hypercortisolism | Measure ACTH and cortisol levels before and after DDAVP stimulation (10 μg IV) | Increase in ACTH > 6 pmol/L supports diagnosis of Cushing disease (note: there are no universally agreed criteria partly because of different ACTH assays) | 75%-87% | 90%-91% |
| Dexamethasone-CRH testing | Hypercortisolism in pseudo-Cushing states is thought to be mediated by CRH and has diminished response to administration of CRH and greater inhibition of cortisol production by glucocorticoids compared with Cushing syndrome | Dexamethasone (0.5 mg every 6 h) given orally for 8 doses, with subsequent administration of CRH (1 μg/kg) in the morning 2 hours after last dexamethasone dose, with measurement of ACTH and cortisol levels pre- and post-CRH administration | Serum cortisol > 1.4 μg/dL (or 38 nmol/L) in response to CRH supports diagnosis of Cushing syndrome | 88%-100% | 50%-100% |
| Test . | Basis . | Technique . | Interpretation of results . | Sensitivity . | Specificity . |
|---|---|---|---|---|---|
| DDAVP stimulation | Corticotroph adenomas have vasopressin receptors. DDAVP will stimulate ACTH secretion in Cushing disease, but response typically absent in physiologic hypercortisolism | Measure ACTH and cortisol levels before and after DDAVP stimulation (10 μg IV) | Increase in ACTH > 6 pmol/L supports diagnosis of Cushing disease (note: there are no universally agreed criteria partly because of different ACTH assays) | 75%-87% | 90%-91% |
| Dexamethasone-CRH testing | Hypercortisolism in pseudo-Cushing states is thought to be mediated by CRH and has diminished response to administration of CRH and greater inhibition of cortisol production by glucocorticoids compared with Cushing syndrome | Dexamethasone (0.5 mg every 6 h) given orally for 8 doses, with subsequent administration of CRH (1 μg/kg) in the morning 2 hours after last dexamethasone dose, with measurement of ACTH and cortisol levels pre- and post-CRH administration | Serum cortisol > 1.4 μg/dL (or 38 nmol/L) in response to CRH supports diagnosis of Cushing syndrome | 88%-100% | 50%-100% |
Secondary testing to differentiate pathologic Cushing syndrome from nonneoplastic physiologic hypercortisolism (pseudo-Cushing syndrome)
| Test . | Basis . | Technique . | Interpretation of results . | Sensitivity . | Specificity . |
|---|---|---|---|---|---|
| DDAVP stimulation | Corticotroph adenomas have vasopressin receptors. DDAVP will stimulate ACTH secretion in Cushing disease, but response typically absent in physiologic hypercortisolism | Measure ACTH and cortisol levels before and after DDAVP stimulation (10 μg IV) | Increase in ACTH > 6 pmol/L supports diagnosis of Cushing disease (note: there are no universally agreed criteria partly because of different ACTH assays) | 75%-87% | 90%-91% |
| Dexamethasone-CRH testing | Hypercortisolism in pseudo-Cushing states is thought to be mediated by CRH and has diminished response to administration of CRH and greater inhibition of cortisol production by glucocorticoids compared with Cushing syndrome | Dexamethasone (0.5 mg every 6 h) given orally for 8 doses, with subsequent administration of CRH (1 μg/kg) in the morning 2 hours after last dexamethasone dose, with measurement of ACTH and cortisol levels pre- and post-CRH administration | Serum cortisol > 1.4 μg/dL (or 38 nmol/L) in response to CRH supports diagnosis of Cushing syndrome | 88%-100% | 50%-100% |
| Test . | Basis . | Technique . | Interpretation of results . | Sensitivity . | Specificity . |
|---|---|---|---|---|---|
| DDAVP stimulation | Corticotroph adenomas have vasopressin receptors. DDAVP will stimulate ACTH secretion in Cushing disease, but response typically absent in physiologic hypercortisolism | Measure ACTH and cortisol levels before and after DDAVP stimulation (10 μg IV) | Increase in ACTH > 6 pmol/L supports diagnosis of Cushing disease (note: there are no universally agreed criteria partly because of different ACTH assays) | 75%-87% | 90%-91% |
| Dexamethasone-CRH testing | Hypercortisolism in pseudo-Cushing states is thought to be mediated by CRH and has diminished response to administration of CRH and greater inhibition of cortisol production by glucocorticoids compared with Cushing syndrome | Dexamethasone (0.5 mg every 6 h) given orally for 8 doses, with subsequent administration of CRH (1 μg/kg) in the morning 2 hours after last dexamethasone dose, with measurement of ACTH and cortisol levels pre- and post-CRH administration | Serum cortisol > 1.4 μg/dL (or 38 nmol/L) in response to CRH supports diagnosis of Cushing syndrome | 88%-100% | 50%-100% |
New Developments: Potential of Hair Cortisol Measurement as a Diagnostic Tool
A relatively novel method of cortisol measurement in patients suspected of CS is scalp hair analysis, a patient-friendly noninvasive method yielding cortisol values representing long-term cortisol exposure of the past months (48, 59-62). This method enables retrospective assessment of glucocorticoid concentrations because both cortisol and its inactive variant cortisone are incorporated in hair (63). It is often compared with measuring glycosylated hemoglobin, which is used to assess mean blood glucose levels over weeks to months. The routine first-line screening tests capture cortisol exposure for up to several days, whereas hair analysis allows assessment of glucocorticoid concentrations in the past months to years. The growth rate of scalp hair is approximately 1 cm/mo. Depending on the length of the collected hair sample, it is possible to make timelines of past glucocorticoid exposure (64). This enables to capture (isolated or recurrent) episodes of hypercortisolism, but also to approximate the beginning and course over time of hypercortisolism. Hence, hair analysis possesses unique features which could further aid in the screening of CS (60). It additionally provides a stable measurement independent of acute stressors that could yield false-positive results with traditional matrices, such as saliva or urine. One of other advantages is that hair sample collection can easily be done at the outpatient clinic at any time of the day.
In the past decade, great progress has been made with the development of scalp hair glucocorticoid analysis, although this method is not yet widely available (65). Hair cortisol has been shown to differentiate between CS patients and healthy controls with high sensitivity and specificity (48, 66). Within CS patients hair cortisol levels have been shown to correlate significantly with UFC (61). We and others also showed high diagnostic efficacy in screening of CS with hair steroid analysis. In these studies, a 3-cm hair sample per patient was used (corresponding to mean glucocorticoid levels of roughly past 3 months) and hair analysis was performed with either immunoassay (59, 61) or liquid chromatography-tandem mass spectrometry (48, 66). Interestingly, we found that hair cortisone has a higher differentiating capacity (sensitivity 87%, specificity 90%) than hair cortisol (sensitivity 81%, specificity 88%) (48). This difference could perhaps be contributed to local metabolism by 11β-HSD enzymes or 5α-reductase; however, further research is needed to confirm those findings (67).
In addition, hair cortisol and cortisone have been shown to contribute to the identification of patients with mild or subclinical CS (66). Because hair can be used as a historical timeline, scalp hair cortisol analysis can also be useful in studying the onset of CS (eg, in ectopic CS) or cyclic CS (59, 68). Regarding the latter group, those patients periodically secrete excess cortisol and thus are less likely to have abnormal results with traditional tests if not screened at moments of actual hypercortisolism. In our previous study with a set of cyclical CS patients, we created historical timelines using hair and indeed demonstrated dynamic cortisol concentrations over time corresponding with clinical cushingoid features (59).
Pituitary apoplexy is another (rare) difficulty in diagnosing Cushing disease because this may induce spontaneous remission of the clinical syndrome when it occurs in an ACTH-overproducing adenoma. In these conditions, it is not possible to biochemically confirm this diagnosis at presentation. We recently reported a patient with a clinical picture of Cushing disease presenting with pituitary apoplexy, who was biochemically in remission at admission. In retrospect, the diagnosis of Cushing disease could be confirmed using hair cortisol analysis. This can be important for clinicians because it enables adequate anticipation of remission of Cushing disease, including potential symptoms reflecting a relative hypocortisolism because previous long-term exposure to hypercortisolism, as well as attention for long-term physical and mental complications and disease recurrence (69).
ACTH-dependent CS
Once CS has been established, plasma ACTH concentrations can help determine whether the cause is ACTH-dependent or ACTH-independent. Because of decreased glucocorticoid negative feedback effects, plasma ACTH levels will be inappropriately normal or elevated (generally > 20 pg/mL) in ACTH-dependent causes and low (generally < 10 pg/mL) in ACTH-independent causes of CS (70, 71). Thirty percent of the patients with CS have ACTH levels in the “gray zone” (5-20 pg/mL) and should have repeat testing and consideration of adrenal imaging to detect possible adrenal pathology (72). ACTH-dependent CS comprises 80% to 85% of all CS cases.
Differentiation Between Cushing disease and Ectopic ACTH Secretion
Cushing disease, the most common cause of ACTH-dependent CS accounting for approximately 80% of cases, occurs when a pituitary adenoma secretes ACTH, which in turn stimulates supraphysiologic secretion of cortisol from the adrenal glands (2, 4, 5, 73). Ectopic ACTH secretion (EAS) accounts for approximately 20% of ACTH-dependent CS. In these cases, most common sources of ACTH secretion are small cell lung carcinomas or pulmonary carcinoid tumors. Other causes can include pancreatic neuroendocrine tumors, thymic neuroendocrine tumors, gastrinomas, medullary thyroid cancer, and pheochromocytomas, as seen in Figure 3 (5). Imaging studies can help to differentiate between Cushing disease and ectopic causes. Pituitary MRI is used for detecting pituitary adenomas. Compared with conventional MRI, which can only detect 36% to 63% of pituitary microadenomas in patients with Cushing disease, high-resolution 3T-MRI with 3-dimensional spoiled gradient-echo sequence is characterized by thinner sections and superior soft-tissue contrast and can detect adenomas as small as 2 mm (74). If a pituitary adenoma > 6 mm is found on MRI, the need for further testing with bilateral inferior petrosal sinus sampling (BIPSS) is not necessary (1, 9, 74, 75). However, pituitary MRI can be negative in up to 40% to 60% of Cushing disease cases. There can also be false-positive pituitary MRI findings in patients with EAS (76). BIPSS is the gold standard to differentiate between Cushing disease and EAS. The test, however, cannot be used to establish the diagnosis of ACTH-dependent CS and the presence of hypercortisolism must be confirmed immediately before and at the time of the procedure (77). During this procedure, plasma ACTH levels are withdrawn simultaneously from each petrosal sinus (venous drainage of the pituitary) and a peripheral vein. Sensitivity can be increased by obtaining ACTH levels under CRH stimulation (1, 76, 78). In ACTH-secreting adenomas, ACTH levels will be higher in the blood samples drawn from the inferior petrosal sinuses (IPS) compared with the periphery and will therefore have elevated IPS-to-peripheral (IPS:P) ACTH ratios: > 2 pre-CRH stimulations or > 3 post-CRH stimulations. A lack of an IPS:P ACTH gradient suggests an ectopic source of ACTH secretion. However, IPS prolactin measurements can be used as a surrogate marker of appropriate catheterization or normal IPS venous efflux to prevent a false-negative result (77). Studies have also shown how prolactin-adjusted intersinus ACTH ratios can be used for tumor lateralization (77, 79). The most common complications of the procedure include groin hematomas and transient headaches. There can also be serious complications, including stroke and subarachnoid hemorrhage, thought to be related to anatomical variations causing transient hypotension and/or vascular injury during the procedure (77).
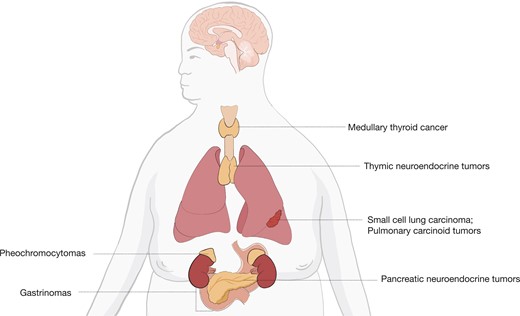
Sources of ectopic ACTH secretion. Based on Lacroix et al (2).
A novel, noninvasive molecular imaging technique that has been described by Walia et al. can help identify corticotroph adenomas using Gallium-68 (68Ga)-tagged CRH combined with positron emission tomography (PET)-CT. 68Ga-tagged CRH can be used to detect CRH receptors, which are upregulated on corticotroph adenomas and can delineate functionality of adenomas. Although the size of the study population was small, 68Ga CRH PET-CT scan was able to correctly identify 100% of Cushing disease cases, including culprit lesions less than 6 mm in size, and was able to provide accurate information regarding lateralization and planning for intraoperative navigation, making it useful in both evaluation and management of ACTH-dependent CS (80). This technique is still investigational and not currently widely available.
Dynamic testing with high-dose DST, CRH test, and desmopressin testing can also be used to help differentiate between Cushing disease and EAS (9, 81). In patients with Cushing disease, glucocorticoid receptors at the pituitary level retain the ability to inhibit ACTH secretion in the presence of high dexamethasone doses (8 mg). In contrast, most ectopic ACTH-secreting tumors do not respond to high-dose dexamethasone (9). The proposed cutoff point for positive response is a decrease in basal cortisol level by 50% or more. The high-dose DST is most often negative in patients with EAS (74). The CRH test is useful because corticotroph pituitary adenomas express CRH receptors and associated downstream cell-signaling pathway molecules, thereby responding to CRH by releasing excess ACTH compared with ectopic ACTH-secreting tumors (81). Therefore, most but not all patients with Cushing disease will have an increase in plasma ACTH by more than 50% and cortisol concentrations by more than 20% after CRH stimulation. Patients with EAS are typically unresponsive although some tumors, in particular bronchial carcinoids can express CRH receptors (9, 74, 81, 82). Desmopressin testing can also be used because type 2 vasopressin receptors, which are generally absent in normal pituitary corticotrophs, have found to be expressed in corticotroph adenomas. Therefore, an increase in plasma ACTH and cortisol can be observed after injection of desmopressin in patients with Cushing disease (81). On the other hand, an absence of both ACTH and cortisol is expected in patients with EAS, although false-positive results can be seen as EAS tumors may express type 3 vasopressin receptors (9, 74, 81). Individually, none of these tests have 100% specificity because if high false-positive rates, and results can be discordant in up to 65% of patients (2) because of a number of factors including differences in type of ectopic tumor, patient age, patient sex, and severity of hypercortisolism (9). In cases of discordant results, BIPSS is needed to determine the disease source (2). However, using these dynamic tests in combination with pituitary MRI can improve clinical accuracy, decreasing the need for BIPSS (9, 75). In cases in which results are inconclusive for Cushing disease, evaluation for EAS should be considered (see also Figure 3 for sources of EAS). Whole-body thin-slice CT scans (cervical, thoracic, abdominal, and pelvic regions) should be performed initially to evaluate for tumors suggestive of EAS (9, 81). Second-line tests include functional imaging using 68Ga-PET/CT or 18FDG PET/CT scans, which can be used to detect occult tumors, reinforce tumors seen on CT scan as being neuroendocrine, or contribute to the workup of metastatic tumors (9, 81).
ACTH-independent CS
ACTH-independent CS is usually caused by an adrenal adenoma and less frequently by bilateral micro- or macronodular adrenal hyperplasia and adrenal carcinoma. Very rare causes include primary pigmented nodular adrenocortical disease, the Carney complex, and McCune-Albright syndrome (2). If after establishment of endogenous hypercortisolism ACTH concentrations are suppressed (< 10 pg/mL or < 2.2 pmol/L), the next diagnostic step is imaging of the adrenal glands with CT or MRI. If the radiological phenotype has worrisome features (eg, tumor size greater than 4 cm, calcifications, irregular tumor margins, Hounsfield units > 20) and/or the plasma steroid profile shows elevated DHEAS and steroid precursors an additional FDG-PET scan can guide the decision on an (open) adrenalectomy with an oncological approach.
In case of intermediate ACTH values, between 10 and 20 pg/mL (2.2 pmol/L and 4.4 pmol/L), the differentiation between an adrenal and a pituitary cause of CS can be difficult, as is illustrated by case 2. In case of an adrenal cause, mild cortisol overproduction may be accompanied by incomplete ACTH suppression. Conversely, in case of more severe clinical and biochemical hypercortisolism an ACTH-dependent cause of CS is more likely. In addition, a cyclical ACTH secretion pattern may explain ACTH values in the lower range. Measurement of DHEAS concentrations and a CRH test can be helpful to differentiate an adrenal from a pituitary cause. DHEAS secretion is partly ACTH driven and low-normal or suppressed DHEAS levels point to an adrenal cause. Corticotroph tumors are sensitive to CRH stimulation and a substantial ACTH and cortisol increase (> 50% of baseline) are compatible with a pituitary cause (83, 84).
Bilateral adrenal hyperplasia, and to a lesser extent adrenal adenomas, is often associated with eutopic or ectopic hormone receptor expression with coupling to steroidogenesis (85). Examples are the vasopressin receptor, LH receptor, and the glucose-dependent insulinotropic polypeptide receptor. Screening for aberrant hormone receptor expression with specific stimulation tests may offer an option for medical therapy via blockade of the receptor or inhibition of secretion of the endogenous ligand (85). Screening of family members of patients with bilateral adrenal hyperplasia with a 1 mg DST is recommended (85).
A subgroup of ACTH-independent hypercortisolism involves patients with uni- or bilateral adrenal incidentaloma(s) and mild autonomous cortisol secretion (MACS). MACS is often accompanied by cushingoid features, in particular, common cardiometabolic and mental complications, such as hypertension, type 2 diabetes, obesity, dyslipidemia, atrial fibrillation, and psychiatric or neurocognitive symptoms (86). MACS is also associated with an increased risk of frailty, osteoporosis, cardiovascular morbidity, and mortality (87). The 1-mg DST is the most sensitive test to detect MACS, whereas UFC and LNSC concentrations are frequently normal (87). It was shown that in patients with adrenal incidentalomas post-DST cortisol levels are related to cardiovascular events and all-cause mortality (88). A cortisol cutoff of 50 nmol/L is used to differentiate MACS from normal physiology. This 1-mg DST is up to 100% sensitive, so it can be used as an optimal first-line screening test (89). However, the specificity at the 50 nmol/L cutoff can be as low as ~60%. Use of other methods such as UFC or LNSC can be necessary to confirm the diagnosis of MACS (89). Low or suppressed ACTH values can further indicate autonomous cortisol production.
Diagnosis of CS in Pregnancy
CS is rarely diagnosed during pregnancy because hypercortisolism inhibits normal follicular development and ovulation. In contrast to nonpregnant patients, the predominant etiology of CS in pregnant patients is adrenal adenomas, found in 40% to 60% of cases (90, 91). Early diagnosis and management of CS during pregnancy are important because of associated fetal and maternal morbidity. Fetal morbidity includes rates of spontaneous abortion, perinatal death, premature birth, and intrauterine growth retardation (see Figure 4). Maternal morbidity caused by CS includes hypertension, preeclampsia, wound breakdown, diabetes, fractures, and opportunistic infections (2, 92).
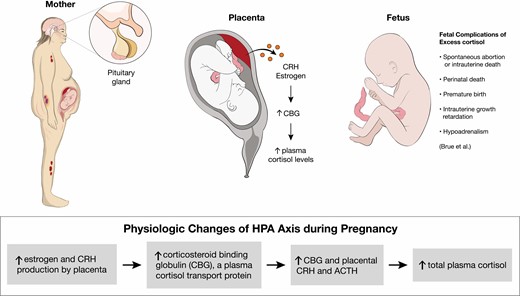
(Patho)physiologic changes of hypothalamus-pituitary-adrenal axis during pregnancy. Based on Brue et al (90). Abbreviation: CRH, corticotropin-releasing hormone.
Clinically, the diagnosis of CS during pregnancy can be more challenging because of overlap in features of hypercortisolism and classic features of pregnancy including fatigue, weight gain, hirsutism, acne, and emotional instability. It has been suggested that when pregnant patients have a triad of hypertension, skin ecchymosis, and muscle atrophy, CS should be considered (90, 93). The biochemical diagnosis of CS during pregnancy can also be more challenging because of normal physiologic changes that occur during pregnancy, including activation of the HPA axis. Starting in the first trimester, there is an increase in estrogen and CRH produced by the placenta, which can lead to an increase in corticosteroid-binding globulin, a plasma cortisol transport protein. This in combination with the rise in placental CRH and ACTH cause an increase in total plasma cortisol levels. Suppression of serum and plasma cortisol by dexamethasone is blunted during pregnancy; therefore, making the DST difficult to interpret in these patients (3, 90, 92). UFC is often the recommended screening test during pregnancy; however, there are challenges to this as well. During the second trimester, UFC also increases, leading to an approximately 1.4-fold increase during the second trimester and a 1.6-fold increase during the third trimester. Therefore, while 24-hour UFC can be unaffected during the first trimester, it may not be a reliable diagnostic test in the second and third trimesters, unless levels are significantly increased, up to 2- to 3-fold the upper limit of normal (3, 90, 92, 93). Although there had previously been fewer data on defining LNSC levels during pregnancy, there have been some studies looking at defining normal threshold values in each trimester of pregnancy, which could lead to increased use in screening these patients (90, 94, 95). In the study of Lopes et al (95), the reference range for the LNSC in each gestational trimester were 0.03 to 0.25 µg/dL (0.8-6.9 nmol/L) in the first trimester, 0.04 to 0.26 µg/dL (1.1-7.2 nmol/L) in the second trimester, and 0.07 to 0.33 µg/dL (1.7-9.1 nmol/L) in the third trimester. The cutoff values for the diagnosis of Cushing disease in the study were 0.255 µg/dL (7.0 nmol/L) for the first trimester, 0.260 µg/dL (7.2 nmol/L) for the second trimester, and 0.285 µg/dL (7.9 nmol/L) for the third trimester (90, 95).
Conclusion
CS is multisystemic disease with serious morbidity and mortality and the diagnosis should preferably be made at an early stage considering long-term complications. Increased awareness of CS among physicians who treat comorbidities (family physicians, neurologists, psychiatrists) could be helpful in this respect. First-line screening tests to establish endogenous hypercortisolism include UFC, 1-mg DST, and LNSC. Hair cortisol/cortisone measurement is a relatively new diagnostic tool with a high sensitivity to diagnose CS and can also be helpful to detect cyclical CS in retrospect. All tests have caveats which should be taken into account when test results are interpreted. In patients with (mild) ACTH-dependent CS, a pseudo-CS should always be considered. In patients with ACTH-dependent CS, a pituitary cause should be differentiated from an ectopic origin. BIPSS has a high diagnostic accuracy for this purpose, but recent development of new noninvasive imaging modalities also shows promising results. The diagnostic workup of ACTH-independent CS is usually straightforward but diagnosing a primary adrenal cause of CS can be difficult when ACTH levels are not fully suppressed. Although diagnostic procedures have improved in the past decades with more accurate hormone measurement and improved imaging techniques, the diagnosis and differential diagnosis of CS can still be extremely challenging.
Abbreviations
- 11β-HSD2
11beta-hydroxysteroid dehydrogenase type 2
- 68Ga
Gallium-68
- BIPSS
bilateral inferior petrosal sinus sampling
- CRH
corticotropin-releasing hormone
- CS
Cushing syndrome
- CT
computed tomography
- DHEAS
dehydroepiandrosterone-sulfate
- DST
dexamethasone suppression test
- EAS
ectopic ACTH secretion
- HPA
hypothalamus-pituitary-adrenal
- IPS
inferior petrosal sinuses
- LNSC
late-night salivary cortisol
- MACS
mild autonomous cortisol secretion
- PET
positron emission tomography
- UFC
urinary free cortisol
- ULN
upper limit of normal
- VTE
venous thromboembolism
Acknowledgments
Figures 1, 3, and 4 were created by Kristen Dancel-Manning.
Disclosures
R.A.F. received consultancy and speakers fees from Recordati. R.A.F. and N.A. received a research grant from Recordati.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Author notes
Mesut Savas and Sonal Mehta Shared first authorship.




