-
PDF
- Split View
-
Views
-
Cite
Cite
Alice Giontella, Luca A Lotta, Aris Baras, Pietro Minuz, Dipender Gill, Olle Melander, Cristiano Fava, Calcium, Its Regulatory Hormones, and Their Causal Role on Blood Pressure: A Two-Sample Mendelian Randomization Study, The Journal of Clinical Endocrinology & Metabolism, Volume 107, Issue 11, November 2022, Pages 3080–3085, https://doi.org/10.1210/clinem/dgac501
Close - Share Icon Share
Abstract
Vitamin D (Vit-D), parathyroid hormone (PTH), and fibroblast growth factor 23 (FGF23) are the major calciotropic hormones involved in the regulation of blood calcium levels from the intestine, kidney, and bone through a tight endocrine feedback loop system. Altered levels of calcium itself or through the effect of its regulatory hormones could affect blood pressure (BP), but the exact mechanisms remain unclear.
To evaluate whether a causal relationship exists between serum calcium level and/or the regulatory hormones involved in its homeostasis with BP, we performed a two-sample Mendelian randomization (MR) study.
From 4 large genome-wide association studies (GWAS) we obtained independent (r2 < 0.001) single nucleotide polymorphisms (SNPs) associated with serum calcium (119 SNPs), Vit-D (78 SNPs), PTH (5 SNPs), and FGF23 (5 SNPs), to investigate through MR their association with systolic BP (SBP) and diastolic BP (DBP) in a Swedish urban-based study, the Malmö Diet and Cancer study (n = 29 298). Causality was evaluated by the inverse variance weighted method (IVW) and weighted median, while MR Egger and MR-PRESSO were used as sensitivity analyses.
Genetically predicted serum calcium level was found to be associated with DBP (IVW: beta = 0.10, SE = 0.04, P = 0.007) and SBP (IVW: beta = 0.07, SE = 0.04, P = 0.04). Genetically predicted Vit-D and PTH showed no association with the traits, while FGF23 was inversely associated with SBP (IVW: beta = −0.11, SE = 0.04, P = 0.01), although this association lost statistical significance in sensitivity analysis.
Our study shows a direct association between genetically predicted calcium level and DBP, and a weaker association with SBP. No such clear association was found for genetically predicted calciotropic hormone levels. It is of interest to detect which target genes involved in calcium homeostasis mediate the effect of calcium on BP, particularly for improving personalized intervention strategies.
Calcium homeostasis relates to the endocrine regulation of calcium concentration among 3 target organs: the intestine, kidney, and bone. Tight control over this endocrine feedback loop is exerted by the concerted action of the calciotropic hormones, which include vitamin D (Vit-D), parathyroid hormone (PTH) and, at least partially, fibroblast growth factor 23 (FGF23) (1, 2).
Low and high serum calcium levels are perceived by calcium-sensing receptors located on chief cells of the parathyroid gland and activate intracellular signals to stimulate or inhibit PTH secretion, respectively (1). The increase in the concentration of PTH level itself regulates the reabsorption of calcium from bones, kidneys, and intestine (3). The effect exerted by PTH on renal absorption is mediated by the control of vitamin D. Vitamin D consists of a functional form, the 1,25 dihydroxy vitamin D [1,25(OH)2D], activated by the enzyme CYP27B1 through hydroxylation of the intermediate form, the 25-hydroxyvitamin D3 (25OHD), which is the form used when assaying vitamin status and the one we used in our analysis and referred to throughout this manuscript as Vit-D. (1). 1,25(OH)2D acts through its nuclear vitamin D receptor (VDR), which is responsible for activating different signaling pathways, including inflammation, cell-mediated immunity, cell cycle progression, and apoptosis, as well as calcium and bone homeostasis (4). The Vit-D has been suggested to influence the cardiovascular system and hypertension by inhibiting the renin/angiotensin system, decreasing coagulation, reducing parathyroid hormone levels, and reducing atherosclerosis (4–6). Additionally, the presence of the vitamin D receptor and CYP27B have been reported in myocytes, endothelial cells, and cardiac fibroblasts (4). Although this association has been widely reported, the results from observational studies are contrasting and randomized controlled trials have failed to demonstrate a causal effect on cardiovascular health (6). FGF23 is predominantly produced in the osteocytes and osteoblasts, stimulated by hypercalcemia and PTH, and is responsible for stimulating an increase in PTH (7). Vit-D stimulates FGF23 release from bones which in turn can decrease Vit-D level (8). FGF23 is mainly known to be involved in bone mineral disorder and has also been largely studied in relation to a plethora of cardiometabolic traits to investigate its possible role as a biomarker or drug target (9).
Vit-D, PTH, and FGF23, along with calcium, therefore, constitute a complex bone-kidney-parathyroid negative feedback loop (7). The role of these hormones in the regulation of blood pressure (BP) has been reported (3, 10–13). Every perturbation of the components responsible to maintain calcium homeostasis can also differently affect BP. Calcium itself can affect vascular tone, an increase in its concentration being a stimulus for vasoconstriction. Moreover, an excess of calcium (and phosphate) can trigger vascular calcification, leading to an increase in arterial stiffness and consequently a rise in pulse pressure. Other mechanisms independent of mineral homeostasis are also suggested, which include influencing the renin/angiotensin or the sympathetic system (11, 14). However, contrasting results have been found in observational studies and meta-analyses, possibly due to confounding factors and incompletely understood mechanisms.
Using Mendelian randomization (MR), we can infer the causality of the relationship between an exposure and an outcome, potentially avoiding the effect of unobserved confounding factors or reverse causation, which is often the issue in conventional observational studies (15). The genetic variants (single nucleotide polymorphisms, SNPs), associated with the exposure, can be considered as randomized variables independent of the environmental factors and could be used to explain which changes in the outcomes, associated to changes in the exposure, are causal (15).
Thus, we used the SNPs associated with calcium, Vit-D, PTH, and FGF23 available from the largest and most recent genome-wide association studies (GWAS) (16–18), as instrumental variables in an MR, to evaluate their possible causal association with BP from a Swedish large-scale urban-based study, the Malmö Diet and Cancer study (MDC) (n = 29 298) (19).
Methods
Study Design and Data Sources
In this work, we present a two-sample MR study in which the summary statistics of the association of the genetic variants with the exposure and the outcomes come from 2 separate GWASs.
We selected the SNPs associated with the GWAS significance level (P < 5 × 10−8) with serum levels of calcium, Vit-D, PTH, and FGF23 selected from the most recent GWAS meta-analyses (16–18, 20) and used as instrumental variables for the MR analysis. Selected SNPs were then clumped based on linkage disequilibrium using a stringent threshold (r2 > 0.001), and SNPs with palindromic and intermediate allele frequencies were excluded from the analysis.
The selected studies included, respectively, 305 349 (calcium), 417 580 (Vit-D), 29 155 (PTH), and 16 624 (FGF23) individuals (Supplementary Table S1 (21)) (16–18, 20).
The summary statistics for the association of the SNPs with BP traits were obtained from genetic data of the Malmö Diet and Cancer study (n = 29 298) (19, 22). The Malmö Diet and Cancer study is a large-scale urban-based cohort that involved 30 447 participants (58 ± 7.6 years of age) from Malmö (Sweden) between 1991 and 1996 (19). Further details about the included populations are presented in Supplementary Table S2 (21). BP was measured manually once in the supine position after a 5-minute rest. BP values were adjusted by taking into account antihypertensive treatment, adding 8/4, 14/10, 20/16, and 26/22 mmHg to systolic BP (SBP)/diastolic BP (DBP) in presence of 1, 2, 3, or 4 antihypertensive medications respectively (23).
Mendelian Randomization
Mendelian randomization validity relies on 3 main assumptions: (i) genetic variants are associated with the exposures of interest; (ii) they should affect the outcome only through the exposure; and (iii) they have not to share causes with the outcome (24).
Inverse variance weighted median (IVW) and weighted median were used to combine the estimates and assess the causal relationship between genetically predicted calcium, PTH, and FGF23 and blood pressure traits. Heterogeneity was evaluated by Cochrane’s Q heterogeneity test.
Palindromic SNPs with intermediate allele frequency were removed from the analysis.
As a sensitivity analysis, we used MR Egger to evaluate the presence of pleiotropy, and we detected outliers by using leave-one-out analysis and MR-PRESSO.
Statistics were performed with R software (Package “TwoSampleMR”) (25) and Plink 1.9 (26).
Results
A total of 119 SNPs were used as proxies for serum calcium level, 78 for Vit-D, 5 for PTH, and 5 for FGF23. The list of the genetics instruments used is shown in Supplementary Table S3 (21).
Genetically predicted serum calcium level was found to be associated to SBP (IVW: beta = 0.07, standard error [SE] = 0.04, P = 0.04) and DBP (IVW: beta = 0.10, SE = 0.04, P = 0.007) as shown in Table 1. FGF23 resulted to be associated to lower SBP (IVW: beta=-0.11, SE = 0.04, P = 0.01), while genetically determined Vit-D and PTH did not show any association with BP. (Table 1, Fig. 1).
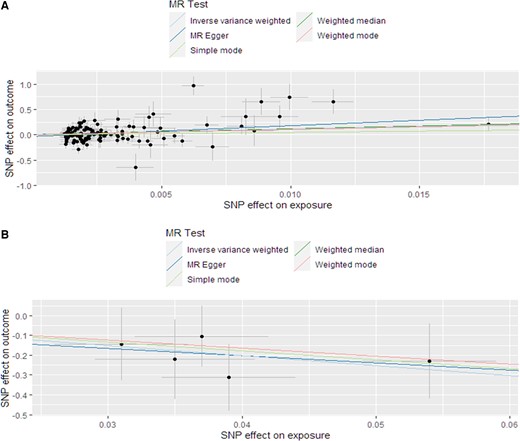
Scatter plot of the association between calcium and DBP. (A) FGF23 and (B) SBP.
Results of the Mendelian randomization analysis of calcium, parathyroid hormone, and FGF23 vs BP traits
| . | . | SBP, mmHg . | . | DBP, mmHg . | . |
|---|---|---|---|---|---|
| . | SNPs, n . | Beta (SE) . | P . | Beta (SE) . | P . |
| Calcium | |||||
| IVW | 119 | 0.07 (0.04) | 0.04 | 0.10 (0.04) | 0.007 |
| Weighted median | 119 | 0.04 (0.06) | 0.50 | 0.11 (0.06) | 0.077 |
| MR Egger | 119 | 0.11 (0.06) | 0.10 | 0.19 (0.07) | 0.003 |
| Vit-D | |||||
| IVW | 78 | 0.06 (0.03) | 0.07 | 0.03 (0.04) | 0.05 |
| Weighted median | 78 | 0.08 (0.04) | 0.06 | 0.04 (0.04) | 0.04 |
| MR Egger | 78 | 0.07 (0.04) | 0.07 | 0.06 (0.04) | 0.21 |
| PTH | |||||
| IVW | 5 | 0.01 (0.07) | 0.9 | −0.01 (0.09) | 0.9 |
| Weighted median | 5 | 0.01 (0.04) | 0.8 | −0.05 (0.04) | 0.2 |
| MR Egger | 5 | −0.06 (0.11) | 0.6 | −0.08 (0.12) | 0.5 |
| FGF23 | |||||
| IVW | 5 | −0.11 (0.04) | 0.01 | −0.08 (0.04) | 0.06 |
| Weighted median | 5 | −0.10 (0.05) | 0.06 | −0.08 (0.05) | 0.1 |
| MR Egger | 5 | −0.08 (0.24) | 0.8 | 0.22 (0.23) | 0.4 |
| . | . | SBP, mmHg . | . | DBP, mmHg . | . |
|---|---|---|---|---|---|
| . | SNPs, n . | Beta (SE) . | P . | Beta (SE) . | P . |
| Calcium | |||||
| IVW | 119 | 0.07 (0.04) | 0.04 | 0.10 (0.04) | 0.007 |
| Weighted median | 119 | 0.04 (0.06) | 0.50 | 0.11 (0.06) | 0.077 |
| MR Egger | 119 | 0.11 (0.06) | 0.10 | 0.19 (0.07) | 0.003 |
| Vit-D | |||||
| IVW | 78 | 0.06 (0.03) | 0.07 | 0.03 (0.04) | 0.05 |
| Weighted median | 78 | 0.08 (0.04) | 0.06 | 0.04 (0.04) | 0.04 |
| MR Egger | 78 | 0.07 (0.04) | 0.07 | 0.06 (0.04) | 0.21 |
| PTH | |||||
| IVW | 5 | 0.01 (0.07) | 0.9 | −0.01 (0.09) | 0.9 |
| Weighted median | 5 | 0.01 (0.04) | 0.8 | −0.05 (0.04) | 0.2 |
| MR Egger | 5 | −0.06 (0.11) | 0.6 | −0.08 (0.12) | 0.5 |
| FGF23 | |||||
| IVW | 5 | −0.11 (0.04) | 0.01 | −0.08 (0.04) | 0.06 |
| Weighted median | 5 | −0.10 (0.05) | 0.06 | −0.08 (0.05) | 0.1 |
| MR Egger | 5 | −0.08 (0.24) | 0.8 | 0.22 (0.23) | 0.4 |
Beta coefficient is expressed as SD unit. Abbreviations: DBP, diastolic blood pressure; FGF23, fibroblast growth factor 23; IVW, inverse weighted median; MR, Mendelian randomization; SBP, systolic blood pressure.
Results of the Mendelian randomization analysis of calcium, parathyroid hormone, and FGF23 vs BP traits
| . | . | SBP, mmHg . | . | DBP, mmHg . | . |
|---|---|---|---|---|---|
| . | SNPs, n . | Beta (SE) . | P . | Beta (SE) . | P . |
| Calcium | |||||
| IVW | 119 | 0.07 (0.04) | 0.04 | 0.10 (0.04) | 0.007 |
| Weighted median | 119 | 0.04 (0.06) | 0.50 | 0.11 (0.06) | 0.077 |
| MR Egger | 119 | 0.11 (0.06) | 0.10 | 0.19 (0.07) | 0.003 |
| Vit-D | |||||
| IVW | 78 | 0.06 (0.03) | 0.07 | 0.03 (0.04) | 0.05 |
| Weighted median | 78 | 0.08 (0.04) | 0.06 | 0.04 (0.04) | 0.04 |
| MR Egger | 78 | 0.07 (0.04) | 0.07 | 0.06 (0.04) | 0.21 |
| PTH | |||||
| IVW | 5 | 0.01 (0.07) | 0.9 | −0.01 (0.09) | 0.9 |
| Weighted median | 5 | 0.01 (0.04) | 0.8 | −0.05 (0.04) | 0.2 |
| MR Egger | 5 | −0.06 (0.11) | 0.6 | −0.08 (0.12) | 0.5 |
| FGF23 | |||||
| IVW | 5 | −0.11 (0.04) | 0.01 | −0.08 (0.04) | 0.06 |
| Weighted median | 5 | −0.10 (0.05) | 0.06 | −0.08 (0.05) | 0.1 |
| MR Egger | 5 | −0.08 (0.24) | 0.8 | 0.22 (0.23) | 0.4 |
| . | . | SBP, mmHg . | . | DBP, mmHg . | . |
|---|---|---|---|---|---|
| . | SNPs, n . | Beta (SE) . | P . | Beta (SE) . | P . |
| Calcium | |||||
| IVW | 119 | 0.07 (0.04) | 0.04 | 0.10 (0.04) | 0.007 |
| Weighted median | 119 | 0.04 (0.06) | 0.50 | 0.11 (0.06) | 0.077 |
| MR Egger | 119 | 0.11 (0.06) | 0.10 | 0.19 (0.07) | 0.003 |
| Vit-D | |||||
| IVW | 78 | 0.06 (0.03) | 0.07 | 0.03 (0.04) | 0.05 |
| Weighted median | 78 | 0.08 (0.04) | 0.06 | 0.04 (0.04) | 0.04 |
| MR Egger | 78 | 0.07 (0.04) | 0.07 | 0.06 (0.04) | 0.21 |
| PTH | |||||
| IVW | 5 | 0.01 (0.07) | 0.9 | −0.01 (0.09) | 0.9 |
| Weighted median | 5 | 0.01 (0.04) | 0.8 | −0.05 (0.04) | 0.2 |
| MR Egger | 5 | −0.06 (0.11) | 0.6 | −0.08 (0.12) | 0.5 |
| FGF23 | |||||
| IVW | 5 | −0.11 (0.04) | 0.01 | −0.08 (0.04) | 0.06 |
| Weighted median | 5 | −0.10 (0.05) | 0.06 | −0.08 (0.05) | 0.1 |
| MR Egger | 5 | −0.08 (0.24) | 0.8 | 0.22 (0.23) | 0.4 |
Beta coefficient is expressed as SD unit. Abbreviations: DBP, diastolic blood pressure; FGF23, fibroblast growth factor 23; IVW, inverse weighted median; MR, Mendelian randomization; SBP, systolic blood pressure.
Only the association between calcium level and DBP remained significant in MR Egger (beta [SE] = 0.19 [0.07] P = 0.003), although the other significant associations (calcium vs SBP; FGF23 vs SBP) showed an effect size that is consistent with that identified with IVW, not contradicting the causal effect identified by the main MR analysis (27).
In the sensitivity leave-one-out analysis, the results have been confirmed for all the significant findings. After having detected 3 outliers with MR-PRESSO test for calcium vs DBP association, as well the presence of heterogeneity (Qp = 0.0001), the analysis was repeated excluding them. Using the updated dataset, no large changes were found in comparison with the previous analysis (IVW: beta [SE] = 0.1 [0.03]; P = 0.004). No outliers or heterogeneity (Qp = 0.08) were detected for FGF23 vs SBP.
Discussion
The present work investigated whether there is a causal relationship between calcium, vitamin D (Vit-D), parathyroid hormone (PTH), and fibroblast growth factor 23 (FGF23) plasma concentration with BP using a two-sample Mendelian randomization approach. Vit-D, PTH, and FGF23 are calciotropic hormones involved in the maintenance of calcium (and phosphate) homeostasis, through the regulation of uptake and retention of serum calcium concentration, through the kidneys, gut, and bones (7).
Genetically predicted serum calcium level was associated with an increased level of DBP and SBP. These results show consistency with several other observational studies (12, 28–30). Serum calcium could affect BP by acting via (i) stimulation of the renin-angiotensin system; (ii) the influence of the vascular smooth cell contractility and consequently the peripheral vascular resistance; and/or (iii) sympathetic stimulation of heart, kidneys, and blood vessels (12, 30).
There are no previous MR studies investigating the causal relationship between serum calcium and BP, but a recent phenome-wide (PheWas)-based Mendelian randomization study has supported a direct association of calcium with hypertension in the UK Biobank (31). Moreover, a wide range of adverse clinical outcomes, such as cardiometabolic diseases, cancer, and neurological conditions have been linked to high calcium in MR studies (32).
Genetically predicted Vit-D did not show a significant association with BP. This null finding is in line with a two-sample MR that used the same set of SNPs used in the present work (20). Another MR, which included 4 SNPs, reported a protective effect on BP, while Kunutsor and colleagues, using the same SNPs, were not able to detect clear evidence of effect (33, 34). The same study performed a pooled meta-analysis of randomized controlled trials that evaluated the effect of vitamin D intervention on BP. While no evidence of significant effect was found, a subgroup analysis identified a reduction in DBP in participants with preexisting cardiometabolic disease (33). An umbrella review summarized the effect of vitamin D supplementation in randomized controlled trials and genetically predicted vitamin D levels, estimated in MR, on different health outcomes. The results supported a beneficial effect of vitamin D intervention in many cardiometabolic outcomes but no association between vitamin D levels and hypertension (35). The difficulty in evaluating a causal role of vitamin D on cardiometabolic health could be due to variability in the nonlinearity of the association between vitamin D and the outcomes (35). Additionally, recent MR studies demonstrated the pivotal role of obesity as a mediator in vitamin D associations (5).
No association was found even between genetically predicted PTH and the explored outcomes. This is, to our knowledge, the first MR exploring this relationship; thus, we cannot provide comparisons with other results. From the literature, there is a wide consensus that there is an association between elevated PTH and higher BP levels (13, 36, 37), but both the causal link and the independency of PTH with respect to calcium level are not clarified (14, 37, 38).
A genetically predicted lower level of FGF23 was found to be associated with increased SBP. Even if our MR Egger analysis result was not significant, the direction and the magnitude of the effect size were consistent with the IVW. Previous studies showing the role of FGF23 in BP rise and hypertension are contrasting. Physiologically, FGF23 could act on hypertension through the activation of the renin-angiotensin-aldosterone system (RAAS) directly, or indirectly mediated by mineral concentrations of phosphate and calcium (39).
Different longitudinal and observational studies showed a higher level of FGF23 associated with a higher risk of hypertension incident (10, 40) or higher concentration in hypertensive individuals (41), but it remains unclear whether this association could be mediated by metabolic cofounders that were unmeasured. The only Mendelian randomization study performing the same analysis reported neither an association with BP nor with markers of renal function. This difference from our results could be because we used BP values adjusted for age, sex, and antihypertensive medication and we also included an additional SNP. In the same previous study, the authors showed FGF23 to be inversely associated with coronary artery disease and myocardial infarction, a direction consistent with our results (42).
Numerous studies have reported an association between increased levels of FGF23 and adverse outcomes in patients with chronic kidney disease and end-stage renal disease (43), supporting a pivotal role of FGF23 in the regulation of phosphate level. Thus, FGF23 is considered a potential early biomarker for disease: its increased level in response to a decrease of glomerular filtration rate has been associated with vascular calcification and cardiovascular diseases (39). However, it is not clear whether it should be considered a cause or a consequence of the increased level of phosphate in renal disease (39). A low level of FGF23 could result in high phosphate and calcium level, as also shown by FGF23−/− knockout models (44), and could represent a risk factor for hypertension and cardiovascular disease.
Strengths and Limitations
Among the strengths of the present study is the use of the MR approach, which allows inferring causality of an association, minimizing the effect of confounding factors. This could be helpful and meaningful in shedding light on complex regulatory systems. The use of a two-sample MR setting could be an advantage in the evaluation of a causal relation; indeed, it allows on one side, the use of genetic variants identified in large cohorts avoiding power issues, and on the other side, the estimation in 2 nonoverlapping samples, as in our case, which would avoid weak instruments bias. Moreover, this is the first MR investigating the role of serum calcium and PTH with BP traits.
There are some limitations that we should consider. First, the instruments we used can explain only a small amount of the variation of the exposures: 5.8% for calcium; 5.7% for Vit-D, 4.2% for PTH, and 3% for FGF23 level, which could be the cause of a false-negative result. Thus, it would be good in the future to update the analysis whenever more genetic variants are identified. Secondly, the two-sample MR has also some intrinsic limitation, for example, some population characteristics that could be different between the 2 groups and the inability to triangulate the causal inference with observational data using the same sample.
Thirdly, when dealing with correlated metabolites, pleiotropy could be present. Finally, further studies should follow the present study to investigate, from a functional point of view, the target genes that could have clinical relevance. This could be done by exploring rare genetic variants or focusing on drug target genes. In this case, it is desirable to perform an MR analysis of drug target perturbation, a method in which cis-SNPs of a drug target gene, are used as instrumental variables to study the effect of the perturbation of the drug target on the outcome (45). A colocalization test could then be performed to assess whether the cis-SNPs in the locus represent independent association with the traits (not colocalization), or they belong to the same shared genetic signal (colocalization) (46).
Conclusion
In conclusion, we provided evidence supporting that genetically predicted higher serum calcium level causally increases BP, especially DBP. Conversely, genetically predicted PTH did not associate with BP, while genetically predicted FGF23 was weakly inversely associated with SBP. Globally, this work highlights the importance of calcium homeostasis in BP regulation and that a genetic predisposition may be potentially relevant in the progression toward hypertension in people with high calcium levels. Further studies are needed to investigate possible candidate genes involved in calcium metabolism and leading to elevated BP.
Acknowledgments
Regeneron Genetics Center Banner Author List and Contribution Statements are available in Supplementary File (21).
Funding
Lund University Infrastructure grant “Malmö population-based cohorts” (STYR 2019/2046).
Disclosure
Dipender Gill is employed part-time by Novo Nordisk.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
alice-gio/JCEM_supplementary_material. Available at: https://github.com/alice-gio/JCEM_supplementary_material. Accessed August 11, 2022.
Abbreviations
- 1,25(OH)2D
1,25-dihydroxyvitamin D (functional vitamin D)
- BP
blood pressure
- DBP
diastolic blood pressure
- FGF23
fibroblast growth factor 23
- GWAS
genome-wide association study
- IVW
inverse-variance weighted
- MR
Mendelian randomization
- PTH
parathyroid hormone
- SBP
systolic blood pressure
- SE
standard error
- SNP
single nucleotide polymorphism
- Vit-D
vitamin D



