-
PDF
- Split View
-
Views
-
Cite
Cite
Xia Li, Yan Chen, Yuting Xie, Yufei Xiang, Xiang Yan, Gan Huang, Zhiguang Zhou, Decline Pattern of Beta-cell Function in Adult-onset Latent Autoimmune Diabetes: an 8-year Prospective Study, The Journal of Clinical Endocrinology & Metabolism, Volume 105, Issue 7, July 2020, Pages 2331–2340, https://doi.org/10.1210/clinem/dgaa205
Close - Share Icon Share
Abstract
To explore the decline pattern and possible determinants of beta-cell function progression in patients with latent-onset autoimmune diabetes in adults (LADA).
In this 8-year prospective study, 106 LADA individuals underwent annual follow-up and their pattern of beta-cell function progression was assessed. Beta-cell function failure was defined by fasting C-peptide (FCP) < 75 pmol/L. Other clinical characteristics, including age of onset, body mass index (BMI), and glutamic acid decarboxylase autoantibody (GADA) titer, were analyzed to find out possible determinants of beta-cell function progression.
The dropout rate was 4.7%. During the 8-year follow-up period, 29 (28.7%) of the 101 subjects developed beta-cell function failure. The decline pattern of C-peptide in LADA was biphasic, showing an initial rapid linear progression and then followed by a stable mode. The declination speed of FCP was 55.19 pmol/L/year (95% CI, −62.54 to −47.84, P < 0.001) during the first 5 years and 4.62 pmol/L/year (95% CI, −69.83 to 60.60, P = 0.790) thereafter. Further analysis showed that GADA titer was the most valuable discriminatory parameter related to a higher risk of development of beta-cell function failure (GADA titer of 173.5 WHO units/mL; area under the curve [AUC], 0.824). Beta-cell function failure occurred in 71.3% of high-GADA titer patients while only 6.2% of low-titer patients.
The decline pattern of C-peptide was a fast-followed-by-slow biphasic mode, with about a quarter of LADA patients developing beta-cell function failure during the first 8 years. GADA titer less than 173.5 WHO units /mL was propitious for the preservation of beta-cell function.
As a slowly progressive form of autoimmune diabetes, latent-onset autoimmune diabetes in adults (LADA) shares clinical and immunogenetic characteristics with either type 1 diabetes or type 2 diabetes (1). LADA is a heterogeneous disease with high prevalence, 3.3 to 12.2 fold higher than that of adult-onset classic type 1 diabetes and 1.5% to 14.2% in phenotypic type 2 diabetes (2, 3). Characterized by latent and slow progression, LADA is also known as slowly progressive insulin-dependent type 1 diabetes (SPIDDM) (4, 5). However, it is not clear how slow it is. The exact progression speed of beta-cell function is unclarified. In addition, the understanding of latent-onset corresponding to slowly progressive is confusing, as most previous studies set the exogenous insulin requirement as the observation endpoint, which was affected by subjective factors and could not illustrate the dynamic changes of beta-cell function (6-8).
It seemed the declination of beta-cell function was not always slow and the speed of progression was quite variable in LADA patients, as reflected by the duration of insulin independence fluctuating from 6 months to over 12 years (6, 9). The treatment strategy of LADA is closely related to the progression of beta-cell function (10). Previously, early insulin intervention was recommended in LADA patients to delay beta-cell function failure (11, 12). With the development of personalized medicine, it has been recommended that individuals who had rapid disease progression should be treated with insulin (13), while people with sluggishly progressing disease could use other potentially beneficial drugs to achieve glycemic control, including glucagon-like peptide-1 receptor agonists (GLP-1RA) and dipeptidyl peptidase-4 inhibitors (DPP-4i) (14-18). Nevertheless, research on evaluating the decline pattern of beta-cell function in LADA is still lacking, hindering the application of precision treatment. This prospective study was aimed to describe the decline pattern and possible determinants of beta-cell function progression in LADA patients during an 8-year follow-up period.
Research Designs and Methods
Patients and designs
From January 2001 to December 2008, LADA patients who received clinical treatment at the Department of Endocrinology of Second Xiangya Hospital were recruited if they fulfilled the following criteria: (1) age ≥ 30 years at onset of diabetes, (2) independence of insulin therapy during the first 6 months after diagnosis, (3) positive glutamic acid decarboxylase autoantibody (GADA) at least twice, 4) duration of diabetes ≤ 24 months, 5) patients with fasting C-peptide (FCP) ≥ 200 pmol/L or postprandial C-peptide (PCP) ≥ 400 pmol/L. Patients who had a history of any severe diseases or malignancy were excluded. Informed consent was obtained from all subjects. This study was conducted in agreement with the Declaration of Helsinki and was approved by local ethics committees.
Out of a total of 163 patients who fulfilled the inclusive criteria, 32 chose not to participate and 25 joined other studies. Of the 106 subjects enrolled in this study, 23 had a disease duration of 12 to 24 months and 83 of less than 12 months. At the time of initial inclusion, data from all subjects, including age of onset, disease duration, body mass index (BMI), waist circumference, systolic blood pressure, and diastolic blood pressure, were collected. Additionally, serum was collected for measurements of glycated hemoglobin A1c (HbA1c), FCP, PCP, triglyceride, and total cholesterol levels. Titers of GADA, as well as human leukocyte antigen DQ (HLA-DQ) genotypes were also tested. In all subjects, 45 patients received oral antidiabetic drugs (OADs), including metformin, sulfonylureas, thiazolidinediones and acarbose, 14 received insulin therapy only, 13 received insulin combined with OADs and 34 were treated with diet. During the follow-up period, 20 patients changed treatment from diets or OADs to insulin therapy.
After the initial visit, patients were followed-up every 12 months to measure their FCP and PCP levels as a reflection of their islet beta-cell function. On each annual visit, tests were started before 9:00 am. Long-acting insulin was not permitted on the visit day. Short-acting insulin and OADs were withdrawn before the end of that visit. After an overnight fast, blood samples were collected from each participant at 0 minutes and 120 minutes after a standard 550 kcal mixed meal tolerance test (57.7% of calories as carbohydrate, 24.2% as fat, and 9.2% as protein). Whole blood samples for genotyping were obtained at baseline.
The dropout rate in our study was 4.7% (n = 5); 2 patients moved out of the city and 3 were unwilling to continue. Of 101 LADA patients who completed the follow-up, 72 were followed for 8 years and 29 were terminated from follow-up after FCP was < 75 pmol/L.
C-peptide and HbA1c assays
FCP and PCP were measured by a chemiluminescence method using the Adiva Centaur Systemakit (Siemens, Munich, Germany). The inter- and intra-assay variation coefficients were 3.7% to 4.1% and 1.0% to 3.3% respectively. The preservation of beta-cell function was defined as FCP ≥ 200 pmol/L, and beta-cell function failure was defined as FCP < 75 pmol/L. HbA1c was detected by automated liquid chromatography (VARIANT-II Hemoglobin Testing System; Bio-Rad Laboratories, Hercules, CA).
Homeostasis model assessment 2 (HOMA2)
HOMA2 of insulin resistance (HOMA2-IR) and beta-cell function (HOMA2-B) were calculated with the HOMA2 calculator, version 2.2 (www.dtu.ox.ac.uk/homacalculator/index.php) using fasting plasma glucose (FPG) and FCP values (19).
Islet autoantibodies assays
Islet autoantibodies were detected by radioligand assays in duplicate, including GADA, insulinoma-associated antigen-2 autoantibody (IA-2A) and zinc-transporter 8 autoantibody (ZnT8A). Based on the 99th percentile observed in 405 healthy participants, the cutoff values of positivity for GADA, IA-2A, and ZnT8A were 0.05 index/mL (18.0 WHO units/mL), 0.02 index/mL (3.3 WHO units/mL), and 0.01 index/mL, respectively, as previous reported (20). The autoantibodies defined as positive were confirmed by repeated assay. In our laboratory, the respective values for sensitivity and specificity were 82% and 98% for GADA, 76% and 100% for IA-2A, and 72% and 100% for ZnT8A. Intra- and inter-assay coefficients of variation for GADA testing were 8.9% and 11.2%, respectively. The results were evaluated in the 2016 Islet Autoantibody Standardization Program.
HLA DR-DQ genotyping
Total genomic DNA was extracted from EDTA‐anticoagulated blood by the phenol‐chloroform method. All subjects were genotyped by sequencing exon 2 of each gene, yielding 2- or 4-digit resolution for HLA-DQA1 and 4-digit resolution for HLA-DQB1. The HLA‐DQ haplotypes were constructed using the PHASE program. The HLA protective (including DQA1*0102-DQB1*0601, DQA1*0102-DQB1*0602 and DQA1*0601-DQB1*0301) and susceptible (including DQA1*03-DQB1*0303, DQA1*03-DQB1*0401 and DQA1*05-DQB1*0201) haplotypes were defined according to the LADA China study reported by Zhou et al and Luo et al (21, 22).
Statistical analysis
Statistical analysis was performed with SPSS 20.0 software. Data were presented as means ± standard deviation (SD) or as indicated. Independent Student t test and Mann-Whitney U test were used, respectively, to compare the means and medians between groups. Frequency differences were compared using the Chi-square test or Fisher’s exact test when appropriate. Multivariate analyses of variance (MANOVA) were performed to compare the FCP levels between 2 groups during the entire follow-up period. Slopes of C-peptide versus time were estimated for subgroups using the linear regression. Kaplan-Meier analyses assessed the time to FCP < 75 pmol/L across the period of follow-up. Multivariable logistic regression analyses were performed to combine variables by the Enter method. The diagnostic accuracy of the single variable and logistic regression models was checked by receiver-operating characteristic (ROC) curves and the area under the ROC curve (AUCROC). Two-sided statistical tests were performed and P < 0.05 was considered significant.
Results
Changes of beta-cell function during the follow-up
No significant differences in baseline characteristics, including age at diagnosis, BMI, FCP, and GADA were found between the group of all eligible patients (n = 163) and those patients who completed the final follow-up (n = 101) (Supplementary Table 1) (23). The decline pattern of FCP was biphasic, demonstrating a linearly rapid decrease with a slope of 55.19 pmol/L/year (95% CI, −62.54, −47.84; P < 0.001) in the first 5 years, and a relatively stable state with a slope of 4.62 pmol/L/year (95% CI, −69.83, 60.60; P = 0.790) thereafter (Fig. 1a). When FCP was log-transformed, data showed that the decline pattern was still a fast-followed-by-slow biphasic mode, with the first 5 years being log-linearly rapid and a much slower decline thereafter (Supplementary Figure 1a) (23).
![Time-dependent changes in FCP levels in (a) all LADA patients, (b) LADA individuals diagnosed at age < 45 years or ≥ 45 years, (c) having BMI < 25 kg/m2 or ≥ 25 kg/m2, (d) with high or low GADA titer. Results are expressed as means with 95% confidence intervals. Abbreviations: Age, age at diagnosis; BMI, body mass index; FCP, fasting C-peptide; GADA, glutamic acid decarboxylase autoantibody; Group [H], patients with high GADA titer; Group [L], patients with low GADA titer; LADA, adult-onset latent autoimmune diabetes.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jcem/105/7/10.1210_clinem_dgaa205/4/m_jcem_105_7_2331_f1.jpeg?Expires=1747986461&Signature=m7PhxB8chuwvTujEzjytLd5BFF4KLIlBR10UpwUAMUwQghqA87-CYXEjExzUpVTwfjlYPulpqAbSP1SwHZwP2GPFCH4kiTRFFV54xBRT1HgnFZyWb9qhyeUx6V6IZ64s2cl4AkXhZ3fpPHcb9HLGXGqHws3QdoAPg7gh2Xt-gBlVoxhV40QoqIthQgyLfGIXugZoRs7LpaBlzPF0rwOq2HvxB37NlxohV9tdg~8j2bogFF690JNJrNGfukKMhSip9vmWYXX~Z8oQ~UCRKZI229g6tK4B8PTOZhh1sI5Ij7EigN0FKSJcYx04Bdr3r~7ks~~j~wS50DC5LM7yJ0r9xA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Time-dependent changes in FCP levels in (a) all LADA patients, (b) LADA individuals diagnosed at age < 45 years or ≥ 45 years, (c) having BMI < 25 kg/m2 or ≥ 25 kg/m2, (d) with high or low GADA titer. Results are expressed as means with 95% confidence intervals. Abbreviations: Age, age at diagnosis; BMI, body mass index; FCP, fasting C-peptide; GADA, glutamic acid decarboxylase autoantibody; Group [H], patients with high GADA titer; Group [L], patients with low GADA titer; LADA, adult-onset latent autoimmune diabetes.
When the participants were divided into 2 groups, according to age of onset or by BMI, results showed that the decline pattern of FCP was similarly biphasic in patients aged < 45 years and ≥ 45 years or BMI < 25kg/m2 and ≥ 25kg/m2. The absolute FCP values were higher in those who were older at diagnosis (P = 0.037) or had higher BMI (P = 0.025) during the entire follow-up period (Fig. 1b and 1c).
The declination of HOMA2-B also showed a biphasic pattern in LADA, regardless of whether analyzed before or after adjusting for HOMA2-IR (Fig. 2a and 2b).
![Time-dependent changes in HOMA2-B levels in (a, b) all LADA patients, and (c, d) LADA individuals with high or low GADA titer. Results are expressed as means with 95% confidence intervals. The HOMA2-B levels in Figure 2b and 2d were adjusted for HOMA2-IR. Abbreviations: GADA, glutamic acid decarboxylase autoantibody; Group [H], patients with high GADA titer; Group [L], patients with low GADA titer; HOMA2-B, homeostatic model assessment 2-beta-cell function; HOMA2-IR, homeostatic model assessment 2-insulin resistance; LADA, adult-onset latent autoimmune diabetes.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jcem/105/7/10.1210_clinem_dgaa205/4/m_jcem_105_7_2331_f2.jpeg?Expires=1747986461&Signature=Xkp~nKVnxRwDoRGObtUyhZEdZaOirtfmt5xGwAdQ-hnxKj4MJ56mSKBMTklbPZm5ppuyEiKUbZJRMvCTadHcvEVbsxeuxxguh9dc0crixwkcZfyd4-uRBR7k5gDy~cHnXpB2RhQlNi2jtBlfsAklWk2xC26~Vp~TBGM6LE456rG2kcgqLmgR6uQ-ClbZs~7op0PHgSz6uuitWUTcMDG-u1qJd76v9BU0ccm3LBV6EHRcIEcvzZFl3jZagWuzrRHnQeUulgkGFkOx2qgTVcMlAYBsqaqfOBxQn4qDfacUI1Cj2zEhSW3r5JznAdj2iSP-EVf1IIIzDQGaLhUQX8zqww__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Time-dependent changes in HOMA2-B levels in (a, b) all LADA patients, and (c, d) LADA individuals with high or low GADA titer. Results are expressed as means with 95% confidence intervals. The HOMA2-B levels in Figure 2b and 2d were adjusted for HOMA2-IR. Abbreviations: GADA, glutamic acid decarboxylase autoantibody; Group [H], patients with high GADA titer; Group [L], patients with low GADA titer; HOMA2-B, homeostatic model assessment 2-beta-cell function; HOMA2-IR, homeostatic model assessment 2-insulin resistance; LADA, adult-onset latent autoimmune diabetes.
Development of beta-cell function failure in LADA
Among all LADA patients, 71.3% preserved beta-cell function and 28.7% developed beta-cell function failure at the end of the 8-year follow-up period. The frequency of patients with failure increased dramatically from 4.0% at the first year to 24.8% at the fifth year, then increased slowly to 28.7% at the eighth year, demonstrating that the changing mode of distinct outcomes in beta-cell function was also biphasic (Fig. 3).
![The proportion of subjects without beta-cell function failure during the 8-year follow-up according to GADA titer. Abbreviations: GADA, glutamic acid decarboxylase autoantibody; Group [H], patients with high GADA titer; Group [L], patients with low GADA titer; HOMA2-B, homeostatic model assessment 2-beta-cell function; LADA, adult-onset latent autoimmune diabetes.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jcem/105/7/10.1210_clinem_dgaa205/4/m_jcem_105_7_2331_f3.jpeg?Expires=1747986461&Signature=Sqe9KNAQ5jFaHCXRgAlBJd6R~uGUhy5XYMQh5v-Xz8cbuVp-bAb0~UC-o2VfQCrkZdT8YhQvod496fFGrED9CNIOigB8jw~QDOSZYtE7qHGcSLQ~zE0NHw7W0phcBaHHcn7HHOluGIIpt4Gc4QZKW0pqgd80m9~wnYWS4PFC89AGtQ7YM-vgQlzrUbUD0P-eBF~Ljf2cMRmyUJ1JITHYwLpywd~9y-uXmpi00nrfOhDqTGxF7Mx9HaR-DI4Cu~yA9nqJdEXPKK8dFox8D4K9hC5Ot5er4QhMtXUahmlmmmHffHjbxxE45fZt41MH3XeehmOdrYPzaVNRec8D7VuSPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
The proportion of subjects without beta-cell function failure during the 8-year follow-up according to GADA titer. Abbreviations: GADA, glutamic acid decarboxylase autoantibody; Group [H], patients with high GADA titer; Group [L], patients with low GADA titer; HOMA2-B, homeostatic model assessment 2-beta-cell function; LADA, adult-onset latent autoimmune diabetes.
Factors associated with the progression of beta-cell failure
LADA patients were divided into 2 groups, those who progressed to beta-cell function failure (progressors, n = 29) and those who did not (nonprogressors, n = 72), to investigate possible predictive factors. As shown in Table 1, the baseline characteristics of younger age at diagnosis, lower BMI, lower initial FCP and PCP levels, higher GADA titer, and greater use of insulin were often present in the progressors group (Table 1). Compared with nonprogressors, the FCP of progressors declined rapidly in the first 5 years after log-transformation (Supplementary Figure 1b) (23).
| . | Progressors (n = 29) . | Nonprogressors (n = 72) . | Data-processing steps . | P value . |
|---|---|---|---|---|
| Age at diagnosis (years) | 45.10 ± 12.32 | 51.40 ± 11.93 | Independent-samples t test | 0.019 |
| Gender (male/female) | 16/13 | 44/28 | Chi-square test | 0.582 |
| Duration (months) | 2 (0, 22) | 2 (0, 24) | Mann-Whitney U test | 0.054 |
| Body mass index (kg/m2) | 21.10 ± 2.53 | 24.39 ± 2.94 | Independent-samples t test | <0.001 |
| Waist circumstance (cm) | 75.86 ± 8.83 | 87.04 ± 8.46 | Independent-samples t test | <0.001 |
| Hemoglobin A1c (%) | 8.39 ± 3.00 | 8.35 ± 2.50 | Independent-samples t test | 0.949 |
| Hemoglobin A1c (mmol/mol) | 68.19 ± 32.76 | 67.83 ± 27.48 | Independent-samples t test | 0.956 |
| Fasting C-peptide (pmol/L) | 455.28 ± 251.66 | 718.59 ± 333.15 | Independent-samples t test | <0.001 |
| Postprandial C-peptide (pmol/L) | 1063.07 ± 853.14 | 2026.93 ± 1446.29 | Independent-samples t test | <0.001 |
| HOMA2-B | 48.30 ± 27.70 | 63.12 ± 30.43 | Independent-samples t test | 0.126 |
| GADA (WHO units/mL) | 775.65 (24.51,1253.74) | 52.76 (18.51,1156.64) | Mann-Whitney U test | <0.001 |
| Metformin (Y/N), n | 3/26 | 18/54 | Chi-square test | 0.101 |
| Acarbose (Y/N), n | 3/26 | 3/69 | Fisher’s exact test | 0.257 |
| Sulfonylureas (Y/N), n | 6/23 | 10/62 | Chi-square test | 0.585 |
| Thiazolidinediones (Y/N), n | 6/23 | 27/45 | Chi-square test | 0.103 |
| Insulin (Y/N), n | 16/13 | 11/61 | Chi-square test | <0.001 |
| ≥ 2 positive DAAs (Y/N), n | 6/23 | 8/64 | Chi-square test | 0.200 |
| HLA protective haplotypes, % (n/N) | 31.8 (7/22) | 25.9 (15/58) | Chi-square test | 0.584 |
| HLA susceptible haplotypes, % (n/N) | 45.4 (10/22) | 51.7 (30/58) | Chi-square test | 0.617 |
| . | Progressors (n = 29) . | Nonprogressors (n = 72) . | Data-processing steps . | P value . |
|---|---|---|---|---|
| Age at diagnosis (years) | 45.10 ± 12.32 | 51.40 ± 11.93 | Independent-samples t test | 0.019 |
| Gender (male/female) | 16/13 | 44/28 | Chi-square test | 0.582 |
| Duration (months) | 2 (0, 22) | 2 (0, 24) | Mann-Whitney U test | 0.054 |
| Body mass index (kg/m2) | 21.10 ± 2.53 | 24.39 ± 2.94 | Independent-samples t test | <0.001 |
| Waist circumstance (cm) | 75.86 ± 8.83 | 87.04 ± 8.46 | Independent-samples t test | <0.001 |
| Hemoglobin A1c (%) | 8.39 ± 3.00 | 8.35 ± 2.50 | Independent-samples t test | 0.949 |
| Hemoglobin A1c (mmol/mol) | 68.19 ± 32.76 | 67.83 ± 27.48 | Independent-samples t test | 0.956 |
| Fasting C-peptide (pmol/L) | 455.28 ± 251.66 | 718.59 ± 333.15 | Independent-samples t test | <0.001 |
| Postprandial C-peptide (pmol/L) | 1063.07 ± 853.14 | 2026.93 ± 1446.29 | Independent-samples t test | <0.001 |
| HOMA2-B | 48.30 ± 27.70 | 63.12 ± 30.43 | Independent-samples t test | 0.126 |
| GADA (WHO units/mL) | 775.65 (24.51,1253.74) | 52.76 (18.51,1156.64) | Mann-Whitney U test | <0.001 |
| Metformin (Y/N), n | 3/26 | 18/54 | Chi-square test | 0.101 |
| Acarbose (Y/N), n | 3/26 | 3/69 | Fisher’s exact test | 0.257 |
| Sulfonylureas (Y/N), n | 6/23 | 10/62 | Chi-square test | 0.585 |
| Thiazolidinediones (Y/N), n | 6/23 | 27/45 | Chi-square test | 0.103 |
| Insulin (Y/N), n | 16/13 | 11/61 | Chi-square test | <0.001 |
| ≥ 2 positive DAAs (Y/N), n | 6/23 | 8/64 | Chi-square test | 0.200 |
| HLA protective haplotypes, % (n/N) | 31.8 (7/22) | 25.9 (15/58) | Chi-square test | 0.584 |
| HLA susceptible haplotypes, % (n/N) | 45.4 (10/22) | 51.7 (30/58) | Chi-square test | 0.617 |
Data are shown as mean ± SD, median (minimum, maximum) or as indicated.
Progressors, subjects who progressed to beta-cell function failure during the follow up; Nonprogressors, subjects who did not progress to failure during the follow-up period.
Abbreviations: DAAs, diabetes autoantibodies (including GADA, insulinoma-associated antigen-2 autoantibody, and zinc-transporter 8 autoantibody); GADA, glutamic acid decarboxylase autoantibody; HOMA2-B, homeostatic model assessment 2-beta-cell function; HLA, human leukocyte antigen; LADA, adult-onset latent autoimmune diabetes; Y/N, yes/no.
Bold indicates P < 0.05.
| . | Progressors (n = 29) . | Nonprogressors (n = 72) . | Data-processing steps . | P value . |
|---|---|---|---|---|
| Age at diagnosis (years) | 45.10 ± 12.32 | 51.40 ± 11.93 | Independent-samples t test | 0.019 |
| Gender (male/female) | 16/13 | 44/28 | Chi-square test | 0.582 |
| Duration (months) | 2 (0, 22) | 2 (0, 24) | Mann-Whitney U test | 0.054 |
| Body mass index (kg/m2) | 21.10 ± 2.53 | 24.39 ± 2.94 | Independent-samples t test | <0.001 |
| Waist circumstance (cm) | 75.86 ± 8.83 | 87.04 ± 8.46 | Independent-samples t test | <0.001 |
| Hemoglobin A1c (%) | 8.39 ± 3.00 | 8.35 ± 2.50 | Independent-samples t test | 0.949 |
| Hemoglobin A1c (mmol/mol) | 68.19 ± 32.76 | 67.83 ± 27.48 | Independent-samples t test | 0.956 |
| Fasting C-peptide (pmol/L) | 455.28 ± 251.66 | 718.59 ± 333.15 | Independent-samples t test | <0.001 |
| Postprandial C-peptide (pmol/L) | 1063.07 ± 853.14 | 2026.93 ± 1446.29 | Independent-samples t test | <0.001 |
| HOMA2-B | 48.30 ± 27.70 | 63.12 ± 30.43 | Independent-samples t test | 0.126 |
| GADA (WHO units/mL) | 775.65 (24.51,1253.74) | 52.76 (18.51,1156.64) | Mann-Whitney U test | <0.001 |
| Metformin (Y/N), n | 3/26 | 18/54 | Chi-square test | 0.101 |
| Acarbose (Y/N), n | 3/26 | 3/69 | Fisher’s exact test | 0.257 |
| Sulfonylureas (Y/N), n | 6/23 | 10/62 | Chi-square test | 0.585 |
| Thiazolidinediones (Y/N), n | 6/23 | 27/45 | Chi-square test | 0.103 |
| Insulin (Y/N), n | 16/13 | 11/61 | Chi-square test | <0.001 |
| ≥ 2 positive DAAs (Y/N), n | 6/23 | 8/64 | Chi-square test | 0.200 |
| HLA protective haplotypes, % (n/N) | 31.8 (7/22) | 25.9 (15/58) | Chi-square test | 0.584 |
| HLA susceptible haplotypes, % (n/N) | 45.4 (10/22) | 51.7 (30/58) | Chi-square test | 0.617 |
| . | Progressors (n = 29) . | Nonprogressors (n = 72) . | Data-processing steps . | P value . |
|---|---|---|---|---|
| Age at diagnosis (years) | 45.10 ± 12.32 | 51.40 ± 11.93 | Independent-samples t test | 0.019 |
| Gender (male/female) | 16/13 | 44/28 | Chi-square test | 0.582 |
| Duration (months) | 2 (0, 22) | 2 (0, 24) | Mann-Whitney U test | 0.054 |
| Body mass index (kg/m2) | 21.10 ± 2.53 | 24.39 ± 2.94 | Independent-samples t test | <0.001 |
| Waist circumstance (cm) | 75.86 ± 8.83 | 87.04 ± 8.46 | Independent-samples t test | <0.001 |
| Hemoglobin A1c (%) | 8.39 ± 3.00 | 8.35 ± 2.50 | Independent-samples t test | 0.949 |
| Hemoglobin A1c (mmol/mol) | 68.19 ± 32.76 | 67.83 ± 27.48 | Independent-samples t test | 0.956 |
| Fasting C-peptide (pmol/L) | 455.28 ± 251.66 | 718.59 ± 333.15 | Independent-samples t test | <0.001 |
| Postprandial C-peptide (pmol/L) | 1063.07 ± 853.14 | 2026.93 ± 1446.29 | Independent-samples t test | <0.001 |
| HOMA2-B | 48.30 ± 27.70 | 63.12 ± 30.43 | Independent-samples t test | 0.126 |
| GADA (WHO units/mL) | 775.65 (24.51,1253.74) | 52.76 (18.51,1156.64) | Mann-Whitney U test | <0.001 |
| Metformin (Y/N), n | 3/26 | 18/54 | Chi-square test | 0.101 |
| Acarbose (Y/N), n | 3/26 | 3/69 | Fisher’s exact test | 0.257 |
| Sulfonylureas (Y/N), n | 6/23 | 10/62 | Chi-square test | 0.585 |
| Thiazolidinediones (Y/N), n | 6/23 | 27/45 | Chi-square test | 0.103 |
| Insulin (Y/N), n | 16/13 | 11/61 | Chi-square test | <0.001 |
| ≥ 2 positive DAAs (Y/N), n | 6/23 | 8/64 | Chi-square test | 0.200 |
| HLA protective haplotypes, % (n/N) | 31.8 (7/22) | 25.9 (15/58) | Chi-square test | 0.584 |
| HLA susceptible haplotypes, % (n/N) | 45.4 (10/22) | 51.7 (30/58) | Chi-square test | 0.617 |
Data are shown as mean ± SD, median (minimum, maximum) or as indicated.
Progressors, subjects who progressed to beta-cell function failure during the follow up; Nonprogressors, subjects who did not progress to failure during the follow-up period.
Abbreviations: DAAs, diabetes autoantibodies (including GADA, insulinoma-associated antigen-2 autoantibody, and zinc-transporter 8 autoantibody); GADA, glutamic acid decarboxylase autoantibody; HOMA2-B, homeostatic model assessment 2-beta-cell function; HLA, human leukocyte antigen; LADA, adult-onset latent autoimmune diabetes; Y/N, yes/no.
Bold indicates P < 0.05.
GADA titer as a valid risk predictor for progression of beta-cell failure
ROC curve showed that GADA was a valid risk predictor for discriminating the progressors out of all LADA patients. The AUC of GADA was 0.824 (0.712, 0.936) and the diagnostic threshold was 173.5 WHO units/mL, which conferred 86% sensitivity and 85% specificity (Fig. 4a). When age at diagnosis, BMI, or initial FCP were analyzed as possible synergic predictors on the basis of GADA, AUC only slightly increased from 0.824 to 0.921 (Fig. 4b), indicating that GADA titer was the most potent predictor for progression of beta-cell function failure in LADA.
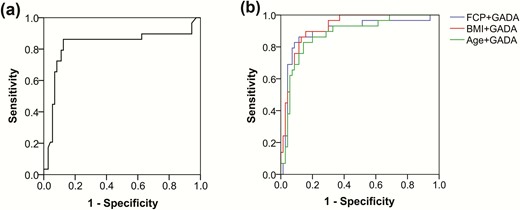
ROC curves showing the relationship between sensitivity and specificity in determining the discriminatory ability of GADA (a) and other features (FCP, BMI or Age) combined with GADA (b) as predictors for the LADA patients with and without failure. The AUCs were: 0.824 (only GADA), 0.893 (FCP+GADA), 0.921 (BMI+GADA), 0.878 (Age+GADA). Abbreviations: Age, age at diagnosis; AUC, the area under the ROC curve BMI, body mass index; FCP, fasting C-peptide; GADA, glutamic acid decarboxylase autoantibody; LADA, adult-onset latent autoimmune diabetes; ROC, receiver operating characteristic.
Long preservation of beta-cell function in patients with low GADA titer
When all subjects were divided into high (Group [H], n = 36) and low (Group [L], n = 65) groups according to the titer of GADA, results demonstrated that the decline patterns of FCP were still biphasic within each subgroup of low and high GADA titer individuals, although mean FCP concentrations in Group [L] were consistently higher than those in Group [H] (P < 0.001). During the first 5 years, the descend rate of FCP in Group [L] was 43.83 pmol/L/year (95% CI, −58.00, −29.65; P = 0.001), lower than that in Group [H] with a slope of 76.09 pmol/L/year (95% CI, −106.00, −46.17; P = 0.002). Subsequently, the FCP levels of Group [L] and [H] was relatively stable, with declination speed being 6.73 pmol/L/year (95% CI, −97.49, 84.03; P = 0.780) and 3.09 pmol/L/year (95% CI: −20.79, 14.62; P = 0.531) respectively. During the follow-up period, the mean FCP values in Group [L] changed from 716.61 pmol/L at baseline to 475.25 pmol/L at the eighth year, still higher than baseline level of 457.06 pmol/L in Group [H] (Figure 1d). The decline patterns of Group [H] and Group [L] did not change after FCP was log-transformed (Supplementary Figure 1c) (23). Similar to FCP, the declination of HOMA2-B in Group[H] was still sharper than Group[L] in the first 5 years, meanwhile the difference was more obvious after adjusting for HOMA2-IR. In the following 3 years, the changes of HOMA2-B were slower in the 2 groups than before, not altered by HOMA2-IR adjustment (Figure 2c and 2d).
In Group [L], 61 out of 65 patients (93.8%) preserved beta-cell function during the entire follow-up period, while the corresponding rate in Group [H] was only 30.5% (11/36) (Fig. 3). Only 4 cases in the low titer group progressed to beta-cell function failure at the first (2 patients), fourth (1 patient) and fifth (1 patient) years, among whom 3 patients had diabetes-susceptible HLA haplotypes (Supplementary Table 2) (23).
Discussion
As the most prevalent autoimmune diabetes among adults, LADA is synonymous with SPIDDM (24). However, it is unknown how slow and what decline pattern it follows, causing inappropriate treatment for some LADA patients, since those who experience slow progression to beta-cell function failure may achieve glycemic control by using alternative antidiabetic drugs other than insulin at the early stage after onset. To the best of our knowledge, this 8-year prospective study was the first report demonstrating the biphasic decline pattern of beta-cell function as rapid-followed-by-slow in LADA patients, with an initial linear fall over the first 5 years, followed by a more stable period where C-peptide levels nearly plateaued. Notably, we found that the development pattern of beta-cell function failure was also biphasic and followed an L-shaped curve. About 28% of LADA patients developed failure, while low GADA titer was propitious for the preservation of beta-cell function.
With reduced genetic load and a less intensive autoimmune process, LADA has been considered as a mild subtype of type 1 diabetes for a long time (22, 25), but whether they shared the same natural history or not has been undefined. The fast-followed-by-slow-or-stable decline pattern has been reported in classic type 1 diabetes with young and acute onset (26-28). Meanwhile, the pattern of beta-cell function in our progressors fitted a log-linear decline as shown for classic type 1 diabetes, indicating that they were similar in progression of beta-cell function. In this sense, the observed biphasic decline might be a composite of rapid and slow decline. Previous results from our group and other research teams demonstrated heterogeneity of LADA, and 2 subtypes with different beta-cell functions, LADA 1 (more similar to classical type 1 diabetes) and LADA 2 (more similar to type 2 diabetes), were identified in some cross-sectional studies (29, 30). Our prospective follow-up study provided data on the decline pattern in LADA as a whole and tried to explore some valid predictive factors for the possible different patterns. Several previous studies showed that the levels of C-peptide in LADA continued to decline rapidly when followed for 3 or 6 years (9, 31), but the subsequent phase with slow decline should be emphasized. Notably, the noninsulin pharmacological therapies were demonstrated to be promising treatments in LADA patients, as proven in several short-term and small-scale clinical trials (14-18, 32). Hals et al discovered that sitagliptin showed similar treatment effects to insulin on LADA in a 21-month randomized trial (16). In this sense, the biphasic pattern of beta-cell function progression would be of great value in deciding on the initiating time of insulin treatment. In our study, the younger LADA patients had lower C-peptide at diagnosis throughout the disease process, which had been well established in classic type 1 diabetes and fitted with the studies that showed the residual insulin-containing islets increased in parallel with age at the onset (33). Age of onset, BMI, initial C-peptide, and GADA titer were all associated with the heterogeneity of beta-cell function progression, as shown in our and other published studies (1, 9, 34).
However, we did find that GADA titer was the most discriminatory parameter for predicting varied outcomes of beta-cell function, and the slight synergic impact of age at diagnosis, BMI, or initial FCP might be a reflection of their relation to GADA titer, as younger onset age, lower BMI, and C-peptide levels were associated with high titer of GADA in previous studies (21, 35). It was unexpected that the number of multiple autoantibodies and HLA haplotypes had no significant differences in the progressors and nonprogressors. Possible reasons include: (1) a relatively small number of enrolled patients; and (2) the roles of GADA titer, multiple autoantibodies, and susceptible HLA haplotypes are interactive, so the impact of multiple autoantibodies and HLA risk might be reflected on GADA titer, thus making GADA titer a mixed predictor for beta-cell function progression (35). A cross-sectional study from Non-insulin Requiring Autoimmune Diabetes (NIRAD) group showed that LADA patients with high GADA titer had more prominent traits of insulin deficiency and a profile of more severe autoimmunity (35). Subsequent longitudinal NIRAD findings demonstrated that high GADA titer accelerated the progression toward insulin requirement in LADA patients (36). Our results confirmed the discriminatory role of GADA titer in identifying 2 distinct subgroups of patients with LADA.
About 95% of our low titer patients preserved beta-cell function and most of them did not need insulin treatment during the entire follow-up. Recently, a Post-Hoc analysis including 3 randomized trials reported that dulaglutide had similar treatment effects in low titer LADA and type 2 diabetes, better than in high titer LADA (17). Low GADA titer patients may achieve glycemic control more easily and respond better to agents which augmented the incretin pathway and insulin secretion (37). It was thus suggested that beta-cell-preserving antidiabetic drugs such as DPP-4i and GLP-1RA, rather than insulin, could be the initial approach in LADA patients with low GADA titer, which would be an important step in the individualized treatment of LADA. In this sense, measurement of serum glucagon and incretin concentrations would be of great value in choosing therapies related to the use of GLP-1RA. Nevertheless, the conclusion should be further evaluated in large-scale and long-term trials in light of the uncertain risks of diabetic complications.
Other than the relation to beta-cell progression, GADA titer had been also reported to be associated with other organ-specific autoimmunity and risks of complications. Zampetti et al demonstrated that high GADA titer was associated with a higher prevalence of autoantibodies for thyroid peroxidase and antiparietal cells in the NIRAD study (38). A regular screening for other organ-specific autoantibodies was recommended in LADA patients according to GADA titer. In the United Kingdom Prospective Diabetes Study (UKPDS), Maddaloni et al described that the risk of microvascular complications was lower in LADA patients with high GADA titer than low titer patients (39). One possible reason was that the presence of severe autoimmunity might accelerate the onset of overt diabetes, thereby avoiding the exposure to the detrimental effects of hyperglycemia for years before diagnosis (39). Indeed, intensive glycemic control was shown to be beneficial in low long-term risk of cardiovascular diseases and microvascular complications (39, 40).
Due to the slow declination of C-peptide during the 8-year follow-up, the rationale that low GADA titer patients were classified as LADA rather than type 2 diabetes, was again questioned. Our team reported that patients with low GADA titer were similar to type 2 diabetes in age of onset, HbA1c, and beta-cell function reserve both in cross-sectional and prospective studies (9, 21). In addition, the transcription factor 7 like 2 (TCF7L2) risk allele for type 2 diabetes was reported to be associated with low GADA titer patients (41, 42). The majority of LADA patients with low GADA titer have preserved beta-cell function for a long time and were unlikely to require insulin, which might make us reconsider the definition of LADA. Although there were concerns that low titer in some LADA patients could be turned into negativity and thus should be considered as real type 2 diabetes (9, 43), we excluded this possibility to the greatest extent by recruiting patients whose GADA was positive for at least twice in one month.
The debate on the exact definition of LADA has been going on for a long time and there have been controversies regarding the diagnostic criteria for age of onset and duration of insulin requirement. Here, our study suggests the possibility of adjusting “GADA positivity” to “GADA positivity with high titer” in the diagnostic criteria. It is worth noting that Emma Ahlqvist et al recently used 6 clinical variables to identify 5 exclusive diabetes subgroups, with severe autoimmune diabetes (SAID) being recognized as cluster 1 (44). For LADA patients in this cluster, GADA positivity was associated with absolute insulin deficiency, although no data on GADA titer was available. The possible reasons for the inconsistency are: (1) ethnic differences; studies from Action LADA in Europe and LADA China showed that Caucasian patients were predominantly high GADA titer (1), unlike Chinese patients; 2) different study design; ours was a prospective study focusing on progression of beta-cell function, while the study of Emma Ahlqvist et al was cross-sectional and they considered GADA positive patients as in the cluster of severe autoimmune diabetes (SAID). The trajectory of beta-cell function progression shown in our study would have great implications for precision treatment in LADA.
This study had several strengths. It was the first prospective report on beta-cell function with a long-term follow-up. To eliminate the confounding effect of some oral drugs and insulin on C-peptide testing, we standardized the follow-up protocol. Our findings indicate that attention should be paid to individuals with high GADA titer, especially during the first 5 years. However, in terms of the current GADA assay, the risk of false positive was inevitable. Furthermore, our sample size was relatively small and the Chinese hospital-based subjects might not be representative of the entire population of patients with diabetes. The parameters for predicting outcomes of beta-cell function and disease patterns may be different in different people of different ethnic/racial backgrounds. Therefore, a large, multicenter study is needed to confirm our observations.
Abbreviations
- BMI
body mass index
- DPP-4i
dipeptidyl peptidase-4 inhibitor
- FCP
fasting C-peptide
- GAD
glutamic acid decarboxylase
- GADA
glutamic acid decarboxylase autoantibody
- GLP-1RA
glucagon-like peptide-1 receptor agonist
- HbA1c
glycated hemoglobin A1c
- HLA-DQ
human leukocyte antigen DQ
- HOMA2-B
homeostasis model assessment 2 for beta-cell function
- HOMA2-IR
homeostasis model assessment 2 for insulin resistance
- IA-2A
insulinoma-associated antigen-2 autoantibody
- LADA
latent-onset autoimmune diabetes in adults
- OAD
oral antidiabetic drug
- PCP
postprandial C-peptide
- ROC
receiver-operating characteristic
- SAID
severe autoimmune diabetes
- ZnT8A
zinc-transporter 8 autoantibody
Acknowledgments
The authors thank Lingjiao Liu for collecting the data, Ting Zhong for providing submission suggestions, Zhiguo Xie and Ying Xia for HLA genotyping, Bingwen Liu for sharing literature, and Guozhi Jiang and Tingting Sha for their advice on statistical methods.
Financial Support: This study was supported by the National Key Technology Research and Development Program of China (Grant NO. 2017YFC1309604, 2016YFC1305000) and the Natural Science Foundation of Hunan Province, China (Grant NO. 2019JJ40419).
Author Contributions: X.L. designed the study and wrote the manuscript. Y.C. collected and analyzed the data and wrote the manuscript. Z.Z. reviewed and edited the manuscript. Y.X., Y.X. and X.Y. collected the data. G.H. contributed to the testing of autoantibody. Z.Z. is the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Additional Information
Disclosure Summary: The authors have nothing to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. No potential conflicts of interest relevant to this article were reported.
Data Availability: The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
References
Author notes
co-first author



