-
PDF
- Split View
-
Views
-
Cite
Cite
Giacomo Rossitto, Laurence Amar, Michel Azizi, Anna Riester, Martin Reincke, Christoph Degenhart, Jiri Widimsky, Mitsuhide Naruse, Jaap Deinum, Leo Schultzekool, Tomaz Kocjan, Aurelio Negro, Ermanno Rossi, Gregory Kline, Akiyo Tanabe, Fumitoshi Satoh, Lars Christian Rump, Oliver Vonend, Holger S Willenberg, Peter Fuller, Jun Yang, Nicholas Yong Nian Chee, Steven B Magill, Zulfiya Shafigullina, Marcus Quinkler, Anna Oliveras, Chin-Chen Chang, Vin Cent Wu, Zusana Somloova, Giuseppe Maiolino, Giulio Barbiero, Michele Battistel, Livia Lenzini, Emilio Quaia, Achille Cesare Pessina, Gian Paolo Rossi, Subtyping of Primary Aldosteronism in the AVIS-2 Study: Assessment of Selectivity and Lateralization, The Journal of Clinical Endocrinology & Metabolism, Volume 105, Issue 6, June 2020, Pages 2042–2052, https://doi.org/10.1210/clinem/dgz017
Close - Share Icon Share
Abstract
Adrenal venous sampling (AVS) is the key test for subtyping primary aldosteronism (PA), but its interpretation varies widely across referral centers and this can adversely affect the management of PA patients.
To investigate in a real-life study the rate of bilateral success and identification of unilateral aldosteronism and their impact on blood pressure outcomes in PA subtyped by AVS.
In a retrospective analysis of the largest international registry of individual AVS data (AVIS-2 study), we investigated how different cut-off values of the selectivity index (SI) and lateralization index (LI) affected rate of bilateral success, identification of unilateral aldosteronism, and blood pressure outcomes.
AVIS-2 recruited 1625 individual AVS studies performed between 2000 and 2015 in 19 tertiary referral centers. Under unstimulated conditions, the rate of biochemically confirmed bilateral AVS success progressively decreased with increasing SI cut-offs; furthermore, with currently used LI cut-offs, the rate of identified unilateral PA leading to adrenalectomy was as low as <25%. A within-patient pairwise comparison of 402 AVS performed both under unstimulated and cosyntropin-stimulated conditions showed that cosyntropin increased the confirmed rate of bilateral selectivity for SI cut-offs ≥ 2.0, but reduced lateralization rates (P < 0.001). Post-adrenalectomy outcomes were not improved by use of cosyntropin or more restrictive diagnostic criteria.
Commonly used SI and LI cut-offs are associated with disappointingly low rates of biochemically defined AVS success and identified unilateral PA. Evidence-based protocols entailing less restrictive interpretative cut-offs might optimize the clinical use of this costly and invasive test. (J Clin Endocrinol Metab XX: 0-0, 2020)
Primary aldosteronism (PA) is incorrectly regarded as a rare condition, despite evidence showing that it is the most common cause of endocrine hypertension (1–4). Failure to identify and subtype PA at an early stage leaves a multitude of patients exposed to life-long hyperaldosteronism, and thus to a high risk of cardiovascular events, particularly atrial fibrillation, as shown in both retrospective and prospective studies (5–8).
In the work-up of PA patients, the subtyping is a fundamental step, because patients with a unilateral form, mostly aldosterone-producing adenoma (APA) and unilateral adrenal hyperplasia (9, 10), benefit from laparoscopic adrenalectomy to obtain definitive correction of the hyperaldosteronism and often cure of arterial hypertension. Conversely, patients with bilateral PA, predominantly bilateral adrenal hyperplasia (also known as idiopathic hyperaldosteronism), require life-long medical treatment with a mineralocorticoid receptor antagonist (MRA), often in combination with multiple other antihypertensive agents.
To distinguish between unilateral and bilateral PA, all current guidelines advocate use of adrenal vein sampling (AVS) (11, 12), a technically demanding test where success is defined as bilateral selectivity, ie, adequate sampling of both adrenal veins. Confirmation of selectivity also serves to minimize the impact of two potential confounders when ascertaining lateralization of aldosterone excess: the degree of proximity of the catheter’s tip to the adrenal cortex, and dilution effect from blood in accessory veins or inferior vena cava.
The criteria to define selectivity and lateralization remain variable, even at major tertiary centers where AVS is performed on a regular basis, as shown by data from a large international survey (AVIS-1) (13) and expert consensus reports (14, 15). This heterogeneity in interpretation can have a profound effect on the clinical decision-making, and thus on the usefulness of AVS.
The Adrenal Vein sampling International Study (AVIS)-2 was planned after completion of AVIS-1(13) with the aim of creating a large international registry of individual AVS data. The results of this study regarding patient outcomes, ie, correction of aldosteronism and rate of cured/improvement of arterial hypertension are reported elsewhere (16): not only did they provide a snapshot of what occurs in real-life and highlight the general outcome benefit of AVS-guided surgical decision-making but also demonstrated the inconsistencies in AVS use and their profound clinical implications (16). Based on those findings, in this study we explored the potential impact and usefulness of more standardized AVS interpretation criteria on management of PA patients. Hence, we herein report on: (i) the potential rate of selective (confirmed successful) AVS studies, (ii) the potential rate of unilateral PA suitable for adrenalectomy; (iii) the post adrenalectomy blood pressure outcomes as a function of the AVS protocol and of commonly advocated diagnostic cut-offs for the indexes defining selectivity (SI) and lateralization (LI).
Methods
The study rationale, design, center recruitment, inclusion/exclusion criteria, population characteristics, and outcome analysis of AVIS-2 were reported in a separate paper (16) and are recapitulated in the Supplementary Methods; all supplementary material and figures are located in a digital research material repository (17). All procedures were carried out according to the Helsinki Declaration. The protocol of the study was approved by the Ethics Committee of both the coordinating center and the participating centers.
In brief, de-identified biochemical data from individual AVS studies were entered in a dedicated web-based platform (https://fm.dmcs.unipd.it) by local investigators who entered the units of measure as per local practice to avoid any conversion errors. The data collection form is shown in the Supplementary Material (17). Post hoc harmonization to conventional units was undertaken in the final database. After database locking, data were checked for internal consistency and standing queries were clarified with the lead investigators from the centers. AVS indices were defined as previously reported and per guidelines (11, 18, 19): (i) the selectivity index (SI) is the ratio between plasma cortisol concentration (PCC) in the adrenal vein (AV) and (infrarenal) inferior vena cava (IVC) and estimates the correct positioning of catheters in the adrenal vein [PCCAV/PCCIVC]; (ii) the “central/peripheral ratio”, originally introduced by Espiner et al. (20) and renamed by some of us (G.R., G.P.R.) “relative aldosterone secretion index” (RASI) (21), is the ratio between the plasma aldosterone concentration (PAC) in each adrenal vein (AV) and inferior vena cava (IVC) divided by the degree of selectivity, ie, dilution, and identifies the contribution of the culprit (dominant) and non-culprit (non-dominant) adrenal to aldosterone secretion [(PACAV/PCCAV)/(PACIVC/PCCIVC)]; in the non-dominant side it is equivalent to the so-called “contralateral suppression index”; (iii) the Lateralization Index (LI) is the ratio between the higher and the lower RASI [or, after simplification: (PACdominant AV/PCCdominant AV)/(PACnon-dominant AV/ PCCnon-dominant AV)] and measures the imbalance in aldosterone secretion between the adrenal glands.
The blood pressure outcomes at follow-up were categorized according to predefined classes as cure, defined as normal blood pressure without any antihypertensive medication, no improvement, defined as lack of blood pressure reduction and/or need for increased number and/or dose of antihypertensive medications, and improvement, the last one split into marked and mild (Supplementary Table 1) (17). These classes are equivalent to those currently used (complete, partial, and absent clinical success) after publication of the clinical outcome (PASO) classification (22). Data were analyzed per protocol according to the original classification, but also by collapsing into a joint definition of partial clinical success the marked and mild improvement, in order to allow for a swift comparison with studies that used the PASO criteria and for a broad generalization of the conclusions.
The diagnostic and outcome impact of different interpretative rules for SI and LI on AVS data obtained with different AVS protocols (non-stimulated, ie, basal, or stimulated) was explored in the entire cohort. Cut-offs used for the analysis were those recommended by guidelines (11, 23) and/or endorsed by expert consensus documents (14, 15).
A paired, within-patient, within-AVS comparison was conducted for cases with available pre- and post-cosyntropin results. ROC (receiver operator characteristic) curve analyses were performed to assess the performance of SI interpretation rules obtained under unstimulated conditions (SI unstimulated) using the post-cosyntropin selectivity data, defined as a SIcosyntropin cut-off = 5.0, as reference standard. This was based on current use and evidence of a clear-cut bimodal separation after cosyntropin (please see results) (17). The Youden index was used to identify the optimal cut-off value for SIunstimulated, ie, the best combination of sensitivity and specificity using post-cosyntropin-ascertained selectivity as classification criterion. The value identified in this analysis (see Results) was included as one of the cut-offs for which the diagnostic/outcome performance was explored.
A sensitivity analysis of the impact of cosyntropin was also performed by repeating the paired comparisons and excluding each individual center stepwise.
The values of SI, LI, and RASI, which showed a skewed distribution at the Kolmogorov–Smirnov test, are reported as median and interquartile range (IQR) and compared across groups with non-parametric Wilcoxon test. The frequency of categorical variables was analyzed with Pearson’s χ 2; McNemar’s test was used for comparison between different diagnostic criteria within the same population. Significance was set at P < 0.05. SPSS for Mac (vers. 25 for Mac, IBM-SPSS Bologna, Italy), GraphPad, Prism (vers. 8.1.1 for Mac, GraphPad Software, La Jolla, CA, USA), and MedCalc (MedCalc Software Ostend Belgium, vers. 15.8) software were used for the statistical analysis.
Results
Study population
The whole database included 1820 individual AVS datasets from consecutive patients studied in 19 centers (Supplementary Table 2) (17). However, to focus upon current AVS practice, the oldest AVS datasets were excluded and the analysis was limited to 1625 individual cases performed from 2000 to 2015 (Fig. 1). The clinical/demographic features of the PA patient population were reported in detail elsewhere (16) and are recapitulated in the Supplementary Table 3 (17).
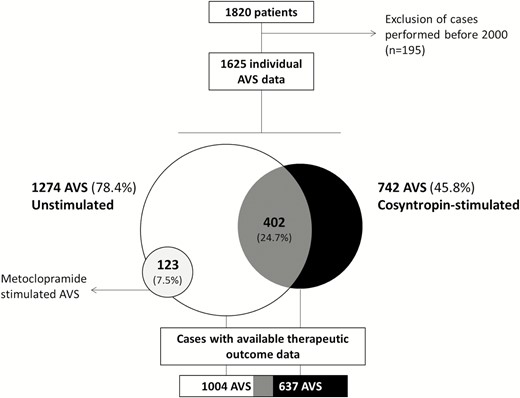
Study population. The flowchart illustrates the distribution of the patients across AVS protocols. In grey, AVS performed under both unstimulated and cosyntropin-stimulated conditions. Abbreviation: AVS, adrenal venous sampling.
Overall, AVS datasets were available in 1274 patients under unstimulated conditions and in 865 cases after pharmacologic stimulation (742 with cosyntropin [85.8%], and 123 with metoclopramide). In 402 patients (24.7% of the total) both unstimulated and cosyntropin-stimulated AVS was performed during the same procedure. This furnished the opportunity for a paired within-patient–within-AVS comparison. Metoclopramide was only used in one center, as already described (19, 24); the results obtained with metoclopramide are reported here simply for comparison with the cosyntropin-stimulated AVS.
In 1004/1274 of AVS performed under unstimulated conditions, in 637/742 after stimulation and in 317/402 AVS after both unstimulated and cosyntropin-stimulated conditions, post-AVS outcome data were available.
Rate of confirmed, successful AVS selectivity at different biochemical SI cut-off definitions
Unstimulated AVS.
The analysis of the success rate of adrenal catheterization under unstimulated conditions showed that increasingly higher biochemical definitions of the SI cut-off up to 5.0 resulted in a progressive fall in the rate of confirmed selective studies on each side and bilaterally (Fig. 2 and Supplementary Table 4) (17). With the most stringent cut-off of 5.0, only 38.3% of the studies were deemed bilaterally selective; however, the rate rose to 52.4% and 67.3% with the commonly used cut-off values of 3.0 and 2.0, respectively.
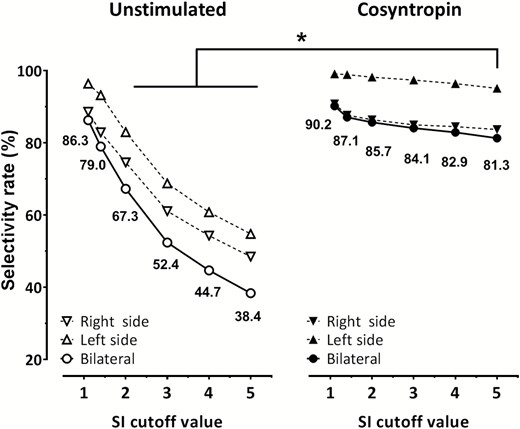
Rate of selectivity as a function of different SI values (definitions). The left panel (empty symbols) shows results under unstimulated conditions (n = 1274); the right panel (closed symbols) shows cosyntropin-stimulated AVS (n = 742) results. Use of increasingly stringent SI cut-off definitions resulted in a progressive fall in the rate of diagnostic studies under unstimulated conditions, but to a much lower extent under cosyntropin stimulation. Please note that a definition of selectivity by SIcosyntropin ≥ 5.0 performed better than any SIunstimulated cut-off ≥ 2.0. *P < 0.001 for SIcosyntropin = 5.0 vs SIunstimulated = 2.0–5.0. Abbreviations: AVS, adrenal venous sampling; LI, lateralization index; SI, selectivity index;
Post-cosyntropin stimulated AVS.
In contrast to the drop in confirmed success observed with increasingly restrictive SI cut-off definitions under unstimulated conditions or during metoclopramide stimulation (Fig. 2 and Supplementary Fig. 1) (17), use of more stringent SI cut-off definitions had less impact on catheterization success when measured after cosyntropin infusion. Despite more stringent interpretation criteria, the proportion of AVS deemed successful decreased only by 9%, ie, from 90.2% at an SI cut-off of 1.1, to 81.3% at 5.0.
Importantly, the currently recommended SI cut-off of 5.0 for cosyntropin-stimulated AVS (11, 15) was associated with a higher rate of bilaterally successful AVS procedures than any SI cut-off definitions ≥ 2.0 under unstimulated conditions (Fig. 2 and Supplementary Table 4) (17).
Use of intraprocedural cortisol assay.
The intraprocedural rapid cortisol assay (IRCA) was used to confirm AVS selectivity under unstimulated conditions only in two centers for a total of 178 patients; it was associated with higher rates of selectivity at each SI cut-off value (Supplementary Table 5) (17).
Comparison of the IRCA cohort with cosyntropin-stimulated AVS showed a similar rate of bilateral selectivity for low SI cut-offs and a better performance of the latter only at SI cut-off values ≥ 3.0 (Supplementary Table 5) (17).
Comparison of unstimulated vs stimulated AVS: determination of the optimal unstimulated SI cut-off
The within-patient–within-AVS pairwise comparison of unstimulated and post-cosyntropin data confirmed increased values of the SI and rate of bilateral selectivity after stimulation (Fig. 3). Notably, the SI values after cosyntropin (Supplementary Fig. 2) showed a bimodal distribution, ie, a clear separation of biochemically successful and non-successful studies, demarcated by the post-cosyntropin SI cut-off definition of 5.0, already in common use (11, 15).
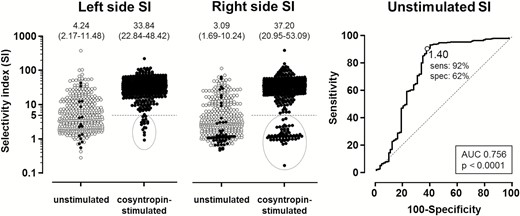
Impact of cosyntropin on SI and identification of optimal unstimulated SI. The left panel shows paired comparison of SI values in the left and right side obtained under unstimulated and post-cosyntropin conditions in 402 patients who were submitted to unstimulated and cosyntropin- stimulated AVS during the same procedure. Medians and interquartile ranges are shown on top. Closed symbols in the unstimulated plots correspond to values that were judged to be unsuccessful, ie, below <5.0, post-cosyntropin. Right panel: ROC curve of unstimulated SI values performed using as golden reference a post-cosyntropin SI ≥ 5.0. Please note that the optimal cut-off for unstimulated SI, ie, Youden index, resulted to be 1.4, which corresponded to a 92% sensitivity and a 62% specificity. Abbreviations: AUC, area under the curve; ROC, receiver operating characteristic; SI, selectivity index.
We used this value as reference for sampling success in a ROC curve analysis to explore the diagnostic performance of unstimulated SI values in this cohort. In general, SI values calculated from unstimulated measurements provided a reasonable accuracy in defining catheterization success (AUC 0.756 (0.724–0.785), P < 0.0001 vs the identity line AUC of 0.50). The Youden Index value of 1.4 from unstimulated measurements generated the highest combined accuracy, with 92% sensitivity and 62% specificity (Fig. 3, Supplementary Table 6) (17). When applied to the entire AVIS-2 database this definition offered a rate of successful selectivity under unstimulated conditions similar to that of a post-cosyntropin SI ≥ 5.0, regardless of IRCA being used or not (Fig. 2, Supplementary Table 5) (17). Results were identical when we used a post-cosyntropin SI cut-off definition of 4.0 (15).
Impact of biochemical diagnostic indices on the diagnosis of unilateral disease and adrenalectomy
Identification of unilateral or bilateral aldosterone excess is possible only after confirmation of technical AVS success according to SI measures. We therefore assessed the rate of patients who could be deemed to have unilateral disease, defined by LI cut-off definitions ranging from 2.0 to 5.0 (11, 14, 15), in concert with SI cut-off definitions of 1.4, 2.0, and 3.0 for unstimulated measurements and 5.0 for post-cosyntropin values. This showed that the rate of lateralization among bilaterally successful studies, dropped significantly with adoption of higher SI cut-off definitions and with each unit increase in LI cut-off definition (Fig. 4, top; Supplementary Table 7 and Supplementary Fig. 3) (17). With commonly used biochemical definitions under unstimulated and cosyntropin-stimulated conditions, ie, ‘SI ≥ 2.0 + LI ≥ 3.0’ and ‘SI ≥ 5.0 + LI ≥ 4.0,’ the proportion of patients deemed to have unilateral disease was 39.8% and 36.6%, respectively (P = 0.174 for comparison). It increased to 55.6% (P < 0.001) with less stringent definitions of unstimulated SI and/or LI, ie, a SI ≥ 1.4 definition for selectivity combined with a cut-off of 2.0 for lateralization.
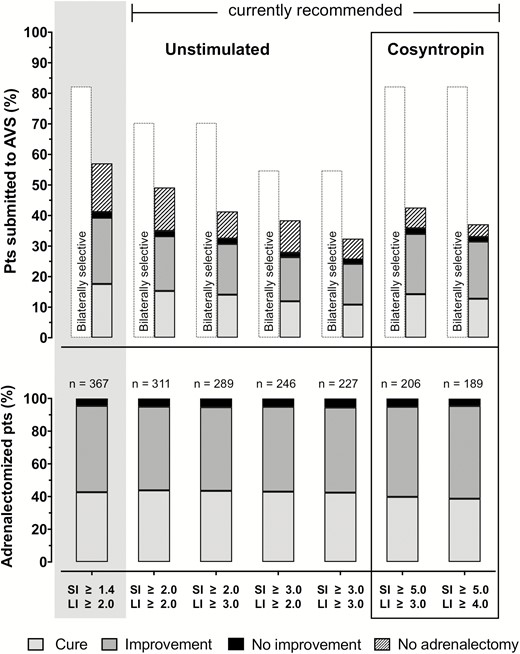
Lateralization, adrenalectomy and blood pressure outcomes according to different sets of diagnostic criteria. Top panel: rate of bilaterally selective (empty bars), no adrenalectomy (dashed bars) and adrenalectomy with ensuing outcomes in the all patients referred to AVS under unstimulated and cosyntropin-stimulated conditions with available clinical outcome data (n = 880/1004 and 580/637, respectively), as a function of set of diagnostic criteria. Those currently recommended are in the mid and right part of the top panel, while a more lenient set (SI > 1.40 and LI > 2.0) based on the above paired-analysis results is shown at the extreme left (grey box). Filled bars indicate adrenalectomized patients, with rates of post-surgical cure, improvement, and no improvement in shades of grey (legend at bottom). The rate of patients fulfilling the same set of homogeneous criteria for selectivity and lateralization but not submitted to surgery are indicated by the superimposed dashed bars. Please note the similar rate of bilateral selectivity at an unstimulated SI > 1.4 and at a post-cosyntropin SI > 5.0, but the lower rate of ascertained lateralization and adrenalectomy if a post-cosyntropin SI > 5.0 were used. Bottom panel: The rate of cure, improvement and no improvement among patients who underwent adrenalectomy meeting the sets of criteria described above, ie, the filled bars from top panel, were similar across sets of criteria. This indicates that use of more restrictive definitions of success and unilateral disease is associated with more patients denied potentially curative surgery (absolute numbers shown on top of each bar) but no better outcomes.
Importantly, there was a lower rate of patients submitted to adrenalectomy after application of more stringent definitions for unilateral disease, to a nadir of less than 25% with an interpretative SI ≥ 3.0 and LI ≥ 4.0 (Supplementary Table 7) (17). However, the proportion of patients referred for adrenalectomy among those with AVS evidence of unilateral disease was higher with more restrictive criteria for lateralization, suggesting more physicians’ confidence in results that meet the stricter definitions (Fig. 4, top, and Supplementary Table 7) (17).
Impact of cosyntropin use upon the determination of unilateral disease
A pairwise comparison of the bilaterally successful AVS under both unstimulated and cosyntropin-stimulated conditions showed a highly significant (P < 0.001) decrease of the LI values between baseline and post cosyntropin, a finding confirmed in the subcohort of those who had a unilateral form of PA unambiguously established by biochemical cure at follow-up post adrenalectomy (n = 149/402). Hence, after cosyntropin stimulation, notwithstanding the higher rate of bilaterally successful AVS studies, a lower proportion of patients was judged to have a unilateral form of PA (Fig. 4 top panel and Supplementary Table 7) (17).
By calculating the relative aldosterone secretion (RASI) to assess the contribution of the culprit and nonculprit adrenal to the LI value (19–21), we could clarify that the LI decreased because of a more prominent drop of RASI in the dominant than the nondominant side (Table 1). This fall was specific to cosyntropin, as it did not occur during metoclopramide stimulation (Supplementary Fig. 4) (17). A sensitivity analysis performed by stepwise elimination of each individual center showed similar results (Supplementary Table 8) (17).
Paired comparison of relative aldosterone secretion index (RASI) by side and lateralization index (LI) between unstimulated and post-cosyntropin conditions
| . | Whole cohort . | Unilateral PA . | ||||||
|---|---|---|---|---|---|---|---|---|
| Indexes . | Unstimulated . | Post cosyntropin . | %Δ RASI (median) . | P . | Unstimulated . | Post cosyntropin . | %Δ RASI (median) . | P . |
| RASI | ||||||||
| Dominant side | 7.26 (2.79–13.00) | 3.93 (2.71–5.32) | –42.0% | <0.001 | 8.41 (5.88–10.90) | 4.25 (2.76–6.69) | –93.2 % | <0.001 |
| Non-dominant side | 1.00 (0.47–2.34) | 1.25 (0.34–2.33) | –2.6% | ns | 0.52 (0.26–1.04) | 0.37 (0.19–0.89) | –2.5 % | 0.020 |
| LI* | 3.81 (1.73–20.40) | 2.52 (1.41–10.80) | — | <0.001 | 17.71 (3.87–33.28) | 9.43 (3.18–19.84) | – | <0.001 |
| . | Whole cohort . | Unilateral PA . | ||||||
|---|---|---|---|---|---|---|---|---|
| Indexes . | Unstimulated . | Post cosyntropin . | %Δ RASI (median) . | P . | Unstimulated . | Post cosyntropin . | %Δ RASI (median) . | P . |
| RASI | ||||||||
| Dominant side | 7.26 (2.79–13.00) | 3.93 (2.71–5.32) | –42.0% | <0.001 | 8.41 (5.88–10.90) | 4.25 (2.76–6.69) | –93.2 % | <0.001 |
| Non-dominant side | 1.00 (0.47–2.34) | 1.25 (0.34–2.33) | –2.6% | ns | 0.52 (0.26–1.04) | 0.37 (0.19–0.89) | –2.5 % | 0.020 |
| LI* | 3.81 (1.73–20.40) | 2.52 (1.41–10.80) | — | <0.001 | 17.71 (3.87–33.28) | 9.43 (3.18–19.84) | – | <0.001 |
Paired comparison in the subcohort of patients with bilaterally selective AVS on both non-stimulated (unstimulated) and post cosyntropin conditions (n = 402). PA = primary aldosteronism. Data presented as median (interquatile range). *SI ≥ 2.0 was used for definition of unstimulated LI; SI ≥ 5.0 was used for definition of post-cosyntropin LI. Results are consistent across different SI cut-off values (not shown). Wilcoxon test, significance set at P < 0.05.
Paired comparison of relative aldosterone secretion index (RASI) by side and lateralization index (LI) between unstimulated and post-cosyntropin conditions
| . | Whole cohort . | Unilateral PA . | ||||||
|---|---|---|---|---|---|---|---|---|
| Indexes . | Unstimulated . | Post cosyntropin . | %Δ RASI (median) . | P . | Unstimulated . | Post cosyntropin . | %Δ RASI (median) . | P . |
| RASI | ||||||||
| Dominant side | 7.26 (2.79–13.00) | 3.93 (2.71–5.32) | –42.0% | <0.001 | 8.41 (5.88–10.90) | 4.25 (2.76–6.69) | –93.2 % | <0.001 |
| Non-dominant side | 1.00 (0.47–2.34) | 1.25 (0.34–2.33) | –2.6% | ns | 0.52 (0.26–1.04) | 0.37 (0.19–0.89) | –2.5 % | 0.020 |
| LI* | 3.81 (1.73–20.40) | 2.52 (1.41–10.80) | — | <0.001 | 17.71 (3.87–33.28) | 9.43 (3.18–19.84) | – | <0.001 |
| . | Whole cohort . | Unilateral PA . | ||||||
|---|---|---|---|---|---|---|---|---|
| Indexes . | Unstimulated . | Post cosyntropin . | %Δ RASI (median) . | P . | Unstimulated . | Post cosyntropin . | %Δ RASI (median) . | P . |
| RASI | ||||||||
| Dominant side | 7.26 (2.79–13.00) | 3.93 (2.71–5.32) | –42.0% | <0.001 | 8.41 (5.88–10.90) | 4.25 (2.76–6.69) | –93.2 % | <0.001 |
| Non-dominant side | 1.00 (0.47–2.34) | 1.25 (0.34–2.33) | –2.6% | ns | 0.52 (0.26–1.04) | 0.37 (0.19–0.89) | –2.5 % | 0.020 |
| LI* | 3.81 (1.73–20.40) | 2.52 (1.41–10.80) | — | <0.001 | 17.71 (3.87–33.28) | 9.43 (3.18–19.84) | – | <0.001 |
Paired comparison in the subcohort of patients with bilaterally selective AVS on both non-stimulated (unstimulated) and post cosyntropin conditions (n = 402). PA = primary aldosteronism. Data presented as median (interquatile range). *SI ≥ 2.0 was used for definition of unstimulated LI; SI ≥ 5.0 was used for definition of post-cosyntropin LI. Results are consistent across different SI cut-off values (not shown). Wilcoxon test, significance set at P < 0.05.
In line with these findings and regardless of the LI definition used, the number of cases judged to be unilateral post cosyntropin decreased significantly compared with the rate determined by unstimulated AVS measures (Table 2). Accordingly, the proportion of PA patients with lateralized AVS results under unstimulated conditions and post-cosyntropin AVS results indicating bilateral disease increased and could exceed 30% depending on the diagnostic criteria used (grey shaded cells in Table 2).
Diagnostic discrepancy between paired unstimulated and cosyntropin-stimulated AVS results
| . | . | . | . | Cosyntropin-stimulated . | |||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | SI ≥ 5.0 . | |||||
| . | . | . | . | LI ≥ 3.0 . | . | LI ≥ 4.0 . | . | ||
| . | . | . | . | Lat . | Bilat . | P McNemar . | Lat . | Bilat . | P McNemar . |
| Unstimulated | SI ≥ 1.4 | LI ≥ 2.0 | Lat | 118 (43.9) | 74 (27.5) | < 0.001 | 106 (39.4) | 86 (32.0) | < 0.001 |
| Bilat | 12 (4.5) | 65 (24.2) | 6 (2.2) | 71 (26.4) | |||||
| LI ≥ 3.0 | Lat | 112 (41.6) | 43 (16) | 0.002 | 102 (37.9) | 53 (19.7) | < 0.001 | ||
| Bilat | 18 (6.7) | 96 (35.7) | 10 (3.7) | 104 (38.7) | |||||
| LI ≥ 4.0 | Lat | — | — | — | 91 (33.8) | 38 (14.1) | 0.037 | ||
| Bilat | — | — | 21 (7.8) | 119 (44.2) | |||||
| SI ≥ 2.0 | LI ≥ 2.0 | Lat | 93 (42.3) | 65 (29.5) | < 0.001 | 83 (37.7) | 75 (34.1) | < 0.001 | |
| Bilat | 8 (3.6) | 54 (24.5) | 3 (1.4) | 59 (26.8) | |||||
| LI ≥ 3.0 | Lat | 88 (40.0) | 39 (17.7) | 0.001 | 80 (36.4) | 47 (21.4) | < 0.001 | ||
| Bilat | 13 (5.9) | 80 (36.4) | 6 (2.7) | 87 (39.5) | |||||
| LI ≥ 4.0 | Lat | — | — | — | 71 (32.3) | 32 (14.5) | 0.020 | ||
| Bilat | — | — | 15 (6.8) | 102 (46.4) | |||||
| SI ≥ 3.0 | LI ≥ 2.0 | Lat | 68 (44.4) | 42 (27.5) | < 0.001 | 62 (40.5) | 48 (31.4) | < 0.001 | |
| Bilat | 4 (2.6) | 39 (25.5) | 2 (1.3) | 41 (26.8) | |||||
| LI ≥ 3.0 | Lat | 64 (41.8) | 25 (16.3) | 0.005 | 59 (38.6) | 30 (19.6) | < 0.001 | ||
| Bilat | 8 (5.2) | 56 (36.6) | 5 (3.3) | 59 (38.6) | |||||
| LI ≥ 4.0 | Lat | — | 52 (34.0) | 19 (12.4) | ns | ||||
| Bilat | — | 12 (7.8) | 70 (45.8) | ||||||
| . | . | . | . | Cosyntropin-stimulated . | |||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | SI ≥ 5.0 . | |||||
| . | . | . | . | LI ≥ 3.0 . | . | LI ≥ 4.0 . | . | ||
| . | . | . | . | Lat . | Bilat . | P McNemar . | Lat . | Bilat . | P McNemar . |
| Unstimulated | SI ≥ 1.4 | LI ≥ 2.0 | Lat | 118 (43.9) | 74 (27.5) | < 0.001 | 106 (39.4) | 86 (32.0) | < 0.001 |
| Bilat | 12 (4.5) | 65 (24.2) | 6 (2.2) | 71 (26.4) | |||||
| LI ≥ 3.0 | Lat | 112 (41.6) | 43 (16) | 0.002 | 102 (37.9) | 53 (19.7) | < 0.001 | ||
| Bilat | 18 (6.7) | 96 (35.7) | 10 (3.7) | 104 (38.7) | |||||
| LI ≥ 4.0 | Lat | — | — | — | 91 (33.8) | 38 (14.1) | 0.037 | ||
| Bilat | — | — | 21 (7.8) | 119 (44.2) | |||||
| SI ≥ 2.0 | LI ≥ 2.0 | Lat | 93 (42.3) | 65 (29.5) | < 0.001 | 83 (37.7) | 75 (34.1) | < 0.001 | |
| Bilat | 8 (3.6) | 54 (24.5) | 3 (1.4) | 59 (26.8) | |||||
| LI ≥ 3.0 | Lat | 88 (40.0) | 39 (17.7) | 0.001 | 80 (36.4) | 47 (21.4) | < 0.001 | ||
| Bilat | 13 (5.9) | 80 (36.4) | 6 (2.7) | 87 (39.5) | |||||
| LI ≥ 4.0 | Lat | — | — | — | 71 (32.3) | 32 (14.5) | 0.020 | ||
| Bilat | — | — | 15 (6.8) | 102 (46.4) | |||||
| SI ≥ 3.0 | LI ≥ 2.0 | Lat | 68 (44.4) | 42 (27.5) | < 0.001 | 62 (40.5) | 48 (31.4) | < 0.001 | |
| Bilat | 4 (2.6) | 39 (25.5) | 2 (1.3) | 41 (26.8) | |||||
| LI ≥ 3.0 | Lat | 64 (41.8) | 25 (16.3) | 0.005 | 59 (38.6) | 30 (19.6) | < 0.001 | ||
| Bilat | 8 (5.2) | 56 (36.6) | 5 (3.3) | 59 (38.6) | |||||
| LI ≥ 4.0 | Lat | — | 52 (34.0) | 19 (12.4) | ns | ||||
| Bilat | — | 12 (7.8) | 70 (45.8) | ||||||
Paired diagnostic comparison in the subcohort of patients with bilaterally selective AVS on both unstimulated and post cosyntropin conditions (n = 402). Data presented as n (%), according to use of different diagnostic criteria. Lat = AVS results suggesting lateralization according to corresponding diagnostic cut-off values; Bilat = AVS results suggesting bilateral PA according to corresponding diagnostic cut-off values. Baseline LI cut-off ≥ 4.0 was not compared with post cosyntopin LI cut-off ≥ 3.0 because there is no published evidence of any center using a more stringent approach under unstimulated conditions. McNemar test, significance set at P < 0.05. Abbreviations: AVS, adrenal venous sampling; LI, lateralization index; ns, not significant; SI, selectivity index.
Diagnostic discrepancy between paired unstimulated and cosyntropin-stimulated AVS results
| . | . | . | . | Cosyntropin-stimulated . | |||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | SI ≥ 5.0 . | |||||
| . | . | . | . | LI ≥ 3.0 . | . | LI ≥ 4.0 . | . | ||
| . | . | . | . | Lat . | Bilat . | P McNemar . | Lat . | Bilat . | P McNemar . |
| Unstimulated | SI ≥ 1.4 | LI ≥ 2.0 | Lat | 118 (43.9) | 74 (27.5) | < 0.001 | 106 (39.4) | 86 (32.0) | < 0.001 |
| Bilat | 12 (4.5) | 65 (24.2) | 6 (2.2) | 71 (26.4) | |||||
| LI ≥ 3.0 | Lat | 112 (41.6) | 43 (16) | 0.002 | 102 (37.9) | 53 (19.7) | < 0.001 | ||
| Bilat | 18 (6.7) | 96 (35.7) | 10 (3.7) | 104 (38.7) | |||||
| LI ≥ 4.0 | Lat | — | — | — | 91 (33.8) | 38 (14.1) | 0.037 | ||
| Bilat | — | — | 21 (7.8) | 119 (44.2) | |||||
| SI ≥ 2.0 | LI ≥ 2.0 | Lat | 93 (42.3) | 65 (29.5) | < 0.001 | 83 (37.7) | 75 (34.1) | < 0.001 | |
| Bilat | 8 (3.6) | 54 (24.5) | 3 (1.4) | 59 (26.8) | |||||
| LI ≥ 3.0 | Lat | 88 (40.0) | 39 (17.7) | 0.001 | 80 (36.4) | 47 (21.4) | < 0.001 | ||
| Bilat | 13 (5.9) | 80 (36.4) | 6 (2.7) | 87 (39.5) | |||||
| LI ≥ 4.0 | Lat | — | — | — | 71 (32.3) | 32 (14.5) | 0.020 | ||
| Bilat | — | — | 15 (6.8) | 102 (46.4) | |||||
| SI ≥ 3.0 | LI ≥ 2.0 | Lat | 68 (44.4) | 42 (27.5) | < 0.001 | 62 (40.5) | 48 (31.4) | < 0.001 | |
| Bilat | 4 (2.6) | 39 (25.5) | 2 (1.3) | 41 (26.8) | |||||
| LI ≥ 3.0 | Lat | 64 (41.8) | 25 (16.3) | 0.005 | 59 (38.6) | 30 (19.6) | < 0.001 | ||
| Bilat | 8 (5.2) | 56 (36.6) | 5 (3.3) | 59 (38.6) | |||||
| LI ≥ 4.0 | Lat | — | 52 (34.0) | 19 (12.4) | ns | ||||
| Bilat | — | 12 (7.8) | 70 (45.8) | ||||||
| . | . | . | . | Cosyntropin-stimulated . | |||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | SI ≥ 5.0 . | |||||
| . | . | . | . | LI ≥ 3.0 . | . | LI ≥ 4.0 . | . | ||
| . | . | . | . | Lat . | Bilat . | P McNemar . | Lat . | Bilat . | P McNemar . |
| Unstimulated | SI ≥ 1.4 | LI ≥ 2.0 | Lat | 118 (43.9) | 74 (27.5) | < 0.001 | 106 (39.4) | 86 (32.0) | < 0.001 |
| Bilat | 12 (4.5) | 65 (24.2) | 6 (2.2) | 71 (26.4) | |||||
| LI ≥ 3.0 | Lat | 112 (41.6) | 43 (16) | 0.002 | 102 (37.9) | 53 (19.7) | < 0.001 | ||
| Bilat | 18 (6.7) | 96 (35.7) | 10 (3.7) | 104 (38.7) | |||||
| LI ≥ 4.0 | Lat | — | — | — | 91 (33.8) | 38 (14.1) | 0.037 | ||
| Bilat | — | — | 21 (7.8) | 119 (44.2) | |||||
| SI ≥ 2.0 | LI ≥ 2.0 | Lat | 93 (42.3) | 65 (29.5) | < 0.001 | 83 (37.7) | 75 (34.1) | < 0.001 | |
| Bilat | 8 (3.6) | 54 (24.5) | 3 (1.4) | 59 (26.8) | |||||
| LI ≥ 3.0 | Lat | 88 (40.0) | 39 (17.7) | 0.001 | 80 (36.4) | 47 (21.4) | < 0.001 | ||
| Bilat | 13 (5.9) | 80 (36.4) | 6 (2.7) | 87 (39.5) | |||||
| LI ≥ 4.0 | Lat | — | — | — | 71 (32.3) | 32 (14.5) | 0.020 | ||
| Bilat | — | — | 15 (6.8) | 102 (46.4) | |||||
| SI ≥ 3.0 | LI ≥ 2.0 | Lat | 68 (44.4) | 42 (27.5) | < 0.001 | 62 (40.5) | 48 (31.4) | < 0.001 | |
| Bilat | 4 (2.6) | 39 (25.5) | 2 (1.3) | 41 (26.8) | |||||
| LI ≥ 3.0 | Lat | 64 (41.8) | 25 (16.3) | 0.005 | 59 (38.6) | 30 (19.6) | < 0.001 | ||
| Bilat | 8 (5.2) | 56 (36.6) | 5 (3.3) | 59 (38.6) | |||||
| LI ≥ 4.0 | Lat | — | 52 (34.0) | 19 (12.4) | ns | ||||
| Bilat | — | 12 (7.8) | 70 (45.8) | ||||||
Paired diagnostic comparison in the subcohort of patients with bilaterally selective AVS on both unstimulated and post cosyntropin conditions (n = 402). Data presented as n (%), according to use of different diagnostic criteria. Lat = AVS results suggesting lateralization according to corresponding diagnostic cut-off values; Bilat = AVS results suggesting bilateral PA according to corresponding diagnostic cut-off values. Baseline LI cut-off ≥ 4.0 was not compared with post cosyntopin LI cut-off ≥ 3.0 because there is no published evidence of any center using a more stringent approach under unstimulated conditions. McNemar test, significance set at P < 0.05. Abbreviations: AVS, adrenal venous sampling; LI, lateralization index; ns, not significant; SI, selectivity index.
Impact of diagnostic cut-off definitions on clinical management and blood pressure outcome
The rate of patients referred to surgery decreased in accordance with the propensity of individual centers to trust more restrictive LI cut-off definitions of unilateral disease or results from cosyntropin-stimulated AVS (Fig. 4, top). However, the relative distribution of blood pressure outcomes (cure, improvement, and no improvement) was remarkably similar despite different diagnostic criteria or protocols used (Fig. 4 bottom panel; Supplementary Table 9) (17). A borderline-significant shift from mild to marked within the class improvement was observed with cosyntropin. However, this occurred at the cost of a much lower rate of adrenalectomies out of the selective (successful) AVS studies. Importantly, in the “paired measurement” cohort with unilateral disease according to unstimulated conditions, but no lateralization post-cosyntropin, only a minority of the patients received adrenalectomy (Supplementary Table 10) (17), possibly due to physicians’ perceived diagnostic uncertainty. While this limited the statistical power of this post-adrenalectomy outcome subanalysis, it is worth emphasizing that all such patients showed at least improvement.
Discussion
Thus far, the performance of AVS protocols and interpretations for defining procedural success and lateralization has only been examined in relatively small PA cohorts with limited geographical representation. We have now reported the performance of widely used SI and LI cut-off definitions and AVS protocols for the identification of unilateral forms of PA in the largest international registry of individual AVS studies. These results confirm and explain, at least in part, the disappointingly low rates of surgical cure of hypertension obtained in the AVIS-2 study by using local and stringent criteria for the interpretation of AVS in real-life clinical practice (16).
By examining the selectivity rate as a function of the SI cut-off definitions recommended by expert consensus under unstimulated conditions (14, 15) we report that approximately one-third of PA patients submitted to AVS would not have gained any diagnostic benefit: even with a more lenient SI cut-off definition of 2.0 approximately one third of AVS were judged to be non-selective (Fig. 2). With a more stringent definition, originally endorsed by the 2008 Endocrine Society guidelines (23) and still used in many centers (15), this rate of “failed” AVS was even higher, confirming previous observations from a smaller study (18).
The second important finding relates to the use of use of cosyntropin (synthetic adrenocorticotropic hormone) stimulation, which has become popular by virtue of its ability to generate higher SI values, thus confirming technical success in sampling. Cosyntropin can maximize the step-up of cortisol between the inferior vena cava (a surrogate for cortisol concentration in peripheral blood) and each adrenal vein. Moreover, it minimizes stress-induced steroid fluctuations and any factitious gradients during sequential (non-simultaneous) AVS. The present results confirmed that cosyntropin infusion can increase the apparent AVS success rate when using very stringent SI cut-off definitions (Supplementary Figs 1, 4) (17); the known secretagogue effect of cosyntropin on cortisol and the selective secretagogue effect of metoclopramide on aldosterone (21) can explain these findings, as suggested also by previous single-center studies (18, 25).
More importantly, the AVIS-2 registry comprised the largest collection of AVS studies performed under both unstimulated and cosyntropin-stimulated conditions in the same patient during the same AVS procedure. As such, it offered a unique opportunity for a paired comparison of both selectivity and lateralization rates achieved with the two protocols. This within-patient and within-AVS analysis revealed a bimodal distribution of SI values post-cosyntropin, with a discriminating level corresponding to the commonly used post-cosyntropin SI cut-off definition of 5.0 (Fig. 3 and Supplementary Fig. 2) (17). Thus, even after stimulation and notwithstanding the aforementioned effect of cosyntropin on the SI, a subset of patients still had non-successful results, indicating that cosyntropin stimulation cannot resolve true catheterization failure due to inadequate catheter’s positioning and/or unfavorable adrenal vein anatomy. The latter is not rare, as it has been documented in approximately 15% of PA patients (26, 27).
By using the post-cosyntropin selectivity as a reference, we found that the unstimulated SI definition that offered the best combination of sensitivity and specificity was ≥ 1.4 (Fig. 3, Suppl. Table 6) (17). This definition corresponds to a rate of bilaterally selective cases similar to that achieved using the post-cosyntropin SI ≥ 5.0 or ≥ 4.0. These findings, along with a substantial loss of diagnostically usable AVS studies (Fig. 2) represent, in our view, a compelling argument against use of stringent SI definitions when measured under unstimulated conditions. The proposal of using less restrictive definitions is also supported by the lack of improvement in clinical outcomes with the use of more restrictive criteria (Fig. 4).
The third main finding of the study was that only a minority of the PA patients submitted to AVS (from one fifth to one third, depending on the combination of currently recommended restrictive SI and LI cut-off definitions) could be eventually referred for unilateral adrenalectomy with currently recommended SI and LI criteria. This low rate is consistent with the rate of AVS-guided adrenalectomy seen in AVIS-2 (31.9%), when the diagnosis was based on the criteria used at each center (16).
Overall, the low rates of biochemically defined apparent success, unilateral diagnoses, and referral for surgery may question the usefulness of a test that, besides being costly and invasive, is supposed to identify candidates for adrenalectomy. However, by applying an estimate of hypertension cure rate equal to that seen in the subset of patients who received AVS-guided adrenalectomy in AVIS-2 (16), ie, 40%, to all those identified as likely affected by a unilateral form following less restrictive criteria (Fig. 3), the rate of patients cured based on AVS results would double, from 1 in every 8 (16) to approximately 1 in every 4.
The increased rate of bilateral catheterization success provided by cosyntropin in the majority, albeit not all patients, could not resolve this disappointingly low diagnostic AVS “yield”, in that the serious drawback of cosyntropin was a consistent drop in the lateralization index, as a result of a greater decrease of the relative aldosterone secretion index in the culprit than the contralateral side (Table 1). This drop, already observed in smaller studies (25, 28), may have led to judging up to 32% of PA patients as having a bilateral disease despite evidence of lateralization under unstimulated conditions. It may also justify the borderline-significant shift from mild to marked improvement observed with cosyntropin and likely due to a preselection of the most florid phenotypes. Unfortunately, the higher rate of adrenalectomy among patients identified as unilateral PA based on cosyntropin stimulation rather than under unstimulated sampling suggests that clinicians used to put higher confidence in post-cosyntropin measures. On the contrary, our data conclusively disprove the contention that cosyntropin stimulation would increase the accuracy of AVS for identifying unilateral PA by maximizing the secretion of aldosterone from an APA (or other unilateral forms of PA), one of the original premises for using cosyntropin: overall, cosyntropin did not result in higher rates of cure or better hard outcomes (Fig. 4).
Some limitations must be acknowledged along with the strengths of the AVIS-2 study, as discussed elsewhere (16). It should be acknowledged that, unlike for the surgically curable unilateral PA, there is still no gold standard for the diagnosis of bilateral adrenal hyperplasia. Therefore, to standardize AVS performance, we had to explore the clinical outcomes associated with currently recommended AVS diagnostic criteria and to compare them with sets of criteria homogeneously applied to the entire cohort, regardless of center-specific preferences.
It might be argued that the observational design and lack of predefined criteria to establish definitions of AVS success and lateralization, which were left to participating centers, can be main limitations of AVIS-2. In truth, these can represent major strengths of this study, as they allowed gathering information of the use of AVS in real-life clinical practice. Additional considerable strengths are a predefined protocol for prospective data acquisition and the comprehensive collection of individual biochemical data from the largest worldwide multicenter cohort of subtyped PA patients.
In summary, this study showed that the full diagnostic potential of AVS is largely unrealized, even in major referral centers with research expertise. The use of cosyntropin during AVS, while increasing the rate of studies judged to be successful, resulted in decreased lateralization, thus masking true unilateral disease in a considerable proportion of cases. It is likely that cosyntropin allows identification of only the most severe PA phenotypes (21), thus explaining why it did not prove superior to a simpler computed tomography scan-only approach in an outcome-based randomized trial (29). Hence, based on data from the largest collection of AVS results generated from around the world, we recommend unstimulated AVS as the optimal AVS protocol, together with the adoption of more lenient diagnostic criteria (eg, SI > 1.4) in concert with expert clinical assessment and other predictors of unilateral disease. The use of the rapid intraprocedural assays and/or of markers of selectivity that have a higher adrenal-to-peripheral blood step-up than cortisol, such as metanephrines or androstenedione (30–32), can be promising strategies to improve the clinical yield of AVS and subsequent patient outcomes.
Abbreviations
- APA
aldosterone-producing adenoma
- AV
adrenal vein
- AVIS
Adrenal Vein sampling International Study
- AVS
adrenal venous sampling
- IVC
inferior vena cava
- LI
lateralization index
- MRA
mineralocorticoid receptor antagonist
- PA
primary aldosteronism
- PAC
plasma aldosterone concentration
- PASO
primary aldosteronism surgery outcome
- PCC
plasma cortisol concentration
- RASI
relative aldosterone secretion index
- SI
selectivity index
Acknowledgments
Financial Support: This study was supported in part by research grants to G.P.R. from FORICA (The Foundation for advanced Research In Hypertension and CArdiovascular diseases) and the Società Italiana dell'Ipertensione Arteriosa; from the Else Kröner-Fresenius-Stiftung to M.R., A.R., M.R. and J.D. received support from the Deutsche Forschungsgemeinschaft (DGE, German Research Foundation, Projektnummer 314061271-TRR 205'.
Clinical Trial Information: The AVIS was registered at clinicaltrials.gov number NCT01234220.
Additional Information
Disclosure Summary: There is no conflict of interest or relationship with industry to be disclosed. All authors have read and approved the manuscript.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to data may be provided.
References



