-
PDF
- Split View
-
Views
-
Cite
Cite
Prapti Singh, Ariela Agress, Vanessa Kasarah Madrigal, Clara Magyar, Nora Ostrzega, Gregorio Daniel Chazenbalk, Daniel Anthony Dumesic, Massive Ovarian Growth in a Woman With Severe Insulin-Resistant Polycystic Ovary Syndrome Receiving GnRH Analogue, The Journal of Clinical Endocrinology & Metabolism, Volume 104, Issue 7, July 2019, Pages 2796–2800, https://doi.org/10.1210/jc.2018-02464
Close - Share Icon Share
Abstract
Ovarian hyperandrogenism from polycystic ovary syndrome (PCOS) and hyperinsulinemia from insulin resistance are modulators of ovarian follicle development. We report on a woman with PCOS and hyperandrogenism and severe insulin resistance from metabolic syndrome who received long-term GnRH analogue therapy preceding bilateral salpingo-oophorectomy for massive ovarian enlargement. Ovarian histological examination showed proliferating granulosa cells within antral follicles coexistent with serous cystadenofibromas, demonstrating a unique link between hyperinsulinemia and granulosa cell mitogenesis.
A 30-year-old woman with PCOS with hyperandrogenism, severe insulin resistance from metabolic syndrome, and nonalcoholic steatohepatitis experienced abdominal pain from bilaterally enlarged ovaries. She had previously experienced a pulmonary embolism while taking oral contraceptives and hepatotoxicity from metformin and spironolactone therapies. Long-term GnRH analogue therapy to induce pituitary desensitization to GnRH successfully decreased gonadotropin-dependent steroidogenesis without improving insulin resistance. Despite GnRH analogue therapy, progressive ovarian enlargement in the presence of hyperinsulinemia from worsening metabolic function eventually required bilateral salpingo-oophorectomy for removal of massively enlarged ovaries. Histological examination showed both ovaries contained proliferating granulosa cells within antral follicles coexistent with serous cystadenofibromas.
In women with PCOS and hyperinsulinemia from severe insulin resistance due to metabolic syndrome, granulosa cell proliferation within antral follicles can occur despite long-term GnRH analogue therapy, implicating hyperinsulinemia as a granulosa cell mitogen in the absence of gonadotropin-dependent ovarian function.
Polycystic ovary syndrome (PCOS) is characterized by ovarian hyperandrogenism, oligo-ovulation, and polycystic ovaries, with hyperinsulinemia from insulin resistance coexisting in more than one-half of these women (1). Underlying PCOS-related reproductive and metabolic dysfunction is hyperandrogenism, which acts through the androgen receptor to upregulate granulosa cell gene expression of the FSH receptor, IGF receptor, and IGF-I (2). Simultaneously, hyperinsulinemia from insulin resistance exerts its own receptor-mediated effects on these cells, causing follicle growth to the small antral stage before eventual follicle arrest (2). Despite these complex androgen-insulin intraovarian interactions, animal studies further suggest that insulin may exert an independent effect on folliculogenesis (3). The present report describes a woman with PCOS and hyperandrogenism and insulin resistance from metabolic syndrome who received long-term GnRH analogue therapy followed by removal of massively enlarged ovaries due to serous cystadenofibromas. Within these ovaries, histological evidence of antral follicles containing proliferating granulosa cells suggests that hyperinsulinemia can act as a granulosa cell mitogen in the absence of gonadotropin-dependent ovarian function.
Case Presentation
A 30-year-old overweight nulliparous woman with PCOS (by National Institutes of Health criteria) and metabolic syndrome (by National Cholesterol Education Program Adult Treatment Panel III guidelines) presented with abdominal pain. Examination showed normal vital signs, a body mass index (BMI) of 27 kg/m2, hirsutism, and abdominal tenderness. She had previously experienced a pulmonary embolism while taking oral contraceptives and hepatotoxicity during metformin and spironolactone therapies. Both of her parents had diabetes and a twin sister had PCOS. Her history included a negative thrombophilia evaluation and previous laparoscopic surgeries for a left ovarian serous cystadenoma and cholecystectomy. Transvaginal ultrasonography showed enlarged multifollicular right and left ovaries measuring 4.5 × 5.9 × 3.1 cm and 7.1 × 3.5 × 6.6 cm, respectively. Serum hormone and fasting lipid levels were as follows: total testosterone (T), 153 ng/dL [normal (nl)< 45 ng/dL]; free T, 26.5 pg/mL (nl < 6.4 pg/mL); 17α-hydroxyprogesterone, 131 ng/dL (nl < 200 ng/dL); dehydroepiandrosterone sulfate, 1730 ng/mL (nl, 400 to 3600 ng/mL); total cholesterol, 177 mg/dL (nl < 200 mg/dL); low-density lipoprotein, 110 mg/dL (nl < 130 mg/dL); high-density lipoprotein, 27 mg/dL (nl > 50 mg/dL); and triglycerides, 197 mg/dL (nl < 150 mg/dL). Fasting plasma glucose and serum insulin levels were 91 mg/dL (nl < 100 mg/dL) and 61 μU/mL (nl < 25 μU/mL), respectively; 2-hour 75-g postprandial plasma glucose and serum insulin levels were 159 mg/dL (nl < 140 mg/dL) and 558 μU/mL, respectively. Serum aspartate aminotransferase and alanine aminotransferase levels were 23 U/L (nl < 36 U/L) and 33 U/L (nl < 45 U/L), respectively.
Over the next 2 years, a GnRH analogue (leuprolide acetate, 3.75 mg intramuscularly monthly) induced pituitary desensitization to GnRH to successfully suppress ovarian steroidogenesis (LH, <0.1 IU/mL; FSH, 2.8 IU/mL; estradiol, <12 pg/mL; total T, 10 ng/dL; free T, 1.4 pg/mL). Simultaneously, amenorrhea was associated with a weakly proliferative endometrium, as determined by transvaginal ultrasonography and endometrial biopsy, whereas bone mineral density remained in the normal range, as measured by dual-energy absorptiometry. Over the same time interval, however, her BMI increased to 30 kg/m2 despite efforts at lifestyle modification and changes in multiple medical therapies, including metformin, phentermine, and bromocriptine mesylate leading to deterioration of metabolic function (i.e., 2-hour postprandial glucose, 187 mg/dL; total cholesterol, 237 mg/dL; low-density lipoprotein, 130 mg/dL; high-density lipoprotein, 29 mg/dL; triglycerides, 388 mg/dL; aspartate aminotransferase, 162 U/L; alanine aminotransferase 339 U/L) (Table 1).
Serum Hormone and Metabolic Levels in a Woman With Insulin-Resistant PCOS and Metabolic Syndrome Who Received Long-Term GnRH Analogue Therapy Before Bilateral Salpingo-Oophorectomy for Massively Enlarged Ovaries
| Laboratory Test . | Patient Value . | Normal Value . |
|---|---|---|
| Aspartate aminotransferase, U/L | 162 | <36 |
| Alanine aminotransferase, U/L | 339 | <45 |
| Fasting glucose, mg/dL | 91 | <100 |
| 2-h postprandial glucose, mg/dL | 187 | <140 |
| Fasting insulin, µU/mL | 56 | <25 |
| 2-h postprandial insulin, µU/mL | 598 | 41 |
| Total cholesterol, normal, mg/dL | 237 | <200 |
| Low-density lipoprotein, mg/dL | 130 | <130 |
| High-density lipoprotein, mg/dL | 29 | >50 |
| Triglycerides, mg/dL | 388 | <150 |
| Total T after GnRH analogue, ng/dL | 10 | <45 |
| Free T after GnRH analogue, pg/mL | 1.4 | <6.4 |
| Laboratory Test . | Patient Value . | Normal Value . |
|---|---|---|
| Aspartate aminotransferase, U/L | 162 | <36 |
| Alanine aminotransferase, U/L | 339 | <45 |
| Fasting glucose, mg/dL | 91 | <100 |
| 2-h postprandial glucose, mg/dL | 187 | <140 |
| Fasting insulin, µU/mL | 56 | <25 |
| 2-h postprandial insulin, µU/mL | 598 | 41 |
| Total cholesterol, normal, mg/dL | 237 | <200 |
| Low-density lipoprotein, mg/dL | 130 | <130 |
| High-density lipoprotein, mg/dL | 29 | >50 |
| Triglycerides, mg/dL | 388 | <150 |
| Total T after GnRH analogue, ng/dL | 10 | <45 |
| Free T after GnRH analogue, pg/mL | 1.4 | <6.4 |
Bold laboratory values are elevated compared to the normal range for each respective category.
Serum Hormone and Metabolic Levels in a Woman With Insulin-Resistant PCOS and Metabolic Syndrome Who Received Long-Term GnRH Analogue Therapy Before Bilateral Salpingo-Oophorectomy for Massively Enlarged Ovaries
| Laboratory Test . | Patient Value . | Normal Value . |
|---|---|---|
| Aspartate aminotransferase, U/L | 162 | <36 |
| Alanine aminotransferase, U/L | 339 | <45 |
| Fasting glucose, mg/dL | 91 | <100 |
| 2-h postprandial glucose, mg/dL | 187 | <140 |
| Fasting insulin, µU/mL | 56 | <25 |
| 2-h postprandial insulin, µU/mL | 598 | 41 |
| Total cholesterol, normal, mg/dL | 237 | <200 |
| Low-density lipoprotein, mg/dL | 130 | <130 |
| High-density lipoprotein, mg/dL | 29 | >50 |
| Triglycerides, mg/dL | 388 | <150 |
| Total T after GnRH analogue, ng/dL | 10 | <45 |
| Free T after GnRH analogue, pg/mL | 1.4 | <6.4 |
| Laboratory Test . | Patient Value . | Normal Value . |
|---|---|---|
| Aspartate aminotransferase, U/L | 162 | <36 |
| Alanine aminotransferase, U/L | 339 | <45 |
| Fasting glucose, mg/dL | 91 | <100 |
| 2-h postprandial glucose, mg/dL | 187 | <140 |
| Fasting insulin, µU/mL | 56 | <25 |
| 2-h postprandial insulin, µU/mL | 598 | 41 |
| Total cholesterol, normal, mg/dL | 237 | <200 |
| Low-density lipoprotein, mg/dL | 130 | <130 |
| High-density lipoprotein, mg/dL | 29 | >50 |
| Triglycerides, mg/dL | 388 | <150 |
| Total T after GnRH analogue, ng/dL | 10 | <45 |
| Free T after GnRH analogue, pg/mL | 1.4 | <6.4 |
Bold laboratory values are elevated compared to the normal range for each respective category.
The patient continued to experience worsening abdominal pain due to progressive bilateral ovarian enlargement [Fig. 1(a) and 1(b)] and eventually underwent a bilateral salpingo-oophorectomy for removal of massively enlarged multifollicular-appearing ovaries (right ovary, 17.5 × 11.4 × 8.1 cm; left ovary, 23.0 × 17.0 × 8.5 cm) [Fig. 2(a)]. Both ovaries contained large serous cystadenofibromas and follicular cysts containing epithelial and granulosa cells, with a proliferation index (PI) of 1.02 ± 0.80% SD (11 separate areas) and 2.42 ± 1.90% SD (12 separate areas), respectively, as determined by Ki-67 staining [Fig. 2(b)]. Within the same ovaries were small antral follicles and a Graafian follicle [Fig. 2(c) and 2(d)], all of which contained proliferating granulosa cells [small antral follicles: PI, 6.85 ± 6.96% SD (three separate areas); Graafian follicle: PI, 99%]. There was a significant difference in cellular PIs among serous cystadenofibromas, follicular cysts, and small antral follicles (P = 0.018; ANOVA). Specifically, the PI of granulosa cells within antral follicles was significantly greater than that of cells within serous cystadenofibromas (P < 0.01) and follicular cysts (P < 0.05), with cellular PIs between the two ovarian pathologies being not significantly different (P = not significant; Tukey post hoc analysis).
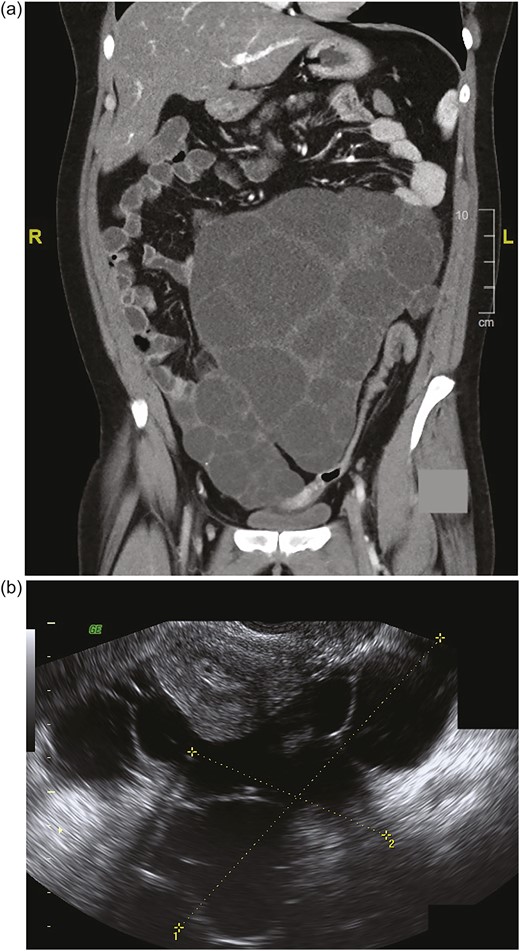
(a) Coronal CT scan shows bilaterally enlarged polyfollicular right and left ovaries measuring 15 cm and 17 cm, respectively. (Largest right ovarian diameter is not shown.) (b) Sagittal transvaginal ultrasonography shows a right ovary measuring 11.3 × 9.5 cm next to the uterus. (Left ovary measuring 18.6 × 14.1 cm is not shown.) L, left; R, right.
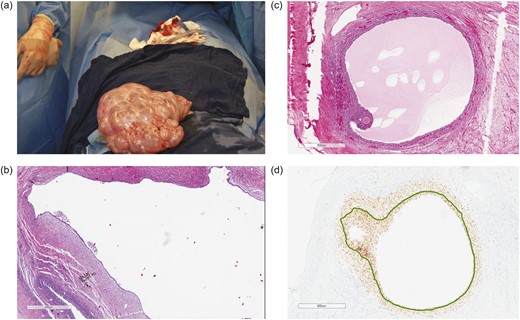
Operative findings of massively enlarged ovaries. (a) Left ovary and fallopian tube at surgery. The left ovary weighed 3219 g (volume 23.0 × 17.0 × 8.5 cm3) and contained multiple benign follicular cysts with clear serous fluid. The right ovary was similar in appearance but was smaller. (b) Hematoxylin and eosin stain of a serous cystadenofibroma (PI by Ki-67 stain, 1.02 ± 0.80%) adjacent to a follicular cyst (PI, 2.42 ± 1.90%). (c) Hematoxylin and eosin stain of a Graafian follicle. (d) PI of granulosa cells within the same Graafian follicle (PI, 99%).
Discussion
The present case report describes a woman with PCOS, hyperandrogenism, and severe insulin resistance from metabolic syndrome in whom granulosa cell proliferation persisted within small antral follicles despite the absence of gonadotropin-dependent ovarian function due to long-term GnRH analogue therapy. The PI of granulosa cells in the small antral follicles of our patient with PCOS receiving GnRH analogue therapy resembled that of granulosa cells in comparable follicles (PI, 9 ± 5% SD) of nonobese women with PCOS and gonadotropin-dependent ovarian function (4); moreover, the granulosa cell PI in the large Graafian follicle approached 100%, consistent with the granulosa cell number determining follicle size in women (5). Such granulosa cell proliferation in the antral follicles was unlikely due to paracrine interactions from coexisting ovarian pathologies because the PI of granulosa cells exceeded that of cells in serous cystadenofibromas of our patient and of other women (3% to 15%) (6, 7) and in adjacent follicular cysts.
It also is unlikely that hypothalamo-pituitary function promoted follicle growth in this patient with PCOS. More than 2 years of successful GnRH analogue therapy should have suppressed gonadotropin-dependent folliculogenesis beyond the 6-month interval required in women for primordial follicle activation and antral follicle growth (8). Furthermore, although sex steroids have been linked with PCOS and ovarian cystadenoma development (9, 10), as has androgen with granulosa cell proliferation and preantral follicle growth (11), long-term GnRH analogue therapy administered to successfully suppress ovarian steroidogenesis in our patient with PCOS eliminated hyperandrogenism as a possible granulosa cell mitogen (2), although estrogen produced by extragonadal aromatase due to the patient’s high BMI likely accounted for her weakly proliferative endometrium (12).
Instead, several lines of evidence suggest that insulin served as a granulosa cell mitogen in our patient with PCOS receiving GnRH analogue therapy, as pituitary desensitization to GnRH does not improve hyperinsulinemia from insulin resistance despite reducing ovarian hyperandrogenism (13). First, hyperinsulinemia from insulin resistance in PCOS results from a postbinding defect in insulin receptor signaling due to increased receptor/insulin receptor substrate-1 serine phosphorylation that selectively impairs the metabolic (PI-3K) but not the mitogenic (MAPK-ERK1/2) pathway in target tissues, including the ovary (12). Second, insulin and IGF receptors are present in human granulosa cells (11), and hyperinsulinemia from insulin resistance accompanies ovarian enlargement in girls despite prepubertal or low gonadotropin levels (14). Third, animal studies confirm that insulin promotes the primordial-to-primary follicle transition, with insulin and IGFs stimulating granulosa cell proliferation through the MAPK signaling pathway to enhance early folliculogenesis (3, 15). Therefore, it is interesting to speculate that in our patient with PCOS and hyperandrogenism and insulin resistance from metabolic syndrome who received long-term GnRH analogue therapy, hyperinsulinemia acted as a granulosa cell mitogen in the absence of gonadotropin-dependent ovarian function.
Our case report concurs with the previous report of a woman with hyperthecosis from type B insulin resistance (16) in whom GnRH analogue therapy successfully suppressed ovarian hyperandrogenism without reversing insulin resistance, implying the importance of gonadotropin-insulin interactions in governing ovarian androgen production in both women. Our case report, however, diverges from this earlier report by raising the additional possibility that severe hyperinsulinism can act alone through its own or hybrid insulin/IGF-I receptors to promote granulosa cell mitogenesis in the absence of gonadotropin-dependent ovarian function.
Nevertheless, primordial follicle recruitment through antral follicle growth requires complex interactions between reproductive and metabolic functions, as well as intraovarian paracrine signals to coordinate granulosa cell proliferation. Although insulin can act alone or as a cogonadotropin to stimulate granulosa cell function, local actions of IGFs and other growth factors may also have contributed to the follicular development observed in our patient. Theoretically, follicle development in this patient could also have been due to adiposity-enhanced serum IGF-I bioactivity through suppressed IGF-binding protein production or to altered actions of oocyte-secreted factors (i.e., growth differentiation factor-9) on IGF-I-stimulated granulosa cells and/or other perturbed oocyte-granulosa cell paracrine interactions (11).
Abbreviations:
- BMI
body mass index
- nl
normal
- PCOS
polycystic ovary syndrome
- PI
proliferation index
- T
testosterone
Acknowledgments
Financial Support: This study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, under award P50HD071836 (to D.A.D.). Additional funding was provided by the Santa Monica Bay Woman’s Club (to D.A.D.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.



