-
PDF
- Split View
-
Views
-
Cite
Cite
Dongyun Zhang, Sarah S R Kim, Daniel F Kelly, Sylvia L Asa, Masoud Movassaghi, Sergey Mareninov, William H Yong, Timothy F Cloughesy, Fausto J Rodriguez, Paul McKeever, Jiang Qian, Jian Yi Li, Qinwen Mao, Kathy L Newell, Richard M Green, Cynthia T Welsh, Zhenggang Xiong, Anthony P Heaney, Somatostatin Receptor Ligand Therapy—A Potential Therapy for Neurocytoma, The Journal of Clinical Endocrinology & Metabolism, Volume 104, Issue 6, June 2019, Pages 2395–2402, https://doi.org/10.1210/jc.2018-02419
Close - Share Icon Share
Abstract
Neurocytoma (NC) is a rare, low-grade tumor of the central nervous system, with a 10-year survival rate of 90% and local control rate of 74%. However, 25% of NCs will be atypical, with an elevated Ki-67 labeling index >2%, and will exhibit a more aggressive course, with a high propensity for local recurrence and/or craniospinal dissemination. Although no standard treatment regimen exists for these atypical cases, adjuvant stereotactic or conventional radiotherapy and/or chemotherapy have been typically offered but have yielded inconsistent results.
We have described the case of a patient with a vasopressin-secreting atypical NC of the sellar and cavernous sinus region. After subtotal resection via endoscopic transsphenoidal surgery, the residual tumor showed increased fluorodeoxyglucose uptake and high somatostatin receptor (SSTR) expression on a 68Ga-DOTA-TATE positron emission tomography/CT scan. Somatostatin receptor ligand (SRL) therapy with lanreotide (120 mg every 28 days) was initiated. Four years later, the residual tumor was stable with decreased fluorodeoxyglucose tumor uptake. Immunocytochemical SSTR2 and SSTR5 expression >80% was further confirmed in a series of NC tissues.
To the best of our knowledge, we have described the first use of SRL therapy for an atypical NC. Our results support consideration of adjuvant SRL therapy for NC refractory to surgical removal. Our findings further raise the possibility of SSTR-directed peptide receptor radionuclide therapy as NC therapy.
Neurocytoma (NC) is a rare low-grade central nervous system (CNS) tumor (World Health Organization grade II), which accounts for 0.1% to 0.5% of all intracranial tumors (1). Usually occurring in adolescents and young adults, with 70% of patients presenting between the second and fourth decade, NCs occur with equal incidence in both sexes (2). NCs appear as well-circumscribed and lobulated CNS masses and are of two types. Central NCs occur within the ventricles, most commonly the lateral ventricle in close proximity to the foramen of Monro. They can attach to the septum pellucidum, causing obstructive hydrocephalus, with symptoms of headache, nausea, and vomiting (2). Also, ~20% will extend into the third and, in rare cases, the fourth ventricles (3). Extraventricular NCs (EVNs) most commonly develop in the cerebral hemispheres, especially the frontal lobe (29.3%), followed by the spinal cord (12.7%), temporal lobe (11.5%), parietal lobe (8.3%), and brainstem (6.4%) (4). EVNs of the sellar region are very uncommon, cause visual impairment due to optic chiasm compression, and hypertensive intracranial syndromes (5–12). Sellar EVNs have often been misdiagnosed as pituitary tumor, craniopharyngioma, or meningioma at presentation (9). Histopathologically, NCs will be characterized by uniform small cells with round to oval nuclei and scant cytoplasm resembling perinuclear halos and neuronal differentiation as evidenced by immunoreactivity for neuronal nuclear antigen and neurofilament (13). We have described a young white man who had presented with profound hyponatremia, normal pituitary endocrine function, and a sellar/suprasellar mass. After subtotal endoscopic transsphenoidal tumor resection, histopathological analysis showed he had a vasopressin-secreting sellar NC. Postoperative 18F-fluorodeoxyglucose (FDG) and 68Ga-DOTA-TATE positron emission tomography (PET)/CT scans showed positive findings with high uptake of radiolabeled somatostatin in the substantial residual tumor. Therefore, he was treated with monthly injections of a long-acting somatostatin receptor ligand (SRL). The residual tumor was radiologically stable at the 4-year follow-up examination. He was largely eunatremic with a judicious fluid balance, fludrocortisone, and increased salt intake. Using immunocytochemistry, we further demonstrated somatostatin receptor (SSTR) 2 and SSTR5 expression in 88% (22 of 25) and 91% (21 of 23) of NC tissues. Our findings of tumor stability with SRL therapy and widespread SSTR expression in NCs suggest that SRL therapy could be a therapeutic option for patients with NC.
Materials and Methods
Histopathological analysis
Formalin-fixed, paraffin-embedded sections from 25 NCs (24 central and 1 extraventricular) were stained with hematoxylin and eosin to confirm NC tumor tissue was present on the samples. Specific antibodies for SSTR2 (anti-SSTR2 antibody; HPA007264; Sigma-Aldrich) and SSTR5 (anti-SSTR2 antibody; SAB2900581; Sigma-Aldrich) were incubated with 5-μm tumor sections. Staining signals were scored using the histoscore (range, 0 to 300), representing the product of staining intensity (absent, 0 to 100; weak, 100 to 200; intermediate, 200 to 300; strong, >300) and the percentage of tumor cell staining (range, 0 to 100). All immunocytochemistry scoring was completed in blinded fashion by our neuropathologist (W.Y).
Copeptin assay
Copeptin was measured by Mayo Medical Laboratories for research purposes only.
Clinical Summary
A previously healthy 17-year-old boy had presented with a history of 10 days of progressive abdominal pain, nausea, and emesis. Laboratory testing revealed profound hyponatremia, with a serum sodium of 104 mmol/L on outside hospital presentation. He denied headache or visual abnormalities, and none was detected. After IV normal saline administration, his serum sodium level had increased to 129 mmol/L over 5 days. After the development of dysarthria, brain MRI was performed and revealed a large sellar/suprasellar mass (45 × 28 × 29 mm) that was causing severe optic chiasmal compression and invading both cavernous sinuses (the left more than the right) with complete encasement of the left internal carotid artery and extending to the superior clivus posteriorly and inferiorly (Fig. 1A–1C). Pituitary function testing demonstrated a normal 8 am serum cortisol [22 µg/L; normal range (NR), 8 to 25 μg/L], prolactin (20 ng/mL; NR, 3 to 30 ng/mL), IGF-1 (269 ng/mL; NR, 207 to 576 ng/mL), and TSH (0.94 µIU/mL; NR, 0.430 to 4.20 µIU/mL), with reduced total testosterone (190 ng/L; NR, 200 to 1100 ng/L), unstimulated LH (2.8 U/L; NR, 0.5 to 10 U/L), and FSH (2.4 U/L; NR, 1.6 to 11.6 U/L), consistent with central hypogonadism (Table 1) 1 week before surgery. At the outside hospital, he was empirically treated with hydrocortisone 10 mg orally three times daily as part of hyponatremia treatment and transferred for endoscopic transsphenoidal resection of the sellar mass. Aggregates of tan-yellow soft tissue fragments were resected and a grade 2 cerebrospinal fluid leak was managed by abdominal fat graft placement. Postoperative imaging revealed an ~90% tumor volume resection with residual tumor present in the bilateral cavernous sinuses, especially on the left, measuring 24 × 9 × 18 mm (transverse by craniocaudal by anteroposterior) and extending along the posterior aspect of the clivus (Fig. 1D–1F). Postoperative pituitary function testing results showed reduced free T4 of 0.74 ng/L (Table 1). He began levothyroxine 75 μg daily.
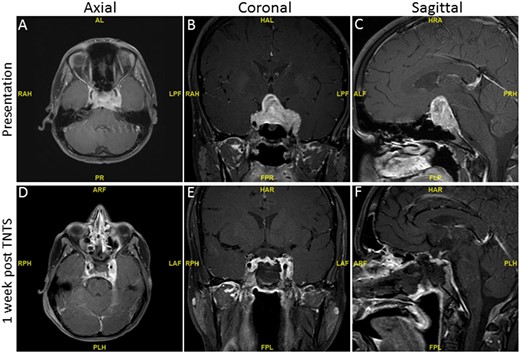
T1-weighted preoperative and postoperative MRI of a large sellar and suprasellar mass with bilateral cavernous extension before and 1 wk after endoscopic transsphenoidal surgery (TNTS). (A, D) Axial view. (B, E) Coronal view. (C, F) Sagittal view.
Pituitary Hormone Levels Before and After Endoscopic Transsphenoidal Surgery
| Test (8 am) . | Reference Range . | Preoperative . | Postoperative . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 Week . | 1 Day . | 2 Days . | 2 Weeks . | 3 Months . | 1 Year . | 2 Years . | 3 Years . | ||
| Serum cortisol | 8–25 µg/L | 22 | HC | 16 | 13 | 10 | |||
| ACTH | 6–59 pg/mL | 49 | 17 | 34 | |||||
| Prolactin | 3–30 ng/mL | 20 | 25 | 11.2 | |||||
| IGF-1 | 207–576 ng/mL | 269 | 100 | ||||||
| TSH | 0.3–4.7 µIU/mL | 0.94 | 1.038 | 0.41 | 1.6 | 1.4 | 1.1 | 0.95 | |
| Free T4 | 0.8–1.6 ng/L | 0.74 | 1.1a | 1 | 1 | 1 | |||
| Total T4 | 4.7–12.4 µg% | 5.6 | |||||||
| LH | 0.5–10 U/L | 2.8 | 3.8 | ||||||
| FSH | 1.6–11.6 U/L | 2.4 | 1.9 | ||||||
| Total testosterone | <1000 ng/L | 190 | 147 | 453b | 343 | ||||
| Free testosterone | 18–111 pg/mL | 52.7 | 53.5 | 43 | |||||
| Test (8 am) . | Reference Range . | Preoperative . | Postoperative . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 Week . | 1 Day . | 2 Days . | 2 Weeks . | 3 Months . | 1 Year . | 2 Years . | 3 Years . | ||
| Serum cortisol | 8–25 µg/L | 22 | HC | 16 | 13 | 10 | |||
| ACTH | 6–59 pg/mL | 49 | 17 | 34 | |||||
| Prolactin | 3–30 ng/mL | 20 | 25 | 11.2 | |||||
| IGF-1 | 207–576 ng/mL | 269 | 100 | ||||||
| TSH | 0.3–4.7 µIU/mL | 0.94 | 1.038 | 0.41 | 1.6 | 1.4 | 1.1 | 0.95 | |
| Free T4 | 0.8–1.6 ng/L | 0.74 | 1.1a | 1 | 1 | 1 | |||
| Total T4 | 4.7–12.4 µg% | 5.6 | |||||||
| LH | 0.5–10 U/L | 2.8 | 3.8 | ||||||
| FSH | 1.6–11.6 U/L | 2.4 | 1.9 | ||||||
| Total testosterone | <1000 ng/L | 190 | 147 | 453b | 343 | ||||
| Free testosterone | 18–111 pg/mL | 52.7 | 53.5 | 43 | |||||
Abbreviation: HC, hydrocortisone replacement.
Thyroid hormone replacement.
Testosterone replacement.
Pituitary Hormone Levels Before and After Endoscopic Transsphenoidal Surgery
| Test (8 am) . | Reference Range . | Preoperative . | Postoperative . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 Week . | 1 Day . | 2 Days . | 2 Weeks . | 3 Months . | 1 Year . | 2 Years . | 3 Years . | ||
| Serum cortisol | 8–25 µg/L | 22 | HC | 16 | 13 | 10 | |||
| ACTH | 6–59 pg/mL | 49 | 17 | 34 | |||||
| Prolactin | 3–30 ng/mL | 20 | 25 | 11.2 | |||||
| IGF-1 | 207–576 ng/mL | 269 | 100 | ||||||
| TSH | 0.3–4.7 µIU/mL | 0.94 | 1.038 | 0.41 | 1.6 | 1.4 | 1.1 | 0.95 | |
| Free T4 | 0.8–1.6 ng/L | 0.74 | 1.1a | 1 | 1 | 1 | |||
| Total T4 | 4.7–12.4 µg% | 5.6 | |||||||
| LH | 0.5–10 U/L | 2.8 | 3.8 | ||||||
| FSH | 1.6–11.6 U/L | 2.4 | 1.9 | ||||||
| Total testosterone | <1000 ng/L | 190 | 147 | 453b | 343 | ||||
| Free testosterone | 18–111 pg/mL | 52.7 | 53.5 | 43 | |||||
| Test (8 am) . | Reference Range . | Preoperative . | Postoperative . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 Week . | 1 Day . | 2 Days . | 2 Weeks . | 3 Months . | 1 Year . | 2 Years . | 3 Years . | ||
| Serum cortisol | 8–25 µg/L | 22 | HC | 16 | 13 | 10 | |||
| ACTH | 6–59 pg/mL | 49 | 17 | 34 | |||||
| Prolactin | 3–30 ng/mL | 20 | 25 | 11.2 | |||||
| IGF-1 | 207–576 ng/mL | 269 | 100 | ||||||
| TSH | 0.3–4.7 µIU/mL | 0.94 | 1.038 | 0.41 | 1.6 | 1.4 | 1.1 | 0.95 | |
| Free T4 | 0.8–1.6 ng/L | 0.74 | 1.1a | 1 | 1 | 1 | |||
| Total T4 | 4.7–12.4 µg% | 5.6 | |||||||
| LH | 0.5–10 U/L | 2.8 | 3.8 | ||||||
| FSH | 1.6–11.6 U/L | 2.4 | 1.9 | ||||||
| Total testosterone | <1000 ng/L | 190 | 147 | 453b | 343 | ||||
| Free testosterone | 18–111 pg/mL | 52.7 | 53.5 | 43 | |||||
Abbreviation: HC, hydrocortisone replacement.
Thyroid hormone replacement.
Testosterone replacement.
Histopathological hematoxylin and eosin staining revealed sheets and vague nests of neoplastic cells with abundant, eosinophilic cytoplasm and round to ovoid, monotonous nuclei containing finely granular chromatin and inconspicuous nucleoli. Dense fibrous stroma and areas of prominent calcification were also present, although no necrosis or anaplasia was seen. The tumor cells were immunoreactive for the neuronal differentiation markers [synaptophysin, chromogranin A, CD56 (neural cell adhesion molecule-1), neurofilament, S-100, and neuronal nuclei antigen) but negative for the astrocyte marker GFAP, pituitary transcription factors (Pit-1, SF-1), and hormones (ACTH, GH, prolactin, TSH, FSH, and LH). Immunoreactivity was also noted for thyroid transcription factor-1 and vasopressin, suggesting the tumor had a hypothalamic origin (12). NB84 immunohistochemistry and cytogenetic examination for MYCN amplification and EWSR1 rearrangement were negative, rendering a diagnosis of neuroblastoma or Ewing sarcoma unlikely. The overall Ki-67 labeling index was 3%, with focal regions of 10%. The final diagnosis was believed to be consistent with an atypical vasopressin-producing neurocytoma.
His immediate postoperative period was complicated by further sodium fluctuations. During surgery, the serum sodium level decreased to 125 mmol/L, with a urine-specific gravity of 1.007, consistent with inappropriate antidiuretic hormone [syndrome of inappropriate antidiuretic hormone secretion (SIADH)], which corrected to 128 mmol/L after 3% hypertonic saline. Within hours after surgery, a single oral dose of tolvaptan 15 mg was given, the serum sodium subsequently increased to 144 mmol/L, with a marked increase in urine output. Despite IV dextrose 5%/half normal saline to match his urine output, his sodium further increased rapidly to 159 mmol/L and later to 163 mmol/L, despite two doses of desmopressin 0.1 mg and additional fluid. He subsequently developed an altered mental status, became less verbal, and experienced seizures, for which he was intubated for airway protection and received antiepileptic medication [levetiracetam (Keppra)] and sedation (propofol). Brain MRI demonstrated new increased T2-weighted and fluid-attenuated inversion recovery signal intensity within the caudate, putamen, and pons, consistent with pontine and extrapontine myelinolysis (data not shown). On transfer to our hospital, his serum sodium was ~129 mmol/L. During the subsequent 10-day hospital course, the patient was treated with careful adjustment of fluid combined with fludrocortisone 0.1 mg daily, resulting in eunatremia, which was maintained during an intensive 15-day rehabilitation phase, during which hydrocortisone was tapered and weekly testosterone injections (80 mg IM) were added to assist musculoskeletal recovery. At the 3-month follow-up examination, no endocrine abnormalities were noted after withdrawal of thyroid and androgen replacement, and his sodium balance was maintained with a 1.5- to 2-L fluid restriction combed with fludrocortisone 0.1 mg daily to maintain normal serum sodium. 68Ga-DOTA-TATE PET scans demonstrated increased tumor uptake (Fig. 2).
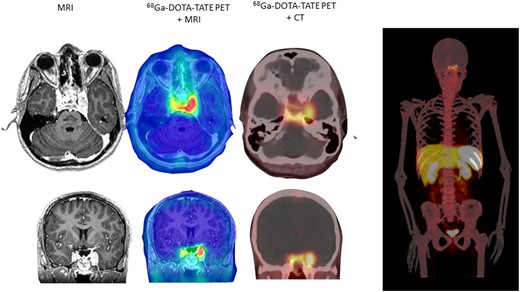
Axial and coronal brain MRI and 68Ga-DOTA-TATE (whole body) image in immediate postoperative period (1 mo postoperatively).
With the tumor SSTR expression, as evidence by the marked positivity of the SSTR-PET 68Ga study and approved use of SRL therapy to control tumor growth in other neuroendocrine tumors, he began taking lanreotide 120 mg every 28 days. Postoperative brain MRI scans at 1, 2, and 7 months and 1.5 and 3 years (Fig. 3A) and functional imaging with FDG-PET scans at 1 month and 1 and 3 years (Fig. 3B) showed stable residual tumor (11 × 9 mm), with virtual disappearance of the T2-weighted and plain signal intensities. Because the patient required ongoing fluid restriction and medication (fludrocortisone and salt tablets) to maintain eunatremia, we reasoned that the tumor remnant was still secreting excess vasopressin. Because vasopressin is quickly sequestered by platelets and unstable in plasma, we measured copeptin, a stable peptide stoichiometrically produced with arginine vasopressin (AVP) from prepro-vasopressin (14). At 1 month postoperatively, the plasma copeptin level was 66.3 (NR, <12.0 pmol/L; 95% confidence interval, 9.8 to 14.3). It had increased to 78.9 pmol/L at 3 months postoperatively and then decreased to 57.5 pmol/L after lanreotide treatment. It has remained elevated but stable for 3.5 years (55 pmol/L at the time of writing; Fig. 4A). Given the high SRL radioligand uptake noted in the index case on SSTR-PET imaging, we used specific antibodies to evaluate immunocytochemical NC SSTR2 and SSTR5 expression in additional NC samples. Using 5-μm tissue sections, we demonstrated strong SSTR2 expression in 2 (8%), moderate expression in 6 (24%), and weak SSTR2 expression in 14 (56%), for a total of 22 of 25 (88%) exhibiting SSTR2 expression. In 23 of 25 NC samples also studied for SSTR5 expression, 21 (91%) exhibited strong SSTR5 immunoreactivity, including 3 NC tissue samples that exhibited strong expression for both SSTR2 and SSTR5. The remaining two NC tissues were negative for SSTR5 expression.
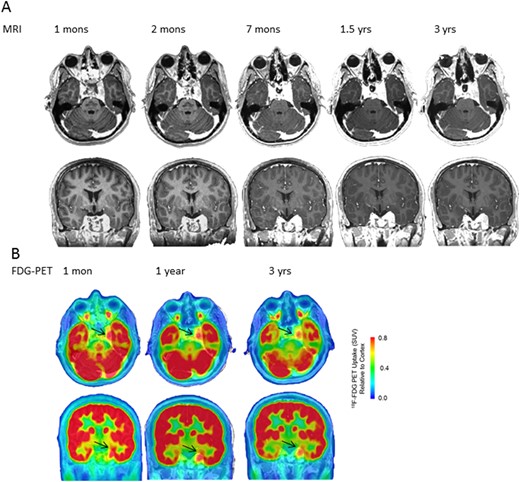
Serial (A) axial and coronal MRI and (B) 18F-FDG-PET/CT images at various postoperative intervals.
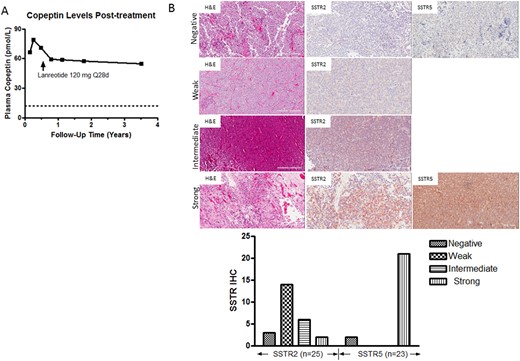
(A) Plasma copeptin levels at 1 mo postoperatively and at intervals during SRL therapy. (B) Representative hematoxylin and eosin (H&E) SSTR2 and SSTR5 immunostaining results (original magnification ×20) in a series of NCs. Immunostaining scores were quantitated using the histoscore (range, 0 to 300), representing the product of staining intensity (absent, 0 to 100; weak, 100 to 200; intermediate, 200 to 300; strong, >300) and percentage of tumor cell staining (range, 0 to 100).
Discussion
As noted in our introduction, NCs are rare tumors, and those in the hypothalamic and sellar region are especially rare. AVP, also known as antidiuretic hormone (ADH), is a member of the highly conserved vasopressin/oxytocin superfamily. The human AVP gene is present in a gene cluster with oxytocin on chromosome 20 spanning ~14 kb. AVP is synthesized as a 164-aa preproprotein, called prepro-vasopressin, in the magnocellular neurons of the hypothalamic paraventricular and supraoptic nuclei (15). Further post-translational processing in neurohypophyseal axons by dipeptidases generates an N-terminus 9-aa AVP, a center neurophysin-II (76 aa), and a C-terminal glycosylated copeptin (39 aa) (16). AVP release from the posterior pituitary into the circulation is stimulated by hypothalamic neuron excitation during high plasma osmolality, a reduced plasma volume, nausea, vomiting, stress, hypoxia, and/or exercise (15). Vasopressin activates four G-protein–coupled receptors, and its action on AVPR2, localized at the basolateral membrane of the renal distal convoluted tubules and collecting ducts, increases cAMP to activate protein kinase A. The latter regulates aquaporin-2 phosphorylation and trafficking to the apical membrane to increase water reabsorption (15). Excessive vasopressin secretion or SIADH can be seen in the setting of head injury, a variety of brain neoplasms, and chest infection and after ingestion of drugs such as carbamazepine, selective serotonin reuptake inhibitors, and various thiazine antipsychotic agents (17). It can also occur as part of a paraneoplastic syndrome with lung tumors (18). Management of acute SIADH includes fluid restriction, hypertonic saline, urea, demeclocycline, and/or vasopressin receptor antagonists (IV conivaptan; oral tolvaptan). A critical aspect of acute SIADH management is the sodium correction rate, which should be between 10 and 12 mmol/L/d to prevent myelin injury (19). The increase in serum sodium observed in our patient after a single dose of tolvaptan was especially rapid and might, in part, have been related to the unusual situation of tumor-derived overproduction of ADH. The present case also highlights that after vasopressin receptor antagonist administration, desmopressin administration will be largely ineffective, and central line placement with aggressive fluid replacement to match urine output will be paramount. In the present case, the patient made a full neurologic recovery despite extensive MRI-confirmed central and extrapontine myelinolysis. Management of chronic SIADH, as in our patient, requires careful monitoring, limiting fluid intake and output, and increased sodium intake. Concomitant administration of fludrocortisone, such as used our patient, to retain sodium or urea to cause osmotic diuresis or loop diuretic therapy to clear free water can also be used. Chronic demeclocycline can also be used; however, given the photosensitivity that can result, this was not a suitable agent for our patient. Chronic tolvaptan use has not been approved but can also be considered. Given his previous experience with the rapid sodium increase, our patient has remained reluctant to consider this therapy.
Both histopathological and functional imaging methods have been investigated to better understand the biological behavior of NCs and guide management decisions but, by and large, have yielded inconsistent findings (20, 21). Some histopathological studies have reported that NCs exhibiting a mean Ki-67 labeling index (LI) of 2.8% are atypical (22) and that a Ki-67 LI >4% and >10% can predict the recurrence of central NCs (21) and EVNs (23), respectively. However, other studies have reported early recurrence of NCs with a Ki-67 LI cutoff >2% (24). Attempts have also been made to correlate FDG-PET measurements such as the minimum apparent diffusion coefficient and/or maximum standardized uptake volume with histopathological indexes. For example, Sakamoto et al. (25) demonstrated a correlation between the maximum standardized uptake volume and Ki-67 LI and concluded that higher FDG uptake was associated with an increased proliferation index (Ki-67 LI >2%) and atypical histological features. Based on these observations, our patient’s overall Ki-67 LI of 3% with focal areas of 10% and positive FDG-PET uptake would be in keeping with a potential for regrowth if left untreated.
Surgical resection of NCs has been the reference standard for therapy, with adjuvant stereotactic radiosurgery or conventional radiotherapy and, occasionally, chemotherapy offered if complete surgical resection is not attainable (3). Somatostatin (SST), derived from a 92-aa precursor, is a cyclic neuropeptide produced by neurons and various endocrine and immune cells. Found as two different biological active forms (SST-14 and SST-28), SST acts via five G-protein–coupled receptors to mediate pleiotropic actions, including inhibition of tumor-derived hormone secretion and tumor growth. Long-acting SRL analogs are extremely effective in controlling excess hormone secretion and inducing tumor shrinkage in various pituitary and pancreatic neuroendocrine tumors (26). Additionally, SSTR-based imaging using radiolabeled SST analogs, such as 111Indium (octreoscan) and, more recently, 68Gallium (NETSPOT; Advanced Accelerator Applications, Novartis) have been routinely used to diagnose, localize, and stage neuroendocrine tumors and some intracranial tumors (27, 28). To the best of our knowledge, the present report is the first to demonstrate neurocytoma uptake of 68Ga-DOTA-TATE, although two previous patients had undergone 111Indium octreoscan scanning (10, 28). Our observed partial tumor response with reduced FDG uptake and copeptin levels after SRL therapy indicated biological activity of NC tumor SSTR. Additionally, our demonstration of widespread SSTR2 expression (88%) and SSTR5 expression (91%) indicated that the use of SRL therapy might be an option for NCs not controlled by standard surgical resection. In support of our findings, SRL-targeted peptide receptor radionuclide therapy with 177Lu-DOTA-TATE was reported to improve the clinical symptoms of headache and visual impairment in one patient with an extraventricular NC, although the tumor size did not change (10). These findings indicate that SSTR-targeted therapies, both cold SRLs and/or SRL-based peptide receptor radionuclide therapy, could be options for NC therapy. Although ectopic vasopressin production is not infrequently encountered in various pulmonary and CNS tumors (29, 30), hypothalamic NCs secreting vasopressin are extremely rare (11, 12).
In conclusion, we have reported the case of an ADH-secreting NC causing severe and, likely, chronic hyponatremia, for which, after subtotal surgical resection, SRL therapy suppressed copeptin levels and was associated with tumor stability for 3.5 years. Longer term follow-up is required to determine the full contribution of SRL therapy to this tumor’s ultimate outcome; however, in the absence of a randomized clinical trial of such a rare disorder, we suggest SRL therapy should be considered in the setting of residual tumor because the risks might outweigh the benefits of other more established therapies, such as radiation treatment.
Abbreviations:
- ADH
antidiuretic hormone
- AVP
arginine vasopressin
- CNS
central nervous system
- EVN
extraventricular neurocytoma
- FDG
18F-fluorodeoxyglucose
- LI
labeling index
- NC
neurocytoma
- NR
normal range
- PET
positron emission tomography
- SIADH
syndrome of inappropriate antidiuretic hormone secretion
- SRL
somatostatin receptor ligand
- SST
somatostatin
- SSTR
somatostatin receptor
Acknowledgments
We are grateful for the NC tissues provided by Marcela White of the Brain Tumor Foundation of Canada, Dr. Brenda Y. Hernandez of the Hawaii Surveillance, Epidemiology, and End Results Program Residual Tissue Repository, and Mark W. Youngblood of Department of Neurosurgery, Yale School of Medicine, and to Mayo Clinic Research Laboratories for the copeptin measurements.
Financial Support: This work was supported by the Warley Trust Foundation (to A.P.H.).
Disclosure Summary: The authors have nothing to disclose.



