-
PDF
- Split View
-
Views
-
Cite
Cite
Sonja Püttgen, Gidon J Bönhof, Alexander Strom, Karsten Müssig, Julia Szendroedi, Michael Roden, Dan Ziegler, Augmented Corneal Nerve Fiber Branching in Painful Compared With Painless Diabetic Neuropathy, The Journal of Clinical Endocrinology & Metabolism, Volume 104, Issue 12, December 2019, Pages 6220–6228, https://doi.org/10.1210/jc.2019-01072
Close - Share Icon Share
Abstract
The factors that determine the development of diabetic sensorimotor polyneuropathy (DSPN) as a painful or painless entity are unknown.
We hypothesized that corneal nerve pathology could be more pronounced in painful DSPN, indicating predominant small nerve fiber damage.
In this cross-sectional study, we assessed 53 patients with painful DSPN, 63 with painless DSPN, and 46 glucose-tolerant volunteers by corneal confocal microscopy (CCM), nerve conduction (NC), and quantitative sensory testing. DSPN was diagnosed according to modified Toronto Consensus criteria. A cutoff at 4 points on the 11-point rating scale was used to differentiate between painful and painless DSPN.
After adjustment for age, sex, body mass index, and smoking, corneal nerve fiber density, corneal nerve fiber length, and corneal nerve branch density (CNBD) were reduced in both DSPN types compared with the control group (P < 0.05). Only CNBD differed between the groups; it was greater in patients with painful DSPN compared with those with painless DSPN [55.8 (SD, 29.9) vs 43.8 (SD, 28.3) branches/mm2; P < 0.05]. Several CCM measures were associated with NC and cold perception threshold in patients with painless DSPN (P < 0.05) but not those with painful DSPN.
Despite a similarly pronounced peripheral nerve dysfunction and corneal nerve fiber loss in patients with painful and painless DSPN, corneal nerve branching was enhanced in those with painful DSPN, pointing to some susceptibility of corneal nerve fibers toward regeneration in this entity, albeit possibly not to a sufficient degree.
Although diabetic sensorimotor polyneuropathy (DSPN) affects ∼30% of patients with diabetes, it remains frequently undiagnosed and undertreated (1, 2). DSPN manifests mainly as a painless entity that may culminate in foot ulceration and a painful variant with neuropathic pain as a hallmark (3). Both entities contribute to increased morbidity, high socioeconomic burden, and reduced quality of life (4, 5). Painful symptoms are encountered in ∼13% to 26% of patients with diabetes (4, 6). On the other hand, up to 50% of diabetic peripheral neuropathies may be asymptomatic, exposing patients to injuries to their insensate feet (7).
Several previous studies attempted to identify distinctive aspects and patterns characterizing painful DSPN compared with painless DSPN, mainly focusing on better understanding the mechanisms of neuropathic pain in DSPN, determining risk markers and factors for the development of pain, or selecting patients who may better respond to analgesic treatments (3). However, although small nerve fiber damage is considered a prerequisite for the presence of neuropathic pain in diabetes, only very few patients actually have pure small fiber neuropathy (8), casting doubt on the exclusive or prominent involvement of small fibers in painful DSPN. A possible contributor is female sex, which was identified in a recent study as the only risk factor for painful DSPN (8). We recently reported that in patients with type 2 diabetes, painful and painless DSPN were associated with a higher and lower body mass index (BMI), respectively (1). Because the presence and severity of neuropathic pain are associated with the severity of DSPN itself (9), it is also conceivable that painful DSPN merely represents the more severe variant of the two entities. Thus, why some individuals with DSPN develop neuropathic pain while others develop the painless entity remains obscure.
In recent years, in vivo corneal confocal microscopy (CCM) has emerged as a noninvasive technique to investigate corneal nerve fibers preferentially in conditions such as DSPN and small fiber neuropathy (10–13), as well as healthy individuals (14). In patients with diabetes, CCM measures such as corneal nerve fiber density (CNFD), corneal nerve fiber length (CNFL), and corneal nerve branch density (CNBD) showed a progressive reduction with increasing severity of DSPN (11), and an early corneal nerve fiber loss was demonstrated in patients recently diagnosed with type 2 diabetes (12). Three studies from one group involving relatively small samples used CCM to compare the extent of corneal nerve fiber loss between painful and painless DSPN (11, 15, 16). These reports had contradictory results, with either no differences in standard CCM measures between the two entities (15), solely lower CNFD but similar CNFL and CNBD (16), or solely lower CNFL but similar CNFD and CNBD (11) in patients with painful compared with those with painless DSPN. Apart from these inconsistent studies, there are no published data using CCM as a potential discriminatory tool between the two entities, to our knowledge. In the present cross-sectional study, we aimed to determine whether painful DSPN may be characterized by more pronounced corneal nerve pathology indicating predominant small nerve fiber damage when compared with the painless entity.
Materials and Methods
Study population
This cross-sectional study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of Heinrich Heine University, Düsseldorf, Germany. After signing written informed consent, 116 patients with diabetes participating in the Probing the Role of Sodium Channels in Painful Neuropathy (PROPANE; ClinicalTrials.gov no. NCT02243475) study and 46 glucose-tolerant volunteers from the baseline cohort of the prospective German Diabetes Study (ClinicalTrials.gov no. NCT01055093) were studied. The study design and cohort profile of the German Diabetes Study have been published elsewhere (17). Inclusion criteria for entry in the PROPANE study for the present patient cohort were age ≥18 years, type 1 or type 2 diabetes according to the American Diabetes Association criteria (18), and diagnosis of sensory, sensorimotor, and/or small fiber DSPN as possible, probable, or confirmed according to the Toronto Consensus criteria (19, 20). Exclusion criteria were other causes for neuropathy such as drugs known to cause neuropathy, alcohol abuse (>5 IU/d), hypothyroidism, vitamin B12 deficiency, monoclonal gammopathy, and concomitant diseases that might interfere with the patients’ ability to complete questionnaires.
Patients with diabetes were allocated to two groups on the basis of the diagnosis of painful DSPN (DSPN+p; n = 53) or painless DSPN (DSPN-p; n = 63). The presence of pain in the distal lower limbs lasting ≥1 year with a pain intensity ≥4 (24-hour average or maximum) on an 11-point numerical rating scale (NRS) in the absence of analgesic treatment, or according to the medical history (recall and/or records) before analgesic treatment, was used to define DSPN+p (20). Patients with DSPN-p reported a pain intensity on the 24-hour average NRS of 0, except for 10 individuals who had an NRS scores ≤2 without analgesic treatment.
Peripheral nerve function
Neurologic examination was performed using the Neuropathy Disability Score (21), whereas neuropathic symptoms were assessed by the Neuropathy Symptom Score (NSS) (21) and neuropathic pain by the 11-point NRS separately for average and maximum pain over 24 hours. Nerve conduction studies and quantitative sensory testing (QST) were performed as previously described (12). In brief, sensory nerve conduction velocity (NCV) was measured in the median, ulnar, and sural nerves; and the sensory nerve action potential (SNAP) was determined in the sural nerve, whereas motor NCV was measured in the peroneal nerve, all at a skin temperature of 33°C to 34°C using surface electrodes (Nicolet VikingQuest; Natus Medical, San Carlos, CA). Vibration perception thresholds (VPTs) were measured at the second metacarpal bone and medial malleolus using the method of limits (Vibrameter; SBMEDIC Electronics, Solna, Sweden). Thermal detection thresholds (TDTs) were measured using the method of limits for warm and cold stimuli at the thenar eminence and the dorsum of the foot (TSA-II NeuroSensory Analyzer; Medoc, Ramat Yishai, Israel).
Corneal confocal microscopy
In vivo CCM was performed using a Heidelberg Retina Tomograph III with the Rostock Cornea Module (Heidelberg Engineering, Heidelberg, Germany), according to the protocol of Tavakoli et al. (22). The distance from the cornea to the microscope was kept constant by a sterile single-use contact element (TomoCap; Heidelberg Engineering). First, the eyes were anesthetized by instilling Proparakain POS 0.5% eye drops (Ursapharm, Saarbrücken, Germany). Then a thin lubricant layer of Vidisic gel (refractive index, 1.35; Bausch & Lomb/Dr. Mann Pharma, Berlin, Germany) was instilled as a coupling agent to the sterile TomoCap to avoid drying of the eyes. Images of the sub-basal nerve plexus were acquired using section mode, which has a resolution of 384 × 384 pixels and a field of view of 0.16 mm2 from the right eye. In subjects with history of, for example, corneal scars or inflammation in one eye, the nonaffected eye was examined. Six representative images of sufficient quality were selected in a masked fashion for analysis. Selection criteria were good contrast of nerves vs background; proper focus of the Bowman’s layer; no motion artifacts, scars, or pressure lines; and overlapping image area not >20%. Ocular surface examination including lid margins, cornea, and conjunctiva was performed to exclude eyes with dry-eye signs and symptoms. Subjects wearing contact lenses were excluded.
Algorithm-based automatic analysis was performed using ACCmetrics (23) and manual morphometric analysis using purpose-written, proprietary software CCMetrics (both from M.A. Dabbah, Imaging Science, University of Manchester, Manchester, UK) as previously published (24). The following CCM parameters were determined using both techniques: CNFL, defined as the total length of all nerve fibers per frame (mm/mm2); CNFD, defined as the number of main nerve fibers per frame (no./mm2); CNBD, defined as the number of branch points on main nerves (no./mm2), and corneal nerve fiber total branch density (CTBD), defined as the total number of all branch points (no./mm2). The tortuosity coefficient was calculated using CCMetrics only. Main nerve fibers were defined as fibers taking up >50% of the frame length (23, 24).
Statistical analysis
Categorical data are expressed as percentages of participants. Continuous data are expressed as mean ± SD. Categorical variables were compared using the χ2 test. For normally distributed data, parametric tests (i.e., Student t test or Pearson correlation) were used; otherwise, nonparametric tests (i.e., Mann-Whitney U test or Spearman rank correlation) were applied. All group comparisons were adjusted for sex, age, BMI, and smoking, except for comparisons between DSPN+p and DSPN-p, which were additionally adjusted for HbA1c, diabetes type, and diabetes duration. The fifth percentiles were computed in the control group to determine the lower limits of normal for each CCM measure, except for the manually analyzed tortuosity coefficient. The level of significance was set at α = 0.05. All analyses were performed using SPSS, version 22.0 (IBM, Armonk, NY), and all graphs were generated using GraphPad Prism, version 6.04 (GraphPad Software, La Jolla, CA).
Results
The demographic and clinical characteristics of the groups are listed in Table 1. Compared with control subjects, patients with DSPN+p and those with DSPN-p had higher BMI, HbA1c, high-sensitivity C-reactive protein level, NSS score, Neuropathy Disability Score, malleolar VPT, and warm TDT. They also had lower total and low-density lipoprotein cholesterol levels; lower median, ulnar, and sural sensory NCV; lower peroneal motor NCV; lower sural SNAP; and lower cold TDT (P < 0.05). In addition, BMI, NSS, average and maximum 24-hour NRS pain, and malleolar VPT values were higher, whereas the percentage of men and high-density lipoprotein cholesterol levels were lower in the DSPN+p group than in the DSPN-p group (P < 0.05). No other differences between the groups were noted.
Demographic, Clinical, and Neurophysiological Data of the Control and Patient Groups in This Study
| . | Control . | DSPN+p . | DSPN-p . |
|---|---|---|---|
| No. of patients | 46 | 53 | 63 |
| Male sex, % | 80.4 | 73.6 | 90.5a |
| Age, y | 66.0 ± 5.2 | 67.2 ± 8.5 | 67.4 ± 9.5 |
| BMI, kg/m2 | 27.3 ± 3.9 | 32.4 ± 5.8b | 29.3 ± 5.4a,b |
| Current smokers, % | 21.7 | 24.5 | 17.5 |
| Type 2 diabetes, % | — | 88.7 | 69.8 |
| Diabetes duration, y | — | 15.6 ± 10.9 | 19.6 ± 15.1 |
| HbA1c, % | 5.44 ± 0.23 | 7.48 ± 1.43b | 7.33 ± 1.14b |
| HbA1c, mmol/mol | 36.0 ± 2.5 | 58.3 ± 15.6b | 56.5 ± 12.5b |
| Cholesterol, mmol/Lc | 5.85 ± 0.61 | 4.98 ± 1.51b | 5.12 ± 1.30b |
| HDL cholesterol, mmol/Lc | 1.55 ± 0.41 | 1.38 ± 0.43 | 1.58 ± 0.54a |
| LDL cholesterol, mmol/Lc | 3.86 ± 0.52 | 3.01 ± 1.04b | 3.06 ± 1.10b |
| hsCRP, nmol/Lc | 16.0 ± 10.9 | 31.5 ± 24.9b | 26.2 ± 25.0b |
| Neuropathy symptom scorec | 0.50 ± 1.51 | 7.92 ± 1.43b | 5.33 ± 2.60a,b |
| Neuropathy disability scorec | 1.33 ± 1.86 | 6.47 ± 2.52b | 6.11 ± 2.86b |
| Average 24-h NRS painc | — | 5.13 ± 2.97 | 0.31 ± 0.74a |
| Maximum 24-h NRS painc | — | 7.24 ± 1.99 | 0.50 ± 1.07a |
| Median sensory NCV, m/secc | 49.49 ± 6.31 | 46.9 ± 6.8b | 46.4 ± 8.4b |
| Ulnar sensory NCV, m/secc | 49.00 ± 5.22 | 45.0 ± 8.1b | 46.3 ± 9.6b |
| Peroneal motor NCV, m/secc | 43.54 ± 5.07 | 35.8 ± 7.8b | 35.5 ± 8.7b |
| Sural sensory NCV, m/secc | 42.41 ± 6.8 | 29.7 ± 7.5b | 29.1 ± 6.8b |
| Sural SNAP, µVc | 9.13 ± 4.63 | 2.40 ± 3.12b | 2.11 ± 2.86b |
| Malleolar VPT, µm† | 3.95 ± 5.19 | 16.5 ± 14.7b | 9.34 ± 9.30a,b |
| Warm TDT foot, °Cc | 39.9 ± 4.8 | 46.9 ± 3.4b | 46.1 ± 4.3b |
| Cold TDT foot, °Cc | 26.04 ± 5.01 | 14.9 ± 11.1b | 16.7 ± 10.6b |
| . | Control . | DSPN+p . | DSPN-p . |
|---|---|---|---|
| No. of patients | 46 | 53 | 63 |
| Male sex, % | 80.4 | 73.6 | 90.5a |
| Age, y | 66.0 ± 5.2 | 67.2 ± 8.5 | 67.4 ± 9.5 |
| BMI, kg/m2 | 27.3 ± 3.9 | 32.4 ± 5.8b | 29.3 ± 5.4a,b |
| Current smokers, % | 21.7 | 24.5 | 17.5 |
| Type 2 diabetes, % | — | 88.7 | 69.8 |
| Diabetes duration, y | — | 15.6 ± 10.9 | 19.6 ± 15.1 |
| HbA1c, % | 5.44 ± 0.23 | 7.48 ± 1.43b | 7.33 ± 1.14b |
| HbA1c, mmol/mol | 36.0 ± 2.5 | 58.3 ± 15.6b | 56.5 ± 12.5b |
| Cholesterol, mmol/Lc | 5.85 ± 0.61 | 4.98 ± 1.51b | 5.12 ± 1.30b |
| HDL cholesterol, mmol/Lc | 1.55 ± 0.41 | 1.38 ± 0.43 | 1.58 ± 0.54a |
| LDL cholesterol, mmol/Lc | 3.86 ± 0.52 | 3.01 ± 1.04b | 3.06 ± 1.10b |
| hsCRP, nmol/Lc | 16.0 ± 10.9 | 31.5 ± 24.9b | 26.2 ± 25.0b |
| Neuropathy symptom scorec | 0.50 ± 1.51 | 7.92 ± 1.43b | 5.33 ± 2.60a,b |
| Neuropathy disability scorec | 1.33 ± 1.86 | 6.47 ± 2.52b | 6.11 ± 2.86b |
| Average 24-h NRS painc | — | 5.13 ± 2.97 | 0.31 ± 0.74a |
| Maximum 24-h NRS painc | — | 7.24 ± 1.99 | 0.50 ± 1.07a |
| Median sensory NCV, m/secc | 49.49 ± 6.31 | 46.9 ± 6.8b | 46.4 ± 8.4b |
| Ulnar sensory NCV, m/secc | 49.00 ± 5.22 | 45.0 ± 8.1b | 46.3 ± 9.6b |
| Peroneal motor NCV, m/secc | 43.54 ± 5.07 | 35.8 ± 7.8b | 35.5 ± 8.7b |
| Sural sensory NCV, m/secc | 42.41 ± 6.8 | 29.7 ± 7.5b | 29.1 ± 6.8b |
| Sural SNAP, µVc | 9.13 ± 4.63 | 2.40 ± 3.12b | 2.11 ± 2.86b |
| Malleolar VPT, µm† | 3.95 ± 5.19 | 16.5 ± 14.7b | 9.34 ± 9.30a,b |
| Warm TDT foot, °Cc | 39.9 ± 4.8 | 46.9 ± 3.4b | 46.1 ± 4.3b |
| Cold TDT foot, °Cc | 26.04 ± 5.01 | 14.9 ± 11.1b | 16.7 ± 10.6b |
Data are reported as mean ± SD unless otherwise indicated.
Abbreviations: HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein.
P < 0.05 vs DSPN+p group.
P < 0.05 vs control group.
Comparisons vs control group were adjusted for age, sex, BMI, and smoking, whereas comparisons between the DSPN-p and DSPN+p groups were adjusted for age, sex, BMI, HbA1c, diabetes type, and diabetes duration.
Demographic, Clinical, and Neurophysiological Data of the Control and Patient Groups in This Study
| . | Control . | DSPN+p . | DSPN-p . |
|---|---|---|---|
| No. of patients | 46 | 53 | 63 |
| Male sex, % | 80.4 | 73.6 | 90.5a |
| Age, y | 66.0 ± 5.2 | 67.2 ± 8.5 | 67.4 ± 9.5 |
| BMI, kg/m2 | 27.3 ± 3.9 | 32.4 ± 5.8b | 29.3 ± 5.4a,b |
| Current smokers, % | 21.7 | 24.5 | 17.5 |
| Type 2 diabetes, % | — | 88.7 | 69.8 |
| Diabetes duration, y | — | 15.6 ± 10.9 | 19.6 ± 15.1 |
| HbA1c, % | 5.44 ± 0.23 | 7.48 ± 1.43b | 7.33 ± 1.14b |
| HbA1c, mmol/mol | 36.0 ± 2.5 | 58.3 ± 15.6b | 56.5 ± 12.5b |
| Cholesterol, mmol/Lc | 5.85 ± 0.61 | 4.98 ± 1.51b | 5.12 ± 1.30b |
| HDL cholesterol, mmol/Lc | 1.55 ± 0.41 | 1.38 ± 0.43 | 1.58 ± 0.54a |
| LDL cholesterol, mmol/Lc | 3.86 ± 0.52 | 3.01 ± 1.04b | 3.06 ± 1.10b |
| hsCRP, nmol/Lc | 16.0 ± 10.9 | 31.5 ± 24.9b | 26.2 ± 25.0b |
| Neuropathy symptom scorec | 0.50 ± 1.51 | 7.92 ± 1.43b | 5.33 ± 2.60a,b |
| Neuropathy disability scorec | 1.33 ± 1.86 | 6.47 ± 2.52b | 6.11 ± 2.86b |
| Average 24-h NRS painc | — | 5.13 ± 2.97 | 0.31 ± 0.74a |
| Maximum 24-h NRS painc | — | 7.24 ± 1.99 | 0.50 ± 1.07a |
| Median sensory NCV, m/secc | 49.49 ± 6.31 | 46.9 ± 6.8b | 46.4 ± 8.4b |
| Ulnar sensory NCV, m/secc | 49.00 ± 5.22 | 45.0 ± 8.1b | 46.3 ± 9.6b |
| Peroneal motor NCV, m/secc | 43.54 ± 5.07 | 35.8 ± 7.8b | 35.5 ± 8.7b |
| Sural sensory NCV, m/secc | 42.41 ± 6.8 | 29.7 ± 7.5b | 29.1 ± 6.8b |
| Sural SNAP, µVc | 9.13 ± 4.63 | 2.40 ± 3.12b | 2.11 ± 2.86b |
| Malleolar VPT, µm† | 3.95 ± 5.19 | 16.5 ± 14.7b | 9.34 ± 9.30a,b |
| Warm TDT foot, °Cc | 39.9 ± 4.8 | 46.9 ± 3.4b | 46.1 ± 4.3b |
| Cold TDT foot, °Cc | 26.04 ± 5.01 | 14.9 ± 11.1b | 16.7 ± 10.6b |
| . | Control . | DSPN+p . | DSPN-p . |
|---|---|---|---|
| No. of patients | 46 | 53 | 63 |
| Male sex, % | 80.4 | 73.6 | 90.5a |
| Age, y | 66.0 ± 5.2 | 67.2 ± 8.5 | 67.4 ± 9.5 |
| BMI, kg/m2 | 27.3 ± 3.9 | 32.4 ± 5.8b | 29.3 ± 5.4a,b |
| Current smokers, % | 21.7 | 24.5 | 17.5 |
| Type 2 diabetes, % | — | 88.7 | 69.8 |
| Diabetes duration, y | — | 15.6 ± 10.9 | 19.6 ± 15.1 |
| HbA1c, % | 5.44 ± 0.23 | 7.48 ± 1.43b | 7.33 ± 1.14b |
| HbA1c, mmol/mol | 36.0 ± 2.5 | 58.3 ± 15.6b | 56.5 ± 12.5b |
| Cholesterol, mmol/Lc | 5.85 ± 0.61 | 4.98 ± 1.51b | 5.12 ± 1.30b |
| HDL cholesterol, mmol/Lc | 1.55 ± 0.41 | 1.38 ± 0.43 | 1.58 ± 0.54a |
| LDL cholesterol, mmol/Lc | 3.86 ± 0.52 | 3.01 ± 1.04b | 3.06 ± 1.10b |
| hsCRP, nmol/Lc | 16.0 ± 10.9 | 31.5 ± 24.9b | 26.2 ± 25.0b |
| Neuropathy symptom scorec | 0.50 ± 1.51 | 7.92 ± 1.43b | 5.33 ± 2.60a,b |
| Neuropathy disability scorec | 1.33 ± 1.86 | 6.47 ± 2.52b | 6.11 ± 2.86b |
| Average 24-h NRS painc | — | 5.13 ± 2.97 | 0.31 ± 0.74a |
| Maximum 24-h NRS painc | — | 7.24 ± 1.99 | 0.50 ± 1.07a |
| Median sensory NCV, m/secc | 49.49 ± 6.31 | 46.9 ± 6.8b | 46.4 ± 8.4b |
| Ulnar sensory NCV, m/secc | 49.00 ± 5.22 | 45.0 ± 8.1b | 46.3 ± 9.6b |
| Peroneal motor NCV, m/secc | 43.54 ± 5.07 | 35.8 ± 7.8b | 35.5 ± 8.7b |
| Sural sensory NCV, m/secc | 42.41 ± 6.8 | 29.7 ± 7.5b | 29.1 ± 6.8b |
| Sural SNAP, µVc | 9.13 ± 4.63 | 2.40 ± 3.12b | 2.11 ± 2.86b |
| Malleolar VPT, µm† | 3.95 ± 5.19 | 16.5 ± 14.7b | 9.34 ± 9.30a,b |
| Warm TDT foot, °Cc | 39.9 ± 4.8 | 46.9 ± 3.4b | 46.1 ± 4.3b |
| Cold TDT foot, °Cc | 26.04 ± 5.01 | 14.9 ± 11.1b | 16.7 ± 10.6b |
Data are reported as mean ± SD unless otherwise indicated.
Abbreviations: HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein.
P < 0.05 vs DSPN+p group.
P < 0.05 vs control group.
Comparisons vs control group were adjusted for age, sex, BMI, and smoking, whereas comparisons between the DSPN-p and DSPN+p groups were adjusted for age, sex, BMI, HbA1c, diabetes type, and diabetes duration.
Table 2 lists the mean values of the CCM parameters in the three groups studied. CNFD and CNFL assessed manually and automatically were lower in the DSPN groups as compared with the control group (P < 0.05). Moreover, CNBD and CTBD measured manually and automatically were lower in the DSPN-p group compared with the control and the DSPN+p groups (P < 0.05). Figure 1 shows representative images of corneal nerve fibers in a control individual with normal CNFD (Fig. 1A) and a DSPN+p patient, showing lower CNBD (Fig. 1B) compared with the control, but markedly higher CNBD in comparison with a patient with DSPN-p (Fig. 1C). The DSPN+p group also had lower manually analyzed CNBD than did the control group (P < 0.05), whereas the manually analyzed tortuosity coefficient was higher in the DSPN groups than in the control group (P < 0.05). No other differences between the groups were observed.
| . | Control . | DSPN+p . | DSPN-p . |
|---|---|---|---|
| mCNFD, no./mm2 | 29.0 ± 6.1 | 23.0 ± 8.01a | 22.2 ± 7.8a |
| aCNFD, no./mm2 | 25.9 ± 6.5 | 19.5 ± 7.9a | 18.6 ± 7.8a |
| mCNFL, mm/mm2 | 22.8 ± 4.9 | 18.6 ± 6.7a | 16.9 ± 6.3a |
| aCNFL, mm/mm2 | 15.1 ± 3.1 | 12.4 ± 4.2a | 11.5 ± 4.0a |
| mCNBD, no./mm2 | 70.5 ± 26.7 | 55. 8 ± 29.9a | 43.8 ± 28.3a,b |
| aCNBD, no./mm2 | 33.2 ± 16.5 | 26.6 ± 14.9 | 21.7 ± 15.9a,b |
| mCTBD, no/mm2 | 96.8 ± 43.6 | 79.0 ± 48.1 | 61.5 ± 39.6a,b |
| aCTBD, no/mm2 | 49.3 ± 23.9 | 41.3 ± 22.0 | 33.7 ± 21.56a,b |
| mTC | 0.15 ± 0.23 | 0.17 ± 0.03a | 0.17 ± 0.03a |
| . | Control . | DSPN+p . | DSPN-p . |
|---|---|---|---|
| mCNFD, no./mm2 | 29.0 ± 6.1 | 23.0 ± 8.01a | 22.2 ± 7.8a |
| aCNFD, no./mm2 | 25.9 ± 6.5 | 19.5 ± 7.9a | 18.6 ± 7.8a |
| mCNFL, mm/mm2 | 22.8 ± 4.9 | 18.6 ± 6.7a | 16.9 ± 6.3a |
| aCNFL, mm/mm2 | 15.1 ± 3.1 | 12.4 ± 4.2a | 11.5 ± 4.0a |
| mCNBD, no./mm2 | 70.5 ± 26.7 | 55. 8 ± 29.9a | 43.8 ± 28.3a,b |
| aCNBD, no./mm2 | 33.2 ± 16.5 | 26.6 ± 14.9 | 21.7 ± 15.9a,b |
| mCTBD, no/mm2 | 96.8 ± 43.6 | 79.0 ± 48.1 | 61.5 ± 39.6a,b |
| aCTBD, no/mm2 | 49.3 ± 23.9 | 41.3 ± 22.0 | 33.7 ± 21.56a,b |
| mTC | 0.15 ± 0.23 | 0.17 ± 0.03a | 0.17 ± 0.03a |
Data are reported as mean ± SD.
Abbreviations: aCNBD, automated analysis of CNDB; aCNFD, automated analysis of CNFD; aCNFL, automated analysis of CNFL; aCTBD, automated analysis of CTBD; mCNBD, manual analysis of CNBD; mCNFD, manual analysis of CNFD; mCNFL, manual analysis of CNFL; mTC, manual analysis of tortuosity coefficient.
P < 0.05 vs control group after adjustment for age, sex, BMI, and smoking.
P < 0.05 vs DSPN+p group after adjustment for age, sex, BMI, HbA1c, diabetes type, and diabetes duration.
| . | Control . | DSPN+p . | DSPN-p . |
|---|---|---|---|
| mCNFD, no./mm2 | 29.0 ± 6.1 | 23.0 ± 8.01a | 22.2 ± 7.8a |
| aCNFD, no./mm2 | 25.9 ± 6.5 | 19.5 ± 7.9a | 18.6 ± 7.8a |
| mCNFL, mm/mm2 | 22.8 ± 4.9 | 18.6 ± 6.7a | 16.9 ± 6.3a |
| aCNFL, mm/mm2 | 15.1 ± 3.1 | 12.4 ± 4.2a | 11.5 ± 4.0a |
| mCNBD, no./mm2 | 70.5 ± 26.7 | 55. 8 ± 29.9a | 43.8 ± 28.3a,b |
| aCNBD, no./mm2 | 33.2 ± 16.5 | 26.6 ± 14.9 | 21.7 ± 15.9a,b |
| mCTBD, no/mm2 | 96.8 ± 43.6 | 79.0 ± 48.1 | 61.5 ± 39.6a,b |
| aCTBD, no/mm2 | 49.3 ± 23.9 | 41.3 ± 22.0 | 33.7 ± 21.56a,b |
| mTC | 0.15 ± 0.23 | 0.17 ± 0.03a | 0.17 ± 0.03a |
| . | Control . | DSPN+p . | DSPN-p . |
|---|---|---|---|
| mCNFD, no./mm2 | 29.0 ± 6.1 | 23.0 ± 8.01a | 22.2 ± 7.8a |
| aCNFD, no./mm2 | 25.9 ± 6.5 | 19.5 ± 7.9a | 18.6 ± 7.8a |
| mCNFL, mm/mm2 | 22.8 ± 4.9 | 18.6 ± 6.7a | 16.9 ± 6.3a |
| aCNFL, mm/mm2 | 15.1 ± 3.1 | 12.4 ± 4.2a | 11.5 ± 4.0a |
| mCNBD, no./mm2 | 70.5 ± 26.7 | 55. 8 ± 29.9a | 43.8 ± 28.3a,b |
| aCNBD, no./mm2 | 33.2 ± 16.5 | 26.6 ± 14.9 | 21.7 ± 15.9a,b |
| mCTBD, no/mm2 | 96.8 ± 43.6 | 79.0 ± 48.1 | 61.5 ± 39.6a,b |
| aCTBD, no/mm2 | 49.3 ± 23.9 | 41.3 ± 22.0 | 33.7 ± 21.56a,b |
| mTC | 0.15 ± 0.23 | 0.17 ± 0.03a | 0.17 ± 0.03a |
Data are reported as mean ± SD.
Abbreviations: aCNBD, automated analysis of CNDB; aCNFD, automated analysis of CNFD; aCNFL, automated analysis of CNFL; aCTBD, automated analysis of CTBD; mCNBD, manual analysis of CNBD; mCNFD, manual analysis of CNFD; mCNFL, manual analysis of CNFL; mTC, manual analysis of tortuosity coefficient.
P < 0.05 vs control group after adjustment for age, sex, BMI, and smoking.
P < 0.05 vs DSPN+p group after adjustment for age, sex, BMI, HbA1c, diabetes type, and diabetes duration.
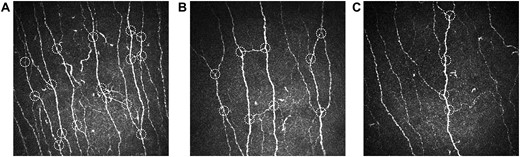
Representative images of corneal nerve fibers in (A) a glucose-tolerant control individual with normal corneal nerve branch density (CNBD) and (B) a patient with painful diabetic polyneuropathy showing reduced CNBD compared with the control, but markedly higher CNBD in comparison with (C) a patient with painless diabetic polyneuropathy. White dotted circles indicate main corneal nerve branches.
The percentages of participants with reduced CCM measures below the fifth percentile of the control group are listed in Table 3. The rates of patients with abnormal CNFD and CNFL measurements assessed manually and automatically were higher in both DSPN groups as compared with the control group (P < 0.05). The percentages of reduced CNBD and CTBD measured manually and automatically were higher in the DSPN-p group as compared with the control and the DSPN+p groups, except for the percentage of reduced automatically analyzed CTBD, which was higher only in DSPN-p patients compared with control subjects (P < 0.05). No other differences in the percentages of reduced CCM measures were noted among the groups.
Percentages of Participants With Abnormal CCM Measures Below the Fifth Percentile of the Control Group
| . | Control (%) . | DSPN+p (%) . | DSPN-p (%) . |
|---|---|---|---|
| mCNFD | 6.5 | 28.3a | 28.6a |
| aCNFD | 4.3 | 34.0a | 36.5a |
| mCNFL | 4.3 | 26.4a | 33.3a |
| aCNFL | 4.3 | 26.4a | 36.5a |
| mCNBD | 4.3 | 9.4 | 23.8a,b |
| aCNBD | 4.3 | 9.4 | 25.4a,b |
| mCTBD | 4.3 | 5.7 | 17.5a,b |
| aCTBD | 4.3 | 13.2 | 22.2a |
| . | Control (%) . | DSPN+p (%) . | DSPN-p (%) . |
|---|---|---|---|
| mCNFD | 6.5 | 28.3a | 28.6a |
| aCNFD | 4.3 | 34.0a | 36.5a |
| mCNFL | 4.3 | 26.4a | 33.3a |
| aCNFL | 4.3 | 26.4a | 36.5a |
| mCNBD | 4.3 | 9.4 | 23.8a,b |
| aCNBD | 4.3 | 9.4 | 25.4a,b |
| mCTBD | 4.3 | 5.7 | 17.5a,b |
| aCTBD | 4.3 | 13.2 | 22.2a |
Abbreviations: aCNBD, automated analysis of CNDB; aCNFD, automated analysis of CNFD; aCNFL, automated analysis of CNFL; aCTBD, automated analysis of CTBD; mCNBD, manual analysis of CNBD; mCNFD, manual analysis of CNFD; mCNFL, manual analysis of CNFL.
P < 0.05 vs control group.
P < 0.05 vs DSPN+p group.
Percentages of Participants With Abnormal CCM Measures Below the Fifth Percentile of the Control Group
| . | Control (%) . | DSPN+p (%) . | DSPN-p (%) . |
|---|---|---|---|
| mCNFD | 6.5 | 28.3a | 28.6a |
| aCNFD | 4.3 | 34.0a | 36.5a |
| mCNFL | 4.3 | 26.4a | 33.3a |
| aCNFL | 4.3 | 26.4a | 36.5a |
| mCNBD | 4.3 | 9.4 | 23.8a,b |
| aCNBD | 4.3 | 9.4 | 25.4a,b |
| mCTBD | 4.3 | 5.7 | 17.5a,b |
| aCTBD | 4.3 | 13.2 | 22.2a |
| . | Control (%) . | DSPN+p (%) . | DSPN-p (%) . |
|---|---|---|---|
| mCNFD | 6.5 | 28.3a | 28.6a |
| aCNFD | 4.3 | 34.0a | 36.5a |
| mCNFL | 4.3 | 26.4a | 33.3a |
| aCNFL | 4.3 | 26.4a | 36.5a |
| mCNBD | 4.3 | 9.4 | 23.8a,b |
| aCNBD | 4.3 | 9.4 | 25.4a,b |
| mCTBD | 4.3 | 5.7 | 17.5a,b |
| aCTBD | 4.3 | 13.2 | 22.2a |
Abbreviations: aCNBD, automated analysis of CNDB; aCNFD, automated analysis of CNFD; aCNFL, automated analysis of CNFL; aCTBD, automated analysis of CTBD; mCNBD, manual analysis of CNBD; mCNFD, manual analysis of CNFD; mCNFL, manual analysis of CNFL.
P < 0.05 vs control group.
P < 0.05 vs DSPN+p group.
The associations of CCM measures with nerve conduction studies and cold TDT in the groups with DSPN+p and DSPN-p are given in Table 4. After adjustment for age, sex, BMI, HbA1c, diabetes type, and known diabetes duration, in the DSPN-p group manually analyzed CNFD and automatically analyzed CNFD were positively associated with peroneal and tibial motor NCV, sural sensory NCV, SNAP, and cold TDT on the foot (P < 0.05). Moreover, manually analyzed CNFL and automatically analyzed CNFL were associated with sural sensory NCV, whereas there was an association of manually analyzed CNFL with sural SNAP and automatically analyzed CNFL with peroneal and tibial motor NCV (P < 0.05). Positive associations were also observed between manually analyzed CNBD and CTBD and cold TDT on the foot, whereas neither automatically analyzed CNBD (aCNBD) nor automatically analyzed CTBD correlated with any of the peripheral nerve function tests after adjustment. No correlations between CCM measures and the NC or TDT parameters listed in Table 4 were found in the DSPN+p group. Correlation analyses including the entire group of patients with DSPN (n = 116) showed correlations between both aCNBD and average 24-hour NRS pain intensity (r = 0.227; P = 0.014) as well as between aCNBD and maximum 24-hour NRS pain intensity (r = 0.207; P = 0.026).
Correlations of CCM Measures With Nerve Conduction Studies and Thermal Detection Threshold for Cold Stimuli in the DSPN+p and DSPN-p Groups
| Parameter, Group | mCNFD | aCNFD | mCNFL | aCNFL | mCNBD | aCNBD | mCTBD | aCTBD | |
| Peroneal motor NCV, DSPN+p | R | 0.202 | 0.213 | 0.146 | 0.193 | 0.121 | 0.224 | 0.090 | 0.129 |
| P | 0.156 | 0.133 | 0.307 | 0.176 | 0.399 | 0.114 | 0.530 | 0.366 | |
| Peroneal motor NCV, DSPN-p | R | 0.298a | 0.286a | 0.248 | 0.306a | 0.232 | 0.259 | 0.163 | 0.245 |
| P | 0.019a | 0.024a | 0.052 | 0.016a | 0.069 | 0.042 | 0.205 | 0.055 | |
| Tibial motor, DSPN+p | R | 0.208 | 0.236 | 0.214 | 0.211 | 0.123 | 0.191 | 0.122 | 0.138 |
| P | 0.135 | 0.089 | 0.123 | 0.130 | 0.378 | 0.170 | 0.383 | 0.324 | |
| Tibial motor NCV, DSPN-p | r | 0.383a | 0.367a | 0.257 | 0.339a | 0.245 | 0.269 | 0.184 | 0.236 |
| P | 0.002a | 0.003a | 0.042 | 0.007a | 0.053 | 0.033 | 0.149 | 0.062 | |
| Sural sensory NCV, DSPN+p | R | −0.107 | −0.143 | −0.132 | −0.117 | −0.181 | −0.111 | −0.191 | −0.107 |
| P | 0.445 | 0.306 | 0.346 | 0.406 | 0.194 | 0.430 | 0.171 | 0.448 | |
| Sural sensory NCV, DSPN-p | R | 0.385a | 0.369a | 0.327a | 0.345a | 0.290 | 0.299 | 0.244 | 0.317 |
| P | 0.002a | 0.003a | 0.010a | 0.006a | 0.023 | 0.019 | 0.058 | 0.013 | |
| Sural SNAP, DSPN+p | R | −0.153 | −0.194 | −0.161 | −0.142 | −0.208 | −0.120 | −0.203 | −0.104 |
| P | 0.275 | 0.163 | 0.250 | 0.311 | 0.136 | 0.391 | 0.145 | 0.460 | |
| Sural SNAP, DSPN-p | R | 0.373a | 0.358a | 0.321a | 0.317a | 0.279 | 0.272 | 0.246 | 0.271 |
| P | 0.003a | 0.005a | 0.012a | 0.013a | 0.030 | 0.034 | 0.056 | 0.035 | |
| Cold TDT foot, DSPN+p | R | 0.161 | 0.149 | 0.127 | 0.090 | 0.081 | 0.058 | 0.103 | 0.042 |
| P | 0.253 | 0.291 | 0.369 | 0.526 | 0.568 | 0.685 | 0.469 | 0.767 | |
| Cold TDT foot, DSPN-p | R | 0.274a | 0.341a | 0.205 | 0.204 | 0.290a | 0.213 | 0.308a | 0.175 |
| P | 0.030a | 0.006a | 0.107 | 0.108 | 0.021a | 0.093 | 0.014a | 0.171 |
| Parameter, Group | mCNFD | aCNFD | mCNFL | aCNFL | mCNBD | aCNBD | mCTBD | aCTBD | |
| Peroneal motor NCV, DSPN+p | R | 0.202 | 0.213 | 0.146 | 0.193 | 0.121 | 0.224 | 0.090 | 0.129 |
| P | 0.156 | 0.133 | 0.307 | 0.176 | 0.399 | 0.114 | 0.530 | 0.366 | |
| Peroneal motor NCV, DSPN-p | R | 0.298a | 0.286a | 0.248 | 0.306a | 0.232 | 0.259 | 0.163 | 0.245 |
| P | 0.019a | 0.024a | 0.052 | 0.016a | 0.069 | 0.042 | 0.205 | 0.055 | |
| Tibial motor, DSPN+p | R | 0.208 | 0.236 | 0.214 | 0.211 | 0.123 | 0.191 | 0.122 | 0.138 |
| P | 0.135 | 0.089 | 0.123 | 0.130 | 0.378 | 0.170 | 0.383 | 0.324 | |
| Tibial motor NCV, DSPN-p | r | 0.383a | 0.367a | 0.257 | 0.339a | 0.245 | 0.269 | 0.184 | 0.236 |
| P | 0.002a | 0.003a | 0.042 | 0.007a | 0.053 | 0.033 | 0.149 | 0.062 | |
| Sural sensory NCV, DSPN+p | R | −0.107 | −0.143 | −0.132 | −0.117 | −0.181 | −0.111 | −0.191 | −0.107 |
| P | 0.445 | 0.306 | 0.346 | 0.406 | 0.194 | 0.430 | 0.171 | 0.448 | |
| Sural sensory NCV, DSPN-p | R | 0.385a | 0.369a | 0.327a | 0.345a | 0.290 | 0.299 | 0.244 | 0.317 |
| P | 0.002a | 0.003a | 0.010a | 0.006a | 0.023 | 0.019 | 0.058 | 0.013 | |
| Sural SNAP, DSPN+p | R | −0.153 | −0.194 | −0.161 | −0.142 | −0.208 | −0.120 | −0.203 | −0.104 |
| P | 0.275 | 0.163 | 0.250 | 0.311 | 0.136 | 0.391 | 0.145 | 0.460 | |
| Sural SNAP, DSPN-p | R | 0.373a | 0.358a | 0.321a | 0.317a | 0.279 | 0.272 | 0.246 | 0.271 |
| P | 0.003a | 0.005a | 0.012a | 0.013a | 0.030 | 0.034 | 0.056 | 0.035 | |
| Cold TDT foot, DSPN+p | R | 0.161 | 0.149 | 0.127 | 0.090 | 0.081 | 0.058 | 0.103 | 0.042 |
| P | 0.253 | 0.291 | 0.369 | 0.526 | 0.568 | 0.685 | 0.469 | 0.767 | |
| Cold TDT foot, DSPN-p | R | 0.274a | 0.341a | 0.205 | 0.204 | 0.290a | 0.213 | 0.308a | 0.175 |
| P | 0.030a | 0.006a | 0.107 | 0.108 | 0.021a | 0.093 | 0.014a | 0.171 |
Boldface indicates P < 0.05.
Abbreviations: aCNBD, automated analysis of CNDB; aCNFD, automated analysis of CNFD; aCNFL, automated analysis of CNFL; aCTBD, automated analysis of CTBD; mCNBD, manual analysis of CNBD; mCNFD, manual analysis of CNFD; mCNFL, manual analysis of CNFL.
Statistically significant after adjustment for age, sex, BMI, HbA1c, diabetes type, and diabetes duration.
Correlations of CCM Measures With Nerve Conduction Studies and Thermal Detection Threshold for Cold Stimuli in the DSPN+p and DSPN-p Groups
| Parameter, Group | mCNFD | aCNFD | mCNFL | aCNFL | mCNBD | aCNBD | mCTBD | aCTBD | |
| Peroneal motor NCV, DSPN+p | R | 0.202 | 0.213 | 0.146 | 0.193 | 0.121 | 0.224 | 0.090 | 0.129 |
| P | 0.156 | 0.133 | 0.307 | 0.176 | 0.399 | 0.114 | 0.530 | 0.366 | |
| Peroneal motor NCV, DSPN-p | R | 0.298a | 0.286a | 0.248 | 0.306a | 0.232 | 0.259 | 0.163 | 0.245 |
| P | 0.019a | 0.024a | 0.052 | 0.016a | 0.069 | 0.042 | 0.205 | 0.055 | |
| Tibial motor, DSPN+p | R | 0.208 | 0.236 | 0.214 | 0.211 | 0.123 | 0.191 | 0.122 | 0.138 |
| P | 0.135 | 0.089 | 0.123 | 0.130 | 0.378 | 0.170 | 0.383 | 0.324 | |
| Tibial motor NCV, DSPN-p | r | 0.383a | 0.367a | 0.257 | 0.339a | 0.245 | 0.269 | 0.184 | 0.236 |
| P | 0.002a | 0.003a | 0.042 | 0.007a | 0.053 | 0.033 | 0.149 | 0.062 | |
| Sural sensory NCV, DSPN+p | R | −0.107 | −0.143 | −0.132 | −0.117 | −0.181 | −0.111 | −0.191 | −0.107 |
| P | 0.445 | 0.306 | 0.346 | 0.406 | 0.194 | 0.430 | 0.171 | 0.448 | |
| Sural sensory NCV, DSPN-p | R | 0.385a | 0.369a | 0.327a | 0.345a | 0.290 | 0.299 | 0.244 | 0.317 |
| P | 0.002a | 0.003a | 0.010a | 0.006a | 0.023 | 0.019 | 0.058 | 0.013 | |
| Sural SNAP, DSPN+p | R | −0.153 | −0.194 | −0.161 | −0.142 | −0.208 | −0.120 | −0.203 | −0.104 |
| P | 0.275 | 0.163 | 0.250 | 0.311 | 0.136 | 0.391 | 0.145 | 0.460 | |
| Sural SNAP, DSPN-p | R | 0.373a | 0.358a | 0.321a | 0.317a | 0.279 | 0.272 | 0.246 | 0.271 |
| P | 0.003a | 0.005a | 0.012a | 0.013a | 0.030 | 0.034 | 0.056 | 0.035 | |
| Cold TDT foot, DSPN+p | R | 0.161 | 0.149 | 0.127 | 0.090 | 0.081 | 0.058 | 0.103 | 0.042 |
| P | 0.253 | 0.291 | 0.369 | 0.526 | 0.568 | 0.685 | 0.469 | 0.767 | |
| Cold TDT foot, DSPN-p | R | 0.274a | 0.341a | 0.205 | 0.204 | 0.290a | 0.213 | 0.308a | 0.175 |
| P | 0.030a | 0.006a | 0.107 | 0.108 | 0.021a | 0.093 | 0.014a | 0.171 |
| Parameter, Group | mCNFD | aCNFD | mCNFL | aCNFL | mCNBD | aCNBD | mCTBD | aCTBD | |
| Peroneal motor NCV, DSPN+p | R | 0.202 | 0.213 | 0.146 | 0.193 | 0.121 | 0.224 | 0.090 | 0.129 |
| P | 0.156 | 0.133 | 0.307 | 0.176 | 0.399 | 0.114 | 0.530 | 0.366 | |
| Peroneal motor NCV, DSPN-p | R | 0.298a | 0.286a | 0.248 | 0.306a | 0.232 | 0.259 | 0.163 | 0.245 |
| P | 0.019a | 0.024a | 0.052 | 0.016a | 0.069 | 0.042 | 0.205 | 0.055 | |
| Tibial motor, DSPN+p | R | 0.208 | 0.236 | 0.214 | 0.211 | 0.123 | 0.191 | 0.122 | 0.138 |
| P | 0.135 | 0.089 | 0.123 | 0.130 | 0.378 | 0.170 | 0.383 | 0.324 | |
| Tibial motor NCV, DSPN-p | r | 0.383a | 0.367a | 0.257 | 0.339a | 0.245 | 0.269 | 0.184 | 0.236 |
| P | 0.002a | 0.003a | 0.042 | 0.007a | 0.053 | 0.033 | 0.149 | 0.062 | |
| Sural sensory NCV, DSPN+p | R | −0.107 | −0.143 | −0.132 | −0.117 | −0.181 | −0.111 | −0.191 | −0.107 |
| P | 0.445 | 0.306 | 0.346 | 0.406 | 0.194 | 0.430 | 0.171 | 0.448 | |
| Sural sensory NCV, DSPN-p | R | 0.385a | 0.369a | 0.327a | 0.345a | 0.290 | 0.299 | 0.244 | 0.317 |
| P | 0.002a | 0.003a | 0.010a | 0.006a | 0.023 | 0.019 | 0.058 | 0.013 | |
| Sural SNAP, DSPN+p | R | −0.153 | −0.194 | −0.161 | −0.142 | −0.208 | −0.120 | −0.203 | −0.104 |
| P | 0.275 | 0.163 | 0.250 | 0.311 | 0.136 | 0.391 | 0.145 | 0.460 | |
| Sural SNAP, DSPN-p | R | 0.373a | 0.358a | 0.321a | 0.317a | 0.279 | 0.272 | 0.246 | 0.271 |
| P | 0.003a | 0.005a | 0.012a | 0.013a | 0.030 | 0.034 | 0.056 | 0.035 | |
| Cold TDT foot, DSPN+p | R | 0.161 | 0.149 | 0.127 | 0.090 | 0.081 | 0.058 | 0.103 | 0.042 |
| P | 0.253 | 0.291 | 0.369 | 0.526 | 0.568 | 0.685 | 0.469 | 0.767 | |
| Cold TDT foot, DSPN-p | R | 0.274a | 0.341a | 0.205 | 0.204 | 0.290a | 0.213 | 0.308a | 0.175 |
| P | 0.030a | 0.006a | 0.107 | 0.108 | 0.021a | 0.093 | 0.014a | 0.171 |
Boldface indicates P < 0.05.
Abbreviations: aCNBD, automated analysis of CNDB; aCNFD, automated analysis of CNFD; aCNFL, automated analysis of CNFL; aCTBD, automated analysis of CTBD; mCNBD, manual analysis of CNBD; mCNFD, manual analysis of CNFD; mCNFL, manual analysis of CNFL.
Statistically significant after adjustment for age, sex, BMI, HbA1c, diabetes type, and diabetes duration.
Discussion
The findings of this study demonstrate enhanced corneal nerve branching in patients with painful DSPN compared with those with painless DSPN, whereas the degree of peripheral nerve dysfunction assessed by NC and TDT and the extent of corneal nerve fiber loss verified by reduced CNFD and CNFL did not differ between the DSPN+p and DSPN-p groups. This pattern of corneal nerve pathology is compatible with the hypothesis that enhanced nerve fiber branching in patients with DSPN who developed neuropathic pain may reflect some preserved susceptibility of corneal nerve fibers toward regeneration, but this repair attempt as yet appears insufficient to culminate in an increased CNFD or CNFL. This pattern resembles our previous finding that dermal nerve fiber regeneration is enhanced in painful DSPN but fails to adequately counteract epidermal nerve fiber loss (20). Moreover, the current study shows associations of CCM measures with small- and large-fiber function tests solely in patients with painless DSPN but not those with painful DSPN in whom there was also no evidence for predominant small nerve fiber loss or dysfunction.
The main finding was increased corneal nerve branching in patients with painful DSPN when compared with those with painless DSPN. This is supported by higher percentages of participants with reduced CNBD below the fifth percentile of the control group among patients with painless DSPN than those with painful DSPN, whereas no differences were noted between the latter group and the control group. Because CNFD and CNFL did not differ between the DSPN+p and DSPN-p groups, the question arises as to whether CNBD could be a more sensitive indicator of corneal nerve fiber regeneration than the other CCM measures. Evidence to support this notion can be derived from three recent intervention trials using CCM measures as primary efficacy outcomes. Indeed, in patients with type 1 diabetes who underwent simultaneous pancreas and kidney transplantation, CNBD was the first CCM parameter to increase after 6 months, whereas CNFL and CNFD increased only after 12 months (25). Likewise, in a 1-year trial using seal oil ω-3 polyunsaturated fatty acid supplementation resulted in an increase of CNBD and CNFL but not CNFD and corneal nerve fiber area in patients with type 1 diabetes (26). In a trial using cibinetide, a peptide that activates cellular repair via innate repair receptor agonism, CNBD and corneal nerve fiber area, but not CNFL and CNFD, were augmented after 4 weeks in patients with sarcoidosis-associated small nerve fiber loss and neuropathic pain (27). Thus, altogether, in line with our results, the findings of these trials support the hypothesis that among the standard CCM measures, CNBD could possess the highest susceptibility to improvement after interventions in DSPN.
Against this background it is tempting to speculate that the increased corneal nerve branching described herein could reflect an enhanced reinnervation of small nerve fibers contributing to neuropathic pain. Peripheral nerve injuries induce functional deficits caused by degeneration of injured axons, which can be recovered by two endogenous mechanisms (i.e., reinnervation of denervated targets by regeneration of injured axons and reinnervation by collateral branching of undamaged axons). Each of these mechanisms is thought to contribute to the generation of neuropathic pain (28). Because, in the current study, the magnitude of corneal nerve branching correlated with 24-hour NRS pain intensity in the entire group with DSPN, it is conceivable that reinnervation processes in corneal nerves mirror those taking place in the distal lower limbs, contributing to the development of neuropathic pain, although more insights can only be gained from a prospective setting.
The present finding of similar CNFD and CNFL levels in patients with painful and painless DSPN is in line with the study by Kalteniece et al. (15) but at variance with the study by Marshall et al. (16) who reported only reduced CNFD, but not reduced CNFL and CNBD, in patients with painful DSPN, and with that of Quattrini et al. (11), who showed only diminished CNFL but not diminished CNFD and CNBD. The apparent discrepancy between the study by Quattrini et al. (11) and ours with respect to CNFL could have several reasons. First, the study by Quattrini et al. (11) had a relatively small sample size (DSPN+p vs DSPN-p: 28 vs 26 patients). Second, the authors did not provide information on neuropathy severity in their subanalysis of painful vs painless DSPN. Therefore, the CNFL reduction could be explained by a more severe underlying neuropathy, because there was a positive correlation between DSPN severity and painful symptoms. Third, their subanalysis included 10 patients without DSPN. Fourth, they used the Tomey Confoscan for CCM, which provides a markedly lower resolution when compared with the Heidelberg Retina Tomograph III used herein (29).
Another finding from our study is the positive association of CCM measures with NC and cold TDT only in patients with painless DSPN but not those with painful DSPN, suggesting widespread small- and large-fiber dysfunction and pathology in the painless entity. Although the reasons for this dichotomy are unclear, the density of intraepithelial nerve fibers of cold thermoreceptors rather than that of polymodal nociceptor nerve fibers is affected in the cornea of diabetic mice, indicating that distinct subpopulations of corneal sensory neurons can be differentially affected by pathology (30), which may explain some of the variance in the correlations observed herein. Nonetheless, the lack of correlation between cold TDT, which is mediated by small Aδ and C fibers, and CCM indices in painful DSPN, as opposed to the painless variant, contradicts the notion of predominant small fiber involvement in painful DSPN. However, in the entire DSPN group, heightened CNBD correlated with the intensity of neuropathic pain, suggesting that reinnervation may take place primarily in patients with DSPN who have neuropathic pain. Thus, neuropathic pain per se, rather than TDT, appears to be an indicator of preserved corneal nerve branching.
Our findings deserve a comment in a broader mechanistic context. Although several risk factors or mechanisms, such as female sex, obesity, DSPN severity, genetic susceptibility, inflammation, regenerative processes, direct hyperalgesic effect of hyperglycemia, and central processing of neuropathic pain, have been proposed to contribute to the generation of neuropathic pain in DSPN, it remains enigmatic why some patients experience distressing pain while others develop the insensate variety or only nonpainful symptoms (3). Previous studies attempting to characterize the extent of nerve fiber loss in painful and painless DSPN using skin biopsy, the gold standard for small fiber neuropathy, did not reveal any differences in intraepidermal nerve fiber density between the two entities (20, 31, 32). In contrast, two recent studies using QST found that despite differing definitions of DSPN and neuropathic pain, sensory deficits were more frequent in patients with painful DSPN than in those with painless DSPN, and the severity of these deficits was associated with the severity of neuropathic pain, suggesting that painful DSPN primarily represents the more severe entity (9, 32). Likewise, studies using bedside tests or clinical scores reported higher severity of sensory deficits in painful compared with painless DSPN (1, 33). However, our results do not support these findings; in our study, malleolar VPT was the only measure that was impaired in the DSPN+p group compared with the DSPN-p group, whereas warm and cold TDT on the foot and all nerve conduction studies did not differ between the groups, in line with a previous study (16). On the other hand, to further complicate the matter, one study demonstrated even less frequently impaired VPT and TDT in painful than painless DSPN (34). Thus, although most studies suggest painful DSPN may represent the more severe entity, some data are at variance with this notion, which might be explained among others by differences in patient number and selection, definitions of DSPN and neuropathic pain, and phenotyping tools. It is also conceivable that further differentiation of painful DSPN into a predominant sensory function–loss phenotype (i.e., insensate painful DSPN) and a sensory function–gain phenotype with relatively preserved sensory function or allodynia/hyperalgesia (i.e., sensate painful DSPN) could be useful. Such subphenotyping using QST (35) or genetic testing (36) could gain clinical attraction for identifying potential responders to analgesic treatment. Moreover, multimodal brain imaging recently revealed lower somatosensory cortical thickness with expansion of the area representing pain in the lower limb to include face and lip regions in insensate compared with sensate painful DSPN (37). On the other hand, it has been suggested that gain-of-function mutations of the voltage-gated sodium channel Nav1.7 may contribute to the painful, but not painless, DSPN phenotype (38), despite similar QST profiles, indicating that QST is more useful to investigate neuropathy rather than neuropathic pain (39, 40), which is in line with our QST findings.
Several limitations of this study should be addressed. First, the cross-sectional study design does not provide any insights into the temporal sequence of the increased CNFB, which will be determined in a prospective, 5-year follow-up. Second, the DSPN groups differed with respect to sex and BMI such that the percentage of women and BMI were higher in the DSPN+p group than the DSPN-p group. Of note, the distribution pattern for sex and BMI among patients with painful and painless DSPN in the current study supports the role of female sex and obesity as risk factors for painful DSPN (1, 8). Although all analyses were adjusted for these variables, possible bias cannot be excluded. Third, an age-matched group of patients with diabetes without DSPN was not included. Fourth, because the manual classification of main nerve fibers is subjective, it may be biased when counting branch points only on main fibers to obtain CNBD. To rule out this possible bias, we additionally computed CTBD, which is independent of main nerve fibers.
In conclusion, we have demonstrated enhanced corneal nerve branching in patients with painful DSPN compared with those with painless DSPN, despite a similar extent of peripheral nerve dysfunction and reduction in CNFD and CNFL. This finding suggests some preserved susceptibility of corneal nerve fibers toward regeneration in the painful entity. Because the degree of corneal nerve branching was related to 24-hour NRS pain intensity in the feet, it is conceivable that reinnervation processes in corneal nerves could mirror those taking place in the distal lower limbs contributing to the development of neuropathic pain. Thus, augmented corneal nerve branching could serve as a particularly suitable end point in clinical trials in DSPN focusing on nerve regeneration as a therapeutic target. A 5-year follow-up of the present cohorts is planned to determine the temporal sequence of enhanced CNBD in painful compared with painless DSPN.
Acknowledgments
We appreciate the voluntary contribution of all study participants. We also thank F. Battiato, M. Schroers-Teuber, J. Schubert, and the staff of the Clinical Research Center and Technical Laboratory of the German Center for Diabetes Research (DDZ) for excellent technical assistance and taking care of the patients.
The GDS Group consists of A.E. Buyken (Department of Sports and Health, Paderborn University, Paderborn, Germany); G. Geerling (Department of Ophthalmology, Medical Faculty, Heinrich Heine University, Düsseldorf, Germany); J. Eckel, H. Al-Hasani, C. Herder, A. Icks, J. Kotzka, O. Kuss, E. Lammert, D. Markgraf, K. Müssig, W. Rathmann, J. Szendroedi, D. Ziegler, and M. Roden (speaker) (DDZ).
Financial Support: The PROPANE study was initiated by the PROPANE consortium and received funding from the European Union Seventh Framework Program FP7/2007-2013 (Grant 602273). This work was supported by the Ministry of Science and Research of the State of North Rhine-Westphalia, the German Federal Ministry of Health, and in part by a grant from the Federal Ministry for Research to the German Center for Diabetes Research and by a grant from the German Center for Diabetes Research.
The funding sources had no influence on the design and conduct of this study; collection, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Clinical Trial Information: ClinicalTrials.gov nos. NCT02243475 (registered 18 September 2014) and NCT01055093 (registered 25 January 2010).
Author Contributions: D.Z. designed the study. S.P. and D.Z. wrote the manuscript. S.P.,G.J.B., and A.S. researched the data. G.J.B., A.S., K.M., J.S., M.R., and D.Z. contributed to the discussion and reviewed and edited the manuscript. M.R. and D.Z. are the guarantors of this study and as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Abbreviations:
- aCNBD
automatically measured corneal nerve branch density
- BMI
body mass index
- CCM
corneal confocal microscopy
- CNBD
corneal nerve branch density
- CNFD
corneal nerve fiber density
- CNFL
corneal nerve fiber length
- CTDB
corneal nerve fiber total branch density
- DSPN
diabetic sensorimotor polyneuropathy
- DSPN+p
painful diabetic sensorimotor polyneuropathy
- DSPN-p
painless diabetic sensorimotor polyneuropathy
- NC
nerve conduction
- NCV
nerve conduction velocity
- NRS
numerical rating scale
- NSS
Neuropathy Symptom Score
- PROPANE
Probing the Role of Sodium Channels in Painful Neuropathy
- QST
quantitative sensory testing
- SNAP
sensory nerve action potential
- TDT
thermal detection threshold
- VPT
vibration perception threshold



