-
PDF
- Split View
-
Views
-
Cite
Cite
Emanuela Medda, Maria Cristina Vigone, Alessandra Cassio, Francesca Calaciura, Pietro Costa, Giovanna Weber, Tiziana de Filippis, Giulia Gelmini, Marianna Di Frenna, Silvana Caiulo, Rita Ortolano, Daniela Rotondi, Monica Bartolucci, Rossella Gelsomino, Simona De Angelis, Marco Gabbianelli, Luca Persani, Antonella Olivieri, Neonatal Screening for Congenital Hypothyroidism: What Can We Learn From Discordant Twins?, The Journal of Clinical Endocrinology & Metabolism, Volume 104, Issue 12, December 2019, Pages 5765–5779, https://doi.org/10.1210/jc.2019-00900
Close - Share Icon Share
Abstract
Newborn screening program for congenital hypothyroidism (CH) adopting rescreening in at-risk neonates.
To estimate the concordance rate for CH in twin pairs discordant at the first screening; to verify whether long-term follow-up of healthy cotwins belonging to CH discordant pairs may be useful to diagnose thyroid hypofunction during development; to evaluate the importance of genetic and environmental influences on liability to permanent and transient CH.
Forty-seven screening discordant twin pairs were investigated. Proband was defined as the twin in the pair with a positive test at the first screening and a confirmed diagnosis of CH.
Seven screening discordant twin pairs became concordant for CH within the first month of life (pairwise concordance of 14.9%) because seven screening negative cotwins showed high TSH values when retested. During long-term follow-up (range, 3 to 21 years), hypothyroidism was diagnosed in two monozygotic screening negative cotwins at the age of 9 months and 12 years, respectively. Furthermore, the twin analysis showed that 95% of liability to transient CH was explained by genetic factors and 5% by environmental (unshared) factors, whereas 64% of phenotypic variance of permanent CH was explained by common environmental factors (shared during the fetal life) and 36% by unshared environmental factors.
This study showed that the introduction of rescreening permits the diagnosis of CH in a greater number of twins. It also showed the importance of long-term follow-up in both twins in the pair, and the role of nongenetic factors in the etiology of permanent CH.
Implementation of newborn screening for primary congenital hypothyroidism (CH) began in the 1970s and has been extraordinarily successful in preventing mental retardation due to this condition. The recent changes in pattern of diagnosis, essentially due to lowering TSH cutoffs and increasing survival rate among preterm babies (1–3), have modified the historical distribution of CH etiologies with 80% to 85% thyroid dysgenesis and 15% to 20% dyshormonogenesis (4). In fact, recent studies have reported that ∼60% to 70% of permanent CH cases are caused by thyroid dysgenesis, whereas the remaining 30% to 40% of patients have eutopic thyroid consistent with functional defects of the gland (5–7).
Thyroid dysgenesis describes a spectrum of defects of thyroid morphogenesis, and some monogenetic forms due to mutations in TSHR, PAX8, NKX2-1, FOXE1, and NKX2-5 have been identified (8, 9). More recently, also variations in JAG1 and BOREALIN genes (10, 11) have been reported. Thyroid dyshormonogenesis comprises defects at every step of thyroid hormone synthesis. Mutations in seven genes are well described causing iodine transport defect (SLC5A5), iodine organification defect (TPO, DUOX2, DUOXA2, SLC26A4), thyroglobulin (TG) synthesis or transport defect, or iodotyrosine deiodinase (IYD/DEHAL1) deficiency (8, 9). Also, mutations in the GLIS3 gene have been reported to be associated with CH with phenotypes from aplasia/dysplasia to in situ thyroid (12), and with the development of a syndrome characterized by CH and neonatal diabetes (13). Finally, a recent study revealed a frequent contribution of oligogenic defects involving rare variations in morphogenetic or functional candidate genes in CH patients regardless of the phenotype, suggesting a possible explanation for the frequent sporadic CH occurrence (14).
Despite these recent findings, genetic causes can explain only a low percentage of cases (<10%). For this reason, great attention has been paid to modifiable environmental risk factors for CH. Among these, iodine deficiency has been shown to have an important role in the etiology of both transient and permanent forms of the disease (15, 16). Furthermore, experimental and human studies have recently suggested that exposure to thyroid-disrupting chemicals during fetal life may also contribute to the etiology of the disease (17–21).
Multiple pregnancies have a risk of CH threefold higher than single ones (22), although a low concordance rate for the disease has been reported. Perry et al. (23) identified 16 twins [5 monozygotic (MZ) and 11 dizygotic (DZ)] in two large CH screening programs, in Quebec and Brussels. The authors found that all of the affected twins were from discordant couples. Similar results were found in another study (22) conducted between 1989 and 2000 on a larger number of CH twins (n = 80) recorded in the Italian National Registry of infants with CH. In this study a very low concordance rate for CH was found (4.3%), and this was due to only three concordant DZ couples with in situ gland. However, in the last two decades a progressive adoption of lower screening TSH cutoffs and special screening procedures for at-risk newborns (2, 5, 24–26), as well as the increased survival rate of a growing number of preterm babies, have led to an increased detection of twin babies with CH (27).
Twins are frequently preterm and/or low birth weight and may have a delayed increase of TSH (28–30). Over the years, to avoid false-negative results at screening, the strategy of the second screening (i.e., rescreening at 2 weeks of life) for twin babies and other at-risk neonates has been adopted by many screening programs worldwide according to available guidelines (25) and country-specific recommendations (31). A second dried blood spot specimen for CH screening at 2 weeks of life in same-sex twins is particularly recommended to avoid delayed or missed diagnoses in MZ twins at risk for fetal blood mixing occurring in 10% to 15% of monochorionic twin pregnancies (32, 33). Fetal blood mixing occurs in twins through placental vascular connections allowing the free transfer of T4 from the euthyroid twin to the hypothyroid one and maintaining TSH in the normal range in the hypothyroid twin until a few days after delivery.
Although the strategy of the second screening has contributed to detect a growing number of twins over the years, no extensive studies have been performed to estimate how many cotwins with a negative test at the first screening (3 to 5 days of life) become positive at rescreening and are diagnosed with CH, and to evaluate the possible benefit of long-term follow-up in healthy cotwins belonging to screening discordant couples or triplets. Additionally, no extensive twin studies have been conducted to disentangle genetic and environmental influences in the etiology of CH. We carried out this multicenter study with the following aims: (i) to estimate the concordance rate for CH in twin pairs or triplets discordant at the first screening; (ii) to verify whether long-term follow-up of healthy cotwins belonging to discordant pairs or triplets may be useful to verify the occurrence of thyroid hypofunction during development; and (iii) to evaluate the importance of genetic and environmental influences on liability to permanent and transient forms of CH.
Subjects and Methods
Four Italian follow-up and clinical pediatric centers for CH took part in this multicenter study (San Raffaele Hospital, Milan, Italy; Sant’Orsola Hospital, Bologna, Italy; University La Sapienza, Rome, Italy; and Garibaldi Hospital, Catania, Italy). The criteria to include twin pairs or triplets in the study were (i) discordance for the results of screening test at 3 to 5 days (one positive twin, one negative), (ii) a confirmed diagnosis of CH in one of the twins of the pair, and (iii) at least 3 years of follow-up in both affected and healthy twins. On the basis of these inclusion criteria, 39 twin pairs and 4 triplets born between 1993 and 2012 were investigated. Proband was defined as a twin in a pair with a positive test at screening (3 to 5 days of life) and a confirmed diagnosis of CH. The recruited pairs and triplets included 43 CH probands (14 boys, 29 girls) and 47 cotwins with a negative screening test (26 boys, 21 girls). The four triplets were transformed in pairs for the analysis, that is, two pairs were obtained from each triplet (one pair for each cotwin of the triplet). Therefore, 47 informative twin pairs were available for the analysis (Fig. 1).
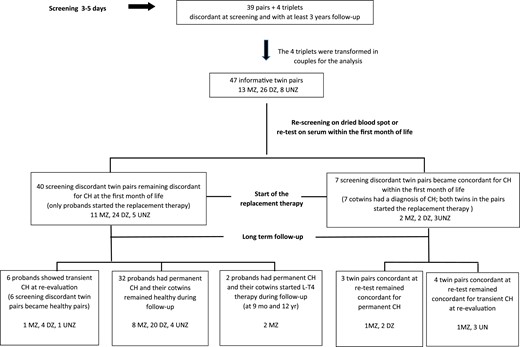
Flow diagram showing outcomes in the 47 informative screening discordant twin pairs.
The Ethical Committee of Istituto Auxologico Italiano approved the study protocol (code RF-2010-2309484), and informed consent for blood sampling for genetic investigations was obtained from the newborn’s parents.
Screening test and confirmation of the diagnosis
All of the twins recruited in the study were screened between the third and the fifth day of life. At the time of the recruitment a screening TSH cutoff of 10.0 to 15.0 mU/L whole blood was adopted by the screening laboratories involved in the study. Since 2002, repeat screening (at 2 weeks of life) in special categories of neonates has been progressively adopted by the Italian screening laboratories. Therefore, during the period in which twins were screened (1993 to 2012) the second screening strategy was not yet regularly applied and not all of the cotwins recruited in this study were rescreened at 2 weeks of life. Nevertheless, during the observation period serum TSH (sTSH) and/or free T4 (sFT4) measurements were carried out within the first month of life in all of the 47 recruited cotwins, with the exception of 3 cotwins who were retested at 12 months of life (sTSH range, 1.2 to 3.1 mU/L). Because these cotwins had remained healthy during follow-up, we excluded that they could be positive when they had been retested within the first month of life. Therefore, these cotwins were assumed to be negative within the first month of life and were included as such in the analysis. Positive results of screening were confirmed by definitive tests of thyroid function on serum; sTSH and sFT4 were considered normal when falling within the age-related reference intervals (34, 35). Serum anti–thyroid peroxidase autoantibodies (TPOAbs) and anti-TG autoantibodies (TGAbs) were also assessed. Thyroid ultrasound (US) and/or scintigraphy were performed to complete the CH diagnosis. The diagnoses of thyroid agenesis obtained by means of scintigraphy were confirmed by thyroid US.
Long-term follow-up and reevaluation of the diagnosis after the age of 3 years
All CH twins were enrolled in a regular hormonal and auxological follow-up every month in the first 3 months of life, every 3 to 6 months in the first 2 years of life, and then every 6 to 12 months. The healthy cotwins were followed up at least once a year in the first 5 years of life, and less frequently in the following years.
Serum samples for thyroid function tests (sTSH, sFT4) were collected according to the age and the status of affected and healthy twins.
When the definitive diagnosis was not established in the neonatal period and a suspicion of transient primary hypothyroidism was present during follow-up, a reevaluation of the diagnosis was performed after the age of 3 years and after a withdrawal of the replacement therapy to ascertain the persistence of CH (36). One month after l-thyroxine withdrawal, sTSH, sFT4, and thyroid antibodies were assessed and US was also performed. Patients with an sTSH value >20 mU/L or sTSH ranging from 10.1 to 20 mU/L and/or low FT4 levels on at least two occasions immediately resumed l-thyroxine therapy. Patients showing normal (<5 mU/L) or slightly elevated sTSH (5.0 to 10 mU/L) at follow-up discontinued levothyroxine therapy.
Genetic analysis
The genomic DNA (gDNA) of each patient was extracted from peripheral blood lymphocytes using a Gene Catcher gDNA 96- × 10-mL automated blood kit (Invitrogen, Life Technologies, Carlsbad, CA). Genetic screening was performed by next-generation sequencing (NGS) of a panel of 14 CH candidate genes: DUOX2, DUOXA2, FOXE1, GLIS3, IYD, JAG1, NKX2-1, NKX2-5, PAX8, SLC26A4, SLC5A5, TG, TPO, and TSHR. The oligonucleotide probes for the TruSeq Custom Amplicon assay (Illumina, San Diego, CA) were generated using Illumina DesignStudio™ software, selecting all exons of each gene and 25 nucleotides upstream and downstream of each exon. The total coverage of the target genes was 97%, with a total of 898 amplicons. All regions not adequately sequenced by the NGS were then recovered by Nextera® DNA library preparation kit (Illumina).
Briefly, the TruSeq NGS libraries were prepared from 250 ng of each gDNA. Each sample library was normalized according to the manufacturer’s instructions, and equal volumes were pooled to generate the final sequencing library. Pooled libraries were sequenced on MiSeq reagent kit v.3 on an Illumina MiSeq sequencer. Obtained sequences were aligned to the reference genome (GRCh37/hg19) using MiSeq Reporter software (Illumina).
Analysis of variants
To determine whether a given variant was situated in a coding or noncoding region, we used Integrative Genomics Viewer, a high-performance visualization tool downloaded from the Web site of the University of California, Santa Cruz (http://genome.ucsc.edu/). All identified variants were filtered with specific criteria to lower the risk to selected variants with a poor functional impact. After careful consultation of free or professional online databases, such as HGMD Professional (https://portal.biobase-international.com/hgmd/pro/start.php), Ensembl genome browser (https://www.ensembl.org/), dbSNP home page–National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/projects/SNP), and OMIM–National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov), we excluded (i) synonymous variants not affecting the splicing site, (ii) intronic variants more than +6 or fewer than −3 nucleotides after or before the exon, respectively, and (iii) common nonsynonymous variants with global minor allele frequency (MAF) >0.01. All of the identified variants were confirmed by the Sanger sequencing method. The purified PCR products were sequenced in both forward and reverse directions using the BigDye Terminator kit and resolved by capillary electrophoresis on an ABI 310 automated sequencer (PE Biosystems, Foster City, CA).
Zygosity
In cases with available DNA, zygosity was assessed by the concordance of single-nucleotide polymorphisms in highly variable gene regions analyzed by NGS. In twins whose parents did not give consent to blood sampling for genetic investigations, zygosity was assessed by the Goldsmith (37) questionnaire at the age of at least 3 years. This consists of items, filled by parents, about physical similarity and frequency of confusion of the twins by family members and strangers during infancy. The questionnaire allows zygosity identification by a mathematical algorithm. This is a well-established procedure in twin studies and is known to be >90% accurate (38).
Statistical analysis
Descriptive analysis was performed on twins as individuals to report means and SDs of principal variables. Additionally, the association between CH condition and birth weight or head circumference was evaluated using a linear regression model taking into account the nonindependence of data within twin pairs.
Means estimated in screening discordant MZ and DZ pairs were compared using Student t test for paired samples, separately for discordant MZ and DZ pairs. Mean intrapair differences were expressed as the mean of the proband–cotwin differences.
Statistical analyses were performed using Stata software for Windows (version 13.1; StataCorp, College Station, TX).
Concordance rates
A pair was defined as concordant for CH when both twins of the pair were affected and as discordant when only one twin was affected. Pairwise concordance (Pp) was used to estimate the probability that both members of a twin pair were affected given that at least one member of the pair was affected (39). Pp is calculated as the proportion of concordant pairs over the sum of concordant and discordant pairs.
The probandwise concordance rate (PBp) is defined as the proportion of affected cotwins of probands (40). It expresses the probability that one twin in a pair is affected given that his/her cotwin is affected (39). PBp is the most important measure of concordance and is directly comparable to disease risk rates in the background population and to estimates of recurrence risk in relatives (41). Owing to its robustness, it is also comparable from study to study. PBp is calculated as twice the number of pairs in which both twins are affected, divided by this number plus the number of pairs in which twins are disease discordant. Pp and PBp were estimated for MZ and DZ pairs separately under the assumption of complete ascertainment.
Tetrachoric correlations (or intrapair correlations) and heritability
Tetrachoric correlations by zygosity were estimated in twins with permanent and transient hypothyroidism. The tetrachoric correlation coefficient (rt) shows how strong (or weak) the association is between variables. A “0” indicates no agreement and a “1” represents a perfect agreement. In general, a coefficient >0.7 indicates a strong correlation between variables. In twin studies a higher correlation in MZ compared with DZ pairs points to genetic influences on liability to the trait.
Because the phenotypic variance of a trait can be attributed to genetic, common environmental, and unique (unshared) environmental effects, a twin analysis can be performed to quantify the importance of these effects (42). Genetic and environmental components of variance were estimated using structural equation modeling and assuming that MZ twins have identical genomes, DZ twins share on average 50% of their segregating genes, and that common environmental factors are shared completely by both zygosities, whereas unshared environmental influences act separately on each twin.
Full ACE models incorporating parameters for additive genetic (A), common environmental (C), and unique environmental influences (including measurements error, E) were considered. The proportions of the total variance that are attributable to the genetic (A, heritability) and environmental components (C, E) of variance and their confidence intervals were estimated by maximum likelihood under a “liability threshold” model (43). These analyses were performed using MX software (44).
Results
Among the 47 screening discordant twin pairs, 13 were MZ and 26 DZ. One MZ pair was derived from a triplet (F-F-F) in which the proband was MZ with a cotwin and DZ with the other. Zygosity was not assessed in eight twin pairs [unknown zygosity (UNZ)] because the newborn’s parents did not give their consent to DNA collection and did not respond to the questionnaire (Fig. 1).
Thirty-three (70.2%) of the 47 twin pairs were born preterm (<37 gestation weeks) and 13 of them (27.7%) were severely premature (≤33 gestation weeks). Information on medically assisted pregnancy (MAP) or spontaneous pregnancy was available in 21 of the 47 informative twin pairs. Eleven (52.4%) of 21 were from MAP and 10 from spontaneous pregnancy.
The median value of whole-blood TSH (bTSH) at screening was 20 mU/L (range, 2.3 to 946) in probands and 1.0 mU/L (range, 0.36 to 13.5) in cotwins. The probands with bTSH at screening less than the cutoff had a positive test at rescreening. Among the 11 pairs from MAP the median value of bTSH was 19.5 mU/L (range, 2.6 to 520) in probands and 1.0 mU/L (range, 0.36 to 1.0) in cotwins, whereas among the 10 pairs from spontaneous pregnancy the median bTSH value was 34.5 mU/L (range, 2.3 to 257) in probands and 1.0 (range, 1.0 to 2.7) in cotwins. Among the 26 couples with no information on the type of pregnancy, probands showed a median bTSH value of 21.7 mU/L (range, 7.0 to 946) and cotwins a median value of 3.4 mU/L (range, 0.4 to 13.5).
At the diagnosis confirmation, among the 54 CH affected twins (47 probands plus 7 cotwins who were diagnosed within the first month of life, see below), 15 were MZ (3 agenesis, 5 thyroid ectopy, 3 hemiagenesis/hypoplastic thyroid, 4 normal/hyperplastic thyroid), 28 were DZ (2 agenesis, 4 ectopy, 8 hemiagenesis, 14 normal/hyperplastic thyroid), and 11 were UNZ (3 hemiagenesis, 8 with normal/hyperplastic thyroid). No positivity for antithyroid autoantibodies was detected in all of the twin pairs.
Extrathyroidal congenital malformations were present in three probands (two DZ with interatrial defects; one UNZ with hypospadia) and in one otherwise healthy DZ cotwin showing anal atresia.
Pairs becoming concordant for CH within the first month of life (seven pairs)
Seven cotwins showed high serum TSH values when retested within the first month of life. Therefore, seven screening discordant twin pairs (two MZ, two DZ, three UNZ) became concordant for CH within the first month of life. Four of these pairs were born preterm (range, 32 to 36 gestational weeks) and three at term. No substantial differences in birth weight (2225 ± 704 and 2283 ± 664 g) and head circumference (31 ± 2.8 and 32 ± 2.9 cm) were observed between probands and their cotwins.
At confirmation of the diagnosis the seven probands (median bTSH, 16.7 mU/L; range, 11.0 to 200) showed a median sTSH value of 23 mU/L (range, 15.3 to 956) and a mean sFT4 concentration of 9.9 ± 3.7 pg/mL (range, 3.1 to 13.0), whereas the seven cotwins with negative screening (median bTSH, 5.1 mU/L; range, 1.0 to 8.2 mU/L) showed a sTSH median value of 13.0 mU/L (range, 8.6 to 113) and a mean sFT4 of 11.4 ± 1.9 pg/mL (range, 8.4 to 13.2) when retested within the first month of life (Table 1).
Blood TSH at Screening, Serum Hormone Values, and Results of Thyroid US and/or Scintigraphy in the Seven Screening Discordant Twin Pairs Becoming Concordant for CH Within the First Month of Life, and in the 40 Screening Discordant Twin Pairs Remaining Discordant at the First Month of Life
| . | Screening Discordant Twin Couples (N = 7) Becoming Concordant for CH Within the First Month of Life (2 MZ, 2 DZ, 3 UNZ) . | Screening Discordant Twin Couples (N = 40) Remaining Discordant for CH at the First Month of Life (11 MZ, 24 DZ, 5 UNZ) . | ||
|---|---|---|---|---|
| Screening Positive Probands . | Screening Negative Cotwins . | Screening Positive Probands . | Screening Negative Cotwins . | |
| Median blood TSH at screening, mU/L (range) | 16.7 (11.0–200) | 5.1 (1.0–8.2) | 22.3 (2.3–946) | 1.0 (0.4–13.5) |
| Median serum TSH, mU/L (range) | 23.0a (15.3–956) | 13.0a (8.6–113) | 85.5a (10.1–1800) | 2.0b (1.0–6.8) |
| Mean ± SD of sFT4, pg/mL (range) | 9.9 ± 3.7a (3.1–13.0) | 11.4 ± 1.9a (8.4–13.2) | 7.3 ± 5.1a (0.4–18.7) | 9.9 ± 3.3b (7.0–16.1) |
| Thyroid US and/or scintigraphy | 1 Hemiagenesis | 1 Hypoplasia | 5 Agenesis | 40 Normal thyroid |
| 1 Ectopy | 6 Normal thyroid | 2 Hemiagenesis | ||
| 5 Normal thyroid | 8 Ectopy | |||
| 10 Hypoplasia | ||||
| 15 Goiter/normal thyroid | ||||
| No. of preterm pairs (<37 wk gestation) | 4 | 29 | ||
| . | Screening Discordant Twin Couples (N = 7) Becoming Concordant for CH Within the First Month of Life (2 MZ, 2 DZ, 3 UNZ) . | Screening Discordant Twin Couples (N = 40) Remaining Discordant for CH at the First Month of Life (11 MZ, 24 DZ, 5 UNZ) . | ||
|---|---|---|---|---|
| Screening Positive Probands . | Screening Negative Cotwins . | Screening Positive Probands . | Screening Negative Cotwins . | |
| Median blood TSH at screening, mU/L (range) | 16.7 (11.0–200) | 5.1 (1.0–8.2) | 22.3 (2.3–946) | 1.0 (0.4–13.5) |
| Median serum TSH, mU/L (range) | 23.0a (15.3–956) | 13.0a (8.6–113) | 85.5a (10.1–1800) | 2.0b (1.0–6.8) |
| Mean ± SD of sFT4, pg/mL (range) | 9.9 ± 3.7a (3.1–13.0) | 11.4 ± 1.9a (8.4–13.2) | 7.3 ± 5.1a (0.4–18.7) | 9.9 ± 3.3b (7.0–16.1) |
| Thyroid US and/or scintigraphy | 1 Hemiagenesis | 1 Hypoplasia | 5 Agenesis | 40 Normal thyroid |
| 1 Ectopy | 6 Normal thyroid | 2 Hemiagenesis | ||
| 5 Normal thyroid | 8 Ectopy | |||
| 10 Hypoplasia | ||||
| 15 Goiter/normal thyroid | ||||
| No. of preterm pairs (<37 wk gestation) | 4 | 29 | ||
At confirmation of the diagnosis.
At 1 mo of life.
Blood TSH at Screening, Serum Hormone Values, and Results of Thyroid US and/or Scintigraphy in the Seven Screening Discordant Twin Pairs Becoming Concordant for CH Within the First Month of Life, and in the 40 Screening Discordant Twin Pairs Remaining Discordant at the First Month of Life
| . | Screening Discordant Twin Couples (N = 7) Becoming Concordant for CH Within the First Month of Life (2 MZ, 2 DZ, 3 UNZ) . | Screening Discordant Twin Couples (N = 40) Remaining Discordant for CH at the First Month of Life (11 MZ, 24 DZ, 5 UNZ) . | ||
|---|---|---|---|---|
| Screening Positive Probands . | Screening Negative Cotwins . | Screening Positive Probands . | Screening Negative Cotwins . | |
| Median blood TSH at screening, mU/L (range) | 16.7 (11.0–200) | 5.1 (1.0–8.2) | 22.3 (2.3–946) | 1.0 (0.4–13.5) |
| Median serum TSH, mU/L (range) | 23.0a (15.3–956) | 13.0a (8.6–113) | 85.5a (10.1–1800) | 2.0b (1.0–6.8) |
| Mean ± SD of sFT4, pg/mL (range) | 9.9 ± 3.7a (3.1–13.0) | 11.4 ± 1.9a (8.4–13.2) | 7.3 ± 5.1a (0.4–18.7) | 9.9 ± 3.3b (7.0–16.1) |
| Thyroid US and/or scintigraphy | 1 Hemiagenesis | 1 Hypoplasia | 5 Agenesis | 40 Normal thyroid |
| 1 Ectopy | 6 Normal thyroid | 2 Hemiagenesis | ||
| 5 Normal thyroid | 8 Ectopy | |||
| 10 Hypoplasia | ||||
| 15 Goiter/normal thyroid | ||||
| No. of preterm pairs (<37 wk gestation) | 4 | 29 | ||
| . | Screening Discordant Twin Couples (N = 7) Becoming Concordant for CH Within the First Month of Life (2 MZ, 2 DZ, 3 UNZ) . | Screening Discordant Twin Couples (N = 40) Remaining Discordant for CH at the First Month of Life (11 MZ, 24 DZ, 5 UNZ) . | ||
|---|---|---|---|---|
| Screening Positive Probands . | Screening Negative Cotwins . | Screening Positive Probands . | Screening Negative Cotwins . | |
| Median blood TSH at screening, mU/L (range) | 16.7 (11.0–200) | 5.1 (1.0–8.2) | 22.3 (2.3–946) | 1.0 (0.4–13.5) |
| Median serum TSH, mU/L (range) | 23.0a (15.3–956) | 13.0a (8.6–113) | 85.5a (10.1–1800) | 2.0b (1.0–6.8) |
| Mean ± SD of sFT4, pg/mL (range) | 9.9 ± 3.7a (3.1–13.0) | 11.4 ± 1.9a (8.4–13.2) | 7.3 ± 5.1a (0.4–18.7) | 9.9 ± 3.3b (7.0–16.1) |
| Thyroid US and/or scintigraphy | 1 Hemiagenesis | 1 Hypoplasia | 5 Agenesis | 40 Normal thyroid |
| 1 Ectopy | 6 Normal thyroid | 2 Hemiagenesis | ||
| 5 Normal thyroid | 8 Ectopy | |||
| 10 Hypoplasia | ||||
| 15 Goiter/normal thyroid | ||||
| No. of preterm pairs (<37 wk gestation) | 4 | 29 | ||
At confirmation of the diagnosis.
At 1 mo of life.
Thyroid US and/or scintigraphy performed before starting the replacement therapy in the seven probands showed one thyroid hemiagenesis, one ectopy, and five normal glands, whereas the seven negative screening cotwins showed six normally located and shaped glands and one hypoplastic thyroid on thyroid US (Table 1). The cotwin with hypoplastic thyroid belonged to a MZ pair (M-M) in which the proband had thyroid hemiagenesis.
The seven probands started the replacement therapy at the mean age of 15.1 ± 6.9 days (range, 8 to 29), whereas the cotwins at the mean age of 24.6 ± 6.7 days (range, 13 to 30).
The reevaluation of the diagnosis was performed in the 11 twins with normal thyroid as shown by thyroid US or scintigraphy. Among these, four probands and their cotwins (one MZ and three UNZ pairs) had transient hypothyroidism, whereas the remaining three reevaluated twins (one MZ and two DZ) had permanent CH (Fig. 1; Table 2).
Details of the Seven Screening Discordant Twin Pairs Becoming Concordant for CH Within the First Month of Life
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|
| PB1 | 3 | F | DZ | Ectopic | Perm | • DUOX2 p.R726W MAF ND |
| • DUOX2 p.P303R MAF 0.0036 | ||||||
| • IYD p.K31E MAF ND | ||||||
| CT1 | M | DZ | Normal | Perm | • DUOX2 p.R726W MAF ND | |
| • IYD p.K31E MAF ND | ||||||
| • TG p.G653D MAF 0.0036 | ||||||
| PB2 | 6 | F | DZ | Normal | Perm | • TPO:p.R584W MAF ND |
| CT2 | M | DZ | Normal | Perm | • TPO:p.R584W MAF ND | |
| PB3 | 5 | M | MZ | Hemiagenesis | Perm | • WT |
| CT3 | M | MZ | Hypoplastic | Perm | • WT | |
| PB4 | 3 | F | MZ | Normal | Trans | • WT |
| CT4 | F | MZ | Normal | Trans | • WT | |
| PB5 | 9 | M | UNZ | Normal | Trans | • No DNA |
| CT5 | M | UNZ | Normal | Trans | • No DNA | |
| PB6 | 4 | M | UNZ | Normal | Trans | • No DNA |
| CT6 | M | UNZ | Normal | Trans | • No DNA | |
| PB7 | 4 | M | UNZ | Normal | Trans | • TSHR p.P68S MAF 0.0004 |
| CT7 | M | UNZ | Normal | Trans | • No DNA |
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|
| PB1 | 3 | F | DZ | Ectopic | Perm | • DUOX2 p.R726W MAF ND |
| • DUOX2 p.P303R MAF 0.0036 | ||||||
| • IYD p.K31E MAF ND | ||||||
| CT1 | M | DZ | Normal | Perm | • DUOX2 p.R726W MAF ND | |
| • IYD p.K31E MAF ND | ||||||
| • TG p.G653D MAF 0.0036 | ||||||
| PB2 | 6 | F | DZ | Normal | Perm | • TPO:p.R584W MAF ND |
| CT2 | M | DZ | Normal | Perm | • TPO:p.R584W MAF ND | |
| PB3 | 5 | M | MZ | Hemiagenesis | Perm | • WT |
| CT3 | M | MZ | Hypoplastic | Perm | • WT | |
| PB4 | 3 | F | MZ | Normal | Trans | • WT |
| CT4 | F | MZ | Normal | Trans | • WT | |
| PB5 | 9 | M | UNZ | Normal | Trans | • No DNA |
| CT5 | M | UNZ | Normal | Trans | • No DNA | |
| PB6 | 4 | M | UNZ | Normal | Trans | • No DNA |
| CT6 | M | UNZ | Normal | Trans | • No DNA | |
| PB7 | 4 | M | UNZ | Normal | Trans | • TSHR p.P68S MAF 0.0004 |
| CT7 | M | UNZ | Normal | Trans | • No DNA |
Abbreviations: ND, not determined; Perm, permanent; Trans, transient.
Details of the Seven Screening Discordant Twin Pairs Becoming Concordant for CH Within the First Month of Life
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|
| PB1 | 3 | F | DZ | Ectopic | Perm | • DUOX2 p.R726W MAF ND |
| • DUOX2 p.P303R MAF 0.0036 | ||||||
| • IYD p.K31E MAF ND | ||||||
| CT1 | M | DZ | Normal | Perm | • DUOX2 p.R726W MAF ND | |
| • IYD p.K31E MAF ND | ||||||
| • TG p.G653D MAF 0.0036 | ||||||
| PB2 | 6 | F | DZ | Normal | Perm | • TPO:p.R584W MAF ND |
| CT2 | M | DZ | Normal | Perm | • TPO:p.R584W MAF ND | |
| PB3 | 5 | M | MZ | Hemiagenesis | Perm | • WT |
| CT3 | M | MZ | Hypoplastic | Perm | • WT | |
| PB4 | 3 | F | MZ | Normal | Trans | • WT |
| CT4 | F | MZ | Normal | Trans | • WT | |
| PB5 | 9 | M | UNZ | Normal | Trans | • No DNA |
| CT5 | M | UNZ | Normal | Trans | • No DNA | |
| PB6 | 4 | M | UNZ | Normal | Trans | • No DNA |
| CT6 | M | UNZ | Normal | Trans | • No DNA | |
| PB7 | 4 | M | UNZ | Normal | Trans | • TSHR p.P68S MAF 0.0004 |
| CT7 | M | UNZ | Normal | Trans | • No DNA |
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|
| PB1 | 3 | F | DZ | Ectopic | Perm | • DUOX2 p.R726W MAF ND |
| • DUOX2 p.P303R MAF 0.0036 | ||||||
| • IYD p.K31E MAF ND | ||||||
| CT1 | M | DZ | Normal | Perm | • DUOX2 p.R726W MAF ND | |
| • IYD p.K31E MAF ND | ||||||
| • TG p.G653D MAF 0.0036 | ||||||
| PB2 | 6 | F | DZ | Normal | Perm | • TPO:p.R584W MAF ND |
| CT2 | M | DZ | Normal | Perm | • TPO:p.R584W MAF ND | |
| PB3 | 5 | M | MZ | Hemiagenesis | Perm | • WT |
| CT3 | M | MZ | Hypoplastic | Perm | • WT | |
| PB4 | 3 | F | MZ | Normal | Trans | • WT |
| CT4 | F | MZ | Normal | Trans | • WT | |
| PB5 | 9 | M | UNZ | Normal | Trans | • No DNA |
| CT5 | M | UNZ | Normal | Trans | • No DNA | |
| PB6 | 4 | M | UNZ | Normal | Trans | • No DNA |
| CT6 | M | UNZ | Normal | Trans | • No DNA | |
| PB7 | 4 | M | UNZ | Normal | Trans | • TSHR p.P68S MAF 0.0004 |
| CT7 | M | UNZ | Normal | Trans | • No DNA |
Abbreviations: ND, not determined; Perm, permanent; Trans, transient.
Pairs remaining discordant for CH at the first month of life (40 pairs)
Forty of the 47 informative twin pairs remained discordant for CH at the first month of life. Among these, 11 were MZ, 24 DZ, and 5 UNZ (Fig. 1).
A linear regression model was performed to compare birth weight and head circumference between the 40 probands and their cotwins. After adjusting by gestational age, the birth weight was significantly lower (β = −182.5; P = 0.003) in probands than in cotwins (1887 ± 621 and 2112 ± 519 g, respectively), whereas no significant difference in head circumference was observed between probands and their cotwins (31.2 ± 2.6 and 32.0 ± 1.8 cm, respectively). A parametric MZ-DZ discordant twin analysis was then carried out to verify whether zygosity might affect differences between probands and cotwins. Birth weight was similar in MZ probands and cotwins (2048.2 ± 459.5 and 2135.5 ± 468.1 g, respectively), whereas it was significantly lower (P = 0.005) in DZ probands than in their cotwins (1863.2 ± 705.7 and 2108.0 ± 541.5 g). No significant difference in head circumference was observed between probands and cotwins even though stratified by zygosity.
At screening, the median bTSH value was 22.3 mU/L (range, 2.3 to 946) in the 40 probands and 1.0 mU/L (range, 0.4 to 13.5) in their cotwins. At confirmation of the diagnosis the 40 probands showed a median sTSH value of 85.5 mU/L (range, 10 to 1800) and a mean sFT4 of 7.3 ± 5.1 pg/mL (range, 0.4 to 18.7), whereas the healthy cotwins showed a median sTSH of 2.0 mU/L (range, 1.0 to 6.8) within the first month of life (Table 1).
Thyroid US and/or scintigraphy were performed before starting the replacement therapy in all probands. Five showed thyroid agenesis (12.5%), 8 ectopic thyroid (20%), 2 hemiagenesis (5%), 10 hypoplasia (25%), and 15 showed normal/hyperplastic thyroid (37.5%). During long-term follow-up at least one thyroid US was performed in the 40 cotwins who were negative at screening and retest. All of these children showed a normally located and shaped gland.
Concordance rates for CH confirmed within the first month of life
Pp for confirmed CH was calculated to estimate the probability to find concordant twin pairs at retest within the first month of life. Pp was 14.9% (95% CI, 4.7 to 25.1) with no significant differences between MZ (15.4%; 95% CI, 0 to 35.0) and DZ twin pairs (7.7%; 95% CI, 0 to 17.9).
PBp for confirmed CH was also calculated to estimate the probability that a cotwin with a negative screening test had a diagnosis of CH within the first month of life. PBp was 25.9% (95% CI, 10.5 to 41.3), and also in this case no significant differences were found between MZ (26.7%; 95% CI, 0 to 56.1) and DZ twin pairs (14.3%; 95% CI, 0 to 31.9).
Long-term follow-up of the 40 twin pairs remaining discordant at the first month of life
Among the 40 twin pairs discordant at the first month of life, 6 probands (1 MZ, 4 DZ, 1 UNZ) showed transient hypothyroidism after reevaluation of the diagnosis (Fig. 1; Table 3), and 32 screening discordant twin pairs (8 MZ, 20 DZ, 4 UNZ) remained discordant for hypothyroidism during long-term follow-up (range, 3 to 21 years) (Fig. 1), whereas hypothyroidism was diagnosed in 2 MZ cotwins at the age of 9 months and 12 years, respectively.
Details of the Six Screening Discordant Twin Pairs Whose Proband Was Diagnosed With Transient Hypothyroidism After Reevaluation of the Diagnosis
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|
| PB8 | 11 | F | MZa | Normal | Trans | • No DNA |
| CT8 | F | MZa | Normal | Healthy | • No DNA | |
| PB9 | 11 | M | DZ | Normal | Trans | • WT |
| CT9 | M | DZ | Normal | Healthy | • WT | |
| PB10 | 4 | F | DZ | Normal | Trans | • GLIS3 p.E515D MAF 0.002 |
| CT10 | M | DZ | Normal | Healthy | • WT | |
| PB11 | 5 | F | DZ | Normal | Trans | • DUOX2 p.A728T MAF 0.0078 |
| • SLC26A4 IVS6+4bp A>C MAF ND | ||||||
| • PAX8 p.K135R MAF 0.0002 | ||||||
| CT11 | F | DZ | Normal | Healthy | • WT | |
| PB12 | 16 | F | DZ | Normal | Trans | • No DNA |
| CT12 | M | DZ | Normal | Healthy | • No DNA | |
| PB13 | 11 | F | UNZ | Normal | Trans | • No DNA |
| CT13 | F | Normal | Healthy | • No DNA |
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|
| PB8 | 11 | F | MZa | Normal | Trans | • No DNA |
| CT8 | F | MZa | Normal | Healthy | • No DNA | |
| PB9 | 11 | M | DZ | Normal | Trans | • WT |
| CT9 | M | DZ | Normal | Healthy | • WT | |
| PB10 | 4 | F | DZ | Normal | Trans | • GLIS3 p.E515D MAF 0.002 |
| CT10 | M | DZ | Normal | Healthy | • WT | |
| PB11 | 5 | F | DZ | Normal | Trans | • DUOX2 p.A728T MAF 0.0078 |
| • SLC26A4 IVS6+4bp A>C MAF ND | ||||||
| • PAX8 p.K135R MAF 0.0002 | ||||||
| CT11 | F | DZ | Normal | Healthy | • WT | |
| PB12 | 16 | F | DZ | Normal | Trans | • No DNA |
| CT12 | M | DZ | Normal | Healthy | • No DNA | |
| PB13 | 11 | F | UNZ | Normal | Trans | • No DNA |
| CT13 | F | Normal | Healthy | • No DNA |
Abbreviations: ND, not determined; Perm, permanent; Trans, transient.
Zygosity ascertained by means of questionnaire.
Details of the Six Screening Discordant Twin Pairs Whose Proband Was Diagnosed With Transient Hypothyroidism After Reevaluation of the Diagnosis
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|
| PB8 | 11 | F | MZa | Normal | Trans | • No DNA |
| CT8 | F | MZa | Normal | Healthy | • No DNA | |
| PB9 | 11 | M | DZ | Normal | Trans | • WT |
| CT9 | M | DZ | Normal | Healthy | • WT | |
| PB10 | 4 | F | DZ | Normal | Trans | • GLIS3 p.E515D MAF 0.002 |
| CT10 | M | DZ | Normal | Healthy | • WT | |
| PB11 | 5 | F | DZ | Normal | Trans | • DUOX2 p.A728T MAF 0.0078 |
| • SLC26A4 IVS6+4bp A>C MAF ND | ||||||
| • PAX8 p.K135R MAF 0.0002 | ||||||
| CT11 | F | DZ | Normal | Healthy | • WT | |
| PB12 | 16 | F | DZ | Normal | Trans | • No DNA |
| CT12 | M | DZ | Normal | Healthy | • No DNA | |
| PB13 | 11 | F | UNZ | Normal | Trans | • No DNA |
| CT13 | F | Normal | Healthy | • No DNA |
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|
| PB8 | 11 | F | MZa | Normal | Trans | • No DNA |
| CT8 | F | MZa | Normal | Healthy | • No DNA | |
| PB9 | 11 | M | DZ | Normal | Trans | • WT |
| CT9 | M | DZ | Normal | Healthy | • WT | |
| PB10 | 4 | F | DZ | Normal | Trans | • GLIS3 p.E515D MAF 0.002 |
| CT10 | M | DZ | Normal | Healthy | • WT | |
| PB11 | 5 | F | DZ | Normal | Trans | • DUOX2 p.A728T MAF 0.0078 |
| • SLC26A4 IVS6+4bp A>C MAF ND | ||||||
| • PAX8 p.K135R MAF 0.0002 | ||||||
| CT11 | F | DZ | Normal | Healthy | • WT | |
| PB12 | 16 | F | DZ | Normal | Trans | • No DNA |
| CT12 | M | DZ | Normal | Healthy | • No DNA | |
| PB13 | 11 | F | UNZ | Normal | Trans | • No DNA |
| CT13 | F | Normal | Healthy | • No DNA |
Abbreviations: ND, not determined; Perm, permanent; Trans, transient.
Zygosity ascertained by means of questionnaire.
In one of the two MZ pairs in which the cotwin developed hypothyroidism during follow-up, both the proband and the cotwin (F-F) had a normally located and shaped thyroid (Table 4). At the age of 9 months the screening negative cotwin showed a stunted growth (body weight at the 10th percentile, height at the 3rd percentile) with a sTSH value of 6.1 mU/L and a sFT4 of 1.1 pg/mL. This twin pair showed two allelic variants (AVs) in two different genes, DUOX2 and TG (Table 4). The cotwin started l-thyroxine treatment at the age of 9 months with a following catch-up growth (body weight at the 10th percentile, height at the 50th percentile steadily during follow-up). Both of the twins were TGAb and TPOAb negative and were still on treatment after 12 years of follow-up, at the time of the study. In the second MZ pair (F-F) the proband showed ectopic thyroid, whereas the cotwin had normal thyroid. Also, this cotwin presented to the pediatric endocrinologist at the age of 12 years with a stunted growth (body weight <3rd percentile, height at the 3rd percentile), normal sTSH (1.91 mU/L), and borderline sFT4 (0.77 pg/mL). This twin pair showed one AV in the GLIS3 gene (Table 4). The cotwin girl started l-thyroxine treatment with a following catch-up growth (body weight and height at the 10th percentile, steadily during follow-up). Also, these girls were TGAb and TPOAb negative and were still on treatment at the time of the study. Both of these MZ pairs were considered discordant for CH when heritability for permanent and transient CH was estimated (see below).
Details of the Two MZ Screening Discordant Twin Pairs Whose Cotwins Were Diagnosed With Hypothyroidism During Long-Term Follow-Up
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Time of the Diagnosis in the Cotwin . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|---|
| PB14 | 12 | F | MZ | Normal | 9 mo | Perm | • DUOX2 p.V1078M MAF 0.0012 |
| • TG p.S523 MAF 0.0010 | |||||||
| CT14 | F | MZ | Normal | Acquired hypothyroidism | • DUOX2 p.V1078M MAF 0.0012 | ||
| • TG p.S523 MAF 0.0010 | |||||||
| PB15 | 21 | F | MZ | Ectopic | 12 y | Perm | • GLIS3 p.G313A MAF 0.0088 |
| CT15 | F | MZ | Normal | Acquired hypothyroidism | • GLIS3 p.G313A MAF 0.0088 |
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Time of the Diagnosis in the Cotwin . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|---|
| PB14 | 12 | F | MZ | Normal | 9 mo | Perm | • DUOX2 p.V1078M MAF 0.0012 |
| • TG p.S523 MAF 0.0010 | |||||||
| CT14 | F | MZ | Normal | Acquired hypothyroidism | • DUOX2 p.V1078M MAF 0.0012 | ||
| • TG p.S523 MAF 0.0010 | |||||||
| PB15 | 21 | F | MZ | Ectopic | 12 y | Perm | • GLIS3 p.G313A MAF 0.0088 |
| CT15 | F | MZ | Normal | Acquired hypothyroidism | • GLIS3 p.G313A MAF 0.0088 |
Abbreviations: Perm, permanent; Trans, transient.
Details of the Two MZ Screening Discordant Twin Pairs Whose Cotwins Were Diagnosed With Hypothyroidism During Long-Term Follow-Up
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Time of the Diagnosis in the Cotwin . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|---|
| PB14 | 12 | F | MZ | Normal | 9 mo | Perm | • DUOX2 p.V1078M MAF 0.0012 |
| • TG p.S523 MAF 0.0010 | |||||||
| CT14 | F | MZ | Normal | Acquired hypothyroidism | • DUOX2 p.V1078M MAF 0.0012 | ||
| • TG p.S523 MAF 0.0010 | |||||||
| PB15 | 21 | F | MZ | Ectopic | 12 y | Perm | • GLIS3 p.G313A MAF 0.0088 |
| CT15 | F | MZ | Normal | Acquired hypothyroidism | • GLIS3 p.G313A MAF 0.0088 |
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Time of the Diagnosis in the Cotwin . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|---|
| PB14 | 12 | F | MZ | Normal | 9 mo | Perm | • DUOX2 p.V1078M MAF 0.0012 |
| • TG p.S523 MAF 0.0010 | |||||||
| CT14 | F | MZ | Normal | Acquired hypothyroidism | • DUOX2 p.V1078M MAF 0.0012 | ||
| • TG p.S523 MAF 0.0010 | |||||||
| PB15 | 21 | F | MZ | Ectopic | 12 y | Perm | • GLIS3 p.G313A MAF 0.0088 |
| CT15 | F | MZ | Normal | Acquired hypothyroidism | • GLIS3 p.G313A MAF 0.0088 |
Abbreviations: Perm, permanent; Trans, transient.
The remaining 32 screening discordant twin pairs (8 MZ, 20 DZ, 4 UNZ) remained discordant for hypothyroidism during follow-up (Fig. 1). Note that in this group of twins there were eight MZ probands showing thyroid dysgenesis (four agenesis, four ectopic thyroid) (Table 5). Among the 20 DZ pairs remaining discordant at follow-up, 2 probands had agenesis, 3 ectopic thyroid, 1 hemiagenesis, 8 hypoplastic thyroid, and 6 had normal thyroid (Table 6). In the 4 CH discordant pairs with unknown zygosity, one proband showed hemiagenesis, one hypoplastic thyroid, and two normal thyroid (Table 7).
Details of the Eight Screening Discordant MZ Twin Pairs Remaining Discordant During Long-Term Follow-Up
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|
| PB16 | 18 | F | MZ | Agenesis | Perm | • DUOX2 p.Q556X MAF ND |
| CT16 | F | MZ | Normal | Healthy | • DUOX2 p.Q556X MAF ND | |
| PB17 | 5 | F | MZ | Agenesis | Perm | • WT |
| CT17 | F | MZ | Normal | Healthy | • WT | |
| PB18 | 21 | M | MZ | Agenesis | Perm | • WT |
| CT18 | M | MZ | Normal | Healthy | • WT | |
| PB19 | 14 | F | MZ | Agenesis | Perm | • WT |
| CT19 | F | MZ | Normal | Healthy | • WT | |
| PB20 | 15 | F | MZ | Ectopic | Perm | • WT |
| CT20 | F | MZ | Normal | Healthy | • WT | |
| PB21 | 8 | F | MZ | Ectopic | Perm | • SLC26A4:p.T410M MAF 0.0002 |
| • SLC26A4:p.V678V MAF ND | ||||||
| CT21 | F | MZ | Normal | Healthy | • SLC26A4:p.T410M MAF 0.0002 | |
| • SLC26A4:p.V678V MAF ND | ||||||
| PB22 | 11 | F | MZa | Ectopic | Perm | • No DNA |
| CT22 | F | MZa | Normal | Healthy | • No DNA | |
| PB23 | F | MZa | Ectopic | Perm | • No DNA | |
| CT23 | F | MZa | Normal | Healthy | • No DNA |
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|
| PB16 | 18 | F | MZ | Agenesis | Perm | • DUOX2 p.Q556X MAF ND |
| CT16 | F | MZ | Normal | Healthy | • DUOX2 p.Q556X MAF ND | |
| PB17 | 5 | F | MZ | Agenesis | Perm | • WT |
| CT17 | F | MZ | Normal | Healthy | • WT | |
| PB18 | 21 | M | MZ | Agenesis | Perm | • WT |
| CT18 | M | MZ | Normal | Healthy | • WT | |
| PB19 | 14 | F | MZ | Agenesis | Perm | • WT |
| CT19 | F | MZ | Normal | Healthy | • WT | |
| PB20 | 15 | F | MZ | Ectopic | Perm | • WT |
| CT20 | F | MZ | Normal | Healthy | • WT | |
| PB21 | 8 | F | MZ | Ectopic | Perm | • SLC26A4:p.T410M MAF 0.0002 |
| • SLC26A4:p.V678V MAF ND | ||||||
| CT21 | F | MZ | Normal | Healthy | • SLC26A4:p.T410M MAF 0.0002 | |
| • SLC26A4:p.V678V MAF ND | ||||||
| PB22 | 11 | F | MZa | Ectopic | Perm | • No DNA |
| CT22 | F | MZa | Normal | Healthy | • No DNA | |
| PB23 | F | MZa | Ectopic | Perm | • No DNA | |
| CT23 | F | MZa | Normal | Healthy | • No DNA |
Abbreviations: ND, not determined; Perm, permanent; Trans, transient.
Zygosity ascertained by means of questionnaire.
Details of the Eight Screening Discordant MZ Twin Pairs Remaining Discordant During Long-Term Follow-Up
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|
| PB16 | 18 | F | MZ | Agenesis | Perm | • DUOX2 p.Q556X MAF ND |
| CT16 | F | MZ | Normal | Healthy | • DUOX2 p.Q556X MAF ND | |
| PB17 | 5 | F | MZ | Agenesis | Perm | • WT |
| CT17 | F | MZ | Normal | Healthy | • WT | |
| PB18 | 21 | M | MZ | Agenesis | Perm | • WT |
| CT18 | M | MZ | Normal | Healthy | • WT | |
| PB19 | 14 | F | MZ | Agenesis | Perm | • WT |
| CT19 | F | MZ | Normal | Healthy | • WT | |
| PB20 | 15 | F | MZ | Ectopic | Perm | • WT |
| CT20 | F | MZ | Normal | Healthy | • WT | |
| PB21 | 8 | F | MZ | Ectopic | Perm | • SLC26A4:p.T410M MAF 0.0002 |
| • SLC26A4:p.V678V MAF ND | ||||||
| CT21 | F | MZ | Normal | Healthy | • SLC26A4:p.T410M MAF 0.0002 | |
| • SLC26A4:p.V678V MAF ND | ||||||
| PB22 | 11 | F | MZa | Ectopic | Perm | • No DNA |
| CT22 | F | MZa | Normal | Healthy | • No DNA | |
| PB23 | F | MZa | Ectopic | Perm | • No DNA | |
| CT23 | F | MZa | Normal | Healthy | • No DNA |
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|
| PB16 | 18 | F | MZ | Agenesis | Perm | • DUOX2 p.Q556X MAF ND |
| CT16 | F | MZ | Normal | Healthy | • DUOX2 p.Q556X MAF ND | |
| PB17 | 5 | F | MZ | Agenesis | Perm | • WT |
| CT17 | F | MZ | Normal | Healthy | • WT | |
| PB18 | 21 | M | MZ | Agenesis | Perm | • WT |
| CT18 | M | MZ | Normal | Healthy | • WT | |
| PB19 | 14 | F | MZ | Agenesis | Perm | • WT |
| CT19 | F | MZ | Normal | Healthy | • WT | |
| PB20 | 15 | F | MZ | Ectopic | Perm | • WT |
| CT20 | F | MZ | Normal | Healthy | • WT | |
| PB21 | 8 | F | MZ | Ectopic | Perm | • SLC26A4:p.T410M MAF 0.0002 |
| • SLC26A4:p.V678V MAF ND | ||||||
| CT21 | F | MZ | Normal | Healthy | • SLC26A4:p.T410M MAF 0.0002 | |
| • SLC26A4:p.V678V MAF ND | ||||||
| PB22 | 11 | F | MZa | Ectopic | Perm | • No DNA |
| CT22 | F | MZa | Normal | Healthy | • No DNA | |
| PB23 | F | MZa | Ectopic | Perm | • No DNA | |
| CT23 | F | MZa | Normal | Healthy | • No DNA |
Abbreviations: ND, not determined; Perm, permanent; Trans, transient.
Zygosity ascertained by means of questionnaire.
Details of the 20 DZ Screening Discordant Twin Pairs Remaining Discordant During Long-Term Follow-Up
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|
| PB24 | 15 | F | DZ | Agenesis | Perm | • WT |
| CT24 | F | DZ | Normal | Healthy | • No DNA | |
| PB25 | 3 | F | DZ | Agenesis | Perm | • No DNA |
| CT25 | M | DZ | Normal | Healthy | • No DNA | |
| PB26 | 20 | F | DZ | Ectopic | Perm | • WT |
| CT26 | M | DZ | Normal | Healthy | • WT | |
| PB27 | 14 | F | DZ | Ectopic | Perm | • DUOX2 p.R726W MAF ND |
| • GLIS3 p.P376S MAF 0.0078 | ||||||
| • GLIS3 p.P364S MAF 0.0022 | ||||||
| CT27 | F | DZ | Normal | Healthy | • GLIS3 p.P376S MAF 0.0078 | |
| PB28 | 15 | F | DZ | Ectopic | Perm | • WT |
| CT28 | F | DZ | Normal | Healthy | • No DNA | |
| PB29 | 6 | M | DZ | Hemiagenesis | Perm | • WT |
| CT29 | M | DZ | Normal | Healthy | • WT | |
| PB30 | 3 | F | DZ | Hypoplastic | Perm | • TPO p.P135H MAF 0.0088 |
| • SLC26A4 p.I455F MAF 0.0076 | ||||||
| CT30 | F | DZ | Normal | Healthy | • WT | |
| PB31 | 11 | F | DZ | Hypoplastic | Perm | • TG p.R2455L MAF 0.0012 |
| CT31 | F | DZ | Normal | Healthy | • WT | |
| PB32 | 11 | F | DZ | Hypoplastic | Perm | • TG p.R2455L MAF 0.0012 |
| CT32 | M | DZ | Normal | Healthy | • WT | |
| PB33 | 4 | F | DZ | Hypoplastic | Perm | • No DNA |
| CT33 | M | DZ | Normal | Healthy | • No DNA | |
| PB34 | 12 | M | DZ | Hypoplastic | Perm | • WT |
| CT34 | M | DZ | Normal | Healthy | • No DNA | |
| PB35 | 11 | F | DZ | Hypoplastic | Perm | • No DNA |
| CT35 | M | DZ | Normal | Healthy | • No DNA | |
| PB36 | 4 | F | DZ | Hypoplastic | Perm | • No DNA |
| CT36 | F | DZ | Normal | Healthy | • NO DNA | |
| PB37 | 6 | M | DZ | Normal | Perm | • WT |
| CT37 | M | DZ | Normal | Healthy | • TPO p.A419E MAF ND | |
| PB38 | 3 | M | DZ | Normal | Perm | • NKX2-1 p.A116D MAF ND |
| CT38 | M | DZ | Normal | Healthy | • WT | |
| PB39 | 10 | F | DZ | Normal | Perm | • No DNA |
| CT39 | M | DZ | Normal | Healthy | • No DNA | |
| PB40 | 3 | F | DZ | Normal | Perm | • IYD p.N108S MAF 0.0094 |
| CT40 | M | DZ | Normal | Healthy | • IYD p.N108S MAF 0.0094 | |
| PB41 | 10 | F | DZ | Hypoplastic | Perm CH | • DUOX2 p.E1546G MAF ND |
| • DUOX2 p.M866R MAF ND | ||||||
| • TG p.P1213L MAF ND | ||||||
| • SLC26A4 p.L597S MAF 0.0086 | ||||||
| CT41 | F | DZ | Normal | Healthy | • TG p.P1213L | |
| • SLC26A4 p.L597S MAF 0.0086 | ||||||
| PB42 | 8 | M | DZ | Normal | Perm | • TSHR p.P162A MAF 0.0004 |
| • TSHR p.S562G MAF ND | ||||||
| CT42 | M | DZ | Normal | Healthy | • TSHR p.S562G MAF ND | |
| PB43 | 8 | M | DZ | Normal | Perm | • TSHR p.P162A MAF 0.0004 |
| • TSHR p.S562G MAF ND | ||||||
| CT43 | M | DZ | Normal | Healthy | • TSHR p.S562G MAF ND |
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|
| PB24 | 15 | F | DZ | Agenesis | Perm | • WT |
| CT24 | F | DZ | Normal | Healthy | • No DNA | |
| PB25 | 3 | F | DZ | Agenesis | Perm | • No DNA |
| CT25 | M | DZ | Normal | Healthy | • No DNA | |
| PB26 | 20 | F | DZ | Ectopic | Perm | • WT |
| CT26 | M | DZ | Normal | Healthy | • WT | |
| PB27 | 14 | F | DZ | Ectopic | Perm | • DUOX2 p.R726W MAF ND |
| • GLIS3 p.P376S MAF 0.0078 | ||||||
| • GLIS3 p.P364S MAF 0.0022 | ||||||
| CT27 | F | DZ | Normal | Healthy | • GLIS3 p.P376S MAF 0.0078 | |
| PB28 | 15 | F | DZ | Ectopic | Perm | • WT |
| CT28 | F | DZ | Normal | Healthy | • No DNA | |
| PB29 | 6 | M | DZ | Hemiagenesis | Perm | • WT |
| CT29 | M | DZ | Normal | Healthy | • WT | |
| PB30 | 3 | F | DZ | Hypoplastic | Perm | • TPO p.P135H MAF 0.0088 |
| • SLC26A4 p.I455F MAF 0.0076 | ||||||
| CT30 | F | DZ | Normal | Healthy | • WT | |
| PB31 | 11 | F | DZ | Hypoplastic | Perm | • TG p.R2455L MAF 0.0012 |
| CT31 | F | DZ | Normal | Healthy | • WT | |
| PB32 | 11 | F | DZ | Hypoplastic | Perm | • TG p.R2455L MAF 0.0012 |
| CT32 | M | DZ | Normal | Healthy | • WT | |
| PB33 | 4 | F | DZ | Hypoplastic | Perm | • No DNA |
| CT33 | M | DZ | Normal | Healthy | • No DNA | |
| PB34 | 12 | M | DZ | Hypoplastic | Perm | • WT |
| CT34 | M | DZ | Normal | Healthy | • No DNA | |
| PB35 | 11 | F | DZ | Hypoplastic | Perm | • No DNA |
| CT35 | M | DZ | Normal | Healthy | • No DNA | |
| PB36 | 4 | F | DZ | Hypoplastic | Perm | • No DNA |
| CT36 | F | DZ | Normal | Healthy | • NO DNA | |
| PB37 | 6 | M | DZ | Normal | Perm | • WT |
| CT37 | M | DZ | Normal | Healthy | • TPO p.A419E MAF ND | |
| PB38 | 3 | M | DZ | Normal | Perm | • NKX2-1 p.A116D MAF ND |
| CT38 | M | DZ | Normal | Healthy | • WT | |
| PB39 | 10 | F | DZ | Normal | Perm | • No DNA |
| CT39 | M | DZ | Normal | Healthy | • No DNA | |
| PB40 | 3 | F | DZ | Normal | Perm | • IYD p.N108S MAF 0.0094 |
| CT40 | M | DZ | Normal | Healthy | • IYD p.N108S MAF 0.0094 | |
| PB41 | 10 | F | DZ | Hypoplastic | Perm CH | • DUOX2 p.E1546G MAF ND |
| • DUOX2 p.M866R MAF ND | ||||||
| • TG p.P1213L MAF ND | ||||||
| • SLC26A4 p.L597S MAF 0.0086 | ||||||
| CT41 | F | DZ | Normal | Healthy | • TG p.P1213L | |
| • SLC26A4 p.L597S MAF 0.0086 | ||||||
| PB42 | 8 | M | DZ | Normal | Perm | • TSHR p.P162A MAF 0.0004 |
| • TSHR p.S562G MAF ND | ||||||
| CT42 | M | DZ | Normal | Healthy | • TSHR p.S562G MAF ND | |
| PB43 | 8 | M | DZ | Normal | Perm | • TSHR p.P162A MAF 0.0004 |
| • TSHR p.S562G MAF ND | ||||||
| CT43 | M | DZ | Normal | Healthy | • TSHR p.S562G MAF ND |
Abbreviations: ND, not determined; Perm, permanent; Trans, transient.
Details of the 20 DZ Screening Discordant Twin Pairs Remaining Discordant During Long-Term Follow-Up
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|
| PB24 | 15 | F | DZ | Agenesis | Perm | • WT |
| CT24 | F | DZ | Normal | Healthy | • No DNA | |
| PB25 | 3 | F | DZ | Agenesis | Perm | • No DNA |
| CT25 | M | DZ | Normal | Healthy | • No DNA | |
| PB26 | 20 | F | DZ | Ectopic | Perm | • WT |
| CT26 | M | DZ | Normal | Healthy | • WT | |
| PB27 | 14 | F | DZ | Ectopic | Perm | • DUOX2 p.R726W MAF ND |
| • GLIS3 p.P376S MAF 0.0078 | ||||||
| • GLIS3 p.P364S MAF 0.0022 | ||||||
| CT27 | F | DZ | Normal | Healthy | • GLIS3 p.P376S MAF 0.0078 | |
| PB28 | 15 | F | DZ | Ectopic | Perm | • WT |
| CT28 | F | DZ | Normal | Healthy | • No DNA | |
| PB29 | 6 | M | DZ | Hemiagenesis | Perm | • WT |
| CT29 | M | DZ | Normal | Healthy | • WT | |
| PB30 | 3 | F | DZ | Hypoplastic | Perm | • TPO p.P135H MAF 0.0088 |
| • SLC26A4 p.I455F MAF 0.0076 | ||||||
| CT30 | F | DZ | Normal | Healthy | • WT | |
| PB31 | 11 | F | DZ | Hypoplastic | Perm | • TG p.R2455L MAF 0.0012 |
| CT31 | F | DZ | Normal | Healthy | • WT | |
| PB32 | 11 | F | DZ | Hypoplastic | Perm | • TG p.R2455L MAF 0.0012 |
| CT32 | M | DZ | Normal | Healthy | • WT | |
| PB33 | 4 | F | DZ | Hypoplastic | Perm | • No DNA |
| CT33 | M | DZ | Normal | Healthy | • No DNA | |
| PB34 | 12 | M | DZ | Hypoplastic | Perm | • WT |
| CT34 | M | DZ | Normal | Healthy | • No DNA | |
| PB35 | 11 | F | DZ | Hypoplastic | Perm | • No DNA |
| CT35 | M | DZ | Normal | Healthy | • No DNA | |
| PB36 | 4 | F | DZ | Hypoplastic | Perm | • No DNA |
| CT36 | F | DZ | Normal | Healthy | • NO DNA | |
| PB37 | 6 | M | DZ | Normal | Perm | • WT |
| CT37 | M | DZ | Normal | Healthy | • TPO p.A419E MAF ND | |
| PB38 | 3 | M | DZ | Normal | Perm | • NKX2-1 p.A116D MAF ND |
| CT38 | M | DZ | Normal | Healthy | • WT | |
| PB39 | 10 | F | DZ | Normal | Perm | • No DNA |
| CT39 | M | DZ | Normal | Healthy | • No DNA | |
| PB40 | 3 | F | DZ | Normal | Perm | • IYD p.N108S MAF 0.0094 |
| CT40 | M | DZ | Normal | Healthy | • IYD p.N108S MAF 0.0094 | |
| PB41 | 10 | F | DZ | Hypoplastic | Perm CH | • DUOX2 p.E1546G MAF ND |
| • DUOX2 p.M866R MAF ND | ||||||
| • TG p.P1213L MAF ND | ||||||
| • SLC26A4 p.L597S MAF 0.0086 | ||||||
| CT41 | F | DZ | Normal | Healthy | • TG p.P1213L | |
| • SLC26A4 p.L597S MAF 0.0086 | ||||||
| PB42 | 8 | M | DZ | Normal | Perm | • TSHR p.P162A MAF 0.0004 |
| • TSHR p.S562G MAF ND | ||||||
| CT42 | M | DZ | Normal | Healthy | • TSHR p.S562G MAF ND | |
| PB43 | 8 | M | DZ | Normal | Perm | • TSHR p.P162A MAF 0.0004 |
| • TSHR p.S562G MAF ND | ||||||
| CT43 | M | DZ | Normal | Healthy | • TSHR p.S562G MAF ND |
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|
| PB24 | 15 | F | DZ | Agenesis | Perm | • WT |
| CT24 | F | DZ | Normal | Healthy | • No DNA | |
| PB25 | 3 | F | DZ | Agenesis | Perm | • No DNA |
| CT25 | M | DZ | Normal | Healthy | • No DNA | |
| PB26 | 20 | F | DZ | Ectopic | Perm | • WT |
| CT26 | M | DZ | Normal | Healthy | • WT | |
| PB27 | 14 | F | DZ | Ectopic | Perm | • DUOX2 p.R726W MAF ND |
| • GLIS3 p.P376S MAF 0.0078 | ||||||
| • GLIS3 p.P364S MAF 0.0022 | ||||||
| CT27 | F | DZ | Normal | Healthy | • GLIS3 p.P376S MAF 0.0078 | |
| PB28 | 15 | F | DZ | Ectopic | Perm | • WT |
| CT28 | F | DZ | Normal | Healthy | • No DNA | |
| PB29 | 6 | M | DZ | Hemiagenesis | Perm | • WT |
| CT29 | M | DZ | Normal | Healthy | • WT | |
| PB30 | 3 | F | DZ | Hypoplastic | Perm | • TPO p.P135H MAF 0.0088 |
| • SLC26A4 p.I455F MAF 0.0076 | ||||||
| CT30 | F | DZ | Normal | Healthy | • WT | |
| PB31 | 11 | F | DZ | Hypoplastic | Perm | • TG p.R2455L MAF 0.0012 |
| CT31 | F | DZ | Normal | Healthy | • WT | |
| PB32 | 11 | F | DZ | Hypoplastic | Perm | • TG p.R2455L MAF 0.0012 |
| CT32 | M | DZ | Normal | Healthy | • WT | |
| PB33 | 4 | F | DZ | Hypoplastic | Perm | • No DNA |
| CT33 | M | DZ | Normal | Healthy | • No DNA | |
| PB34 | 12 | M | DZ | Hypoplastic | Perm | • WT |
| CT34 | M | DZ | Normal | Healthy | • No DNA | |
| PB35 | 11 | F | DZ | Hypoplastic | Perm | • No DNA |
| CT35 | M | DZ | Normal | Healthy | • No DNA | |
| PB36 | 4 | F | DZ | Hypoplastic | Perm | • No DNA |
| CT36 | F | DZ | Normal | Healthy | • NO DNA | |
| PB37 | 6 | M | DZ | Normal | Perm | • WT |
| CT37 | M | DZ | Normal | Healthy | • TPO p.A419E MAF ND | |
| PB38 | 3 | M | DZ | Normal | Perm | • NKX2-1 p.A116D MAF ND |
| CT38 | M | DZ | Normal | Healthy | • WT | |
| PB39 | 10 | F | DZ | Normal | Perm | • No DNA |
| CT39 | M | DZ | Normal | Healthy | • No DNA | |
| PB40 | 3 | F | DZ | Normal | Perm | • IYD p.N108S MAF 0.0094 |
| CT40 | M | DZ | Normal | Healthy | • IYD p.N108S MAF 0.0094 | |
| PB41 | 10 | F | DZ | Hypoplastic | Perm CH | • DUOX2 p.E1546G MAF ND |
| • DUOX2 p.M866R MAF ND | ||||||
| • TG p.P1213L MAF ND | ||||||
| • SLC26A4 p.L597S MAF 0.0086 | ||||||
| CT41 | F | DZ | Normal | Healthy | • TG p.P1213L | |
| • SLC26A4 p.L597S MAF 0.0086 | ||||||
| PB42 | 8 | M | DZ | Normal | Perm | • TSHR p.P162A MAF 0.0004 |
| • TSHR p.S562G MAF ND | ||||||
| CT42 | M | DZ | Normal | Healthy | • TSHR p.S562G MAF ND | |
| PB43 | 8 | M | DZ | Normal | Perm | • TSHR p.P162A MAF 0.0004 |
| • TSHR p.S562G MAF ND | ||||||
| CT43 | M | DZ | Normal | Healthy | • TSHR p.S562G MAF ND |
Abbreviations: ND, not determined; Perm, permanent; Trans, transient.
Details of the Four Screening Discordant Twin Pairs With UNZ Remaining Discordant During Long-Term Follow-Up
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|
| PB44 | 4 | M | UNZ | Normal | Perm | • No DNA |
| CT44 | M | Normal | Healthy | • No DNA | ||
| PB45 | 8 | M | UNZ | Normal | Perm | • TSHR P162A MAF 0.0004 |
| • SLC26A4 p.G6V MAF 0.008 | ||||||
| CT45 | M | Normal | Healthy | • No DNA | ||
| PB46 | 14 | F | UNZ | Hemiagenesis | Perm | • No DNA |
| CT46 | F | Normal | Healthy | • No DNA | ||
| PB47 | 3 | F | UNZ | Hypoplastic | Perm | • No DNA |
| CT47 | F | Normal | Healthy | • No DNA |
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|
| PB44 | 4 | M | UNZ | Normal | Perm | • No DNA |
| CT44 | M | Normal | Healthy | • No DNA | ||
| PB45 | 8 | M | UNZ | Normal | Perm | • TSHR P162A MAF 0.0004 |
| • SLC26A4 p.G6V MAF 0.008 | ||||||
| CT45 | M | Normal | Healthy | • No DNA | ||
| PB46 | 14 | F | UNZ | Hemiagenesis | Perm | • No DNA |
| CT46 | F | Normal | Healthy | • No DNA | ||
| PB47 | 3 | F | UNZ | Hypoplastic | Perm | • No DNA |
| CT47 | F | Normal | Healthy | • No DNA |
Abbreviations: Perm, permanent; Trans, transient.
Details of the Four Screening Discordant Twin Pairs With UNZ Remaining Discordant During Long-Term Follow-Up
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|
| PB44 | 4 | M | UNZ | Normal | Perm | • No DNA |
| CT44 | M | Normal | Healthy | • No DNA | ||
| PB45 | 8 | M | UNZ | Normal | Perm | • TSHR P162A MAF 0.0004 |
| • SLC26A4 p.G6V MAF 0.008 | ||||||
| CT45 | M | Normal | Healthy | • No DNA | ||
| PB46 | 14 | F | UNZ | Hemiagenesis | Perm | • No DNA |
| CT46 | F | Normal | Healthy | • No DNA | ||
| PB47 | 3 | F | UNZ | Hypoplastic | Perm | • No DNA |
| CT47 | F | Normal | Healthy | • No DNA |
| Twin Pairs . | Follow-Up, y . | Sex . | Zygosity . | CH Diagnosis . | Perm/Trans CH . | NGS Analysis . |
|---|---|---|---|---|---|---|
| PB44 | 4 | M | UNZ | Normal | Perm | • No DNA |
| CT44 | M | Normal | Healthy | • No DNA | ||
| PB45 | 8 | M | UNZ | Normal | Perm | • TSHR P162A MAF 0.0004 |
| • SLC26A4 p.G6V MAF 0.008 | ||||||
| CT45 | M | Normal | Healthy | • No DNA | ||
| PB46 | 14 | F | UNZ | Hemiagenesis | Perm | • No DNA |
| CT46 | F | Normal | Healthy | • No DNA | ||
| PB47 | 3 | F | UNZ | Hypoplastic | Perm | • No DNA |
| CT47 | F | Normal | Healthy | • No DNA |
Abbreviations: Perm, permanent; Trans, transient.
Intrapair (tetrachoric) correlations and heritability
Similar tetrachoric coefficients were found in MZ (rt = 0.64; 95% CI, 0.28 to 0.87) and DZ pairs (rt = 0.64; 95% CI, 0.38 to 0.82) with permanent CH, whereas the coefficient was higher in MZ than in DZ twin pairs with transient CH (rt = 0.95; 95% CI, 0.60 to 0.99 and rt=0; 95% CI, 0 to 0.91), indicating the presence of genetic effects in this form of the disease (Table 8).
Estimates of Tetrachoric Correlations by Zygosity and Additive Genetic (A), Common Environmental (C), and Unshared Environmental (E) Variance Components In CH Liability
| . | Frequencies (Pairs) . | rt (95% CI) . | Variance Component Under Full Model(95% CI) . | |||||
|---|---|---|---|---|---|---|---|---|
| MZ . | DZ . | MZ . | DZ . | Testa . | A . | C . | E . | |
| Total sample (permanent + transient CH) | Concordant = 2 | Concordant = 2 | 0.72 | 0.60 | P = 0.47 | 0.24 | 0.48 | 0.28 |
| Discordant = 11 | Discordant = 24 | (0.44–0.90) | (0.34–0.79) | (0.00–0.70) | (0.00–0.78) | (0.10–0.52) | ||
| Transient CH | Concordant = 1 | Concordant = 0 | 0.95 | 0 | P = 0.11 | 0.95 | 0.00 | 0.05 |
| Discordant = 1 | Discordant = 4 | (0.60–0.99) | (0–0.91) | (0.00–0.96) | (0.00–0.91) | (0.001–0.09) | ||
| Permanent CH | Concordant = 1 | Concordant = 2 | 0.64 | 0.64 | P = 1.00 | 0 | 0.64 | 0.36 |
| Discordant= 10b | Discordant = 20 | (0.28–0.87) | (0.38–0.82) | (0.00–0.93) | (0.06–0.79) | (0.13–0.57) | ||
| . | Frequencies (Pairs) . | rt (95% CI) . | Variance Component Under Full Model(95% CI) . | |||||
|---|---|---|---|---|---|---|---|---|
| MZ . | DZ . | MZ . | DZ . | Testa . | A . | C . | E . | |
| Total sample (permanent + transient CH) | Concordant = 2 | Concordant = 2 | 0.72 | 0.60 | P = 0.47 | 0.24 | 0.48 | 0.28 |
| Discordant = 11 | Discordant = 24 | (0.44–0.90) | (0.34–0.79) | (0.00–0.70) | (0.00–0.78) | (0.10–0.52) | ||
| Transient CH | Concordant = 1 | Concordant = 0 | 0.95 | 0 | P = 0.11 | 0.95 | 0.00 | 0.05 |
| Discordant = 1 | Discordant = 4 | (0.60–0.99) | (0–0.91) | (0.00–0.96) | (0.00–0.91) | (0.001–0.09) | ||
| Permanent CH | Concordant = 1 | Concordant = 2 | 0.64 | 0.64 | P = 1.00 | 0 | 0.64 | 0.36 |
| Discordant= 10b | Discordant = 20 | (0.28–0.87) | (0.38–0.82) | (0.00–0.93) | (0.06–0.79) | (0.13–0.57) | ||
Test for difference between MZ and DZ twins.
The two MZ pairs whose probands had permanent CH and cotwins developed hypothyroidism after the first month of life (at 9 mo and 12 y) were considered discordant for CH.
Estimates of Tetrachoric Correlations by Zygosity and Additive Genetic (A), Common Environmental (C), and Unshared Environmental (E) Variance Components In CH Liability
| . | Frequencies (Pairs) . | rt (95% CI) . | Variance Component Under Full Model(95% CI) . | |||||
|---|---|---|---|---|---|---|---|---|
| MZ . | DZ . | MZ . | DZ . | Testa . | A . | C . | E . | |
| Total sample (permanent + transient CH) | Concordant = 2 | Concordant = 2 | 0.72 | 0.60 | P = 0.47 | 0.24 | 0.48 | 0.28 |
| Discordant = 11 | Discordant = 24 | (0.44–0.90) | (0.34–0.79) | (0.00–0.70) | (0.00–0.78) | (0.10–0.52) | ||
| Transient CH | Concordant = 1 | Concordant = 0 | 0.95 | 0 | P = 0.11 | 0.95 | 0.00 | 0.05 |
| Discordant = 1 | Discordant = 4 | (0.60–0.99) | (0–0.91) | (0.00–0.96) | (0.00–0.91) | (0.001–0.09) | ||
| Permanent CH | Concordant = 1 | Concordant = 2 | 0.64 | 0.64 | P = 1.00 | 0 | 0.64 | 0.36 |
| Discordant= 10b | Discordant = 20 | (0.28–0.87) | (0.38–0.82) | (0.00–0.93) | (0.06–0.79) | (0.13–0.57) | ||
| . | Frequencies (Pairs) . | rt (95% CI) . | Variance Component Under Full Model(95% CI) . | |||||
|---|---|---|---|---|---|---|---|---|
| MZ . | DZ . | MZ . | DZ . | Testa . | A . | C . | E . | |
| Total sample (permanent + transient CH) | Concordant = 2 | Concordant = 2 | 0.72 | 0.60 | P = 0.47 | 0.24 | 0.48 | 0.28 |
| Discordant = 11 | Discordant = 24 | (0.44–0.90) | (0.34–0.79) | (0.00–0.70) | (0.00–0.78) | (0.10–0.52) | ||
| Transient CH | Concordant = 1 | Concordant = 0 | 0.95 | 0 | P = 0.11 | 0.95 | 0.00 | 0.05 |
| Discordant = 1 | Discordant = 4 | (0.60–0.99) | (0–0.91) | (0.00–0.96) | (0.00–0.91) | (0.001–0.09) | ||
| Permanent CH | Concordant = 1 | Concordant = 2 | 0.64 | 0.64 | P = 1.00 | 0 | 0.64 | 0.36 |
| Discordant= 10b | Discordant = 20 | (0.28–0.87) | (0.38–0.82) | (0.00–0.93) | (0.06–0.79) | (0.13–0.57) | ||
Test for difference between MZ and DZ twins.
The two MZ pairs whose probands had permanent CH and cotwins developed hypothyroidism after the first month of life (at 9 mo and 12 y) were considered discordant for CH.
These findings were consistent with outcomes of the ACE model including both genetic and environmental factors (shared and unshared). The model showed that 95% of the liability to transient CH is attributable to genetic factors, whereas individual specific environmental factors (not shared by the twins) account for the remaining 5%. The twin analysis also showed that 64% of liability to permanent CH is attributable to common environmental factors (shared during the intrauterine life), whereas individual specific environmental factors (e.g., epigenetic events) account for the remaining 36%.
NGS analysis
DNA was available in 36 of the 54 CH affected twins (47 probands plus 7 cotwins with a diagnosis of CH within the first month of life) and in 23 of the 40 screening negative cotwins. NGS analysis of the 14 genes identified 51 AVs. No significant difference was observed in the frequency of children with the wild-type (WT) genotype, one AV, and two or more AVs (χ2 = 5.72; P = 0.221) among screening negative cotwins and CH affected twins stratified by diagnosis (Fig. 2). Detailed results of NGS analysis are reported in Tables 2–7.

Percentage distribution of children with AVs in screening-negative cotwins and in CH-affected twins stratified by diagnosis.
Parental DNA was available only in a small number of parents, and therefore it was not possible to estimate the frequency of de novo and inherited variants.
Discussion
Twins have a high risk to develop CH, although twin pairs frequently show discordant results at screening (22, 23, 45). Nevertheless, the progressive adoption of repeat screening in at-risk categories of neonates has provided new insights over the years. Our multicenter study showed that the risk for CH in twins may be higher than previously reported and that the strategy of the second screening can unravel a hidden concordance. Specifically, the likelihood of both twins being affected was higher (Pp = 14.9%) than that estimated (4.3%) when the repeat screening strategy was not yet adopted (22). This finding is particularly relevant if we consider that the observed Pp value (14.9%) can be underestimated because the study design did not include screening concordant twin pairs. In this regard, a new study on data of the Italian National Registry of infants with CH is currently ongoing in our country to estimate the risk of CH in twin and singleton pregnancies after the adoption of repeat screening.
This multicenter study also showed a probability of 25.9% (PBp) that the initially screening negative cotwin becomes positive at retesting and is diagnosed with CH. Interestingly, no significant difference was found between MZ (26.7%; 95% CI, 0 to 56.1) and DZ twin pairs (14.3%; 95% CI, 0 to 31.9). Taken together, these findings suggest that not only fetal blood mixing in MZ twins but also other factors can be responsible for the high screening discordance rate in both MZ and DZ twin pairs. European guidelines on CH report that repeat screening is particularly recommended in the same sex twins, assuming them as MZ twins at risk for fetal blood mixing (25). Nevertheless, our results show that repeat sampling can be useful also in DZ twins, suggesting that this special procedure can be recommended also for twins with different sex.
This study allowed us to evaluate the contribution of genetic and environmental influences on liability to permanent and transient CH on the basis of follow-up results of discordant twin pairs. Both intrapair (tetrachoric) correlations and the etiological model, including additive genetic and environmental (shared and nonshared) factors, suggested the presence of genetic effects in transient CH. In fact, the intrapair correlations showed a significantly higher coefficient in MZ than in DZ pairs with transient CH. Accordingly, ACE model results showed that genetic factors account for 95% of liability to transient CH, whereas environmental factors explained the remaining 5% of the variance. These findings are consistent with previous studies reporting familial cases of transient CH, mostly due to mutations in the DUOX2 gene (46–48). In our study, one of the five twins with transient CH and NGS results was found to harbor oligogenic heterozygous defects not only in DUOX2, but also in PAX8 and SLC26A4 genes. These findings suggest that genetic defects with a minor loss of function can be associated with neonatal phenotype, but when the thyroid functional requirements diminish along life a spontaneous recovery may be possible.
Tetrachoric correlations and the ACE model also revealed the important role of nongenetic factors in the etiology of permanent CH. Particularly, our results showed that in screening discordant twin pairs common environmental factors (shared during the fetal life) explain the majority (64%) of the phenotypic variance of permanent CH, whereas about one third of the variance was explained by unshared environmental factors. Among factors shared during the fetal life, maternal thyroid status and iodine nutrition appear to be important risk factors in the etiology of permanent CH. Recent studies showed that maternal thyroid function may affect fetal growth and neonatal TSH concentration (49, 50). Additionally, a lower incidence of permanent CH has been reported in iodine-sufficient areas in comparison with mildly deficient ones (16). There is also evidence that exposure to chemical compounds with thyroid-disrupting activity during pregnancy may play a role in the etiology of the disease by acting at different levels (17, 21). Importantly, however, note that in our multicenter study heritability of permanent and transient CH was estimated using a relatively small sample of screening discordant twin pairs. This implies that the results obtained in this study are for guidance, and further studies are necessary to more accurately estimate the proportions of the total variance of permanent and transient CH attributable to genetic and environmental influences.
Among the 32 twin pairs remaining discordant for CH during follow-up, there were 8 MZ probands with thyroid dysgenesis (4 with agenesis and 4 with ectopic thyroid) that were negative at the NGS analysis. In fact, the genetic variants identified by NGS appeared completely devoid of potential effect in causing CH among the long-term discordant MZ twins. In these cases, only defects of thyroid morphogenesis were seen, thus giving a strong indication that thyroid dysgenesis does not frequently involve classic mechanisms of genetic inheritance. However, discordance in MZ twins is not exclusive of CH, being also reported for other medical conditions (51–54). In a recent study, Magne et al. (55) searched for genetic variants of thyroid dysgenesis under the hypothesis that high penetrance somatic mutations in protein-coding regions of the genome were responsible for the disorder. To test this hypothesis, they performed whole-exome sequencing in DNA from three MZ twin pairs discordant for CH with ectopic thyroid. This analysis showed an identical-coding genome in both probands and their cotwins, suggesting that molecular causes of thyroid dysgenesis might be either due to genomic changes outside the coding regions of the genome or due to yet unidentified epigenetic differences. Although MZ twins are called “genetically identical,” evidence for genetic and epigenetic differences within MZ twin pairs has accumulated (56), as well as for gene expression differences that are not dependent on genetic variants (57).
Results obtained in this study also indicate a beneficial effect of hormonal and auxological follow-up in the screening negative healthy cotwins, particularly when they are MZ. In fact, in 2 of the 10 MZ twin pairs discordant for permanent CH, both of the cotwins (girls) showed a delayed growth and borderline hormonal values. In both cases a catch-up growth was observed after starting levothyroxine therapy. Although these two MZ cotwins were not diagnosed with CH, a thyroid hypofunction in these girls was ascertained, as well as a genotype showing genetic variants in DUOX2, TG, and GLIS3 genes. These findings are particularly intriguing, raising the question of whether CH may represent a complex condition with different phenotypic expressions due to possible existing protective or predisposing nongenetic factors for CH at birth.
No positivity for antithyroid autoantibodies was detected in all of the twin pairs both in the first month of life and during the follow-up. Although autoimmune thyroiditis is the most common thyroid disorder in pediatric age (58), this finding can be explained by the small number of twins included in the study.
In conclusion, the lesson learned from screening discordant twins is important and has implications for public health. Although twin pairs are frequently discordant at screening, repeat sampling allows the identification of a greater number of positive twins. Therefore, the frequency of CH concordant twin pairs was higher than that reported when repeat screening was not yet adopted. We also learned that hormonal and auxological long-term follow-up is important in both probands and healthy cotwins, particularly when they are MZ, and that nongenetic still-undefined factors account for thyroid dygenesis frequently observed in MZ twin pairs discordant for CH.
Acknowledgments
We are grateful to Dr. Viviana Cordeddu for technical assistance.
Financial Support: This work was supported by funds from the Italian Ministry of Health, Rome, Italy (Grant RF-2010-2309484 to L.P.) and from the Istituto Superiore di Santà, Rome, Italy (Grant FASC.1ACQ-cap524-EF20111 to A.O.).
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Abbreviations:
- AV
allelic variant
- CH
congenital hypothyroidism
- DZ
dizygotic
- gDNA
genomic DNA
- MAF
minor allele frequency
- MAP
medically assisted pregnancy
- MZ
monozygotic
- NGS
next-generation sequencing
- PBp
probandwise concordance rate
- Pp
pairwise concordance
- rt
tetrachoric correlation coefficient
- sFT4
serum free T4
- sTSH
serum TSH
- TG
thyroglobulin
- TGAb
TG autoantibody
- TPOAb
thyroid peroxidase autoantibody
- UNZ
unknown zygosity
- US
ultrasound
- WT
wild-type



