-
PDF
- Split View
-
Views
-
Cite
Cite
Yee-Ming Chan, Amalia Feld, Elfa Jonsdottir-Lewis, Effects of the Timing of Sex-Steroid Exposure in Adolescence on Adult Health Outcomes, The Journal of Clinical Endocrinology & Metabolism, Volume 104, Issue 10, October 2019, Pages 4578–4586, https://doi.org/10.1210/jc.2019-00569
Close - Share Icon Share
Abstract
Variation in pubertal timing is associated with a wide range of adult risks and outcomes, but it is unclear whether these associations are causal, and it is largely unknown whether these associations can be modified by treatment.
We conducted PubMed searches to identify Mendelian randomization (MR) studies on the influence of pubertal timing on adult health and studies on sex-steroid treatment of the following conditions associated with reduced reproductive endocrine function in adolescence: constitutional delay, Turner syndrome, and Klinefelter syndrome.
Results of MR studies suggest that earlier pubertal timing increases body mass index; increases risk for breast, ovarian, endometrial, and prostate cancers; elevates fasting glucose levels and blood pressure; impairs lung capacity and increases risk for asthma; leads to earlier sexual intercourse and first birth; decreases time spent in education; and increases depressive symptoms in adolescence. Later pubertal timing appears to lower bone mineral density (BMD). Although studies of constitutional delay have not shown that sex-steroid treatment alters adult height or BMD, studies of girls with Turner syndrome and boys with Klinefelter syndrome suggest that earlier initiation of sex-steroid treatment improves physical and neurocognitive outcomes.
Despite having some limitations, MR studies suggest that pubertal timing causally influences many adult conditions and disease risks. Studies of Turner syndrome and Klinefelter syndrome suggest that earlier sex-steroid exposure may have short- and long-term benefits. The mechanisms underlying these findings and the effects of trends and treatments affecting pubertal timing remain to be determined.
Puberty is arguably the most dramatic transformation in postnatal human life. Normal pubertal timing varies across a broad range, and this range is extended even further by extremes of physiologic variation and by pathologic conditions (1–3). In addition to directly affecting an individual’s adolescent experience, pubertal timing is associated with risk for various adult conditions and diseases (4). Some key questions arise from these associations: Are the associations causal, that is, does pubertal timing directly affect the risk for adult conditions? If so, would treatment modify the effect of pubertal timing on adult risk? In this review, we summarize literature addressing these questions, with a focus on Mendelian randomization (MR) studies and on clinical studies assessing sex-steroid treatment of the following conditions that affect reproductive endocrine function in adolescence: constitutional delay, Turner syndrome, and Klinefelter syndrome.
Evidence That Pubertal Timing Influences Adult Outcomes
Epidemiological studies have demonstrated clear associations between pubertal timing and various adult outcomes (4). However, these studies only demonstrate association and cannot establish causation. MR is a method that can demonstrate causality using cross-sectional data. The method uses genetic variants that influence the hypothesized risk factor (in this case, pubertal timing) and enables assessment of the effects of those genetic variants on the outcome of interest to infer a causal relationship between the two [Fig. 1A (5)]. A known issue with the MR approach is pleiotropy, in which a genetic variant has direct effects on both the risk factor and the outcome. Most studies address this issue by omitting known pleiotropic factors and/or by applying regression techniques to identify and correct for pleiotropic effects within the data (at the cost of a reduction in statistical power). In this section, we review MR studies that have examined whether pubertal timing directly causes changes in body mass index (BMI); bone health; oncologic, cardiovascular, and respiratory disease risk; reproductive behaviors; and educational and mental health outcomes (Table 1).
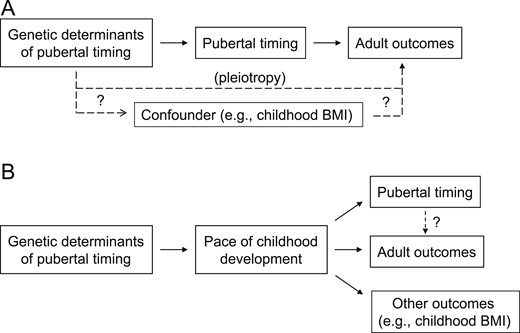
Models for interpretation of MR studies on pubertal timing. (A) MR studies of pubertal timing are used to analyze the effect of genetic factors that influence pubertal timing on an outcome of interest. After correcting for pleiotropic effects (i.e., direct effects of the genetic factors on the outcome), which could be mediated by confounders, MR infers that any effect of the genetic factors on the outcome is mediated by pubertal timing. (B) An alternative model is that genetic factors influence pubertal timing through yet-to-be-identified mediators that influence the pace of childhood development. It may be these mediators, rather than pubertal timing itself, that affect the adult outcomes of interest (and potentially other outcomes) in MR studies. BMI, body mass index.
| Outcome Studied . | Subjects . | Findings . | P Value . | Reference . |
|---|---|---|---|---|
| BMI | 151,157 women | Adult BMI decreased by 0.38 kg/m2 per y of later menarche | 6 × 10−9 | (6) |
| 2053 women | BMI at age 18 years decreased by 1.38 kg/m2 per y of later puberty | 1 × 10−8 | (7) | |
| 322,154 women and men | Adult BMI decreased by 0.81 kg/m2 per y of later puberty | 4 × 10−15 | (7) | |
| BMD | 53,236 women and men | Areal BMD at the femoral neck and lumbar spine decreased by 0.072–0.074 g/cm2 per y of later puberty in women and by 0.113–0.119 g/cm2 per y of later puberty in men | ≤3 × 10−4 | (8, 9) |
| Breast cancer | 47,800 cases, 40,302 control women | Odds of breast cancer decreased by 6.5% per y of later menarche | 3 × 10−3 | (10) |
| Ovarian cancer | 18,175 cases, 26,134 control women | Odds of ovarian cancer decreased by 7.0% per y of later menarche | 9 × 10−3 | (10) |
| Endometrial cancer | 4,401 cases, 28,758 control women | Odds of endometrial cancer decreased by 22% per y of later menarche | 1 × 10−5 | (10) |
| Prostate cancer | 20,219 cases, 20,440 control men | Odds of prostate cancer decreased by 7.5% per y of later voice breaking | 4 × 10−3 | (10) |
| 1135 men | Odds of more severe prostate cancer (Gleason score ≥7) decreased by 24% per tertile increase in genetic puberty score | 1 × 10−3 | (11) | |
| Fasting glucose | 12,229 women | Fasting glucose decreased by 0.39 mmol/L (7 mg/dL) per y of later menarche | 95% CI 0.001–0.78 mmol/L | (12) |
| Blood pressure | 3303 women and men | Systolic blood pressure at age 18 y decreased by 3.9 mm Hg per y of later puberty | 2 × 10−5 | (7) |
| Lung function | 46,944 women | Forced vital capacity increased by 25 mL per y of later puberty | 4 × 10−2 | (13) |
| Asthma | 435,383 women and men | Odds of asthma decreased by 7%–8% for late vs. normal or normal vs. early puberty | < 3 × 10−4 (women), 0.03-0.06 (men) | (14) |
| Reproductive behaviors | 142,630 women and men | Age at first sexual intercourse increased by 0.081–0.095 y per y of later puberty | 1 × 10−19 | (15) |
| ∼71,000 women | Age at first birth increased by 0.37 y per y of later menarche | 6 × 10−8 | (15) | |
| Educational attainment | 97,643 women and men | Odds of staying in school after age 16 y increased by 2% per y of later puberty | 6 × 10−4 | (15) |
| 118,443 women | Time spent in education increased by ∼53 d per y of later menarche | 4 × 10−8 | (16) | |
| Depressive symptoms | 2404 girls | Prevalence of significant depressive symptoms at age 14 y decreased by 0.6% per month of later menarche | 1 × 10−2 | (17) |
| Outcome Studied . | Subjects . | Findings . | P Value . | Reference . |
|---|---|---|---|---|
| BMI | 151,157 women | Adult BMI decreased by 0.38 kg/m2 per y of later menarche | 6 × 10−9 | (6) |
| 2053 women | BMI at age 18 years decreased by 1.38 kg/m2 per y of later puberty | 1 × 10−8 | (7) | |
| 322,154 women and men | Adult BMI decreased by 0.81 kg/m2 per y of later puberty | 4 × 10−15 | (7) | |
| BMD | 53,236 women and men | Areal BMD at the femoral neck and lumbar spine decreased by 0.072–0.074 g/cm2 per y of later puberty in women and by 0.113–0.119 g/cm2 per y of later puberty in men | ≤3 × 10−4 | (8, 9) |
| Breast cancer | 47,800 cases, 40,302 control women | Odds of breast cancer decreased by 6.5% per y of later menarche | 3 × 10−3 | (10) |
| Ovarian cancer | 18,175 cases, 26,134 control women | Odds of ovarian cancer decreased by 7.0% per y of later menarche | 9 × 10−3 | (10) |
| Endometrial cancer | 4,401 cases, 28,758 control women | Odds of endometrial cancer decreased by 22% per y of later menarche | 1 × 10−5 | (10) |
| Prostate cancer | 20,219 cases, 20,440 control men | Odds of prostate cancer decreased by 7.5% per y of later voice breaking | 4 × 10−3 | (10) |
| 1135 men | Odds of more severe prostate cancer (Gleason score ≥7) decreased by 24% per tertile increase in genetic puberty score | 1 × 10−3 | (11) | |
| Fasting glucose | 12,229 women | Fasting glucose decreased by 0.39 mmol/L (7 mg/dL) per y of later menarche | 95% CI 0.001–0.78 mmol/L | (12) |
| Blood pressure | 3303 women and men | Systolic blood pressure at age 18 y decreased by 3.9 mm Hg per y of later puberty | 2 × 10−5 | (7) |
| Lung function | 46,944 women | Forced vital capacity increased by 25 mL per y of later puberty | 4 × 10−2 | (13) |
| Asthma | 435,383 women and men | Odds of asthma decreased by 7%–8% for late vs. normal or normal vs. early puberty | < 3 × 10−4 (women), 0.03-0.06 (men) | (14) |
| Reproductive behaviors | 142,630 women and men | Age at first sexual intercourse increased by 0.081–0.095 y per y of later puberty | 1 × 10−19 | (15) |
| ∼71,000 women | Age at first birth increased by 0.37 y per y of later menarche | 6 × 10−8 | (15) | |
| Educational attainment | 97,643 women and men | Odds of staying in school after age 16 y increased by 2% per y of later puberty | 6 × 10−4 | (15) |
| 118,443 women | Time spent in education increased by ∼53 d per y of later menarche | 4 × 10−8 | (16) | |
| Depressive symptoms | 2404 girls | Prevalence of significant depressive symptoms at age 14 y decreased by 0.6% per month of later menarche | 1 × 10−2 | (17) |
| Outcome Studied . | Subjects . | Findings . | P Value . | Reference . |
|---|---|---|---|---|
| BMI | 151,157 women | Adult BMI decreased by 0.38 kg/m2 per y of later menarche | 6 × 10−9 | (6) |
| 2053 women | BMI at age 18 years decreased by 1.38 kg/m2 per y of later puberty | 1 × 10−8 | (7) | |
| 322,154 women and men | Adult BMI decreased by 0.81 kg/m2 per y of later puberty | 4 × 10−15 | (7) | |
| BMD | 53,236 women and men | Areal BMD at the femoral neck and lumbar spine decreased by 0.072–0.074 g/cm2 per y of later puberty in women and by 0.113–0.119 g/cm2 per y of later puberty in men | ≤3 × 10−4 | (8, 9) |
| Breast cancer | 47,800 cases, 40,302 control women | Odds of breast cancer decreased by 6.5% per y of later menarche | 3 × 10−3 | (10) |
| Ovarian cancer | 18,175 cases, 26,134 control women | Odds of ovarian cancer decreased by 7.0% per y of later menarche | 9 × 10−3 | (10) |
| Endometrial cancer | 4,401 cases, 28,758 control women | Odds of endometrial cancer decreased by 22% per y of later menarche | 1 × 10−5 | (10) |
| Prostate cancer | 20,219 cases, 20,440 control men | Odds of prostate cancer decreased by 7.5% per y of later voice breaking | 4 × 10−3 | (10) |
| 1135 men | Odds of more severe prostate cancer (Gleason score ≥7) decreased by 24% per tertile increase in genetic puberty score | 1 × 10−3 | (11) | |
| Fasting glucose | 12,229 women | Fasting glucose decreased by 0.39 mmol/L (7 mg/dL) per y of later menarche | 95% CI 0.001–0.78 mmol/L | (12) |
| Blood pressure | 3303 women and men | Systolic blood pressure at age 18 y decreased by 3.9 mm Hg per y of later puberty | 2 × 10−5 | (7) |
| Lung function | 46,944 women | Forced vital capacity increased by 25 mL per y of later puberty | 4 × 10−2 | (13) |
| Asthma | 435,383 women and men | Odds of asthma decreased by 7%–8% for late vs. normal or normal vs. early puberty | < 3 × 10−4 (women), 0.03-0.06 (men) | (14) |
| Reproductive behaviors | 142,630 women and men | Age at first sexual intercourse increased by 0.081–0.095 y per y of later puberty | 1 × 10−19 | (15) |
| ∼71,000 women | Age at first birth increased by 0.37 y per y of later menarche | 6 × 10−8 | (15) | |
| Educational attainment | 97,643 women and men | Odds of staying in school after age 16 y increased by 2% per y of later puberty | 6 × 10−4 | (15) |
| 118,443 women | Time spent in education increased by ∼53 d per y of later menarche | 4 × 10−8 | (16) | |
| Depressive symptoms | 2404 girls | Prevalence of significant depressive symptoms at age 14 y decreased by 0.6% per month of later menarche | 1 × 10−2 | (17) |
| Outcome Studied . | Subjects . | Findings . | P Value . | Reference . |
|---|---|---|---|---|
| BMI | 151,157 women | Adult BMI decreased by 0.38 kg/m2 per y of later menarche | 6 × 10−9 | (6) |
| 2053 women | BMI at age 18 years decreased by 1.38 kg/m2 per y of later puberty | 1 × 10−8 | (7) | |
| 322,154 women and men | Adult BMI decreased by 0.81 kg/m2 per y of later puberty | 4 × 10−15 | (7) | |
| BMD | 53,236 women and men | Areal BMD at the femoral neck and lumbar spine decreased by 0.072–0.074 g/cm2 per y of later puberty in women and by 0.113–0.119 g/cm2 per y of later puberty in men | ≤3 × 10−4 | (8, 9) |
| Breast cancer | 47,800 cases, 40,302 control women | Odds of breast cancer decreased by 6.5% per y of later menarche | 3 × 10−3 | (10) |
| Ovarian cancer | 18,175 cases, 26,134 control women | Odds of ovarian cancer decreased by 7.0% per y of later menarche | 9 × 10−3 | (10) |
| Endometrial cancer | 4,401 cases, 28,758 control women | Odds of endometrial cancer decreased by 22% per y of later menarche | 1 × 10−5 | (10) |
| Prostate cancer | 20,219 cases, 20,440 control men | Odds of prostate cancer decreased by 7.5% per y of later voice breaking | 4 × 10−3 | (10) |
| 1135 men | Odds of more severe prostate cancer (Gleason score ≥7) decreased by 24% per tertile increase in genetic puberty score | 1 × 10−3 | (11) | |
| Fasting glucose | 12,229 women | Fasting glucose decreased by 0.39 mmol/L (7 mg/dL) per y of later menarche | 95% CI 0.001–0.78 mmol/L | (12) |
| Blood pressure | 3303 women and men | Systolic blood pressure at age 18 y decreased by 3.9 mm Hg per y of later puberty | 2 × 10−5 | (7) |
| Lung function | 46,944 women | Forced vital capacity increased by 25 mL per y of later puberty | 4 × 10−2 | (13) |
| Asthma | 435,383 women and men | Odds of asthma decreased by 7%–8% for late vs. normal or normal vs. early puberty | < 3 × 10−4 (women), 0.03-0.06 (men) | (14) |
| Reproductive behaviors | 142,630 women and men | Age at first sexual intercourse increased by 0.081–0.095 y per y of later puberty | 1 × 10−19 | (15) |
| ∼71,000 women | Age at first birth increased by 0.37 y per y of later menarche | 6 × 10−8 | (15) | |
| Educational attainment | 97,643 women and men | Odds of staying in school after age 16 y increased by 2% per y of later puberty | 6 × 10−4 | (15) |
| 118,443 women | Time spent in education increased by ∼53 d per y of later menarche | 4 × 10−8 | (16) | |
| Depressive symptoms | 2404 girls | Prevalence of significant depressive symptoms at age 14 y decreased by 0.6% per month of later menarche | 1 × 10−2 | (17) |
Clear associations have been demonstrated between BMI and the timing of puberty (18–20). MR studies have now assessed the extent to which the contribution of pubertal timing to adult BMI is causal. Because of considerable overlap between the genetics of pubertal timing and the genetics of BMI, these studies applied methods to correct for pleiotropy. In one study, earlier timing of puberty appeared to cause an increase in adult BMI of 0.38 kg/m2 per year of earlier menarche in women after correcting for pleiotropy (6). Another study suggested an increase in adult BMI of 0.81 kg/m2 per year of earlier puberty (7). The same study analyzed another cohort and found that BMI at age 18 years increased by 1.38 kg/m2 per year of earlier puberty; however, this association was greatly attenuated after adjusting for BMI at age 8 years (well before puberty is expected to have an effect), and the authors concluded that age at menarche has a minimal effect on BMI at age 18 years (7). An alternative interpretation is that the effect of genetic variants that influence age at menarche on BMI at age 18 years is mediated not through pubertal timing per se but through some other, as-yet-unidentified factor that affects not only pubertal timing and BMI at age 18 years but also BMI at age 8 years (Fig. 1B).
Bone acquisition accelerates greatly during pubertal development, and the timing of pubertal onset has been associated with bone health later in life (4, 21). Results of MR studies now suggest that the relationship between pubertal timing and bone mineralization [as assessed by areal bone mineral density (BMD) with appropriate corrections for body size] is causal, with a 0.072 to 0.074 g/cm2 decrease in BMD per year of pubertal delay at both the lumbar spine and femoral neck in women and a decrease of 0.113 to 0.119 g/cm2 per year of pubertal delay at these sites in men (8, 9).
Pubertal timing has long been associated with risks for various reproductive cancers in women and men (22–26). Results of MR studies suggest that pubertal timing directly causes these differences in cancer risk. Each year of earlier age at menarche appears to cause increased risk of breast cancer (OR, 1.07), ovarian cancer (OR, 1.08), and endometrial cancer (OR, 1.28) (10). Similarly, MR studies have revealed that men who had earlier age at voice breaking are at increased risk for prostate cancer (OR, 1.08 per year of earlier voice breaking) (10). Another study among men with prostate cancer showed a 32% increased odds of more severe cancer (Gleason score ≥7) per tertile of genetic puberty score (11). Thus, earlier pubertal timing appears to causally increase the risks for reproductive hormone–sensitive cancers in both women and men.
Association studies have demonstrated a link between earlier and later pubertal timing and increased risk for cardiovascular disease and cardiovascular risk factors (22, 27). MR studies now have demonstrated likely causal links for a subset of these associations. One study in which several risk factors were examined found an effect for fasting glucose, which was higher by 0.39 mmol/L (7 mg/dL) per year of earlier age at menarche, but there was limited power to detect associations for other cardiovascular risk factors (12). Results of another study suggested a causal effect of pubertal timing on systolic blood pressure at age 18 years, with an increase of 3.9 mm Hg for every year of earlier puberty (7). We did not identify any MR studies that directly examined causal links between pubertal timing and actual cardiovascular outcomes.
Intriguing links have been demonstrated between pubertal timing and respiratory function, with earlier menarche associated with decreased performance on pulmonary function tests (28) and both earlier and later puberty associated with increased risk for asthma (22, 29). MR studies now have demonstrated a decrease in forced vital capacity of 25 mL with each year of earlier puberty (13). Pubertal onset also has causal effects on asthma risk, with a 7% to 8% increase in the odds of asthma in women and men when comparing late or normal timing to early timing (14). This finding differs from that of previous association studies demonstrating an increased risk of asthma with late puberty. These differences were no longer statistically significant after adjusting for pleiotropy, but the effect sizes remained similar, raising the possibility that the loss of significance was due to the penalty to statistical power rather than actual effects of pleiotropy.
Reproductive behavior and sexual outcomes in adolescents may be affected by pubertal timing (30), and an MR study examined the relationship between pubertal timing and these outcomes in men and women (15). With each year of earlier puberty, the age at first sexual intercourse was advanced by 0.081 and 0.095 years in men and women, respectively. This same study also showed an advancement of age at first birth by 0.37 years per year of earlier menarche in women (15).
Educational attainment and performance can be influenced by multiple factors, one of them being the timing of puberty. MR studies have now demonstrated modest causal links between the timing of puberty and time spent in education, with less time spent in education [decreased by 0.04 SD (∼53 days)] per year of earlier menarche (16). Men and women are also less likely to stay in school after 16 years of age (OR, 0.98) per year of earlier pubertal onset (15).
Mental health was examined in one MR study of 2404 girls, in which each month of earlier menarche appeared to cause a 0.6% increase in prevalence of significant depressive symptoms at age 14 years (Short Moods and Feelings Questionnaire score ≥11) (17). However, this effect was not statistically significant at age 17 or 19 years, potentially due to the relatively small sample sizes, as the effect estimates at these ages were comparable to those for depressive symptoms at age 14 years.
As with all MR studies, these studies have limitations. Although the studies incorporated appropriate covariates, it is always possible that not all covariates are represented and that confounding may have contributed to the observed findings (Fig. 1A). Furthermore, as noted previously, because pubertal timing is associated with other aspects of childhood development, such as growth rate (1–3), it is unclear whether it is pubertal timing itself or one of these other aspects that primarily influences the adult outcomes studied (Fig. 1B). Many MR studies use a linear model, which assumes that early and delayed puberty exert opposite effects, and thus may not identify associations in which both early and delayed puberty increase (or decrease) adult disease risk. Despite these limitations, taken together, results of these MR studies strongly suggest that the timing of puberty has direct effects on a variety of adult outcomes.
One question raised by these studies is the mechanism by which puberty affects adult outcomes. One potential mechanism that has been proposed is that the number of years of exposure to sex steroids may influence adult conditions. Although this mechanism might explain a shift in the incidence of such conditions by a few years (proportional to the degree puberty is earlier or later), it is unlikely to be sufficient to account for the effect sizes seen with many of the associations. Furthermore, this mechanism does not explain associations in which both earlier and later puberty confer increased risk. An additional potential mechanism is that the effects of puberty may differ across critical developmental windows in childhood and adolescence. These critical windows may be physiological, with sex steroids exerting quantitatively or qualitatively different effects on target organs at different ages, and they may also be psychosocial, with the changes of puberty producing different effects depending on the stage of a child’s cognitive, emotional, and social development, as well as the child’s social environment.
Another question raised by these studies is whether intervention can modify the effects of pubertal timing on adult disease risk. For early pubertal onset, pubertal timing in the early-normal range is not typically treated. Long-term effects of treatment of frankly precocious puberty with GnRH analogs are reviewed by Guaraldi et al. (31). Briefly, no long-term negative effects on BMI or BMD have been observed. The remainder of this review focuses on studies that examined outcomes of sex-steroid treatment of constitutional delay, which is generally considered a developmental variant, and then focuses on two causes of primary gonadal insufficiency: Turner syndrome and Klinefelter syndrome.
Studies of Sex-Steroid Treatment of Constitutional Delay
Children with constitutional delay have a delayed onset of puberty but eventually achieve normal adult reproductive endocrine function (1–3). Though constitutional delay is a self-limited condition, some (but not all) studies have suggested that this delay can have lasting consequences. Treatment with sex steroids is often offered to children with delayed puberty, and here we review studies that have examined whether sex-steroid treatment affects long-term outcomes.
Several retrospective studies have demonstrated a decrease in adult height in men with a history of delayed puberty, though this has not been observed in all studies; the reported height deficits range from 0 to 7 cm (32–43). Wehkalampi et al. (41, 43) identified reductions in adult height only in a subset of girls and boys who had a slow rate of childhood growth, suggesting that constitutional delay may encompass multiple conditions characterized by distinct rates of prepubertal growth and distinct height outcomes. Even in the studies that demonstrated decreased adult height, no difference was seen between those who had been treated with sex steroids and those who had not. Because all these studies were retrospective, sex-steroid treatment was not standardized with respect to timing of treatment, dose, or duration, and these variations in practice may have limited the ability to detect effects of treatment on adult height.
BMD may also be lower in adult men with a history of constitutional delay, by ∼10% in some studies but not others (44–50). As with adult height, no difference in BMD was observed between those who had been treated with testosterone and those who had not.
Few randomized studies of androgen treatment have been conducted in boys with delayed puberty (51–53). These studies show short-term increases in growth velocity as well as increases in predicted adult height but have not reported data on adult height or peak BMD. We found no reports of comparable randomized studies in girls. Given the paucity of studies of constitutional delay, we turned to studies of sex-steroid treatment of other conditions that affect reproductive endocrine activity in adolescence: Turner syndrome and Klinefelter syndrome.
Timing of Estrogen Treatment in Girls With Turner Syndrome
Questions about the timing of sex-steroid treatment in individuals with delayed puberty may be illuminated by research on the timing of estrogen treatment in girls with Turner syndrome. Because of concerns that earlier estrogen exposure may promote epiphyseal closure and compromise adult height, it had been common practice to delay initiation of estrogen treatment past the ages when puberty would typically occur. In this section, we first present evidence supporting treatment initiation at a more physiologic age and then review studies that extend the treatment window even earlier into childhood.
A randomized study of girls with Turner syndrome examined height outcomes in girls assigned to start estrogen treatment between ages 12 and 12.9 years or between ages 14 and 14.9 years; all had begun GH therapy before pubertal induction (54). The early-treatment group grew about 1.6 cm more than the late-treatment group, though there was no statistically significant difference in adult height between the groups, at least in part because the late-treatment group was taller at baseline. Guidelines for management of Turner syndrome now recommend starting estrogen treatment between 11 and 12 years of age (55).
Estrogen deficiency can be seen in girls with Turner syndrome even in the prepubertal years (56), and studies have examined starting estrogen treatment before the typical pubertal window. In a double-blind, placebo-controlled trial, 149 girls with Turner syndrome, ages 5 to 12.5 years, were assigned to receive a combination of either oral low-dose ethinyl estradiol or placebo and either GH or placebo (57). The participants who received both ethinyl estradiol and GH had an increase in height SD score of 0.49 compared with an increase of 0.22 in those who received GH alone, with an even larger difference in the subset followed to near-adult height (increase of height SD score of 0.58 vs 0.26, respectively).
A retrospective study examined BMD in a cohort of 799 women with Turner syndrome and found lower BMD at the hip and spine in women who had started estrogen treatment later (58). The negative correlation between age and BMD was roughly linear for estrogen initiation at ages throughout the adolescent years.
Outcomes beyond growth have also been examined in randomized, double-blind, placebo-controlled trials. One study of girls with Turner syndrome, ages 5 to 11 years, showed that girls treated with low-dose ethinyl estradiol showed improvements on the order of one-half to one full SD on spatially mediated motor tasks and nonverbal processing speed at age 12 years (59). Another study of 7- to 9-year-old girls with Turner syndrome found improved verbal and nonverbal memory in those treated with low-dose ethinyl estradiol compared with those treated with placebo (60).
Collectively, these studies provide evidence that starting estrogen treatment at physiological ages or even earlier improves growth, BMD, and aspects of neurocognitive function and illustrate the importance of the timing of sex-steroid exposure for these outcomes. These findings lend support to the concept that exposure to estradiol at different developmental stages may have differential effects on the bones and on cognitive development.
Studies of Androgen Treatment in Boys With Klinefelter Syndrome
Boys with Klinefelter syndrome typically have normal onset of and early progress through puberty, then experience a progressive decline in testicular function starting in midpuberty or later (61–64). Physical and psychosocial benefits of testosterone treatment have been demonstrated for adolescents and adults (64, 65), but few studies have examined the role of timing of treatment. One cross-sectional study of BMD in 42 individuals with Klinefelter syndrome showed higher areal BMD in those who had started testosterone treatment before age 20 years than for those who started treatment after 20 years (66), but results from prospective, randomized studies (e.g., ClinicalTrials.gov no. NCT01585831) are needed to inform the optimal timing of testosterone treatment in adolescent boys with Klinefelter syndrome.
As with Turner syndrome, sex-steroid treatment has been proposed for prepubertal children with Klinefelter syndrome. Though, to our knowledge, no randomized trials have been published on the use of testosterone in prepubertal boys with Klinefelter syndrome, a double-blind, placebo-controlled trial of oxandrolone in 4- to 12-year-old boys found that those who received 0.06 mg/kg oxandrolone daily for 2 years saw an improvement in their visual-motor capacities, moods, and social behaviors (67). Earlier treatment was not without risk, however; those treated with oxandrolone experienced earlier gonadarche, and precocious puberty developed in 23% of those younger than 9 years (68).
Some (but not all) studies have demonstrated evidence of hypogonadism in boys with Klinefelter syndrome as early as infancy (69, 70), raising the possibility that treatment of Klinefelter syndrome in infancy may be beneficial—a possibility examined in several retrospective, nonrandomized studies. A group of 10 boys with 49,XXXXY syndrome treated with testosterone in infancy had improved verbal and nonverbal communication at age ∼5 years compared with a group of seven untreated boys with 49,XXXXY syndrome (71). According to results of another study, after testosterone treatment in infancy, 34 boys with Klinefelter syndrome had superior measures of neurodevelopment, intellect, reading, and verbal capacities, and fewer behavioral issues when assessed at 36 and 72 months of age as compared with an untreated group of 67 boys (72, 73).
Though these studies in infants do not directly address the question of whether the timing of sex-steroid treatment in adolescence affects adult disease risk, they suggest there may be unique developmental windows for the effects of sex steroids in infancy, childhood, and adolescence. However, this suggestion is tempered by the nonrandomized nature of the studies and the possibility that sex-steroid administration may represent pharmacologic treatment rather than physiologic replacement.
Summary
The technique of MR has enhanced our understanding of the long-recognized associations between pubertal timing and adult health outcomes by demonstrating that it is likely pubertal timing itself that causally affects these adult health outcomes. Furthermore, results of clinical studies in individuals with constitutional delay, Turner syndrome, and Klinefelter syndrome have suggested that earlier exposure to sex steroids may be beneficial for short- and long-term outcomes. However, many questions remain: Given the ongoing trend toward earlier pubertal timing (74–76), to what degree does this trend affect adult disease risk? What other adult conditions are influenced by pubertal timing, and by what mechanisms? To what degree can the association between pubertal timing and these adult conditions be modified by interventions to delay early pubertal onset and to prevent late pubertal onset? And what interventions are realistic either for individuals or at the population scale? The answers to these questions will require updated epidemiological studies to capture recent trends, expanded MR studies with larger cohorts and improved genetic prediction scores (and hence greater statistical power), additional clinical trials on modifying the timing of pubertal changes and sex-steroid exposure, and basic studies of the effects of sex steroids at different developmental stages.
Acknowledgments
We thank Jeremy Cunniff for contributions to the manuscript.
Financial Support: This work was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant R01 HD090071 to Y.-M.C.).
Additional Information
Disclosure Summary: Y.-M.C. was previously a medical advisory board member for Endo Pharmaceuticals. The remaining authors have nothing to disclose.
Data Availability:
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.



