-
PDF
- Split View
-
Views
-
Cite
Cite
Marie Lindhardt Ljubicic, Anne Jørgensen, Carlo Acerini, Juliana Andrade, Antonio Balsamo, Silvano Bertelloni, Martine Cools, Rieko Tadokoro Cuccaro, Feyza Darendeliler, Christa E Flück, Romina P Grinspon, Andrea Maciel-Guerra, Tulay Guran, Sabine E Hannema, Angela K Lucas-Herald, Olaf Hiort, Paul Martin Holterhus, Corina Lichiardopol, Leendert H J Looijenga, Rita Ortolano, Stefan Riedl, S Faisal Ahmed, Anders Juul, Clinical but Not Histological Outcomes in Males With 45,X/46,XY Mosaicism Vary Depending on Reason for Diagnosis, The Journal of Clinical Endocrinology & Metabolism, Volume 104, Issue 10, October 2019, Pages 4366–4381, https://doi.org/10.1210/jc.2018-02752
Close - Share Icon Share
Abstract
Larger studies on outcomes in males with 45,X/46,XY mosaicism are rare.
To compare health outcomes in males with 45,X/46,XY diagnosed as a result of either genital abnormalities at birth or nongenital reasons later in life.
A retrospective, multicenter study.
Sixteen tertiary centers.
Sixty-three males older than 13 years with 45,X/46,XY mosaicism.
Health outcomes, such as genital phenotype, gonadal function, growth, comorbidities, fertility, and gonadal histology, including risk of neoplasia.
Thirty-five patients were in the genital group and 28 in the nongenital. Eighty percent of all patients experienced spontaneous pubertal onset, significantly more in the nongenital group (P = 0.023). Patients were significantly shorter in the genital group with median adult heights of 156.7 cm and 164.5 cm, respectively (P = 0.016). Twenty-seven percent of patients received recombinant human GH. Forty-four patients had gonadal histology evaluated. Germ cells were detected in 42%. Neoplasia in situ was found in five patients. Twenty-five percent had focal spermatogenesis, and another 25.0% had arrested spermatogenesis. Fourteen out of 17 (82%) with semen analyses were azoospermic; three had motile sperm.
Patients diagnosed as a result of genital abnormalities have poorer health outcomes than those diagnosed as a result of nongenital reasons. Most patients, however, have relatively good endocrine gonadal function, but most are also short statured. Patients have a risk of gonadal neoplasia, and most are azoospermic, but almost one-half of patients has germ cells present histologically and up to one-quarter has focal spermatogenesis, providing hope for fertility treatment options.
The 45,X/46,XY karyotype and its variants are rare, with a previously reported incidence of one of 15,000 live births (1). The resulting phenotype spans across a wide range of effects, including genital anomalies, impaired growth, altered gonadal function and histology, and infertility. The karyotype is covered by the umbrella term differences (or disorders) of sex development (DSD), referring to diagnoses in which anatomical, gonadal, and/or chromosomal sex are affected (2). The 45,X cell line in these patients probably stems from the loss of a normal or structurally abnormal Y-chromosome in early embryonic mitosis, which produces the mosaicism (3–7).
The phenotypic spectrum of 45,X/46,XY patients varies greatly from females with Turner syndrome to normally androgenized males. Moreover, several studies have reported that 80% to 95% of prenatally diagnosed cases with a 45,X/46,XY karyotype are born as normally androgenized males (3, 5, 8, 9), whereas postnatally diagnosed pediatric cases present more varied phenotypes, including ambiguous genitalia (5, 10, 11). Furthermore, normally androgenized male patients with a 45,X/46,XY karyotype diagnosed in adulthood are now more frequently identified as a result of male infertility work-ups, including genetic screening (12). Thus, severity of the patient’s phenotype often appears to be directly related to the age at diagnosis and reason for referral.
The wide spectrum of phenotypes in these patients is also reflected in health outcomes, such as growth, gonadal function, risk of gonadal neoplasia, and comorbidities, which are all reported with varying incidences and severities, both within the same centers and between centers (5, 7, 10, 13–16). It seems intuitive that the severity of the genital phenotype may be considered a read-out for other health outcomes. Nevertheless, even normally androgenized males diagnosed in adulthood have been reported to have short stature and declining testicular function with age and infertility, likely related to histologically dysgenetic testes (5, 6). However, there is a lack of studies with direct comparisons of outcomes in terms of growth, gonadal function, and comorbidities between patients diagnosed at birth as a result of genital abnormalities and those diagnosed later in life as a result of other reasons, such as short stature, pubertal delay, and infertility.
The risk of gonadal neoplasia in patients with 45,X/46,XY mosaicism is reported to be relatively high, at ∼10%–15% (5, 16–19). The current practice of early (prepubertal) gonadectomy in girls renders it impossible to evaluate gonadal function and possible fertility potential in women. Moreover, single-center studies on histological outcomes are limited by numbers, thereby making thorough pathohistological evaluations of larger datasets rare.
Thus, we wanted to investigate and compare health outcomes, such as growth, gonadal function, comorbidities, fertility, and histology, including risk of neoplasia in males with 45,X/46,XY mosaicism and variants diagnosed as a result of the following different reasons: (i) genital abnormalities and (ii) other reasons, such as stunted growth, lack of pubertal onset, undervirilization, and infertility, in a large multicenter study with 16 participating centers, including a total of 63 male patients with 45,X/46,XY mosaicism.
Materials and Methods
Subjects
Patients were identified using the International DSD (I-DSD) Registry, which contains pseudoanonymized information on patients with DSD. Information on the registry is available at https://www.gla.ac.uk/schools/medicine/research/childhealth/researchinterests/i-dsdproject/, and recent uses of the registry have previously been published (14, 20).
We identified centers in the registry that had included patients with 45,X/46,XY and its variants [including different aberrations to the Y-chromosome, such as deletions and isodicentricism, and a single patient with a 45,X/46,XX (sex-determining region Y-positive) karyotype] uploaded to the registry. Through the European Cooperation in Science and Technology (COST) network, DSDnet (http://www.dsdnet.eu/cost.html), three additional centers with patients not yet uploaded to the registry were identified. A total of 22 centers were contacted, of which 19 centers responded, and 16 centers supplied data on a total of 63 male patients. The inclusion criteria were the following: male sex of rearing age and an age old enough to evaluate height and gonadal function (>13 years of age).
Patients were stratified into two groups, according to whether they were diagnosed as a result of genital abnormalities or other reasons (hereafter, “genital” and “nongenital,” respectively). Other reasons included prenatal screening (fetal and maternal factors), growth retardation, gynecomastia, lack of spontaneous pubertal onset, lack of virilization in adulthood, and infertility. Two patients in the nongenital group underwent hypospadias repairs and thus, had genital abnormalities but were not diagnosed as a result of these and were thus included in the nongenital group.
Data collection
Following identification of suitable cases in the I-DSD Registry, each center was contacted to complete a detailed questionnaire that collected the following information: age at presentation; reason for diagnosis; karyotype; sex of rearing; birth weight and length; genital phenotype, including external masculinization score [EMS; as described by Ahmed et al. (21)]; renal and cardiac comorbidities; growth, including target height and recombinant human GH (rhGH) treatment; pubertal onset; gynecomastia; testosterone (T) treatment; genital and gonadal surgery; and gonadal histology, including neoplasia, fertility workups, and endocrine biochemistry at presentation and at last available follow-up. Histopathological evaluations were translated locally and added to a predefined table by each participating center. In a few cases, attempts were made to get further evaluations, images, and/or tissue blocks. However, this was not always possible. Thus, an image of a gonadoblastoma from a patient not included in this study (with a 46,XX/47,XYY/48,XXYY karyotype from the BioBank at the Department of Growth and Reproduction, Copenhagen University Hospital, Copenhagen, Denmark) is used.
Hormone assays
Sixteen centers participated in this study, and different commercially available assays were used to measure follicle-stimulating hormone (FSH), luteinizing hormone (LH), and T. All FSH and LH concentrations were reported using international units per liter. T concentrations were reported in nanomoles per liter, nanograms per milliliter, and picograms per milliliter. Based on the molar mass of T of 288 daltons, all values were standardized to nanomoles per liter (nanomoles per liter = nanograms per milliliter × 3.47 M). Reference ranges for FSH and LH were based on measurements using the time-resolved immunoflourometric assays (Delfia; PerkinElmer, Boston, MA). The limits of detection (LODs) were 0.05 IU/L and 0.05 IU/L, respectively. The intra- and interassay coefficients of variation were <5% in both assays. Reference range values for T were measured using the DPC Coat-A-Count radioimmunoassay kit (Diagnostic Products Corp., Los Angeles, CA). LOD was 0.23 nM. Intra- and interassay coefficients of variation were 7.6% and 8.6%, respectively. As a result of the retrospective and historic nature of the study, assay details were not available from all participating centers.
Hormone reference ranges
Reference ranges and LODs plotted were based on the assays used at the Department of Growth and Reproduction, Copenhagen University Hospital, where this study was based. Any values below the LODs were plotted as (LOD/2). All reference ranges of reproductive hormones and testicular volumes are based on a total of 2095 healthy boys recruited for a cross-sectional study of healthy Danish children (The COPENHAGEN Puberty Study), as previously published (22, 23). The plotting of the reference ranges, despite being from a single center only, was done to enable comparison of normative data with the patient data.
Statistical analyses
The genital and nongenital groups were compared using the Mann-Whitney U test in terms of external genitalia (EMS), age at referral, final height, and height SD scores (SDSs), whereas Pearson’s χ2 or Fisher’s exact test (wherever appropriate) was used to compare the binary outcomes of spontaneous puberty, renal and cardiac malformations and disease, genital and gonadal surgeries, fertility, gonadal neoplasia, spermatogenesis, presence of germ cells, and distribution (presence) of testicles and streak gonads.
Height was standardized using height-for-age SDSs and plotted against reference ranges from the World Health Organization (WHO) (24).
Ethical considerations
The I-DSD registry is approved by the National Research Ethics Service of the United Kingdom for collection of routine clinical data. In addition, each center obtained necessary approvals and adhered to local laws regarding data collection from patient files. Patient and/or parental consent was obtained before registration of cases in the I-DSD Registry. The database from this study is based in Copenhagen, Denmark, and has received appropriated approvals from the Danish Data Protection Agency (RH-2015-235, I-Suite No. 04204) and the Danish Health and Medicinal Authorities (3-3013-1376/1). The COPENHAGEN Puberty Study was approved by the Danish Data Protection Agency (2015-41-4494) and by the regional Ethics Committee (KF 01 282214 and V200.1996/90).
Results
In total, 63 males from 16 different centers were included in this study. All patients had missing values for one or more variables, but all were included in the study.
Age and phenotype at diagnosis
Thirty-five (55.6%) patients were diagnosed as a result of genital abnormalities, and 28 (44.4%) were diagnosed as a result of other reasons. Ages at first presentation were [median (range)] 0.0 years (0.0 to 42.7 years) and 24.0 years (0.0 to 49.0 years) in the two groups (n = 34 genital, n = 27 nongenital), respectively (Table 1).
Differences Between the Genital and Nongenital Group in Terms of Age, Genital Phenotype, Growth, Comorbidities, Surgeries, and Gonadal Neoplasia
| . | Diagnosed Due to: . | . | |||
|---|---|---|---|---|---|
| Genital Anomalies . | Nongenital Reasons . | P Value . | |||
| Median (Range) or Yes/No . | n . | Median (Range) or Yes/No . | n . | ||
| Age | |||||
| Age at presentation, y, median (range) | 0.00 (0.0 to 42.7) | 35 | 24.0 (0.0 to 49.00) | 25 | <0.001a |
| Age at last evaluation, y, median (range) | 18.9 (14.0 to 70.2) | 34 | 30.6 (13.4 to 58.6) | 27 | 0.039 |
| Genital phenotype | |||||
| EMS,b median (range) | 4.00 (1.0 to 9.5) | 24 | 12 (10.0 to 12.0) | 20 | <0.001a |
| Spontaneous pubertal onset, yes/no | 22/10 | 32 | 25/2 | 27 | 0.023a |
| T treatment, yes/no | 17/15 | 32 | 4/17 | 21 | 0.013a |
| Growth | |||||
| Final height, cm, median (range) | 156.7 (143.0 to 169.2) | 31 | 164.5 (141.1 to 187.7) | 21 | 0.001a |
| Height, SDS, median (range) | −2.29 (−4.6 to −0.28) | 29 | −1.53 (−3.09 to 1.53) | 18 | 0.016a |
| H – TH, SDS,c median (range) | −2.49 (−4.18 to −1.22) | 23 | −2.21 (−3.44 to −0.96) | 13 | 0.296 |
| GH treatment, yes/no | 13/19 | 32 | 4/19 | 23 | 0.066 |
| Comorbidity | |||||
| Renal malformations, yes/no | 5/27 | 32 | 2/23 | 25 | 0.450 |
| Cardiac malformations, yes/no | 9/24 | 33 | 4/21 | 25 | 0.308 |
| Surgery | |||||
| Hypospadias repair | 29/4 | 33 | 2/23 | 25 | <0.001a |
| Orchidopexy, uni- or bilateral | 14/16 | 30 | 3/22 | 25 | 0.006a |
| Gonadectomy, uni- or bilateral | 28/8 | 36 | 2/24 | 26 | <0.001a |
| Neoplasia | |||||
| Gonadal neoplasia,d yes/no | 4/20 | 24 | 1/16 | 17 | 0.382 |
| . | Diagnosed Due to: . | . | |||
|---|---|---|---|---|---|
| Genital Anomalies . | Nongenital Reasons . | P Value . | |||
| Median (Range) or Yes/No . | n . | Median (Range) or Yes/No . | n . | ||
| Age | |||||
| Age at presentation, y, median (range) | 0.00 (0.0 to 42.7) | 35 | 24.0 (0.0 to 49.00) | 25 | <0.001a |
| Age at last evaluation, y, median (range) | 18.9 (14.0 to 70.2) | 34 | 30.6 (13.4 to 58.6) | 27 | 0.039 |
| Genital phenotype | |||||
| EMS,b median (range) | 4.00 (1.0 to 9.5) | 24 | 12 (10.0 to 12.0) | 20 | <0.001a |
| Spontaneous pubertal onset, yes/no | 22/10 | 32 | 25/2 | 27 | 0.023a |
| T treatment, yes/no | 17/15 | 32 | 4/17 | 21 | 0.013a |
| Growth | |||||
| Final height, cm, median (range) | 156.7 (143.0 to 169.2) | 31 | 164.5 (141.1 to 187.7) | 21 | 0.001a |
| Height, SDS, median (range) | −2.29 (−4.6 to −0.28) | 29 | −1.53 (−3.09 to 1.53) | 18 | 0.016a |
| H – TH, SDS,c median (range) | −2.49 (−4.18 to −1.22) | 23 | −2.21 (−3.44 to −0.96) | 13 | 0.296 |
| GH treatment, yes/no | 13/19 | 32 | 4/19 | 23 | 0.066 |
| Comorbidity | |||||
| Renal malformations, yes/no | 5/27 | 32 | 2/23 | 25 | 0.450 |
| Cardiac malformations, yes/no | 9/24 | 33 | 4/21 | 25 | 0.308 |
| Surgery | |||||
| Hypospadias repair | 29/4 | 33 | 2/23 | 25 | <0.001a |
| Orchidopexy, uni- or bilateral | 14/16 | 30 | 3/22 | 25 | 0.006a |
| Gonadectomy, uni- or bilateral | 28/8 | 36 | 2/24 | 26 | <0.001a |
| Neoplasia | |||||
| Gonadal neoplasia,d yes/no | 4/20 | 24 | 1/16 | 17 | 0.382 |
n, total count of individuals with available information.
Significance by a 0.05 level.
EMS (0 to 12 points).
Height (H) − target height (TH), SDS.
Gonadal neoplasia is defined as germ-cell neoplasia in situ (GCNIS), gonadoblastoma, and/or invasive gonadal tumors.
Differences Between the Genital and Nongenital Group in Terms of Age, Genital Phenotype, Growth, Comorbidities, Surgeries, and Gonadal Neoplasia
| . | Diagnosed Due to: . | . | |||
|---|---|---|---|---|---|
| Genital Anomalies . | Nongenital Reasons . | P Value . | |||
| Median (Range) or Yes/No . | n . | Median (Range) or Yes/No . | n . | ||
| Age | |||||
| Age at presentation, y, median (range) | 0.00 (0.0 to 42.7) | 35 | 24.0 (0.0 to 49.00) | 25 | <0.001a |
| Age at last evaluation, y, median (range) | 18.9 (14.0 to 70.2) | 34 | 30.6 (13.4 to 58.6) | 27 | 0.039 |
| Genital phenotype | |||||
| EMS,b median (range) | 4.00 (1.0 to 9.5) | 24 | 12 (10.0 to 12.0) | 20 | <0.001a |
| Spontaneous pubertal onset, yes/no | 22/10 | 32 | 25/2 | 27 | 0.023a |
| T treatment, yes/no | 17/15 | 32 | 4/17 | 21 | 0.013a |
| Growth | |||||
| Final height, cm, median (range) | 156.7 (143.0 to 169.2) | 31 | 164.5 (141.1 to 187.7) | 21 | 0.001a |
| Height, SDS, median (range) | −2.29 (−4.6 to −0.28) | 29 | −1.53 (−3.09 to 1.53) | 18 | 0.016a |
| H – TH, SDS,c median (range) | −2.49 (−4.18 to −1.22) | 23 | −2.21 (−3.44 to −0.96) | 13 | 0.296 |
| GH treatment, yes/no | 13/19 | 32 | 4/19 | 23 | 0.066 |
| Comorbidity | |||||
| Renal malformations, yes/no | 5/27 | 32 | 2/23 | 25 | 0.450 |
| Cardiac malformations, yes/no | 9/24 | 33 | 4/21 | 25 | 0.308 |
| Surgery | |||||
| Hypospadias repair | 29/4 | 33 | 2/23 | 25 | <0.001a |
| Orchidopexy, uni- or bilateral | 14/16 | 30 | 3/22 | 25 | 0.006a |
| Gonadectomy, uni- or bilateral | 28/8 | 36 | 2/24 | 26 | <0.001a |
| Neoplasia | |||||
| Gonadal neoplasia,d yes/no | 4/20 | 24 | 1/16 | 17 | 0.382 |
| . | Diagnosed Due to: . | . | |||
|---|---|---|---|---|---|
| Genital Anomalies . | Nongenital Reasons . | P Value . | |||
| Median (Range) or Yes/No . | n . | Median (Range) or Yes/No . | n . | ||
| Age | |||||
| Age at presentation, y, median (range) | 0.00 (0.0 to 42.7) | 35 | 24.0 (0.0 to 49.00) | 25 | <0.001a |
| Age at last evaluation, y, median (range) | 18.9 (14.0 to 70.2) | 34 | 30.6 (13.4 to 58.6) | 27 | 0.039 |
| Genital phenotype | |||||
| EMS,b median (range) | 4.00 (1.0 to 9.5) | 24 | 12 (10.0 to 12.0) | 20 | <0.001a |
| Spontaneous pubertal onset, yes/no | 22/10 | 32 | 25/2 | 27 | 0.023a |
| T treatment, yes/no | 17/15 | 32 | 4/17 | 21 | 0.013a |
| Growth | |||||
| Final height, cm, median (range) | 156.7 (143.0 to 169.2) | 31 | 164.5 (141.1 to 187.7) | 21 | 0.001a |
| Height, SDS, median (range) | −2.29 (−4.6 to −0.28) | 29 | −1.53 (−3.09 to 1.53) | 18 | 0.016a |
| H – TH, SDS,c median (range) | −2.49 (−4.18 to −1.22) | 23 | −2.21 (−3.44 to −0.96) | 13 | 0.296 |
| GH treatment, yes/no | 13/19 | 32 | 4/19 | 23 | 0.066 |
| Comorbidity | |||||
| Renal malformations, yes/no | 5/27 | 32 | 2/23 | 25 | 0.450 |
| Cardiac malformations, yes/no | 9/24 | 33 | 4/21 | 25 | 0.308 |
| Surgery | |||||
| Hypospadias repair | 29/4 | 33 | 2/23 | 25 | <0.001a |
| Orchidopexy, uni- or bilateral | 14/16 | 30 | 3/22 | 25 | 0.006a |
| Gonadectomy, uni- or bilateral | 28/8 | 36 | 2/24 | 26 | <0.001a |
| Neoplasia | |||||
| Gonadal neoplasia,d yes/no | 4/20 | 24 | 1/16 | 17 | 0.382 |
n, total count of individuals with available information.
Significance by a 0.05 level.
EMS (0 to 12 points).
Height (H) − target height (TH), SDS.
Gonadal neoplasia is defined as germ-cell neoplasia in situ (GCNIS), gonadoblastoma, and/or invasive gonadal tumors.
EMSs at first examination were [median (range)] 4.0 (1.0 to 9.5) and 12.0 (10.0 to 12.0) in each group (n = 24 genital, n = 20 nongenital; Table 1), respectively, and significantly differed between the groups (P < 0.001).
The prevalence of hypospadias repairs (P < 0.001), orchidopexies (P = 0.006), and gonadectomies (P < 0.001) was higher in the genital group (Table 1).
Spontaneous puberty, reproductive hormones, and T replacement
The majority of patients in both groups entered puberty spontaneously [n = 47 patients (79.7%)], with a significantly higher prevalence in the nongenital group (n=22 genital, n = 25 nongenital; P = 0.023; Table 1). Twenty-one patients (39.6%) were treated with T at some point or continuously during the follow-up period, with significantly more in the genital group (17 genital, 4 nongenital; P = 0.013). Regardless of the reason for diagnosis, most patients had FSH and LH concentrations at the higher end of the normal reference range (Fig. 1). Likewise, T levels were mostly within the normal reference range. Testicular volumes were typically normal or low within the reference range, independent of diagnosis group (Fig. 1).
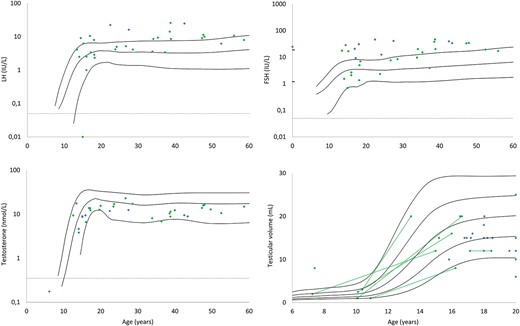
LH, FSH, and T values, along with testicular volumes (largest testicle), at last evaluation according to age. Blue dots represent the genital group, and green dots represent the nongenital group. Solid lines are reference ranges (means; and ±2 SD for the hormones and means; ±1 SD and ±2 SD for testicular volumes). Dotted lines signify LODs.
Growth
Final heights were reduced in the genital compared with the nongenital group [median (range) 156.7 cm (143.0 to 169.2 cm) and 164.5 cm (141.1 to 187.7 cm); P = 0.001; Table 1 and Fig. 2a]. However, when the genetic potential was accounted for, there was no significant difference. Patients in neither group grew according to genetic potential [height SDS – target height SDS; median SDS (range): −2.5 (−4.2 to −1.2) and −2.2 (−3.4 to −1.0); Table 1 and Fig. 2b].
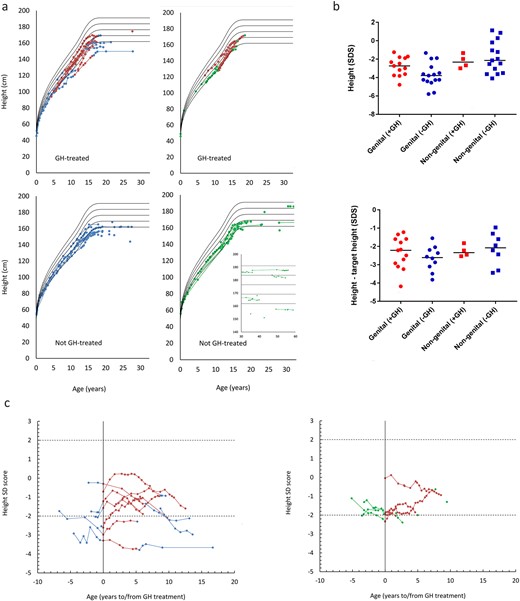
(a) Height (centimeters), according to age, stratified according to rhGH treatment and group. Red dots represent rhGH treatment, blue dots represent the genital group, and green dots represent the nongenital. Solid lines represent WHO reference ranges (means; ±1 SD and ±2 SD). (b) Height and height according to genetic potential (height – target height), expressed in SDSs, according to group and rhGH treatment. Dots represent patients in the genital group, red dots have received rhGH treatment, and blue dots have not. Squares represent patients in the nongenital group, red squares have received rhGH treatment, and blue squares have not. Solid lines represent group medians. (c) Height SDSs according to rhGH treatment and stratified according to group. Red dots represent rhGH treatment, blue dots represent the genital group, and green dots represent the nongenital group. Dotted lines represent ±2 SD.
Seventeen patients (27.0%) were treated with rhGH, with no significant difference in the number of treated patients between the two groups (P = 0.066). There was no difference in height SDSs 1 year before and 1 or 5 years following treatment of all patients overall and when subdivided into the two diagnosis groups or grouped based on treatment (no treatment vs treated; all P > 0.05; Fig. 2c).
Comorbidities
Cardiac malformations were more frequent (13 patients, 22.4%) than renal malformations [seven patients (12.3%); Table 1]. There was no difference in frequencies between the groups.
Gonadal histology, spermatogenesis, and neoplasia
Histological features from gonadal biopsies and/or gonadectomies from 44 patients (65.0%), including a total of 61 gonads, are summarized in Table 2 and Figs. 3 and 4. In total, 31 patients from the genital group (88.6%) and 13 from the nongenital group (46.4%) had histological data available.
Histological Findings in Samples From 61 Gonads Grouped According to Reason for Diagnosis and Including Ages at the Time of Biopsy/Gonadectomy and EMS Scores
| ID . | Biopsy or Gonadectomy: Age in Years (L/R) . | Overall Morphology . | Male Characteristics . | Female Characteristics . | Germ Cells . | Spermatogenesis/Follicles . | Internal Genitalia . | EMS . | Group (N/NG) . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Testicular Tubules . | Leydig Cells . | SCO, Y/N . | ||||||||||
| Female . | Male . | |||||||||||
| 1 | G: 15 (l) | Dysgenetic testis | Present, thick basal membrane, fibrosis; branched tubules, calcification. | Hyperplasia | Y | — | Absent | — | UT; FT | EP | 4 | G |
| G: 15 (r) | Müllerian derivatives | — | — | — | — | — | — | |||||
| 2 | G: 0.3 (l) | Dysgenetic testis | Prepubertal pattern | Absent | Y | — | Absent | NA due to age | UT | EP | 4 | G |
| G: 0.3 (r) | Dysgenetic testis | Prepubertal pattern | Absent | Y | — | Absent | NA due to age | |||||
| 3 | G: 4 (l) | Testis | Prepubertal pattern | — | — | NA | — | NA due to age | UT; FT UL | EP; VD UL | 1 | G |
| G: 4 (r) | Streak gonad | NA | NA | NA | Ovarian-like tissue | Absent | NA due to age | |||||
| 4 | G: 2 (l) | Wolffian and Müllerian derivatives | — | — | — | — | — | NA due to age | FT | EP | 9.5 | G |
| 5 | B: 5 (l) | Dysgenetic testis | Prepubertal testis | Absent | N | Present | NA due to age | UT; FT BL | EP BL | 8 | G | |
| G: 5 (r) | Streak gonad | NA | NA | NA | Ovarian-like tissue with primitive sex cord structures | — | NA due to age | |||||
| 6 | B: 4.8 (l) | Dysgenetic testis | Normal | Present | Y | — | Absent | NA due to age | FT UL | VD, EP UL | 5.5 | G |
| G: 4.8 (r) | Ovarian-like tissue | NA | NA | NA | Ovarian-like tissue | Absent | NA due to age | |||||
| 7 | B: 0.1 (l) | Dysgenetic testis | Irregular, abnormal | — | N | — | Rare absent | NA due to age, absent | UT UL | VD, EP UL | 5.5 | G |
| B: 15 (l) | Dysgenetic testis | Irregular, abnormal | — | Y | — | |||||||
| G: 1.0 (r) | Ovarian-like tissue | NA | NA | NA | Ovarian-like tissue with germ cells present | Present, gonadoblastoma in situ | NA due to age | FT UL | ||||
| 8 | B: 1 (r) | Prepubertal testis; abnormal area with peripheral microlithiasis | Normal | Normal | N | — | — | NA due to age | — | Normal | — | G |
| 9 | G: childhood (l) | Prepubertal testis | — | — | — | — | — | NA due to age | — | Agenesis of SV | — | G |
| G: childhood (r) | Streak gonad | NA | NA | NA | Streak gonad | Absent | NA due to age | — | ||||
| 10 | B: 13.2 (l) | Fibrous tissue without testicular morphology | Absent | Absent | — | — | Absent | Absent | FT, UT UL | VD, EP UL | 3 | G |
| B: 13.2 (r) | Testis without spermatogenesis | Normal | Rare | Y | Fibrous ovarian-like tissue | Absent | Absent | |||||
| G: 13.9 (r) | ||||||||||||
| 11 | B: 0.75 (l) | Prepubertal testis | Normal | Absent | N | None | Normal | NA due to age | UT, FT UL | WR UL | 5.5 | G |
| B: 16 | Testis with partial atrophy and SCO; other parts with incomplete spermatogenesis | Mixed abnormal and normal | Normal | Y/N | None | Strongly reduced number, no malignancy | Incomplete spermatogenesis, round elongated spermatids, no mature sperm (sperm present in semen sample) | |||||
| G: 0.75 (r) | No gonadal tissue | NA | NA | NA | NA | NA | NA | UT, FT UL | WR | |||
| 12 | B: 3 (l) | Dysgenetic testis | Dichotomized seminiferous tubules predominantly with Sertoli cells | — | N | — | Few spermatogonia | NA due to age | UT-like structure | WR | 3 | G |
| G: 6 (l) | Hypoplastic TA | Prepubertal seminiferous tubules invading TA | Rare | N | — | — | NA due to age | |||||
| Dysgenetic testis; thickened TA | ||||||||||||
| B: 6 (r) | Prepubertal testis | Normal | Normal | N | — | — | NA due to age | |||||
| 13 | B: 2 (l) | Prepubertal testis | — | — | — | — | — | NA due to age | UT, FT | — | G | |
| 14 | B: 1.5 | Streak gonad | NA | NA | NA | Ovarian-like tissue | Absent | NA due to age | UT, FT | — | G | |
| G: 6 | NA due to age | |||||||||||
| 15 | G: 1 (l) | Streak gonad | NA | NA | NA | Streak gonad | Absent | NA due to age | FT UL | VD UL | 2.5 | G |
| G: 2 (r) | Dysgenetic testis | — | — | — | — | — | NA due to age | |||||
| 16 | B:21 (l) | Hypotrophic testis | Hyalinized | Rare | — | NA | Absent | Absent | UT, FT UL | VD, EP UL | 2.5 | G |
| G:3 (r) | Atrophic testis | — | — | — | — | — | NA due to age | |||||
| 17 | G: 3 (l) | Dysgenetic testis and ovarian tissue | Seminiferous tubules without germ cells | — | Y | Ovarian tissue | Absent, no malignancy | NA due to age | — | — | 4 | G |
| B: 3 (r) | Dysgenetic testis | — | — | — | — | No malignancy | NA due to age | — | — | |||
| 18 | G: 1 (l) | Steak gonad | NA | NA | NA | Streak gonad | Absent, no malignancy | NA due to age | — | — | 8.5 | G |
| B: 1 (r) | Dysgenetic testis | — | — | — | — | No malignancy | NA due to age | — | — | |||
| B: 5 (r) | Dysgenetic testis | — | — | — | — | — | NA due to age | |||||
| 19 | G: 4 | Dysgenetic gonad | — | — | — | — | No malignancy | NA due to age | — | — | 5 | G |
| B: 11 | — | — | — | — | — | — | — | |||||
| 20 | B/G: 5 | Fibrous tissue | — | — | — | — | No malignancy | NA due to age | — | — | 6 | G |
| 21 | B: 3.3 (r) | Prepubertal testis | — | — | — | — | Presence of germ cells uncertain | NA due to age | — | EP | — | G |
| 22 | G: 1.9 (l) | Dysgenetic gonad | Few nests of sex cords | — | — | Ovarian- like tissue | Absent | NA due to age | FT | WR | — | G |
| 23 | G: 1.5 (l) | Dysgenetic gonad UGT | Abnormal | — | Y | Ovarian-like tissue with germ cells present | Present, presence of primordial follicles uncertain | NA due to age | Rudimentary UT, FT | — | — | G |
| B: 9.5 (r) | No gonadal tissue | NA | NA | NA | NA | NA | NA | Fimbriae with edema | — | |||
| 24 | G: 1.3 (l) | Streak gonad | NA | NA | NA | Ovarian-like tissue | Absent | NA due to age | FT | — | — | G |
| 25 | B: 14.0 (l) | No gonadal tissue | NA | NA | NA | NA | NA | Rudimentary UT, FT BL | VD BL | 8 | G | |
| B: 21.3 (l) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Hyperplasia | Y/N | Absent | Present, no malignancy | Spermatocytic arrest | |||||
| G: 14.0 (r) | Ovotesticular remnant | Present | — | — | Ovotesticular remnant | Absent, no malignancy | Absent | |||||
| 26 | B: 1.9 (l) | Prepubertal testis | Normal | Presence uncertain, fibrosis | N | Absent | Present, no malignancy | NA due to age | Rudimentary UT, FL BL | VD UL | 4 | G |
| G: 14.5 (l) | Dysgenetic testis | Present, thick basal membrane | Hyperplasia | Y/N | Absent | Present, GCNIS | Overall spermatocytic arrest, some spermatids present | |||||
| G: 1.9 (r) | Streak gonad | NA | NA | NA | Ovarian-like tissue | Absent, no malignancy | Absent | |||||
| 27 | G: 2.2 (l) | Fibrotic ovarian-like tissue | NA | NA | NA | Ovarian-like tissue | Absent | NA | MR, rudimentary UT, FT BL | VD, EP BL | 1 | G |
| B: 2.2 (r) | Dysgenetic testis | Normal | Present | Y/N | Absent | Spermatogonia and gonocytes present | NA due to age | |||||
| G: 14.2 (r) | Prepubertal testis | Normal | Present | Y/N | Absent | Present, GCNIS | NA due to age | |||||
| 28 | B: 43.7 (l) | Dysgenetic testis | Present, some hyalinized, fibrosis | Hyperplasia | N | Absent | Absent, no malignancy | Absent | 9.5 | G | ||
| G: 42.8 (r) | Streak gonad | NA | NA | NA | Streak gonad | Absent, no malignancy | Absent | Rudimentary UT, FT UL | ||||
| 29 | B: 16.1 (l) | Dysgenetic testis | Present | — | Y | Absent | Absent, no malignancy | Absent | — | — | 7 | G |
| 30 | B: 1.5 (l) | Dysgenetic testis | Present | Unknown | Y | Absent | Absent, no malignancy | NA due to age | — | — | 4 | G |
| G: 12.0 (r) | Streak gonad | NA | NA | NA | Streak gonad | Absent, no malignancy | Absent | |||||
| 31 | G: 22 (r) | Dysgenetic testis | — | — | — | — | Present, GCNIS | — | — | — | — | G |
| 32 | G: 0.08 (l) | Streak gonad | NA | NA | NA | Streak gonad | Absent | NA due to age | — | VD UL | — | NG |
| B: 0.08 (r) | Dysgenetic testis | Few, abnormal, hyalinized | Rare | N | — | Absent | NA due to age | |||||
| 33 | B: 0.42 (l) | Dysgenetic testis | Few, abnormal, hyalinized | Rare | N | — | Absent | NA due to age | — | VD BL | 10 | NG |
| B: 0.42 (r) | Dysgenetic testis | Few, abnormal, hyalinized | Rare | N | — | Absent | NA due to age | |||||
| 34 | B: 34.4 (l) | Dysgenetic testis | Present, thick basal membrane | Present | Y | Absent | Absent, no malignancy | Absent | 12 | NG | ||
| B: 34.4 (r) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Present | Y/N | Absent | Present, no malignancy | Spermatocytic arrest | |||||
| 35 | B: 28.2 (l) | Dysgenetic testis | Present, thick basal membrane | Normal | Y | Absent | Absent, no malignancy | Absent | 12 | NG | ||
| B: 28.2 (r) | Dysgenetic testis | Present, thick basal membrane | Normal | Y | Absent | Absent, no malignancy | Absent | |||||
| 36 | B: 30.1 (l) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Normal | Y | Absent | Absent, no malignancy | Absent | 11 | NG | ||
| B: 30.1 (r) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Hyperplasia | Y | Absent | Absent, no malignancy | Absent | |||||
| 37 | B: 18.77 (l) | Dysgenetic testis | Present, thick basal membrane | Normal | N | Absent | Present, no malignancy | Overall spermatocytic arrest, few spermatids present | 12 | NG | ||
| B: 18.77 (r) | Dysgenetic testis | Present, thick basal membrane | Normal | N | Absent | Present, no malignancy | Spermatocytic arrest | |||||
| 38 | B: 36.3 (l) | Dysgenetic testis | Present, thick basal membrane | Hyperplasia | Y/N | Absent | Present, no malignancy | Only spermatogonia present | — | NG | ||
| B: 36.3 (r) | Dysgenetic testis | Present, thick basal membrane | Hyperplasia | Y/N | Absent | Present, no malignancy | Only spermatogonia present | |||||
| 39 | B: 49.4 (l) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Hyperplasia | Y/N | Absent | Present, no malignancy | Spermatocytic arrest | 12 | NG | ||
| B: 49.4 (r) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Hyperplasia | Y/N | Absent | Present, no malignancy | Only spermatogonia present | |||||
| 40 | B: 19.1 (l) | Dysgenetic testis | Present, thick basal membrane | Hyperplasia | Y | Absent | Absent, no malignancy | Absent | — | — | 12 | NG |
| B: 19.1 (r) | Dysgenetic testis | Present, thick basal membrane | Hyperplasia | Y | Absent | Absent, no malignancy | Absent | |||||
| 41 | B: 34.1 (l) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Present, fibrosis | Y/N | Absent | Present, no malignancy | Spermatocytic arrest | — | — | 11.5 | NG |
| B: 34.1 (r) | Dysgenetic testis | Present, thick basal membrane | Present | Y | Absent | Absent, no malignancy | Absent | |||||
| 42 | B: 17.1 (l) | Normal testis | — | — | — | — | Present, no malignancy | Normal spermatogenesis (small vol semen sample with conc of 114 mio/mL) | Rudimentary UT, FT BL | — | 11.5 | NG |
| G: 15.6 (r) | Streak gonad | NA | NA | NA | Ovarian-like tissue | Absent, no malignancy | Absent | |||||
| 43 | B: 28.6 (l) | Dysgenetic testis | Present, thick basal membrane, fibrosis | Present | N | Absent | Present, GCNIS | — | — | — | 12 | NG |
| B: 28.6 (r) | Slightly dysgenetic testis | Present, slightly thick basal membrane | Normal | N | Absent | Present, no malignancy | Preserved spermatogenesis (semen sample with conc of 0.06 mio/mL, few progressively motile) | |||||
| 44 | B: 47.5 (l) | Dysgenetic testis | Present, some atrophic and hyalinized | Hyperplasia | Y | Absent | Absent | Absent | — | EP BL | 10.5 | NG |
| B: 47.5 (r) | Dysgenetic testis | Present, some atrophic and hyalinized | Hyperplasia | Y | Absent | Absent, no malignancy | Absent | |||||
| ID . | Biopsy or Gonadectomy: Age in Years (L/R) . | Overall Morphology . | Male Characteristics . | Female Characteristics . | Germ Cells . | Spermatogenesis/Follicles . | Internal Genitalia . | EMS . | Group (N/NG) . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Testicular Tubules . | Leydig Cells . | SCO, Y/N . | ||||||||||
| Female . | Male . | |||||||||||
| 1 | G: 15 (l) | Dysgenetic testis | Present, thick basal membrane, fibrosis; branched tubules, calcification. | Hyperplasia | Y | — | Absent | — | UT; FT | EP | 4 | G |
| G: 15 (r) | Müllerian derivatives | — | — | — | — | — | — | |||||
| 2 | G: 0.3 (l) | Dysgenetic testis | Prepubertal pattern | Absent | Y | — | Absent | NA due to age | UT | EP | 4 | G |
| G: 0.3 (r) | Dysgenetic testis | Prepubertal pattern | Absent | Y | — | Absent | NA due to age | |||||
| 3 | G: 4 (l) | Testis | Prepubertal pattern | — | — | NA | — | NA due to age | UT; FT UL | EP; VD UL | 1 | G |
| G: 4 (r) | Streak gonad | NA | NA | NA | Ovarian-like tissue | Absent | NA due to age | |||||
| 4 | G: 2 (l) | Wolffian and Müllerian derivatives | — | — | — | — | — | NA due to age | FT | EP | 9.5 | G |
| 5 | B: 5 (l) | Dysgenetic testis | Prepubertal testis | Absent | N | Present | NA due to age | UT; FT BL | EP BL | 8 | G | |
| G: 5 (r) | Streak gonad | NA | NA | NA | Ovarian-like tissue with primitive sex cord structures | — | NA due to age | |||||
| 6 | B: 4.8 (l) | Dysgenetic testis | Normal | Present | Y | — | Absent | NA due to age | FT UL | VD, EP UL | 5.5 | G |
| G: 4.8 (r) | Ovarian-like tissue | NA | NA | NA | Ovarian-like tissue | Absent | NA due to age | |||||
| 7 | B: 0.1 (l) | Dysgenetic testis | Irregular, abnormal | — | N | — | Rare absent | NA due to age, absent | UT UL | VD, EP UL | 5.5 | G |
| B: 15 (l) | Dysgenetic testis | Irregular, abnormal | — | Y | — | |||||||
| G: 1.0 (r) | Ovarian-like tissue | NA | NA | NA | Ovarian-like tissue with germ cells present | Present, gonadoblastoma in situ | NA due to age | FT UL | ||||
| 8 | B: 1 (r) | Prepubertal testis; abnormal area with peripheral microlithiasis | Normal | Normal | N | — | — | NA due to age | — | Normal | — | G |
| 9 | G: childhood (l) | Prepubertal testis | — | — | — | — | — | NA due to age | — | Agenesis of SV | — | G |
| G: childhood (r) | Streak gonad | NA | NA | NA | Streak gonad | Absent | NA due to age | — | ||||
| 10 | B: 13.2 (l) | Fibrous tissue without testicular morphology | Absent | Absent | — | — | Absent | Absent | FT, UT UL | VD, EP UL | 3 | G |
| B: 13.2 (r) | Testis without spermatogenesis | Normal | Rare | Y | Fibrous ovarian-like tissue | Absent | Absent | |||||
| G: 13.9 (r) | ||||||||||||
| 11 | B: 0.75 (l) | Prepubertal testis | Normal | Absent | N | None | Normal | NA due to age | UT, FT UL | WR UL | 5.5 | G |
| B: 16 | Testis with partial atrophy and SCO; other parts with incomplete spermatogenesis | Mixed abnormal and normal | Normal | Y/N | None | Strongly reduced number, no malignancy | Incomplete spermatogenesis, round elongated spermatids, no mature sperm (sperm present in semen sample) | |||||
| G: 0.75 (r) | No gonadal tissue | NA | NA | NA | NA | NA | NA | UT, FT UL | WR | |||
| 12 | B: 3 (l) | Dysgenetic testis | Dichotomized seminiferous tubules predominantly with Sertoli cells | — | N | — | Few spermatogonia | NA due to age | UT-like structure | WR | 3 | G |
| G: 6 (l) | Hypoplastic TA | Prepubertal seminiferous tubules invading TA | Rare | N | — | — | NA due to age | |||||
| Dysgenetic testis; thickened TA | ||||||||||||
| B: 6 (r) | Prepubertal testis | Normal | Normal | N | — | — | NA due to age | |||||
| 13 | B: 2 (l) | Prepubertal testis | — | — | — | — | — | NA due to age | UT, FT | — | G | |
| 14 | B: 1.5 | Streak gonad | NA | NA | NA | Ovarian-like tissue | Absent | NA due to age | UT, FT | — | G | |
| G: 6 | NA due to age | |||||||||||
| 15 | G: 1 (l) | Streak gonad | NA | NA | NA | Streak gonad | Absent | NA due to age | FT UL | VD UL | 2.5 | G |
| G: 2 (r) | Dysgenetic testis | — | — | — | — | — | NA due to age | |||||
| 16 | B:21 (l) | Hypotrophic testis | Hyalinized | Rare | — | NA | Absent | Absent | UT, FT UL | VD, EP UL | 2.5 | G |
| G:3 (r) | Atrophic testis | — | — | — | — | — | NA due to age | |||||
| 17 | G: 3 (l) | Dysgenetic testis and ovarian tissue | Seminiferous tubules without germ cells | — | Y | Ovarian tissue | Absent, no malignancy | NA due to age | — | — | 4 | G |
| B: 3 (r) | Dysgenetic testis | — | — | — | — | No malignancy | NA due to age | — | — | |||
| 18 | G: 1 (l) | Steak gonad | NA | NA | NA | Streak gonad | Absent, no malignancy | NA due to age | — | — | 8.5 | G |
| B: 1 (r) | Dysgenetic testis | — | — | — | — | No malignancy | NA due to age | — | — | |||
| B: 5 (r) | Dysgenetic testis | — | — | — | — | — | NA due to age | |||||
| 19 | G: 4 | Dysgenetic gonad | — | — | — | — | No malignancy | NA due to age | — | — | 5 | G |
| B: 11 | — | — | — | — | — | — | — | |||||
| 20 | B/G: 5 | Fibrous tissue | — | — | — | — | No malignancy | NA due to age | — | — | 6 | G |
| 21 | B: 3.3 (r) | Prepubertal testis | — | — | — | — | Presence of germ cells uncertain | NA due to age | — | EP | — | G |
| 22 | G: 1.9 (l) | Dysgenetic gonad | Few nests of sex cords | — | — | Ovarian- like tissue | Absent | NA due to age | FT | WR | — | G |
| 23 | G: 1.5 (l) | Dysgenetic gonad UGT | Abnormal | — | Y | Ovarian-like tissue with germ cells present | Present, presence of primordial follicles uncertain | NA due to age | Rudimentary UT, FT | — | — | G |
| B: 9.5 (r) | No gonadal tissue | NA | NA | NA | NA | NA | NA | Fimbriae with edema | — | |||
| 24 | G: 1.3 (l) | Streak gonad | NA | NA | NA | Ovarian-like tissue | Absent | NA due to age | FT | — | — | G |
| 25 | B: 14.0 (l) | No gonadal tissue | NA | NA | NA | NA | NA | Rudimentary UT, FT BL | VD BL | 8 | G | |
| B: 21.3 (l) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Hyperplasia | Y/N | Absent | Present, no malignancy | Spermatocytic arrest | |||||
| G: 14.0 (r) | Ovotesticular remnant | Present | — | — | Ovotesticular remnant | Absent, no malignancy | Absent | |||||
| 26 | B: 1.9 (l) | Prepubertal testis | Normal | Presence uncertain, fibrosis | N | Absent | Present, no malignancy | NA due to age | Rudimentary UT, FL BL | VD UL | 4 | G |
| G: 14.5 (l) | Dysgenetic testis | Present, thick basal membrane | Hyperplasia | Y/N | Absent | Present, GCNIS | Overall spermatocytic arrest, some spermatids present | |||||
| G: 1.9 (r) | Streak gonad | NA | NA | NA | Ovarian-like tissue | Absent, no malignancy | Absent | |||||
| 27 | G: 2.2 (l) | Fibrotic ovarian-like tissue | NA | NA | NA | Ovarian-like tissue | Absent | NA | MR, rudimentary UT, FT BL | VD, EP BL | 1 | G |
| B: 2.2 (r) | Dysgenetic testis | Normal | Present | Y/N | Absent | Spermatogonia and gonocytes present | NA due to age | |||||
| G: 14.2 (r) | Prepubertal testis | Normal | Present | Y/N | Absent | Present, GCNIS | NA due to age | |||||
| 28 | B: 43.7 (l) | Dysgenetic testis | Present, some hyalinized, fibrosis | Hyperplasia | N | Absent | Absent, no malignancy | Absent | 9.5 | G | ||
| G: 42.8 (r) | Streak gonad | NA | NA | NA | Streak gonad | Absent, no malignancy | Absent | Rudimentary UT, FT UL | ||||
| 29 | B: 16.1 (l) | Dysgenetic testis | Present | — | Y | Absent | Absent, no malignancy | Absent | — | — | 7 | G |
| 30 | B: 1.5 (l) | Dysgenetic testis | Present | Unknown | Y | Absent | Absent, no malignancy | NA due to age | — | — | 4 | G |
| G: 12.0 (r) | Streak gonad | NA | NA | NA | Streak gonad | Absent, no malignancy | Absent | |||||
| 31 | G: 22 (r) | Dysgenetic testis | — | — | — | — | Present, GCNIS | — | — | — | — | G |
| 32 | G: 0.08 (l) | Streak gonad | NA | NA | NA | Streak gonad | Absent | NA due to age | — | VD UL | — | NG |
| B: 0.08 (r) | Dysgenetic testis | Few, abnormal, hyalinized | Rare | N | — | Absent | NA due to age | |||||
| 33 | B: 0.42 (l) | Dysgenetic testis | Few, abnormal, hyalinized | Rare | N | — | Absent | NA due to age | — | VD BL | 10 | NG |
| B: 0.42 (r) | Dysgenetic testis | Few, abnormal, hyalinized | Rare | N | — | Absent | NA due to age | |||||
| 34 | B: 34.4 (l) | Dysgenetic testis | Present, thick basal membrane | Present | Y | Absent | Absent, no malignancy | Absent | 12 | NG | ||
| B: 34.4 (r) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Present | Y/N | Absent | Present, no malignancy | Spermatocytic arrest | |||||
| 35 | B: 28.2 (l) | Dysgenetic testis | Present, thick basal membrane | Normal | Y | Absent | Absent, no malignancy | Absent | 12 | NG | ||
| B: 28.2 (r) | Dysgenetic testis | Present, thick basal membrane | Normal | Y | Absent | Absent, no malignancy | Absent | |||||
| 36 | B: 30.1 (l) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Normal | Y | Absent | Absent, no malignancy | Absent | 11 | NG | ||
| B: 30.1 (r) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Hyperplasia | Y | Absent | Absent, no malignancy | Absent | |||||
| 37 | B: 18.77 (l) | Dysgenetic testis | Present, thick basal membrane | Normal | N | Absent | Present, no malignancy | Overall spermatocytic arrest, few spermatids present | 12 | NG | ||
| B: 18.77 (r) | Dysgenetic testis | Present, thick basal membrane | Normal | N | Absent | Present, no malignancy | Spermatocytic arrest | |||||
| 38 | B: 36.3 (l) | Dysgenetic testis | Present, thick basal membrane | Hyperplasia | Y/N | Absent | Present, no malignancy | Only spermatogonia present | — | NG | ||
| B: 36.3 (r) | Dysgenetic testis | Present, thick basal membrane | Hyperplasia | Y/N | Absent | Present, no malignancy | Only spermatogonia present | |||||
| 39 | B: 49.4 (l) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Hyperplasia | Y/N | Absent | Present, no malignancy | Spermatocytic arrest | 12 | NG | ||
| B: 49.4 (r) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Hyperplasia | Y/N | Absent | Present, no malignancy | Only spermatogonia present | |||||
| 40 | B: 19.1 (l) | Dysgenetic testis | Present, thick basal membrane | Hyperplasia | Y | Absent | Absent, no malignancy | Absent | — | — | 12 | NG |
| B: 19.1 (r) | Dysgenetic testis | Present, thick basal membrane | Hyperplasia | Y | Absent | Absent, no malignancy | Absent | |||||
| 41 | B: 34.1 (l) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Present, fibrosis | Y/N | Absent | Present, no malignancy | Spermatocytic arrest | — | — | 11.5 | NG |
| B: 34.1 (r) | Dysgenetic testis | Present, thick basal membrane | Present | Y | Absent | Absent, no malignancy | Absent | |||||
| 42 | B: 17.1 (l) | Normal testis | — | — | — | — | Present, no malignancy | Normal spermatogenesis (small vol semen sample with conc of 114 mio/mL) | Rudimentary UT, FT BL | — | 11.5 | NG |
| G: 15.6 (r) | Streak gonad | NA | NA | NA | Ovarian-like tissue | Absent, no malignancy | Absent | |||||
| 43 | B: 28.6 (l) | Dysgenetic testis | Present, thick basal membrane, fibrosis | Present | N | Absent | Present, GCNIS | — | — | — | 12 | NG |
| B: 28.6 (r) | Slightly dysgenetic testis | Present, slightly thick basal membrane | Normal | N | Absent | Present, no malignancy | Preserved spermatogenesis (semen sample with conc of 0.06 mio/mL, few progressively motile) | |||||
| 44 | B: 47.5 (l) | Dysgenetic testis | Present, some atrophic and hyalinized | Hyperplasia | Y | Absent | Absent | Absent | — | EP BL | 10.5 | NG |
| B: 47.5 (r) | Dysgenetic testis | Present, some atrophic and hyalinized | Hyperplasia | Y | Absent | Absent, no malignancy | Absent | |||||
Ovarian-like tissue does not contain follicles unless specifically indicated.
Abbreviations: —, information not available; B, biopsy; BL, bilateral; EP, epididymis; FT, fallopian tubes; G, genital (last column); G, gonadectomy (column 2); ID, identification; l, left; mio/mL, million per milliliter; MR, Müllerian remnants; N, no; NA, not applicable; NG, nongenital; r, right; SCO, Sertoli cell only; SV, seminal vesicle; TA, tunica albuginea; UGT, undifferentiated; UL, unilateral; UT, uterus; VD, vas deferens; vol, volume; WR, Wolffian duct remnants; Y, yes.
Histological Findings in Samples From 61 Gonads Grouped According to Reason for Diagnosis and Including Ages at the Time of Biopsy/Gonadectomy and EMS Scores
| ID . | Biopsy or Gonadectomy: Age in Years (L/R) . | Overall Morphology . | Male Characteristics . | Female Characteristics . | Germ Cells . | Spermatogenesis/Follicles . | Internal Genitalia . | EMS . | Group (N/NG) . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Testicular Tubules . | Leydig Cells . | SCO, Y/N . | ||||||||||
| Female . | Male . | |||||||||||
| 1 | G: 15 (l) | Dysgenetic testis | Present, thick basal membrane, fibrosis; branched tubules, calcification. | Hyperplasia | Y | — | Absent | — | UT; FT | EP | 4 | G |
| G: 15 (r) | Müllerian derivatives | — | — | — | — | — | — | |||||
| 2 | G: 0.3 (l) | Dysgenetic testis | Prepubertal pattern | Absent | Y | — | Absent | NA due to age | UT | EP | 4 | G |
| G: 0.3 (r) | Dysgenetic testis | Prepubertal pattern | Absent | Y | — | Absent | NA due to age | |||||
| 3 | G: 4 (l) | Testis | Prepubertal pattern | — | — | NA | — | NA due to age | UT; FT UL | EP; VD UL | 1 | G |
| G: 4 (r) | Streak gonad | NA | NA | NA | Ovarian-like tissue | Absent | NA due to age | |||||
| 4 | G: 2 (l) | Wolffian and Müllerian derivatives | — | — | — | — | — | NA due to age | FT | EP | 9.5 | G |
| 5 | B: 5 (l) | Dysgenetic testis | Prepubertal testis | Absent | N | Present | NA due to age | UT; FT BL | EP BL | 8 | G | |
| G: 5 (r) | Streak gonad | NA | NA | NA | Ovarian-like tissue with primitive sex cord structures | — | NA due to age | |||||
| 6 | B: 4.8 (l) | Dysgenetic testis | Normal | Present | Y | — | Absent | NA due to age | FT UL | VD, EP UL | 5.5 | G |
| G: 4.8 (r) | Ovarian-like tissue | NA | NA | NA | Ovarian-like tissue | Absent | NA due to age | |||||
| 7 | B: 0.1 (l) | Dysgenetic testis | Irregular, abnormal | — | N | — | Rare absent | NA due to age, absent | UT UL | VD, EP UL | 5.5 | G |
| B: 15 (l) | Dysgenetic testis | Irregular, abnormal | — | Y | — | |||||||
| G: 1.0 (r) | Ovarian-like tissue | NA | NA | NA | Ovarian-like tissue with germ cells present | Present, gonadoblastoma in situ | NA due to age | FT UL | ||||
| 8 | B: 1 (r) | Prepubertal testis; abnormal area with peripheral microlithiasis | Normal | Normal | N | — | — | NA due to age | — | Normal | — | G |
| 9 | G: childhood (l) | Prepubertal testis | — | — | — | — | — | NA due to age | — | Agenesis of SV | — | G |
| G: childhood (r) | Streak gonad | NA | NA | NA | Streak gonad | Absent | NA due to age | — | ||||
| 10 | B: 13.2 (l) | Fibrous tissue without testicular morphology | Absent | Absent | — | — | Absent | Absent | FT, UT UL | VD, EP UL | 3 | G |
| B: 13.2 (r) | Testis without spermatogenesis | Normal | Rare | Y | Fibrous ovarian-like tissue | Absent | Absent | |||||
| G: 13.9 (r) | ||||||||||||
| 11 | B: 0.75 (l) | Prepubertal testis | Normal | Absent | N | None | Normal | NA due to age | UT, FT UL | WR UL | 5.5 | G |
| B: 16 | Testis with partial atrophy and SCO; other parts with incomplete spermatogenesis | Mixed abnormal and normal | Normal | Y/N | None | Strongly reduced number, no malignancy | Incomplete spermatogenesis, round elongated spermatids, no mature sperm (sperm present in semen sample) | |||||
| G: 0.75 (r) | No gonadal tissue | NA | NA | NA | NA | NA | NA | UT, FT UL | WR | |||
| 12 | B: 3 (l) | Dysgenetic testis | Dichotomized seminiferous tubules predominantly with Sertoli cells | — | N | — | Few spermatogonia | NA due to age | UT-like structure | WR | 3 | G |
| G: 6 (l) | Hypoplastic TA | Prepubertal seminiferous tubules invading TA | Rare | N | — | — | NA due to age | |||||
| Dysgenetic testis; thickened TA | ||||||||||||
| B: 6 (r) | Prepubertal testis | Normal | Normal | N | — | — | NA due to age | |||||
| 13 | B: 2 (l) | Prepubertal testis | — | — | — | — | — | NA due to age | UT, FT | — | G | |
| 14 | B: 1.5 | Streak gonad | NA | NA | NA | Ovarian-like tissue | Absent | NA due to age | UT, FT | — | G | |
| G: 6 | NA due to age | |||||||||||
| 15 | G: 1 (l) | Streak gonad | NA | NA | NA | Streak gonad | Absent | NA due to age | FT UL | VD UL | 2.5 | G |
| G: 2 (r) | Dysgenetic testis | — | — | — | — | — | NA due to age | |||||
| 16 | B:21 (l) | Hypotrophic testis | Hyalinized | Rare | — | NA | Absent | Absent | UT, FT UL | VD, EP UL | 2.5 | G |
| G:3 (r) | Atrophic testis | — | — | — | — | — | NA due to age | |||||
| 17 | G: 3 (l) | Dysgenetic testis and ovarian tissue | Seminiferous tubules without germ cells | — | Y | Ovarian tissue | Absent, no malignancy | NA due to age | — | — | 4 | G |
| B: 3 (r) | Dysgenetic testis | — | — | — | — | No malignancy | NA due to age | — | — | |||
| 18 | G: 1 (l) | Steak gonad | NA | NA | NA | Streak gonad | Absent, no malignancy | NA due to age | — | — | 8.5 | G |
| B: 1 (r) | Dysgenetic testis | — | — | — | — | No malignancy | NA due to age | — | — | |||
| B: 5 (r) | Dysgenetic testis | — | — | — | — | — | NA due to age | |||||
| 19 | G: 4 | Dysgenetic gonad | — | — | — | — | No malignancy | NA due to age | — | — | 5 | G |
| B: 11 | — | — | — | — | — | — | — | |||||
| 20 | B/G: 5 | Fibrous tissue | — | — | — | — | No malignancy | NA due to age | — | — | 6 | G |
| 21 | B: 3.3 (r) | Prepubertal testis | — | — | — | — | Presence of germ cells uncertain | NA due to age | — | EP | — | G |
| 22 | G: 1.9 (l) | Dysgenetic gonad | Few nests of sex cords | — | — | Ovarian- like tissue | Absent | NA due to age | FT | WR | — | G |
| 23 | G: 1.5 (l) | Dysgenetic gonad UGT | Abnormal | — | Y | Ovarian-like tissue with germ cells present | Present, presence of primordial follicles uncertain | NA due to age | Rudimentary UT, FT | — | — | G |
| B: 9.5 (r) | No gonadal tissue | NA | NA | NA | NA | NA | NA | Fimbriae with edema | — | |||
| 24 | G: 1.3 (l) | Streak gonad | NA | NA | NA | Ovarian-like tissue | Absent | NA due to age | FT | — | — | G |
| 25 | B: 14.0 (l) | No gonadal tissue | NA | NA | NA | NA | NA | Rudimentary UT, FT BL | VD BL | 8 | G | |
| B: 21.3 (l) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Hyperplasia | Y/N | Absent | Present, no malignancy | Spermatocytic arrest | |||||
| G: 14.0 (r) | Ovotesticular remnant | Present | — | — | Ovotesticular remnant | Absent, no malignancy | Absent | |||||
| 26 | B: 1.9 (l) | Prepubertal testis | Normal | Presence uncertain, fibrosis | N | Absent | Present, no malignancy | NA due to age | Rudimentary UT, FL BL | VD UL | 4 | G |
| G: 14.5 (l) | Dysgenetic testis | Present, thick basal membrane | Hyperplasia | Y/N | Absent | Present, GCNIS | Overall spermatocytic arrest, some spermatids present | |||||
| G: 1.9 (r) | Streak gonad | NA | NA | NA | Ovarian-like tissue | Absent, no malignancy | Absent | |||||
| 27 | G: 2.2 (l) | Fibrotic ovarian-like tissue | NA | NA | NA | Ovarian-like tissue | Absent | NA | MR, rudimentary UT, FT BL | VD, EP BL | 1 | G |
| B: 2.2 (r) | Dysgenetic testis | Normal | Present | Y/N | Absent | Spermatogonia and gonocytes present | NA due to age | |||||
| G: 14.2 (r) | Prepubertal testis | Normal | Present | Y/N | Absent | Present, GCNIS | NA due to age | |||||
| 28 | B: 43.7 (l) | Dysgenetic testis | Present, some hyalinized, fibrosis | Hyperplasia | N | Absent | Absent, no malignancy | Absent | 9.5 | G | ||
| G: 42.8 (r) | Streak gonad | NA | NA | NA | Streak gonad | Absent, no malignancy | Absent | Rudimentary UT, FT UL | ||||
| 29 | B: 16.1 (l) | Dysgenetic testis | Present | — | Y | Absent | Absent, no malignancy | Absent | — | — | 7 | G |
| 30 | B: 1.5 (l) | Dysgenetic testis | Present | Unknown | Y | Absent | Absent, no malignancy | NA due to age | — | — | 4 | G |
| G: 12.0 (r) | Streak gonad | NA | NA | NA | Streak gonad | Absent, no malignancy | Absent | |||||
| 31 | G: 22 (r) | Dysgenetic testis | — | — | — | — | Present, GCNIS | — | — | — | — | G |
| 32 | G: 0.08 (l) | Streak gonad | NA | NA | NA | Streak gonad | Absent | NA due to age | — | VD UL | — | NG |
| B: 0.08 (r) | Dysgenetic testis | Few, abnormal, hyalinized | Rare | N | — | Absent | NA due to age | |||||
| 33 | B: 0.42 (l) | Dysgenetic testis | Few, abnormal, hyalinized | Rare | N | — | Absent | NA due to age | — | VD BL | 10 | NG |
| B: 0.42 (r) | Dysgenetic testis | Few, abnormal, hyalinized | Rare | N | — | Absent | NA due to age | |||||
| 34 | B: 34.4 (l) | Dysgenetic testis | Present, thick basal membrane | Present | Y | Absent | Absent, no malignancy | Absent | 12 | NG | ||
| B: 34.4 (r) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Present | Y/N | Absent | Present, no malignancy | Spermatocytic arrest | |||||
| 35 | B: 28.2 (l) | Dysgenetic testis | Present, thick basal membrane | Normal | Y | Absent | Absent, no malignancy | Absent | 12 | NG | ||
| B: 28.2 (r) | Dysgenetic testis | Present, thick basal membrane | Normal | Y | Absent | Absent, no malignancy | Absent | |||||
| 36 | B: 30.1 (l) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Normal | Y | Absent | Absent, no malignancy | Absent | 11 | NG | ||
| B: 30.1 (r) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Hyperplasia | Y | Absent | Absent, no malignancy | Absent | |||||
| 37 | B: 18.77 (l) | Dysgenetic testis | Present, thick basal membrane | Normal | N | Absent | Present, no malignancy | Overall spermatocytic arrest, few spermatids present | 12 | NG | ||
| B: 18.77 (r) | Dysgenetic testis | Present, thick basal membrane | Normal | N | Absent | Present, no malignancy | Spermatocytic arrest | |||||
| 38 | B: 36.3 (l) | Dysgenetic testis | Present, thick basal membrane | Hyperplasia | Y/N | Absent | Present, no malignancy | Only spermatogonia present | — | NG | ||
| B: 36.3 (r) | Dysgenetic testis | Present, thick basal membrane | Hyperplasia | Y/N | Absent | Present, no malignancy | Only spermatogonia present | |||||
| 39 | B: 49.4 (l) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Hyperplasia | Y/N | Absent | Present, no malignancy | Spermatocytic arrest | 12 | NG | ||
| B: 49.4 (r) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Hyperplasia | Y/N | Absent | Present, no malignancy | Only spermatogonia present | |||||
| 40 | B: 19.1 (l) | Dysgenetic testis | Present, thick basal membrane | Hyperplasia | Y | Absent | Absent, no malignancy | Absent | — | — | 12 | NG |
| B: 19.1 (r) | Dysgenetic testis | Present, thick basal membrane | Hyperplasia | Y | Absent | Absent, no malignancy | Absent | |||||
| 41 | B: 34.1 (l) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Present, fibrosis | Y/N | Absent | Present, no malignancy | Spermatocytic arrest | — | — | 11.5 | NG |
| B: 34.1 (r) | Dysgenetic testis | Present, thick basal membrane | Present | Y | Absent | Absent, no malignancy | Absent | |||||
| 42 | B: 17.1 (l) | Normal testis | — | — | — | — | Present, no malignancy | Normal spermatogenesis (small vol semen sample with conc of 114 mio/mL) | Rudimentary UT, FT BL | — | 11.5 | NG |
| G: 15.6 (r) | Streak gonad | NA | NA | NA | Ovarian-like tissue | Absent, no malignancy | Absent | |||||
| 43 | B: 28.6 (l) | Dysgenetic testis | Present, thick basal membrane, fibrosis | Present | N | Absent | Present, GCNIS | — | — | — | 12 | NG |
| B: 28.6 (r) | Slightly dysgenetic testis | Present, slightly thick basal membrane | Normal | N | Absent | Present, no malignancy | Preserved spermatogenesis (semen sample with conc of 0.06 mio/mL, few progressively motile) | |||||
| 44 | B: 47.5 (l) | Dysgenetic testis | Present, some atrophic and hyalinized | Hyperplasia | Y | Absent | Absent | Absent | — | EP BL | 10.5 | NG |
| B: 47.5 (r) | Dysgenetic testis | Present, some atrophic and hyalinized | Hyperplasia | Y | Absent | Absent, no malignancy | Absent | |||||
| ID . | Biopsy or Gonadectomy: Age in Years (L/R) . | Overall Morphology . | Male Characteristics . | Female Characteristics . | Germ Cells . | Spermatogenesis/Follicles . | Internal Genitalia . | EMS . | Group (N/NG) . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Testicular Tubules . | Leydig Cells . | SCO, Y/N . | ||||||||||
| Female . | Male . | |||||||||||
| 1 | G: 15 (l) | Dysgenetic testis | Present, thick basal membrane, fibrosis; branched tubules, calcification. | Hyperplasia | Y | — | Absent | — | UT; FT | EP | 4 | G |
| G: 15 (r) | Müllerian derivatives | — | — | — | — | — | — | |||||
| 2 | G: 0.3 (l) | Dysgenetic testis | Prepubertal pattern | Absent | Y | — | Absent | NA due to age | UT | EP | 4 | G |
| G: 0.3 (r) | Dysgenetic testis | Prepubertal pattern | Absent | Y | — | Absent | NA due to age | |||||
| 3 | G: 4 (l) | Testis | Prepubertal pattern | — | — | NA | — | NA due to age | UT; FT UL | EP; VD UL | 1 | G |
| G: 4 (r) | Streak gonad | NA | NA | NA | Ovarian-like tissue | Absent | NA due to age | |||||
| 4 | G: 2 (l) | Wolffian and Müllerian derivatives | — | — | — | — | — | NA due to age | FT | EP | 9.5 | G |
| 5 | B: 5 (l) | Dysgenetic testis | Prepubertal testis | Absent | N | Present | NA due to age | UT; FT BL | EP BL | 8 | G | |
| G: 5 (r) | Streak gonad | NA | NA | NA | Ovarian-like tissue with primitive sex cord structures | — | NA due to age | |||||
| 6 | B: 4.8 (l) | Dysgenetic testis | Normal | Present | Y | — | Absent | NA due to age | FT UL | VD, EP UL | 5.5 | G |
| G: 4.8 (r) | Ovarian-like tissue | NA | NA | NA | Ovarian-like tissue | Absent | NA due to age | |||||
| 7 | B: 0.1 (l) | Dysgenetic testis | Irregular, abnormal | — | N | — | Rare absent | NA due to age, absent | UT UL | VD, EP UL | 5.5 | G |
| B: 15 (l) | Dysgenetic testis | Irregular, abnormal | — | Y | — | |||||||
| G: 1.0 (r) | Ovarian-like tissue | NA | NA | NA | Ovarian-like tissue with germ cells present | Present, gonadoblastoma in situ | NA due to age | FT UL | ||||
| 8 | B: 1 (r) | Prepubertal testis; abnormal area with peripheral microlithiasis | Normal | Normal | N | — | — | NA due to age | — | Normal | — | G |
| 9 | G: childhood (l) | Prepubertal testis | — | — | — | — | — | NA due to age | — | Agenesis of SV | — | G |
| G: childhood (r) | Streak gonad | NA | NA | NA | Streak gonad | Absent | NA due to age | — | ||||
| 10 | B: 13.2 (l) | Fibrous tissue without testicular morphology | Absent | Absent | — | — | Absent | Absent | FT, UT UL | VD, EP UL | 3 | G |
| B: 13.2 (r) | Testis without spermatogenesis | Normal | Rare | Y | Fibrous ovarian-like tissue | Absent | Absent | |||||
| G: 13.9 (r) | ||||||||||||
| 11 | B: 0.75 (l) | Prepubertal testis | Normal | Absent | N | None | Normal | NA due to age | UT, FT UL | WR UL | 5.5 | G |
| B: 16 | Testis with partial atrophy and SCO; other parts with incomplete spermatogenesis | Mixed abnormal and normal | Normal | Y/N | None | Strongly reduced number, no malignancy | Incomplete spermatogenesis, round elongated spermatids, no mature sperm (sperm present in semen sample) | |||||
| G: 0.75 (r) | No gonadal tissue | NA | NA | NA | NA | NA | NA | UT, FT UL | WR | |||
| 12 | B: 3 (l) | Dysgenetic testis | Dichotomized seminiferous tubules predominantly with Sertoli cells | — | N | — | Few spermatogonia | NA due to age | UT-like structure | WR | 3 | G |
| G: 6 (l) | Hypoplastic TA | Prepubertal seminiferous tubules invading TA | Rare | N | — | — | NA due to age | |||||
| Dysgenetic testis; thickened TA | ||||||||||||
| B: 6 (r) | Prepubertal testis | Normal | Normal | N | — | — | NA due to age | |||||
| 13 | B: 2 (l) | Prepubertal testis | — | — | — | — | — | NA due to age | UT, FT | — | G | |
| 14 | B: 1.5 | Streak gonad | NA | NA | NA | Ovarian-like tissue | Absent | NA due to age | UT, FT | — | G | |
| G: 6 | NA due to age | |||||||||||
| 15 | G: 1 (l) | Streak gonad | NA | NA | NA | Streak gonad | Absent | NA due to age | FT UL | VD UL | 2.5 | G |
| G: 2 (r) | Dysgenetic testis | — | — | — | — | — | NA due to age | |||||
| 16 | B:21 (l) | Hypotrophic testis | Hyalinized | Rare | — | NA | Absent | Absent | UT, FT UL | VD, EP UL | 2.5 | G |
| G:3 (r) | Atrophic testis | — | — | — | — | — | NA due to age | |||||
| 17 | G: 3 (l) | Dysgenetic testis and ovarian tissue | Seminiferous tubules without germ cells | — | Y | Ovarian tissue | Absent, no malignancy | NA due to age | — | — | 4 | G |
| B: 3 (r) | Dysgenetic testis | — | — | — | — | No malignancy | NA due to age | — | — | |||
| 18 | G: 1 (l) | Steak gonad | NA | NA | NA | Streak gonad | Absent, no malignancy | NA due to age | — | — | 8.5 | G |
| B: 1 (r) | Dysgenetic testis | — | — | — | — | No malignancy | NA due to age | — | — | |||
| B: 5 (r) | Dysgenetic testis | — | — | — | — | — | NA due to age | |||||
| 19 | G: 4 | Dysgenetic gonad | — | — | — | — | No malignancy | NA due to age | — | — | 5 | G |
| B: 11 | — | — | — | — | — | — | — | |||||
| 20 | B/G: 5 | Fibrous tissue | — | — | — | — | No malignancy | NA due to age | — | — | 6 | G |
| 21 | B: 3.3 (r) | Prepubertal testis | — | — | — | — | Presence of germ cells uncertain | NA due to age | — | EP | — | G |
| 22 | G: 1.9 (l) | Dysgenetic gonad | Few nests of sex cords | — | — | Ovarian- like tissue | Absent | NA due to age | FT | WR | — | G |
| 23 | G: 1.5 (l) | Dysgenetic gonad UGT | Abnormal | — | Y | Ovarian-like tissue with germ cells present | Present, presence of primordial follicles uncertain | NA due to age | Rudimentary UT, FT | — | — | G |
| B: 9.5 (r) | No gonadal tissue | NA | NA | NA | NA | NA | NA | Fimbriae with edema | — | |||
| 24 | G: 1.3 (l) | Streak gonad | NA | NA | NA | Ovarian-like tissue | Absent | NA due to age | FT | — | — | G |
| 25 | B: 14.0 (l) | No gonadal tissue | NA | NA | NA | NA | NA | Rudimentary UT, FT BL | VD BL | 8 | G | |
| B: 21.3 (l) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Hyperplasia | Y/N | Absent | Present, no malignancy | Spermatocytic arrest | |||||
| G: 14.0 (r) | Ovotesticular remnant | Present | — | — | Ovotesticular remnant | Absent, no malignancy | Absent | |||||
| 26 | B: 1.9 (l) | Prepubertal testis | Normal | Presence uncertain, fibrosis | N | Absent | Present, no malignancy | NA due to age | Rudimentary UT, FL BL | VD UL | 4 | G |
| G: 14.5 (l) | Dysgenetic testis | Present, thick basal membrane | Hyperplasia | Y/N | Absent | Present, GCNIS | Overall spermatocytic arrest, some spermatids present | |||||
| G: 1.9 (r) | Streak gonad | NA | NA | NA | Ovarian-like tissue | Absent, no malignancy | Absent | |||||
| 27 | G: 2.2 (l) | Fibrotic ovarian-like tissue | NA | NA | NA | Ovarian-like tissue | Absent | NA | MR, rudimentary UT, FT BL | VD, EP BL | 1 | G |
| B: 2.2 (r) | Dysgenetic testis | Normal | Present | Y/N | Absent | Spermatogonia and gonocytes present | NA due to age | |||||
| G: 14.2 (r) | Prepubertal testis | Normal | Present | Y/N | Absent | Present, GCNIS | NA due to age | |||||
| 28 | B: 43.7 (l) | Dysgenetic testis | Present, some hyalinized, fibrosis | Hyperplasia | N | Absent | Absent, no malignancy | Absent | 9.5 | G | ||
| G: 42.8 (r) | Streak gonad | NA | NA | NA | Streak gonad | Absent, no malignancy | Absent | Rudimentary UT, FT UL | ||||
| 29 | B: 16.1 (l) | Dysgenetic testis | Present | — | Y | Absent | Absent, no malignancy | Absent | — | — | 7 | G |
| 30 | B: 1.5 (l) | Dysgenetic testis | Present | Unknown | Y | Absent | Absent, no malignancy | NA due to age | — | — | 4 | G |
| G: 12.0 (r) | Streak gonad | NA | NA | NA | Streak gonad | Absent, no malignancy | Absent | |||||
| 31 | G: 22 (r) | Dysgenetic testis | — | — | — | — | Present, GCNIS | — | — | — | — | G |
| 32 | G: 0.08 (l) | Streak gonad | NA | NA | NA | Streak gonad | Absent | NA due to age | — | VD UL | — | NG |
| B: 0.08 (r) | Dysgenetic testis | Few, abnormal, hyalinized | Rare | N | — | Absent | NA due to age | |||||
| 33 | B: 0.42 (l) | Dysgenetic testis | Few, abnormal, hyalinized | Rare | N | — | Absent | NA due to age | — | VD BL | 10 | NG |
| B: 0.42 (r) | Dysgenetic testis | Few, abnormal, hyalinized | Rare | N | — | Absent | NA due to age | |||||
| 34 | B: 34.4 (l) | Dysgenetic testis | Present, thick basal membrane | Present | Y | Absent | Absent, no malignancy | Absent | 12 | NG | ||
| B: 34.4 (r) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Present | Y/N | Absent | Present, no malignancy | Spermatocytic arrest | |||||
| 35 | B: 28.2 (l) | Dysgenetic testis | Present, thick basal membrane | Normal | Y | Absent | Absent, no malignancy | Absent | 12 | NG | ||
| B: 28.2 (r) | Dysgenetic testis | Present, thick basal membrane | Normal | Y | Absent | Absent, no malignancy | Absent | |||||
| 36 | B: 30.1 (l) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Normal | Y | Absent | Absent, no malignancy | Absent | 11 | NG | ||
| B: 30.1 (r) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Hyperplasia | Y | Absent | Absent, no malignancy | Absent | |||||
| 37 | B: 18.77 (l) | Dysgenetic testis | Present, thick basal membrane | Normal | N | Absent | Present, no malignancy | Overall spermatocytic arrest, few spermatids present | 12 | NG | ||
| B: 18.77 (r) | Dysgenetic testis | Present, thick basal membrane | Normal | N | Absent | Present, no malignancy | Spermatocytic arrest | |||||
| 38 | B: 36.3 (l) | Dysgenetic testis | Present, thick basal membrane | Hyperplasia | Y/N | Absent | Present, no malignancy | Only spermatogonia present | — | NG | ||
| B: 36.3 (r) | Dysgenetic testis | Present, thick basal membrane | Hyperplasia | Y/N | Absent | Present, no malignancy | Only spermatogonia present | |||||
| 39 | B: 49.4 (l) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Hyperplasia | Y/N | Absent | Present, no malignancy | Spermatocytic arrest | 12 | NG | ||
| B: 49.4 (r) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Hyperplasia | Y/N | Absent | Present, no malignancy | Only spermatogonia present | |||||
| 40 | B: 19.1 (l) | Dysgenetic testis | Present, thick basal membrane | Hyperplasia | Y | Absent | Absent, no malignancy | Absent | — | — | 12 | NG |
| B: 19.1 (r) | Dysgenetic testis | Present, thick basal membrane | Hyperplasia | Y | Absent | Absent, no malignancy | Absent | |||||
| 41 | B: 34.1 (l) | Dysgenetic testis | Present, thick basal membrane, some hyalinized | Present, fibrosis | Y/N | Absent | Present, no malignancy | Spermatocytic arrest | — | — | 11.5 | NG |
| B: 34.1 (r) | Dysgenetic testis | Present, thick basal membrane | Present | Y | Absent | Absent, no malignancy | Absent | |||||
| 42 | B: 17.1 (l) | Normal testis | — | — | — | — | Present, no malignancy | Normal spermatogenesis (small vol semen sample with conc of 114 mio/mL) | Rudimentary UT, FT BL | — | 11.5 | NG |
| G: 15.6 (r) | Streak gonad | NA | NA | NA | Ovarian-like tissue | Absent, no malignancy | Absent | |||||
| 43 | B: 28.6 (l) | Dysgenetic testis | Present, thick basal membrane, fibrosis | Present | N | Absent | Present, GCNIS | — | — | — | 12 | NG |
| B: 28.6 (r) | Slightly dysgenetic testis | Present, slightly thick basal membrane | Normal | N | Absent | Present, no malignancy | Preserved spermatogenesis (semen sample with conc of 0.06 mio/mL, few progressively motile) | |||||
| 44 | B: 47.5 (l) | Dysgenetic testis | Present, some atrophic and hyalinized | Hyperplasia | Y | Absent | Absent | Absent | — | EP BL | 10.5 | NG |
| B: 47.5 (r) | Dysgenetic testis | Present, some atrophic and hyalinized | Hyperplasia | Y | Absent | Absent, no malignancy | Absent | |||||
Ovarian-like tissue does not contain follicles unless specifically indicated.
Abbreviations: —, information not available; B, biopsy; BL, bilateral; EP, epididymis; FT, fallopian tubes; G, genital (last column); G, gonadectomy (column 2); ID, identification; l, left; mio/mL, million per milliliter; MR, Müllerian remnants; N, no; NA, not applicable; NG, nongenital; r, right; SCO, Sertoli cell only; SV, seminal vesicle; TA, tunica albuginea; UGT, undifferentiated; UL, unilateral; UT, uterus; VD, vas deferens; vol, volume; WR, Wolffian duct remnants; Y, yes.
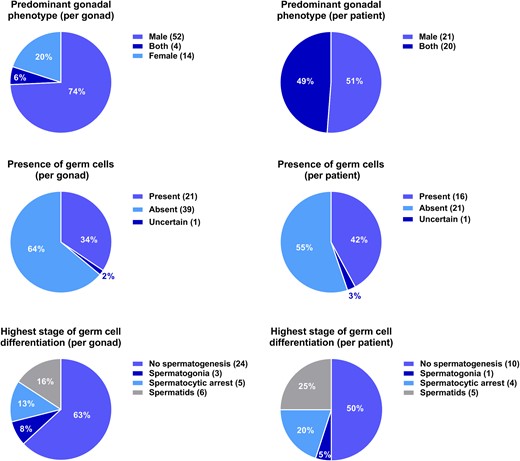
Key histology findings in terms of phenotype, presence of germ cells, and germ-cell differentiation counted by patients and gonads, respectively.
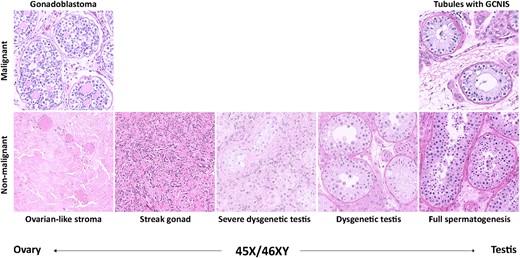
The histological spectrum found in the gonadal samples from males with 45,X/46,XY mosaicism. All images show hematoxylin–eosin-stained sections.
Patients either had bilateral testicular tissue (51.2%, 10 genital, 11 nongenital) or testicular tissue on one side and more undifferentiated, ovarian-like tissue on the contralateral side, most often in the form of streak gonads (48.8%, 18 genital, 2 nongenital; Figs. 3 and 4).
Sertoli cell-only (SCO) pattern was evident in 30 patients with available pre- and/or postpubertal histological samples (66%). In seven of the postpubertal patients (35%), the SCO pattern was also associated with spermatocytic arrest in other tubules and in a single case with tubules containing germ-cell neoplasia in situ (GCNIS; Tables 2 and 3).
Tally of Patients With SCO Pattern Alongside Spermatocytic Arrest, Full Spermatogenesis, and/or GCNIS
| . | SCO Pattern . | Total n, Gonads . | Percentagea . |
|---|---|---|---|
| Prepubertal | SCO | 7 | 87.5 |
| SCO and GCNIS | 1 | 12.5 | |
| Postpubertal | SCO | 14 | 63.6 |
| SCO and tubules with spermatocytic arrest | 7 | 31.8 | |
| SCO and tubules with full spermatogenesis | 0 | 0 | |
| SCO and tubules with spermatocytic arrest and GCNIS | 1 | 4.5 | |
| SCO and tubules with full spermatogenesis and GCNIS | 0 | 0 |
| . | SCO Pattern . | Total n, Gonads . | Percentagea . |
|---|---|---|---|
| Prepubertal | SCO | 7 | 87.5 |
| SCO and GCNIS | 1 | 12.5 | |
| Postpubertal | SCO | 14 | 63.6 |
| SCO and tubules with spermatocytic arrest | 7 | 31.8 | |
| SCO and tubules with full spermatogenesis | 0 | 0 | |
| SCO and tubules with spermatocytic arrest and GCNIS | 1 | 4.5 | |
| SCO and tubules with full spermatogenesis and GCNIS | 0 | 0 |
According to age (pre- or postpubertal).
Tally of Patients With SCO Pattern Alongside Spermatocytic Arrest, Full Spermatogenesis, and/or GCNIS
| . | SCO Pattern . | Total n, Gonads . | Percentagea . |
|---|---|---|---|
| Prepubertal | SCO | 7 | 87.5 |
| SCO and GCNIS | 1 | 12.5 | |
| Postpubertal | SCO | 14 | 63.6 |
| SCO and tubules with spermatocytic arrest | 7 | 31.8 | |
| SCO and tubules with full spermatogenesis | 0 | 0 | |
| SCO and tubules with spermatocytic arrest and GCNIS | 1 | 4.5 | |
| SCO and tubules with full spermatogenesis and GCNIS | 0 | 0 |
| . | SCO Pattern . | Total n, Gonads . | Percentagea . |
|---|---|---|---|
| Prepubertal | SCO | 7 | 87.5 |
| SCO and GCNIS | 1 | 12.5 | |
| Postpubertal | SCO | 14 | 63.6 |
| SCO and tubules with spermatocytic arrest | 7 | 31.8 | |
| SCO and tubules with full spermatogenesis | 0 | 0 | |
| SCO and tubules with spermatocytic arrest and GCNIS | 1 | 4.5 | |
| SCO and tubules with full spermatogenesis and GCNIS | 0 | 0 |
According to age (pre- or postpubertal).
Germ cells were detected in 42.1% of patients (nine genital, seven nongenital), whereas no germ cells were found in 55.3% of patients (15 genital, 6 nongenital). Seven patients did not have information on germ cells available. There was no significant difference between ages at biopsy/gonadectomy in patients with detectable germ cells and patients without detectable germ cells [median (range), 18.77 years (0.10 to 49.40) vs 13.55 years (0.30 to 47.50); P = 0.154].
Folliculogenesis was not detected in any of the included gonadal samples. Moreover, three patients were originally labeled with ovotestes, but upon thorough re-examination of the original slides, no follicles could be detected, and therefore, the presence of ovotestes could not be confirmed in any of the samples from the current study.
Spermatogenesis was evaluated by histology in 20 postpubertal patients. Spermatids were present in 25.0% (two genital, three nongenital), whereas in 50.0% (six genital, four nongenital), no germ cells were observed (Fig. 3). In the remaining 25.0%, germ cells were present but with arrest of spermatogenesis at different stages. The five patients with spermatids present had a median EMS of 11.5 (4.0 to 12.0; Table 2).
GCNIS was present in four patients (two prepubertal, two postpubertal) and gonadoblastoma in one (total n = 5, 11.4%, four genital, one nongenital; Tables 1 and 2). The median EMS in these patients was 4.8 (1.0 to 12.0; Table 2).
Tubules with preserved spermatogenesis, including the presence of spermatids, were present alongside tubules with GCNIS in both of the postpubertal patients (one patient with an EMS of four, one patient with an EMS of 12).
Overall, with the comparison of the included histology parameters between the genital and the nongenital groups, no differences were found (all P > 0.05), except for a significant difference in the distribution of predominantly male-only and mixed gonads in the two groups, with predominantly male gonads more frequent in the nongenital group (P = 0.009).
Fertility
Complete azoospermia was observed in 14 (82.4%, 2 genital, 12 nongenital) of 17 patients who had undergone clinical semen analyses (Table 4). Three (17.6%, one genital, two nongenital) had evidence of live spermatozoa (one sample with some live spermatozoa, one sample with a concentration of 0.06 million/mL, few progressively motile, and lastly, 1 small volume sample with a concentration of 114 million/mL). In all of these three cases, spermatids had also been identified in the histological evaluation. One azoospermic patient had spermatids present in a histological sample (Table 4).
Reproductive Hormones, Clinical Features, and Gonadal Histology in Patients With Available Semen Analyses
| ID . | Semen Analysis . | LH, IU/L . | FSH, IU/L . | T, nM . | Tvol, mL . | EMS . | Tx, y/n . | Histology, Right . | Histology, Left . | Group, G/NG . |
|---|---|---|---|---|---|---|---|---|---|---|
| — | Azoospermia | — | — | — | — | — | y | — | — | NG |
| — | Azoospermia | — | — | — | — | — | n | — | — | NG |
| — | Azoospermia | — | — | — | — | — | n | — | — | NG |
| Azoospermia | — | 0.17 | 23.0 | 8 | 12 | n | — | — | NG | |
| 4 | Azoospermia | 7.19 | 21.25 | 2.07 | 15 | 9.5 | n | Wolffian and Müllerian derivatives | G | |
| 34 | Azoospermia | 8.56 | 20.10 | 12.26 | 13.5 | 12 | n | DT, SA, SCO | DT, SCO | NG |
| 35 | Azoospermia | 9.35 | 15.20 | 6.84 | — | 12 | n | DT, SCO | DT, SCO | NG |
| 36 | Azoospermia | 3.59 | 6.69 | 8.12 | — | 11 | n | DT, SCO | DT, SCO | NG |
| — | Azoospermia | 4.18 | 2.32 | 16.94 | — | 12 | n | — | — | NG |
| 39 | Azoospermia | 7.99 | — | 14.87 | — | 12 | y | DT, SCO | DT, SCO, SA | NG |
| — | Azoospermia | 6.19 | 19.40 | 10.55 | — | 12 | n | — | — | NG |
| 26 | Azoospermia | 7.63 | 5.89 | 11.18 | — | 4 | y | SA, GCNIS, SP | Streak gonad | G |
| 41 | Azoospermia | 8.56 | 20.10 | 12.26 | 12 | 11.5 | n | DT, SCO | DT, SCO, SA | NG |
| 44 | Azoospermia | — | — | — | 8 | 10.5 | n | DT, SCO | DT, SCO | NG |
| 11 | Sperm present | 3.2 | 3.7 | 13.0 | 20 | 5.5 | n | — | Atrophy, SCO, SP | G |
| 42 | 114 mio/mL | 2.34 | 5.01 | 12.61 | 15 | 11.5 | y | Streak gonad | Normal testis, SP | NG |
| 43 | 0.06 mio/mL | — | — | — | 25 | 12 | n | DT, SP | DT, GCNIS | NG |
| ID . | Semen Analysis . | LH, IU/L . | FSH, IU/L . | T, nM . | Tvol, mL . | EMS . | Tx, y/n . | Histology, Right . | Histology, Left . | Group, G/NG . |
|---|---|---|---|---|---|---|---|---|---|---|
| — | Azoospermia | — | — | — | — | — | y | — | — | NG |
| — | Azoospermia | — | — | — | — | — | n | — | — | NG |
| — | Azoospermia | — | — | — | — | — | n | — | — | NG |
| Azoospermia | — | 0.17 | 23.0 | 8 | 12 | n | — | — | NG | |
| 4 | Azoospermia | 7.19 | 21.25 | 2.07 | 15 | 9.5 | n | Wolffian and Müllerian derivatives | G | |
| 34 | Azoospermia | 8.56 | 20.10 | 12.26 | 13.5 | 12 | n | DT, SA, SCO | DT, SCO | NG |
| 35 | Azoospermia | 9.35 | 15.20 | 6.84 | — | 12 | n | DT, SCO | DT, SCO | NG |
| 36 | Azoospermia | 3.59 | 6.69 | 8.12 | — | 11 | n | DT, SCO | DT, SCO | NG |
| — | Azoospermia | 4.18 | 2.32 | 16.94 | — | 12 | n | — | — | NG |
| 39 | Azoospermia | 7.99 | — | 14.87 | — | 12 | y | DT, SCO | DT, SCO, SA | NG |
| — | Azoospermia | 6.19 | 19.40 | 10.55 | — | 12 | n | — | — | NG |
| 26 | Azoospermia | 7.63 | 5.89 | 11.18 | — | 4 | y | SA, GCNIS, SP | Streak gonad | G |
| 41 | Azoospermia | 8.56 | 20.10 | 12.26 | 12 | 11.5 | n | DT, SCO | DT, SCO, SA | NG |
| 44 | Azoospermia | — | — | — | 8 | 10.5 | n | DT, SCO | DT, SCO | NG |
| 11 | Sperm present | 3.2 | 3.7 | 13.0 | 20 | 5.5 | n | — | Atrophy, SCO, SP | G |
| 42 | 114 mio/mL | 2.34 | 5.01 | 12.61 | 15 | 11.5 | y | Streak gonad | Normal testis, SP | NG |
| 43 | 0.06 mio/mL | — | — | — | 25 | 12 | n | DT, SP | DT, GCNIS | NG |
Abbreviations: DT, dysgenetic testis; SA, spermatocytic arrest; SP, spermatids present; Tvol, T volume at last evaluation (largest testis); Tx, previous or ongoing T treatment.
Reproductive Hormones, Clinical Features, and Gonadal Histology in Patients With Available Semen Analyses
| ID . | Semen Analysis . | LH, IU/L . | FSH, IU/L . | T, nM . | Tvol, mL . | EMS . | Tx, y/n . | Histology, Right . | Histology, Left . | Group, G/NG . |
|---|---|---|---|---|---|---|---|---|---|---|
| — | Azoospermia | — | — | — | — | — | y | — | — | NG |
| — | Azoospermia | — | — | — | — | — | n | — | — | NG |
| — | Azoospermia | — | — | — | — | — | n | — | — | NG |
| Azoospermia | — | 0.17 | 23.0 | 8 | 12 | n | — | — | NG | |
| 4 | Azoospermia | 7.19 | 21.25 | 2.07 | 15 | 9.5 | n | Wolffian and Müllerian derivatives | G | |
| 34 | Azoospermia | 8.56 | 20.10 | 12.26 | 13.5 | 12 | n | DT, SA, SCO | DT, SCO | NG |
| 35 | Azoospermia | 9.35 | 15.20 | 6.84 | — | 12 | n | DT, SCO | DT, SCO | NG |
| 36 | Azoospermia | 3.59 | 6.69 | 8.12 | — | 11 | n | DT, SCO | DT, SCO | NG |
| — | Azoospermia | 4.18 | 2.32 | 16.94 | — | 12 | n | — | — | NG |
| 39 | Azoospermia | 7.99 | — | 14.87 | — | 12 | y | DT, SCO | DT, SCO, SA | NG |
| — | Azoospermia | 6.19 | 19.40 | 10.55 | — | 12 | n | — | — | NG |
| 26 | Azoospermia | 7.63 | 5.89 | 11.18 | — | 4 | y | SA, GCNIS, SP | Streak gonad | G |
| 41 | Azoospermia | 8.56 | 20.10 | 12.26 | 12 | 11.5 | n | DT, SCO | DT, SCO, SA | NG |
| 44 | Azoospermia | — | — | — | 8 | 10.5 | n | DT, SCO | DT, SCO | NG |
| 11 | Sperm present | 3.2 | 3.7 | 13.0 | 20 | 5.5 | n | — | Atrophy, SCO, SP | G |
| 42 | 114 mio/mL | 2.34 | 5.01 | 12.61 | 15 | 11.5 | y | Streak gonad | Normal testis, SP | NG |
| 43 | 0.06 mio/mL | — | — | — | 25 | 12 | n | DT, SP | DT, GCNIS | NG |
| ID . | Semen Analysis . | LH, IU/L . | FSH, IU/L . | T, nM . | Tvol, mL . | EMS . | Tx, y/n . | Histology, Right . | Histology, Left . | Group, G/NG . |
|---|---|---|---|---|---|---|---|---|---|---|
| — | Azoospermia | — | — | — | — | — | y | — | — | NG |
| — | Azoospermia | — | — | — | — | — | n | — | — | NG |
| — | Azoospermia | — | — | — | — | — | n | — | — | NG |
| Azoospermia | — | 0.17 | 23.0 | 8 | 12 | n | — | — | NG | |
| 4 | Azoospermia | 7.19 | 21.25 | 2.07 | 15 | 9.5 | n | Wolffian and Müllerian derivatives | G | |
| 34 | Azoospermia | 8.56 | 20.10 | 12.26 | 13.5 | 12 | n | DT, SA, SCO | DT, SCO | NG |
| 35 | Azoospermia | 9.35 | 15.20 | 6.84 | — | 12 | n | DT, SCO | DT, SCO | NG |
| 36 | Azoospermia | 3.59 | 6.69 | 8.12 | — | 11 | n | DT, SCO | DT, SCO | NG |
| — | Azoospermia | 4.18 | 2.32 | 16.94 | — | 12 | n | — | — | NG |
| 39 | Azoospermia | 7.99 | — | 14.87 | — | 12 | y | DT, SCO | DT, SCO, SA | NG |
| — | Azoospermia | 6.19 | 19.40 | 10.55 | — | 12 | n | — | — | NG |
| 26 | Azoospermia | 7.63 | 5.89 | 11.18 | — | 4 | y | SA, GCNIS, SP | Streak gonad | G |
| 41 | Azoospermia | 8.56 | 20.10 | 12.26 | 12 | 11.5 | n | DT, SCO | DT, SCO, SA | NG |
| 44 | Azoospermia | — | — | — | 8 | 10.5 | n | DT, SCO | DT, SCO | NG |
| 11 | Sperm present | 3.2 | 3.7 | 13.0 | 20 | 5.5 | n | — | Atrophy, SCO, SP | G |
| 42 | 114 mio/mL | 2.34 | 5.01 | 12.61 | 15 | 11.5 | y | Streak gonad | Normal testis, SP | NG |
| 43 | 0.06 mio/mL | — | — | — | 25 | 12 | n | DT, SP | DT, GCNIS | NG |
Abbreviations: DT, dysgenetic testis; SA, spermatocytic arrest; SP, spermatids present; Tvol, T volume at last evaluation (largest testis); Tx, previous or ongoing T treatment.
One of the three patients with live spermatozoa in their semen sample underwent testicular sperm cell extraction during orchiectomy, following a biopsy showing GCNIS. However, none of the patients included in this study fathered biological offspring during the follow-up period.
There was no significant difference in fertility (azoospermia vs live spermatozoa) between the genital and nongenital groups (P = 0.42).
Discussion
This large multicenter study of male patients with 45,X/46,XY mosaicism found that most patients are short with varying degrees of gonadal function. Moreover, gonadal histology revealed that the risk of preneoplasia was relatively high but also that the presence of ongoing spermatogenesis was common. Lastly, the risk of preneoplasia and presence of spermatogenesis appear to be independent of genital phenotype (degree of virilization).
In general, almost 80% of males with 45,X/46,XY mosaicism had sufficient gonadal function to enter puberty spontaneously, although almost 40% needed subsequent T treatment. However, patients in the genital group had lower rates of spontaneous puberty and higher rates of T treatment. Interestingly, we found that most of the patients appeared to have normal serum concentrations of T. It has previously been reported that many patients with scrotal gonads have some hormone production (25), but it was unexpected that most patients had T levels in the normal range despite their genital phenotype and overall fairly small testicular volumes. However, gonadotropin levels were relatively high, indicative of some degree of (early) gonadal failure. Altogether, our findings are in accordance with previous reports on gonadal function in males with a 45,X/46,XY karyotype (5, 7, 10, 16, 26).
Most patients in this cohort were short and did not grow according to their genetic potential. Patients in the genital group were significantly shorter than those in the nongenital, probably reflecting that patients with more severe genital phenotypes (genital group) are also more likely to have affected growth. This could, in theory, be a result of a larger degree of 45,X cells and thus, a larger degree of short stature homeobox haploinsufficiency, as seen in classic Turner syndrome. Some of the growth trajectories in the adolescent patients in this study appeared to lack the expected pubertal growth spurt. Theoretically, hypogonadism in adolescence could potentiate the effects of the 45,X cell line on growth (5–7, 10, 15, 27, 28), thus producing this growth pattern. It is noteworthy that a recent study reports that XY-mosaic Turner patients have less affected growth than classic Turner girls (29).
One-third of patients in this study received rhGH treatment, and the percentage was not influenced by group. Final height SDSs were similar in groups, with and without rhGH treatment, but the retrospective nature of this study does not allow firm conclusions on the efficacy of rhGH treatment. In general, the literature shows contradictory findings on the effects of rhGH treatment on growth (25), and our results, as well as those from previous studies, raise the critical need for well-designed studies to examine the possible benefits of rhGH in these children. However, none of the existing studies are randomized controlled trials, and the opposing results could be a result of study design and perhaps also the underlying mechanisms of the growth retardation.
Theoretically, earlier intervention could improve several of the aforementioned clinical outcomes compared with patients with delayed or no intervention. However, a conclusive study will require a larger sample size, as well as detailed information on T therapy.
The vast histological material in this study (44 patients, 61 gonads) showed a broad phenotypic spectrum, confirming that some patients had one (often scrotal) testis and one intra-abdominal streak gonad (48.8%), whereas others had two testes (often bilaterally scrotal; 51.2%). The distribution was significantly different between the genital and nongenital groups. The 45,X/46,XY karyotype alone therefore does not allude to the histology or the location of the gonads, and thus, the application of the term “mixed gonadal dysgenesis” to this patient population could be deemed inappropriate.
Most gonads in this study were dysgenetic testes, but there was a wide range from relatively normal testes containing tubules with complete/full spermatogenesis to streak gonads at each end of the spectrum. We did not detect the presence of follicles in any of the gonad samples evaluated. This could be a result of oogonial loss before the formation of primordial follicles or breakdown of formed follicles already in early (fetal) life. Consensus understanding is that it takes two X-chromosomes for primordial follicles to develop. This notion is supported by a study examining the presence of primordial follicles (and number of germ cells) in ovaries from Turner fetuses, aged 17 to 37 weeks, which reported that no primordial follicles could be detected (30). The etiology behind the 45,X/46,XY karyotype has been suggested as one where larger structural aberrations, such as deletions, isodicentricism, etc., or minor molecular abnormalities to the Y-chromosome may cause its loss in some cells (3–7). Thus, it appears plausible that the vast majority of patients with 45,X/46,XY mosaicism never has had two X-chromosomes present, and the development of follicles therefore seems unlikely, supported by our current findings. Moreover, it has previously been reported that gonads are relatively often mislabeled, as ovotestes in 45,X/46,XY patients (31). The morphology in these samples resembles undifferentiated gonadal tissue and/or streak-like tissue with scattered germ cells. In such cases, a higher risk of neoplasia, namely gonadoblastoma instead of GCNIS, has been reported (32). Given all of the above, it seems possible that ovotesticular DSD in 45,X/46,XY patients is a rarity or maybe even a misconception, as has also previously been suggested (31). Additionally, regarding future fertility preservation potential, the focus should lie on spermatogenesis rather than folliculogenesis in these patients.
In 11.4% of patients, we found gonadal neoplasia, specifically four patients with GCNIS and one patient with gonadoblastoma. Given the majority of (dysgenetic) testes in this series, GCNIS is the more likely neoplasia in this group of patients, which is in line with previous reports of gonadoblastoma being more frequent in patients with 45,X/46,XY mosaicism raised as females (18). Interestingly, we found that both postpubertal patients with GCNIS had spermatogenesis alongside their neoplasia. This is a very important point for clinicians, as testicular sperm cell extraction or aspiration should be considered before gonadectomy in these patients, as was done in one patient in this study. It also indicates that the presence of spermatogenesis should not be interpreted as the absence of testicular dysgenesis and also poses the question of when to biopsy postpubertal patients in particular.
Interestingly, there did not appear to be a correlation between EMS and risk of germ-cell malignancy. A higher risk of neoplasia has previously been found in patients with greater genital ambiguity (5, 16), but our current findings are not completely in accordance with this notion. This may be explained by the fact that the study population of this study differs from previous studies, with only males and not females with a Turner syndrome phenotype included. It probably also highlights a dual relationship in which severely dysgenetic gonads do not sufficiently support germ cells regardless of whether these are normal or potentially malignant. Conversely, a low EMS in men with 45,X/46,XY mosaicism is highly suggestive of dysgenetic testes or undifferentiated gonadal tissue, which if germ cells are present, has a high risk of GCNIS. Thus, clinicians should be aware of the risk of malignant germ cells in all patients regardless of virilization status.
Surprisingly, one-quarter of postpubertal patients had focal spermatogenesis, whereas another one-quarter had spermatogenesis arrested at different stages of germ-cell differentiation. Altogether, this demonstrates a future fertility potential in up to one-half of the postpubertal patients. It was noteworthy that many patients demonstrated focal SCO, along with spermatocytic arrest, at different stages, highlighting that each gonad may be heterogenous, and focal spermatogenesis cannot be ruled out based on a single biopsy. Moreover, one azoospermic male had histological evidence of spermatids emphasizing that even males with azoospermia may have focal spermatogenesis.
The implications of the high proportion of patients with spermatogenesis and spermatocytic arrest are important; in vitro spermatogenesis, in which germ cells are differentiated in vitro, may provide a future fertility treatment option for these patients. However, no verified protocol is currently available for human testis tissue, despite few previous reports of successful in vitro maturation of germ cells in human tissue (33–36) and even early-stage germ cells (prepubertal) in mice (37). The possibility that a protocol for human in vitro spermatogenesis may be established in the near future also raises the question of whether attempts to cryopreserve testicular tissue from 45,X/46,XY patients should be considered. It does, nonetheless, also provide the clinician with ethical dilemmas, such as the inclusion of patients in experimental protocols where the outcome and timeline are still unknown (current patients may not benefit), as well as possible transmission of an aberrant Y-chromosome to offspring.
No patients fathered offspring during the follow-up period, and >80% had complete azoospermia (assessed by their semen samples). However, almost 20% did produce semen samples with live spermatozoa. Both findings are in accordance with previous studies reporting low or no fertility in patients with 45,X/46,XY mosaicism (6, 12, 26, 38). It is important to note that the males with live spermatozoa in this study were diagnosed at different ages from birth into adulthood and with varying degrees of genital androgenization. Clinicians should therefore be aware of fertility-preservation methods, also in the pediatric setting, and semen sampling should be considered in all patients once they enter a mature age in late adolescence or early adulthood.
The strengths of this study include the following: (i) the multicenter design that has made it possible to collect data on a large series of males, (ii) the inclusion of numerous outcomes in a single study, both clinical and histological, which allow for a thorough understanding of the outcomes and how they relate (or do not) to gonadal histology and karyotypic etiology, (iii) all patients are old enough to have long-term outcomes, such as gonadal function and final height assessed, and (iv) all patients are raised as males, allowing for a unified evaluation of their outcomes. There are, however, also limitations and they include the following: (i) the retrospective design that has led to missing data for some variables for all patients, (ii) histological data were not available in all patients, and conclusions may be skewed by the fact that the most severely affected individuals are far more likely to have gonadal biopsies, (iii) the use of reference ranges for LH, FSH, T, testicular volumes, and growth, based on a Danish population and the WHO growth curves, respectively, which do not reflect the composition of this study population, although allow for the comparison of healthy background populations with the patients studied, (iv) some of the patients included in this study have been included in studies previously published (5, 16, 27), which may alter conclusions if drawn across published data, (v) most patients were diagnosed postpubertally, which may skew conclusions toward poorer outcomes than if they had been prenatally diagnosed, and (vi) patients were followed at multiple centers, and consequently, follow-up schemes varied considerably.
In conclusion, in this large multicenter study of males with 45,X/46,XY mosaicism, we find that patients diagnosed as a result of genital abnormalities have poorer health outcomes than those diagnosed as a result of other reasons, such as short stature, lack of puberty, and infertility. Overall, patients do, however, have relatively good endocrine gonadal function, but most are also short statured. Moreover, patients, regardless of reason for referral, have a relatively high risk of gonadal neoplasia, and most are azoospermic. Nevertheless, almost one-half of patients has germ cells present, and up to one-quarter has focal spermatogenesis, which provides hope for fertility treatment in some patients and future treatment options in many. In general, the data indicate the importance of highly personalized medical management.
Acknowledgments
The authors thank Niels Erik Skakkebæk (Department of Growth and Reproduction, Rigshospitalet, University of Copenhagen, Denmark), Sukran Poyrazoğlu (Istanbul University, Turkey), and Lutz Wuensch (Department of Paediatric Surgery, University of Luebeck, Germany) for their help. The authors also thank Jillian Bryce (Developmental Endocrinology Research Group, University of Glasgow, United Kingdom) for administrative support.
Financial Support: The I-DSD Registry was supported by Medical Research Council partnership award G1100236 and was initially developed under project grants from the Seventh European Union Framework Program (201444) and the Research Unit of the European Society for Paediatric Endocrinology. Several of the authors participated in this study as part of the COST Action BM1303 DSDnet, supported by COST, under the European Union framework program Horizon 2020. M.L.L. is funded by Copenhagen University Hospital’s Research Foundation (Rigshospitalets Forskningsudvalg; R85-A3105) through a 3-year stipend.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations:
- COST
European Cooperation in Science and Technology
- DSD
disorders of sex development
- EMS
external masculinization score
- FSH
follicle-stimulating hormone
- GCNIS
germ-cell neoplasia in situ
- I-DSD
International Disorders of Sex Development
- LH
luteinizing hormone
- LOD
limit of detection
- rhGH
recombinant human GH
- SCO
Sertoli cell only
- SDS
SD score
- T
testosterone
- WHO
World Health Organization



