-
PDF
- Split View
-
Views
-
Cite
Cite
Katarzyna Krzyzanowska-Mittermayer, Anders F Mattsson, Dominique Maiter, Ulla Feldt-Rasmussen, Cecilia Camacho-Hübner, Anton Luger, Roger Abs, New Neoplasm During GH Replacement in Adults With Pituitary Deficiency Following Malignancy: A KIMS Analysis, The Journal of Clinical Endocrinology & Metabolism, Volume 103, Issue 2, February 2018, Pages 523–531, https://doi.org/10.1210/jc.2017-01899
Close - Share Icon Share
Abstract
Data on the association between growth hormone (GH) replacement in patients with GH deficiency (GHD) after malignancies and new neoplasms show conflicting results.
To clarify the incidence of new malignant neoplasm in childhood-onset (CO) and adult-onset (AO) adult cancer survivors (CSs).
Retrospective comparison of CO-CS and AO-CS with CO idiopathic GHD (IGHD) and AO nonfunctioning pituitary adenoma (NFPA) patients and with the general population [standardized incidence ratio (SIR)].
Data from the Pfizer International Metabolic Database study (KIMS).
CO-CS [n = 349; 50.4% females; mean baseline (MBL) IGF-I standard deviation score (SDS), −2.4], IGHD (n = 619; 35.7% females; MBL IGF-I SDS, −3.4), AO-CS (n = 174; 42.5% females; MBL IGF-I SDS, −1.4), and NFPA (n = 2449; 38.1% females; MBL IGF-I SDS, −1.0).
SIRs of malignant neoplasms.
After a median follow-up of 5.9 years (2192 patient-years), 15 CO-CS (4.3%) had developed 16 new neoplasms. The SIR was 10.4 [95% confidence interval (CI), 5.9 to 16.9] and 6.5 (95% CI, 3.0 to 12.4) after exclusion of seven patients with skin cancers. In IGHD, three malignant neoplasms (0.5%) were observed after a median follow-up of 5.4 years (3908 patient-years; SIR, 0.47; 95% CI, 0.09 to 1.37). New malignant neoplasms occurred in three AO-CS (1.7%; SIR, 1.1; 95% CI, 0.2 to 3.2) and 146 NFPA patients (153 cases, 6.0%; SIR, 1.1; 95% CI, 0.9 to 1.2) after a median follow-up of 4.9 (1024 patient-years) and 5.6 years (15,215 patient-years).
The risk of second malignant neoplasms was increased in CO-CS but not in AO-CS, which illustrates the need to closely follow patients on GH replacement because of a prior malignancy.
More than 60% of childhood cancers are tumors of the central nervous system (CNS) or malignancies of hematological origin (1). Although it is unclear whether their incidence is stable or has slightly increased over the past few decades, the mortality rate is decreasing because of advancing therapeutic possibilities (1, 2). This implies that a prolonged follow-up is required to detect late-onset complications and associated disorders. It has indeed been shown that adult survivors of childhood cancer have an increased risk of adverse health outcomes, including an increased risk for second neoplasms (2). Moreover, as a consequence of the primary lesion and its treatment, the prevalence of secondary hormonal pituitary dysfunction is very high (3). The adverse clinical expression of growth hormone deficiency (GHD) has been characterized in large cohorts of patients in observational studies, and premature mortality from cardiovascular disease has been reported in such patients (4, 5). A large number of publications have subsequently shown that growth hormone (GH) replacement induces beneficial changes, such as improvement of quality of life, reduction of cardiovascular risk factors, and possibly reduction of the increased mortality associated with GHD (6, 7).
On the other hand, in view of its direct and IGF-I–mediated mitotic properties, GH administration is not recommended in patients with an active malignant disease (8, 9). Two postmarketing surveillance studies, Pfizer International Metabolic Database (KIMS; formerly the Kabi International Metabolic Survey) and Hypopituitary Control and Complications Study (HypoCCS), have been conducted to ensure long-term safety information (10, 11). Published data are very reassuring because no increased risk for the development of malignancy has been reported after 20 years of follow-up (12–15). Moreover, GH replacement in nonmalignant tumoral lesions, such as craniopharyngiomas or nonfunctioning pituitary adenomas (NFPAs), is apparently not associated with an increased risk of recurrence or induction of a second tumor (13, 16, 17).
Recent publications have emphasized that patients who survived childhood cancer are at risk for developing a subsequent CNS neoplasm, necessitating further extensive and coordinated research (18). Although cranial irradiation for CNS tumors and leukemia has been implicated as the most probable causative agent, a facilitating carcinogenic effect by GH replacement had also to be considered (19, 20). Studies have been investigating the association between GH exposure and the development of a subsequent neoplasm (21, 22). Recently, two meta-analyses have also been presented that yielded conflicting data (23, 24). The present KIMS study intends to further clarify the safety of GH replacement in a large group of adult survivors of both childhood-onset (CO) and adult-onset (AO) cancer.
Materials and Methods
Patients
Adults who had survived malignancy and had developed hypopituitarism in relation to the primary cancer or as a consequence of its treatment were retrieved from KIMS. These cancer survivors (CSs) all had an established diagnosis of severe GHD confirmed by an adequate GH stimulatory test (25) and were either of CO-CS (n = 349, 2192 patient-years, 5.9-year median follow-up time) or AO-CS (n = 174, 1024 patient-years, 4.9-year median follow-up time) according to a cutoff for onset fixed at 18 years.
The cancer origin of GHD in the CO-CS cohort was germ cell tumor (n = 112), medulloblastoma (n = 68), astrocytoma (n = 53), glioma (n = 49), leukemia or lymphoma (n = 47), nasopharyngeal tumor (n = 10), sarcoma (n = 7), and chordoma (n = 3). The cancer origin of GHD in the AO-CS cohort was germ cell tumor (n = 59), leukemia or lymphoma (n = 35), astrocytoma (n = 29), glioma (n = 18), nasopharyngeal tumor (n = 13), medulloblastoma (n = 9), chordoma (n = 7), and sarcoma (n = 4).
The two cohorts were compared with a group of adults with hypopituitarism resulting from a single etiology and of comparable onset: the CO-CS patients with CO congenital or idiopathic GHD (IGHD, n = 619, 3908 patient-years, 5.4 year median follow-up time) and the AO-CS patients with AO NFPA (n = 2449, 15,215 patient-years, 5.6-year median follow-up time).
Methods
Characteristics of the four cohorts were assessed at baseline (KIMS entry). Background data included sex, age at primary diagnosis (either onset of the primary malignancy for CS or age at GHD diagnosis for IGHD and NFPA), age at entry into KIMS, modalities of treatment of primary malignancy, additional pituitary hormone deficits, presence of comorbidities and naivety to GH replacement (seminaivety corresponding to latest GH replacement ≥6 months before KIMS entry). Baseline body mass index (BMI) and centrally measured IGF-I standard deviation score (SDS) were recorded. To assess whether the effectiveness of GH replacement could be considered comparable in the different study cohorts, GH dose and changes in serum IGF-I were determined after 1 year.
Furthermore, the occurrence of an additional neoplasm before KIMS entry was recorded as was the development of a second malignancy or a meningioma during follow-up in KIMS and the cause of death during follow-up.
The data collection into KIMS was approved by the institutional review boards/ethical committees as required by local regulations in each participating country. Written informed consent was obtained from all patients before any data were entered into KIMS. The study was performed in accordance with The Declaration of Helsinki (26).
Statistical methods
For descriptive statistics values are expressed as mean ± standard deviation or proportions, depending on type of variable. The unadjusted mean comparisons between groups were performed by t tests for numerical variables (PROC TTEST, SAS, version 9.2). For nominal or categorical variables (proportions) χ2 tests were performed (PROC FREQ, SAS, version 9.2).
Standardized incidence ratios (SIRs) were calculated and compared between groups by using the indirect method of standardization with external reference rates from the general population with stratification for attained age, sex, and country (27). These ratios compare observed number of cases in the patient group and the expected number of cases. The expected number of cases quantifies the number of expected cases in the patient group, if the patient group had the same specific rates as the external reference population. Patient-years were calculated from the date of KIMS entry or GH start (if later than KIMS entry) to the date of studied event, or if no event, the date of last visit or death. In the comparisons between patient groups, ratios of SIRs [risk ratios (RRs)] were further adjusted for attained age and sex using Poisson regression methods. The 95% two-sided confidence intervals (CIs) were calculated with the Byar approximation formula or, in Poisson regression models, with likelihood-based methods (28). P values < 0.05 were considered statistically significant; a two-sided significance level was applied.
Results
Characteristics of the cohorts at baseline
In the CO group, 349 CO-CS were compared with 619 IGHD; in the adult-onset group, 174 AO-CS were compared with 2449 NFPA. The background data, the baseline characteristics of the cohorts, and the statistically significant differences between CO-CS and IGHD and between AO-CS and NFPA are reported in Table 1.
Baseline Characteristics (Mean ± Standard Deviation) and Statistical Comparison of CO-CS, IGHD or Congenital GHD, AO-CS, and NFPA Patients
| . | CO-CS . | CO-CS vs IGHD, P . | IGHD . | AO-CS . | AO-CS vs NFPA, P . | NFPA . |
|---|---|---|---|---|---|---|
| n | 349 | 619 | 174 | 2449 | ||
| Sex (% males) | 50 | <0.0001 | 64 | 57 | NS | 62 |
| Age at primary diagnosis (y) | 10.4 ± 4.4 | <0.0001 | 9.0 ± 4.7 | 30.1 ± 10.4 | <0.0001 | 46.6 ± 12.9 |
| Age at KIMS entry (y) | 24.5 ± 6.4 | <0.0001 | 28.8 ± 10.4 | 36.0 ± 11.6 | <0.0001 | 53.2 ± 11.7 |
| Treatment modality (%) | ||||||
| Surgery | 48 | NA | 0 | 36 | <0.0001 | 92 |
| Radiotherapy | 68 | NA | 0 | 59 | <0.0001 | 34 |
| Chemotherapy | 26 | NA | 0 | 11 | <0.0001 | 0.1 |
| Pituitary deficiency (%) | ||||||
| TSH | 64 | NS | 66 | 60 | <0.0001 | 77 |
| ACTH | 52 | NS | 48 | 61 | <0.0001 | 75 |
| LH/FSH | 61 | 0.025 | 68 | 67 | <0.0001 | 82 |
| ADH | 33 | <0.0001 | 6 | 33 | <0.0001 | 19 |
| Comorbidity (%) | ||||||
| Hypertension | 2 | NS | 3 | 7 | <0.0001 | 22 |
| Peripheral vascular | 0 | NS | 0 | 2 | NS | 1 |
| Cardiovascular | 0 | 0.046 | 1 | 1 | 0.015 | 5 |
| Cerebrovascular | 2 | 0.022 | 0 | 2 | NS | 3 |
| Epilepsy | 8 | 0.010 | 4 | 7 | 0.0012 | 2 |
| Diabetes mellitus | 4 | NS | 4 | 8 | NS | 9 |
| GH naivety at KIMS entry (%) | <0.0001 | NS | ||||
| Nonnaive | 32 | 27 | 19 | 23 | ||
| Seminaive | 44 | 64 | 6 | 4 | ||
| True naive | 24 | 9 | 75 | 73 | ||
| BMI (kg/m2) | 26.6 ± 5.9 | 0.002 | 24.9 ± 8.4 | 29.4 ± 6.0 | NS | 28.9 ± 4.9 |
| IGF1 SDS | −2.4 ± 1.9 | <0.0001 | −3.4 ± 2.6 | −1.4 ± 1.7 | 0.035 | −1.0 ± 1.9 |
| . | CO-CS . | CO-CS vs IGHD, P . | IGHD . | AO-CS . | AO-CS vs NFPA, P . | NFPA . |
|---|---|---|---|---|---|---|
| n | 349 | 619 | 174 | 2449 | ||
| Sex (% males) | 50 | <0.0001 | 64 | 57 | NS | 62 |
| Age at primary diagnosis (y) | 10.4 ± 4.4 | <0.0001 | 9.0 ± 4.7 | 30.1 ± 10.4 | <0.0001 | 46.6 ± 12.9 |
| Age at KIMS entry (y) | 24.5 ± 6.4 | <0.0001 | 28.8 ± 10.4 | 36.0 ± 11.6 | <0.0001 | 53.2 ± 11.7 |
| Treatment modality (%) | ||||||
| Surgery | 48 | NA | 0 | 36 | <0.0001 | 92 |
| Radiotherapy | 68 | NA | 0 | 59 | <0.0001 | 34 |
| Chemotherapy | 26 | NA | 0 | 11 | <0.0001 | 0.1 |
| Pituitary deficiency (%) | ||||||
| TSH | 64 | NS | 66 | 60 | <0.0001 | 77 |
| ACTH | 52 | NS | 48 | 61 | <0.0001 | 75 |
| LH/FSH | 61 | 0.025 | 68 | 67 | <0.0001 | 82 |
| ADH | 33 | <0.0001 | 6 | 33 | <0.0001 | 19 |
| Comorbidity (%) | ||||||
| Hypertension | 2 | NS | 3 | 7 | <0.0001 | 22 |
| Peripheral vascular | 0 | NS | 0 | 2 | NS | 1 |
| Cardiovascular | 0 | 0.046 | 1 | 1 | 0.015 | 5 |
| Cerebrovascular | 2 | 0.022 | 0 | 2 | NS | 3 |
| Epilepsy | 8 | 0.010 | 4 | 7 | 0.0012 | 2 |
| Diabetes mellitus | 4 | NS | 4 | 8 | NS | 9 |
| GH naivety at KIMS entry (%) | <0.0001 | NS | ||||
| Nonnaive | 32 | 27 | 19 | 23 | ||
| Seminaive | 44 | 64 | 6 | 4 | ||
| True naive | 24 | 9 | 75 | 73 | ||
| BMI (kg/m2) | 26.6 ± 5.9 | 0.002 | 24.9 ± 8.4 | 29.4 ± 6.0 | NS | 28.9 ± 4.9 |
| IGF1 SDS | −2.4 ± 1.9 | <0.0001 | −3.4 ± 2.6 | −1.4 ± 1.7 | 0.035 | −1.0 ± 1.9 |
Abbreviations: ACTH, adrenocorticotropic hormone; ADH, antidiuretic hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone; NA, not available; NS, not significant.
Baseline Characteristics (Mean ± Standard Deviation) and Statistical Comparison of CO-CS, IGHD or Congenital GHD, AO-CS, and NFPA Patients
| . | CO-CS . | CO-CS vs IGHD, P . | IGHD . | AO-CS . | AO-CS vs NFPA, P . | NFPA . |
|---|---|---|---|---|---|---|
| n | 349 | 619 | 174 | 2449 | ||
| Sex (% males) | 50 | <0.0001 | 64 | 57 | NS | 62 |
| Age at primary diagnosis (y) | 10.4 ± 4.4 | <0.0001 | 9.0 ± 4.7 | 30.1 ± 10.4 | <0.0001 | 46.6 ± 12.9 |
| Age at KIMS entry (y) | 24.5 ± 6.4 | <0.0001 | 28.8 ± 10.4 | 36.0 ± 11.6 | <0.0001 | 53.2 ± 11.7 |
| Treatment modality (%) | ||||||
| Surgery | 48 | NA | 0 | 36 | <0.0001 | 92 |
| Radiotherapy | 68 | NA | 0 | 59 | <0.0001 | 34 |
| Chemotherapy | 26 | NA | 0 | 11 | <0.0001 | 0.1 |
| Pituitary deficiency (%) | ||||||
| TSH | 64 | NS | 66 | 60 | <0.0001 | 77 |
| ACTH | 52 | NS | 48 | 61 | <0.0001 | 75 |
| LH/FSH | 61 | 0.025 | 68 | 67 | <0.0001 | 82 |
| ADH | 33 | <0.0001 | 6 | 33 | <0.0001 | 19 |
| Comorbidity (%) | ||||||
| Hypertension | 2 | NS | 3 | 7 | <0.0001 | 22 |
| Peripheral vascular | 0 | NS | 0 | 2 | NS | 1 |
| Cardiovascular | 0 | 0.046 | 1 | 1 | 0.015 | 5 |
| Cerebrovascular | 2 | 0.022 | 0 | 2 | NS | 3 |
| Epilepsy | 8 | 0.010 | 4 | 7 | 0.0012 | 2 |
| Diabetes mellitus | 4 | NS | 4 | 8 | NS | 9 |
| GH naivety at KIMS entry (%) | <0.0001 | NS | ||||
| Nonnaive | 32 | 27 | 19 | 23 | ||
| Seminaive | 44 | 64 | 6 | 4 | ||
| True naive | 24 | 9 | 75 | 73 | ||
| BMI (kg/m2) | 26.6 ± 5.9 | 0.002 | 24.9 ± 8.4 | 29.4 ± 6.0 | NS | 28.9 ± 4.9 |
| IGF1 SDS | −2.4 ± 1.9 | <0.0001 | −3.4 ± 2.6 | −1.4 ± 1.7 | 0.035 | −1.0 ± 1.9 |
| . | CO-CS . | CO-CS vs IGHD, P . | IGHD . | AO-CS . | AO-CS vs NFPA, P . | NFPA . |
|---|---|---|---|---|---|---|
| n | 349 | 619 | 174 | 2449 | ||
| Sex (% males) | 50 | <0.0001 | 64 | 57 | NS | 62 |
| Age at primary diagnosis (y) | 10.4 ± 4.4 | <0.0001 | 9.0 ± 4.7 | 30.1 ± 10.4 | <0.0001 | 46.6 ± 12.9 |
| Age at KIMS entry (y) | 24.5 ± 6.4 | <0.0001 | 28.8 ± 10.4 | 36.0 ± 11.6 | <0.0001 | 53.2 ± 11.7 |
| Treatment modality (%) | ||||||
| Surgery | 48 | NA | 0 | 36 | <0.0001 | 92 |
| Radiotherapy | 68 | NA | 0 | 59 | <0.0001 | 34 |
| Chemotherapy | 26 | NA | 0 | 11 | <0.0001 | 0.1 |
| Pituitary deficiency (%) | ||||||
| TSH | 64 | NS | 66 | 60 | <0.0001 | 77 |
| ACTH | 52 | NS | 48 | 61 | <0.0001 | 75 |
| LH/FSH | 61 | 0.025 | 68 | 67 | <0.0001 | 82 |
| ADH | 33 | <0.0001 | 6 | 33 | <0.0001 | 19 |
| Comorbidity (%) | ||||||
| Hypertension | 2 | NS | 3 | 7 | <0.0001 | 22 |
| Peripheral vascular | 0 | NS | 0 | 2 | NS | 1 |
| Cardiovascular | 0 | 0.046 | 1 | 1 | 0.015 | 5 |
| Cerebrovascular | 2 | 0.022 | 0 | 2 | NS | 3 |
| Epilepsy | 8 | 0.010 | 4 | 7 | 0.0012 | 2 |
| Diabetes mellitus | 4 | NS | 4 | 8 | NS | 9 |
| GH naivety at KIMS entry (%) | <0.0001 | NS | ||||
| Nonnaive | 32 | 27 | 19 | 23 | ||
| Seminaive | 44 | 64 | 6 | 4 | ||
| True naive | 24 | 9 | 75 | 73 | ||
| BMI (kg/m2) | 26.6 ± 5.9 | 0.002 | 24.9 ± 8.4 | 29.4 ± 6.0 | NS | 28.9 ± 4.9 |
| IGF1 SDS | −2.4 ± 1.9 | <0.0001 | −3.4 ± 2.6 | −1.4 ± 1.7 | 0.035 | −1.0 ± 1.9 |
Abbreviations: ACTH, adrenocorticotropic hormone; ADH, antidiuretic hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone; NA, not available; NS, not significant.
The CO-CS cohort comprised significantly fewer males compared with the IGHD cohort. CO-CS patients were older at diagnosis and younger at start of adult GH replacement. BMI was higher in CO-CS compared with IGHD. The AO-CS cohort was younger at diagnosis and at start of GH replacement compared with the NFPA cohort, whereas there was no difference in sex ratio or BMI between these subgroups. Regarding the treatment modalities, AO-CS patients had less frequent surgery but more frequent radiotherapy and chemotherapy compared with NFPA.
The 1-year GH dose was similar in CO-CS compared with IGHD (0.48 mg; 95% CI, 0.45 to 0.51 vs 0.51 mg, 95% CI, 0.48 to 0.53; P = 0.26) and between NFPA and AO-CS (0.35 mg, 95% CI, 0.34 to 0.36 vs 0.38 mg, 95% CI, 0.34 to 0.42; P = NS); likewise, the ΔIGF-1 SDS was comparable between CO-CS and IGHD (1.75; 95% CI, 1.46 to 2.04 vs 2.19; 95% CI, 1.86 to 2.51; P = 0.05) and between NFPA and AO-CS (1.60; 95% CI, 1.49 to 1.70 vs 1.50, 95% CI, 1.14 to 1.85; P = NS).
Additional neoplasms before KIMS entry
Within the CO-CS cohort, additional neoplasms, of which six basal cell carcinomas and three meningiomas, were reported in 11 patients before inclusion into KIMS (Table 2). All these patients had received radiotherapy prior to KIMS entry. Only one neoplasm (testis cancer) was reported in the IGHD cohort.
Characteristics of the 11 CO-CS and 6 AO-CS Who Presented With ≥1 Additional Neoplasms Before Adult GH Replacement (KIMS Entry)
| . | Sex . | Age at Surgery/Radiotherapy (y) . | Additional Neoplasm Before Adult GH Replacement . | Age at Adult GH Replacement (y) . |
|---|---|---|---|---|
| Primary malignancy CO-CS | ||||
| Glioma | M | 7/7 | Basal cell carcinoma | 18 |
| Medulloblastoma | F | 3/3 | Basal cell carcinoma | 20 |
| Medulloblastoma | F | 1/1 | Basal cell carcinoma | 20 |
| Medulloblastoma | F | 5/5 | Basal cell carcinoma | 24 |
| Medulloblastoma | F | 14/14 | Basal cell carcinoma | 24 |
| Germ cell tumor | F | 13/13 | Basal cell carcinoma | 52 |
| Germ cell tumor | F | 11/11 | Ovarian carcinoma | 21 |
| Lymphatic leukemia | M | NA/13 | Testis carcinoma | 33 |
| Lymphatic leukemia | M | NA/11 | Meningioma | 32 |
| Medulloblastoma | F | 7/8 | Meningioma | 26 |
| Astrocytoma | F | 15/15 | Meningioma | 39 |
| Primary malignancy AO-CS | ||||
| Lymphatic leukemia | F | NA/36 | Basal cell carcinoma salivary gland carcinoma cervical carcinoma in situ | 38 |
| Astrocytoma | F | 35/35 | Cervical carcinoma in situ | 47 |
| Glioma | F | 43/52 | Breast carcinoma | 57 |
| Germ cell tumor | F | 25/25 | Meningioma | 31 |
| . | Sex . | Age at Surgery/Radiotherapy (y) . | Additional Neoplasm Before Adult GH Replacement . | Age at Adult GH Replacement (y) . |
|---|---|---|---|---|
| Primary malignancy CO-CS | ||||
| Glioma | M | 7/7 | Basal cell carcinoma | 18 |
| Medulloblastoma | F | 3/3 | Basal cell carcinoma | 20 |
| Medulloblastoma | F | 1/1 | Basal cell carcinoma | 20 |
| Medulloblastoma | F | 5/5 | Basal cell carcinoma | 24 |
| Medulloblastoma | F | 14/14 | Basal cell carcinoma | 24 |
| Germ cell tumor | F | 13/13 | Basal cell carcinoma | 52 |
| Germ cell tumor | F | 11/11 | Ovarian carcinoma | 21 |
| Lymphatic leukemia | M | NA/13 | Testis carcinoma | 33 |
| Lymphatic leukemia | M | NA/11 | Meningioma | 32 |
| Medulloblastoma | F | 7/8 | Meningioma | 26 |
| Astrocytoma | F | 15/15 | Meningioma | 39 |
| Primary malignancy AO-CS | ||||
| Lymphatic leukemia | F | NA/36 | Basal cell carcinoma salivary gland carcinoma cervical carcinoma in situ | 38 |
| Astrocytoma | F | 35/35 | Cervical carcinoma in situ | 47 |
| Glioma | F | 43/52 | Breast carcinoma | 57 |
| Germ cell tumor | F | 25/25 | Meningioma | 31 |
Abbreviations: F, female; M, male.
Characteristics of the 11 CO-CS and 6 AO-CS Who Presented With ≥1 Additional Neoplasms Before Adult GH Replacement (KIMS Entry)
| . | Sex . | Age at Surgery/Radiotherapy (y) . | Additional Neoplasm Before Adult GH Replacement . | Age at Adult GH Replacement (y) . |
|---|---|---|---|---|
| Primary malignancy CO-CS | ||||
| Glioma | M | 7/7 | Basal cell carcinoma | 18 |
| Medulloblastoma | F | 3/3 | Basal cell carcinoma | 20 |
| Medulloblastoma | F | 1/1 | Basal cell carcinoma | 20 |
| Medulloblastoma | F | 5/5 | Basal cell carcinoma | 24 |
| Medulloblastoma | F | 14/14 | Basal cell carcinoma | 24 |
| Germ cell tumor | F | 13/13 | Basal cell carcinoma | 52 |
| Germ cell tumor | F | 11/11 | Ovarian carcinoma | 21 |
| Lymphatic leukemia | M | NA/13 | Testis carcinoma | 33 |
| Lymphatic leukemia | M | NA/11 | Meningioma | 32 |
| Medulloblastoma | F | 7/8 | Meningioma | 26 |
| Astrocytoma | F | 15/15 | Meningioma | 39 |
| Primary malignancy AO-CS | ||||
| Lymphatic leukemia | F | NA/36 | Basal cell carcinoma salivary gland carcinoma cervical carcinoma in situ | 38 |
| Astrocytoma | F | 35/35 | Cervical carcinoma in situ | 47 |
| Glioma | F | 43/52 | Breast carcinoma | 57 |
| Germ cell tumor | F | 25/25 | Meningioma | 31 |
| . | Sex . | Age at Surgery/Radiotherapy (y) . | Additional Neoplasm Before Adult GH Replacement . | Age at Adult GH Replacement (y) . |
|---|---|---|---|---|
| Primary malignancy CO-CS | ||||
| Glioma | M | 7/7 | Basal cell carcinoma | 18 |
| Medulloblastoma | F | 3/3 | Basal cell carcinoma | 20 |
| Medulloblastoma | F | 1/1 | Basal cell carcinoma | 20 |
| Medulloblastoma | F | 5/5 | Basal cell carcinoma | 24 |
| Medulloblastoma | F | 14/14 | Basal cell carcinoma | 24 |
| Germ cell tumor | F | 13/13 | Basal cell carcinoma | 52 |
| Germ cell tumor | F | 11/11 | Ovarian carcinoma | 21 |
| Lymphatic leukemia | M | NA/13 | Testis carcinoma | 33 |
| Lymphatic leukemia | M | NA/11 | Meningioma | 32 |
| Medulloblastoma | F | 7/8 | Meningioma | 26 |
| Astrocytoma | F | 15/15 | Meningioma | 39 |
| Primary malignancy AO-CS | ||||
| Lymphatic leukemia | F | NA/36 | Basal cell carcinoma salivary gland carcinoma cervical carcinoma in situ | 38 |
| Astrocytoma | F | 35/35 | Cervical carcinoma in situ | 47 |
| Glioma | F | 43/52 | Breast carcinoma | 57 |
| Germ cell tumor | F | 25/25 | Meningioma | 31 |
Abbreviations: F, female; M, male.
In the AO-CS cohort, six new neoplasms in addition to the primary cancer were reported in four patients before KIMS entry, of which three occurred in the same patient. One meningioma was observed. All of these patients had received radiotherapy before KIMS entry. In the NFPA cohort, 44 additional neoplasms were reported (nine prostate cancers, five basal cell carcinomas, four breast cancers, four meningiomas, three kidney cancers, three papillary thyroid cancers, three testis cancers, two bladder cancers, two lung cancers, two melanomas, one cervical cancer in situ, one gastric cancer, one nasopharyngeal cancer, one ovarian cancer, one rectal cancer in situ, one salivary gland cancer, and one uterine cancer).
Second neoplasm during GH replacement in KIMS
In the CO-CS cohort, 15 of 349 (4.3%) patients developed 16 new neoplasms, mainly basal cell carcinomas of the skin (n = 7) and brain neoplasms (n = 6). The median duration from start of GH replacement to development of the second neoplasm was 2.3 years (minimum, 0.3; maximum, 14.0) for the 15 patients and 2.3 years (minimum, 0.3; maximum, 14.0) for the nine patients when excluding the basal cell carcinomas. Details on the individual cases are reported in Table 3. None of the patients had an IGF-1 SDS above normality at the time of diagnosis of the second neoplasm.
Characteristics of the 16 CO-CS and the 3 AO-CS Who Developed a Second Neoplasm During Adult GH Replacement as Reported in KIMS
| . | Sex . | Age at Surgery/Radiotherapy (y) . | Age of Childhood GH Replacement (y) . | Age at Start — Duration of Adult GH Replacement (y) . | Second Neoplasm . |
|---|---|---|---|---|---|
| Primary malignancy CO-CS | |||||
| Germ cell tumor | M | 15.8/15.8 | None | 18.4 — 0.3 | Cerebral carcinoma |
| Glioma | F | 17.7/25.7 | None | 30.7 — 2.1 | Cerebral lymphoma |
| Chordoma | F | 16.0/17.4 | None | 20.1 — 14.0 | Cerebral sarcoma |
| Medulloblastoma | F | 10.2/10.2 | 13.1 → 15.1 | 18.9 — 2.4 | Astrocytoma |
| Medulloblastoma | F | 7.3/8.2 | 10.3 → 14.3 | 22.4 — 11.3 | Diffuse gliomatosis |
| Lymphatic leukemia | F | NA/7.0 | 8.1 → 16.1 | 17.1 — 0.8 | Cerebral, not specified |
| Germ cell tumor | F | 5.8/6.3 | 6.3 → 16.3 | 30.1 — 2.3 | Cervical carcinoma |
| Lymphatic leukemia | F | NA/9.3 | None | 35.1 — 4.4 | Cervical carcinoma papillary thyroid |
| Lymphatic leukemia | F | NA/12.9 | 13.7 → 14.7 | 32.1 — 3.3 | Basal cell carcinoma |
| Astrocytoma | M | 13.1/13.3 | None | 31.9 — 0.9 | Basal cell carcinoma |
| Germ cell tumor | F | 12.9/13.8 | None | 52.0 — 1.6 | Basal cell carcinoma |
| Medulloblastoma | M | 7.2/9.0 | 11.9 → 16.9 | 26.9 — 1.9 | Basal cell carcinoma |
| Medulloblastoma | M | 9.4/10.0 | 11.5 → 14.5 | 27.1 — 2.0 | Basal cell carcinoma |
| Medulloblastoma | M | 17.4/17.6 | None | 41.5 — 4.6 | Basal cell carcinoma |
| Germ cell tumor | M | 13.7/13.7 | 15.7 → 17.7 | 29.1 — 5.7 | Basal cell carcinoma |
| Primary malignancy AO-CS | |||||
| Germ cell tumor | M | 24.9/38.8 | NA | 33.0 — 1.6 | Malignant histiocytosis |
| Nasopharyngeal tumor | M | ND/56.0 | NA | 65.3 — 4.1 | Prostate carcinoma |
| Astrocytoma | F | 34.7/28.1 | NA | 47.2 — 3.4 | Liver carcinoma |
| . | Sex . | Age at Surgery/Radiotherapy (y) . | Age of Childhood GH Replacement (y) . | Age at Start — Duration of Adult GH Replacement (y) . | Second Neoplasm . |
|---|---|---|---|---|---|
| Primary malignancy CO-CS | |||||
| Germ cell tumor | M | 15.8/15.8 | None | 18.4 — 0.3 | Cerebral carcinoma |
| Glioma | F | 17.7/25.7 | None | 30.7 — 2.1 | Cerebral lymphoma |
| Chordoma | F | 16.0/17.4 | None | 20.1 — 14.0 | Cerebral sarcoma |
| Medulloblastoma | F | 10.2/10.2 | 13.1 → 15.1 | 18.9 — 2.4 | Astrocytoma |
| Medulloblastoma | F | 7.3/8.2 | 10.3 → 14.3 | 22.4 — 11.3 | Diffuse gliomatosis |
| Lymphatic leukemia | F | NA/7.0 | 8.1 → 16.1 | 17.1 — 0.8 | Cerebral, not specified |
| Germ cell tumor | F | 5.8/6.3 | 6.3 → 16.3 | 30.1 — 2.3 | Cervical carcinoma |
| Lymphatic leukemia | F | NA/9.3 | None | 35.1 — 4.4 | Cervical carcinoma papillary thyroid |
| Lymphatic leukemia | F | NA/12.9 | 13.7 → 14.7 | 32.1 — 3.3 | Basal cell carcinoma |
| Astrocytoma | M | 13.1/13.3 | None | 31.9 — 0.9 | Basal cell carcinoma |
| Germ cell tumor | F | 12.9/13.8 | None | 52.0 — 1.6 | Basal cell carcinoma |
| Medulloblastoma | M | 7.2/9.0 | 11.9 → 16.9 | 26.9 — 1.9 | Basal cell carcinoma |
| Medulloblastoma | M | 9.4/10.0 | 11.5 → 14.5 | 27.1 — 2.0 | Basal cell carcinoma |
| Medulloblastoma | M | 17.4/17.6 | None | 41.5 — 4.6 | Basal cell carcinoma |
| Germ cell tumor | M | 13.7/13.7 | 15.7 → 17.7 | 29.1 — 5.7 | Basal cell carcinoma |
| Primary malignancy AO-CS | |||||
| Germ cell tumor | M | 24.9/38.8 | NA | 33.0 — 1.6 | Malignant histiocytosis |
| Nasopharyngeal tumor | M | ND/56.0 | NA | 65.3 — 4.1 | Prostate carcinoma |
| Astrocytoma | F | 34.7/28.1 | NA | 47.2 — 3.4 | Liver carcinoma |
Abbreviation: ND, no data.
Characteristics of the 16 CO-CS and the 3 AO-CS Who Developed a Second Neoplasm During Adult GH Replacement as Reported in KIMS
| . | Sex . | Age at Surgery/Radiotherapy (y) . | Age of Childhood GH Replacement (y) . | Age at Start — Duration of Adult GH Replacement (y) . | Second Neoplasm . |
|---|---|---|---|---|---|
| Primary malignancy CO-CS | |||||
| Germ cell tumor | M | 15.8/15.8 | None | 18.4 — 0.3 | Cerebral carcinoma |
| Glioma | F | 17.7/25.7 | None | 30.7 — 2.1 | Cerebral lymphoma |
| Chordoma | F | 16.0/17.4 | None | 20.1 — 14.0 | Cerebral sarcoma |
| Medulloblastoma | F | 10.2/10.2 | 13.1 → 15.1 | 18.9 — 2.4 | Astrocytoma |
| Medulloblastoma | F | 7.3/8.2 | 10.3 → 14.3 | 22.4 — 11.3 | Diffuse gliomatosis |
| Lymphatic leukemia | F | NA/7.0 | 8.1 → 16.1 | 17.1 — 0.8 | Cerebral, not specified |
| Germ cell tumor | F | 5.8/6.3 | 6.3 → 16.3 | 30.1 — 2.3 | Cervical carcinoma |
| Lymphatic leukemia | F | NA/9.3 | None | 35.1 — 4.4 | Cervical carcinoma papillary thyroid |
| Lymphatic leukemia | F | NA/12.9 | 13.7 → 14.7 | 32.1 — 3.3 | Basal cell carcinoma |
| Astrocytoma | M | 13.1/13.3 | None | 31.9 — 0.9 | Basal cell carcinoma |
| Germ cell tumor | F | 12.9/13.8 | None | 52.0 — 1.6 | Basal cell carcinoma |
| Medulloblastoma | M | 7.2/9.0 | 11.9 → 16.9 | 26.9 — 1.9 | Basal cell carcinoma |
| Medulloblastoma | M | 9.4/10.0 | 11.5 → 14.5 | 27.1 — 2.0 | Basal cell carcinoma |
| Medulloblastoma | M | 17.4/17.6 | None | 41.5 — 4.6 | Basal cell carcinoma |
| Germ cell tumor | M | 13.7/13.7 | 15.7 → 17.7 | 29.1 — 5.7 | Basal cell carcinoma |
| Primary malignancy AO-CS | |||||
| Germ cell tumor | M | 24.9/38.8 | NA | 33.0 — 1.6 | Malignant histiocytosis |
| Nasopharyngeal tumor | M | ND/56.0 | NA | 65.3 — 4.1 | Prostate carcinoma |
| Astrocytoma | F | 34.7/28.1 | NA | 47.2 — 3.4 | Liver carcinoma |
| . | Sex . | Age at Surgery/Radiotherapy (y) . | Age of Childhood GH Replacement (y) . | Age at Start — Duration of Adult GH Replacement (y) . | Second Neoplasm . |
|---|---|---|---|---|---|
| Primary malignancy CO-CS | |||||
| Germ cell tumor | M | 15.8/15.8 | None | 18.4 — 0.3 | Cerebral carcinoma |
| Glioma | F | 17.7/25.7 | None | 30.7 — 2.1 | Cerebral lymphoma |
| Chordoma | F | 16.0/17.4 | None | 20.1 — 14.0 | Cerebral sarcoma |
| Medulloblastoma | F | 10.2/10.2 | 13.1 → 15.1 | 18.9 — 2.4 | Astrocytoma |
| Medulloblastoma | F | 7.3/8.2 | 10.3 → 14.3 | 22.4 — 11.3 | Diffuse gliomatosis |
| Lymphatic leukemia | F | NA/7.0 | 8.1 → 16.1 | 17.1 — 0.8 | Cerebral, not specified |
| Germ cell tumor | F | 5.8/6.3 | 6.3 → 16.3 | 30.1 — 2.3 | Cervical carcinoma |
| Lymphatic leukemia | F | NA/9.3 | None | 35.1 — 4.4 | Cervical carcinoma papillary thyroid |
| Lymphatic leukemia | F | NA/12.9 | 13.7 → 14.7 | 32.1 — 3.3 | Basal cell carcinoma |
| Astrocytoma | M | 13.1/13.3 | None | 31.9 — 0.9 | Basal cell carcinoma |
| Germ cell tumor | F | 12.9/13.8 | None | 52.0 — 1.6 | Basal cell carcinoma |
| Medulloblastoma | M | 7.2/9.0 | 11.9 → 16.9 | 26.9 — 1.9 | Basal cell carcinoma |
| Medulloblastoma | M | 9.4/10.0 | 11.5 → 14.5 | 27.1 — 2.0 | Basal cell carcinoma |
| Medulloblastoma | M | 17.4/17.6 | None | 41.5 — 4.6 | Basal cell carcinoma |
| Germ cell tumor | M | 13.7/13.7 | 15.7 → 17.7 | 29.1 — 5.7 | Basal cell carcinoma |
| Primary malignancy AO-CS | |||||
| Germ cell tumor | M | 24.9/38.8 | NA | 33.0 — 1.6 | Malignant histiocytosis |
| Nasopharyngeal tumor | M | ND/56.0 | NA | 65.3 — 4.1 | Prostate carcinoma |
| Astrocytoma | F | 34.7/28.1 | NA | 47.2 — 3.4 | Liver carcinoma |
Abbreviation: ND, no data.
In the IGHD cohort, three of 619 (0.5%) patients developed a malignant neoplasm after a median follow-up of 1.6 years (minimum, 0.8; maximum, 13.2): one breast cancer, one myeloid leukemia, and one skin melanoma.
SIRs for the occurrence of a new malignancy in the CO-CS and IGHD cohorts are reported in Fig. 1. SIR for CO-CS and IGHD were significantly different (P < 0.0001). This was independent of the inclusion (RR = 22.3; 95% CI, 6.48 to 76.30) or exclusion of basal cell carcinoma (RR = 12.9; 95% CI, 3.49 to 47.60). Additionally, the SIR for malignant brain tumors (International Classification of Diseases 10: C70 to C72) associated with radiotherapy was 135 (5 cases vs 0.04 expected; 95% CI, 43.4 to 315.0), whereas no case of malignant brain tumor was observed in the group without radiotherapy, compared with 0.02 expected.
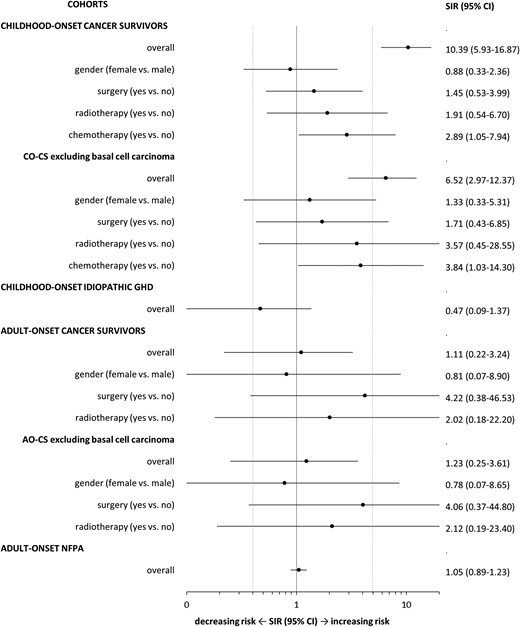
SIRs for the occurrence of a new malignancy in CO CSs and patients with IGHD as well as in AO-CS and patients with AO NFPA.
In the AO-CS cohort, three patients of 174 (1.7%) developed a malignant neoplasm after a median follow-up of 3.4 years (minimum, 1.6; maximum, 4.1): one liver carcinoma, one prostate carcinoma, and one histiocytosis. Details on the individual cases are reported in Table 3. One of the patients had IGF-1 SDS outside normality (IGF-1 SDS 2.1) at the time of diagnosis of the second neoplasm.
In the NFPA cohort, 146 patients of 2449 (6.0%) developed one (n = 139) or two malignant neoplasms (n = 7), after a median follow-up of 4.6 years (minimum, 0.0; maximum, 14.9). The organ affected or type of cancer was prostate (n = 35), skin (n = 34, of which 15 were melanoma), gastrointestinal (n = 20, of which 8 were colon), leukemia/lymphoma (n = 15), gynecologic/urologic (n = 14), lung (n = 13), breast (n = 8), brain (n = 7), and other (n = 7).
SIRs for the occurrence of a new malignancy in the AO-CS and NFPA cohorts are reported in Fig. 1 and were not significantly different (RR = 1.05; 95% CI, 0.33 to 3.37).
Cause of death during follow-up in KIMS
In the CO-CS cohort, 18 patients (5.2%) died during follow-up. The causes of death were malignancy (n = 5), infectious disease (n = 3), cerebrovascular accident (n = 2), injury (n = 2), neuropsychiatric disorder (n = 2), and unknown etiology (n = 4). Of the five patients dying from a malignancy, four died of a second cerebral neoplasm, whereas one died of the primary malignancy. In the IGHD cohort, seven patients (1.1%) died either from an infectious disease (n = 2), injury (n = 2), neuropsychiatric disorder (n = 1), or an unknown cause (n = 2).
In the AO-CS cohort, 12 patients (6.9%) died during follow-up. The causes of death were malignancy (n = 2), cardiovascular disease (n = 3), cerebrovascular accident (n = 1), injury (n = 1), endocrine disorder (n = 1), and unknown (n = 4). In the NFPA cohort, 114 patients (4.7%) died either from a malignancy (n = 31), cardiovascular disease (n = 29), cerebrovascular accident (n = 12), infectious disease (n = 12), or from other (n = 8) or unknown cause (n = 22).
Discussion
The paucity of information on the risk to develop a second neoplasm during GH replacement in CS is due to the still-recent introduction of this treatment and the considerable caution adopted in such patients. The most relevant information can be retrieved from the Childhood Cancer Survival Study (CCSS), a large retrospective study with prospective follow-up, initiated 20 years ago and now regrouping 14,358 patients from 26 institutions in the United States and Canada (29). The recruitment is strict: a follow-up of at least 5 years is required before allowing any analysis. The initial study in 361 CO-CS receiving GH had shown an increased relative risk for a second solid neoplasm of 3.21 (95% CI, 1.88 to 5.46; P < 0.0001), but no increased relative risk of recurrence (RR = 0.83, 95% CI, 0.37 to 1.86; P = 0.65) (19). However, a later study with prolonged follow-up and addition of new patients showed a less important relative risk for a second neoplasm of 2.15 (95% CI, 1.3 to 3.5; P < 0.002) (20). Remarkably, meningioma was the most common diagnosis, representing nine of 20 cases, all occurring in irradiated patients. In the postmarketing HypoCCS study of 252 GH-replaced adult CO-CS patients with a follow-up duration of 2.9 years, 15 developed a second neoplasm, resulting in a proportion of 6.0% (95% CI, 3.4 to 9.6) (21). The estimated cumulative incidence at 5-year follow-up was calculated at 4.8% (standard error of the mean, 1.6%). In the most recent CCSS analysis, the association between GH replacement and the occurrence of a second CNS neoplasm was also investigated (22). Of 338 CO-CS GH-replaced patients, 16 (4.7%) developed a second CNS neoplasm, consisting of 10 meningiomas and 6 gliomas. The overall risk for a second CNS neoplasm was not increased during GH replacement, with the adjusted ratio to GH-untreated patients being 0.8 (95% CI, 0.4 to 1.7; P = 0.61) for meningiomas and 1.9 (95% CI, 0.7 to 4.8; P = 0.21) for gliomas.
The KIMS registry has a different concept compared with the HypoCCS and CCSS studies. The HypoCCS cohort included no control group and the CCSS cohort did not include GHD patients with other causes of GHD than CSs (21, 22). Although the KIMS database permitted the inclusion of a control group of GH-untreated patients, it was primarily conceived to analyze the safety of GH replacement, resulting in a limited number of control patients. A comparative analysis between groups with or without GH substitution was thus not feasible. To overcome this shortcoming, two control groups of GH-treated patients, CO IGHD and AO NFPA, were selected; these bore similarities regarding age of onset with the CO-CS and AO-CS, respectively. Although evaluating the data from another perspective, the present KIMS study adds further information regarding the development of a second neoplasm during adulthood in GH-replaced CO-CS, but it also analyzes this risk factor in AO-CS.
Regarding the occurrence of a second neoplasm during GH replacement in CO-CS, the present analysis was compared with the literature and appeared to be in line with the published information. Although HypoCCS estimated the 5-year cumulative incidence as 4.8% and CCSS indicated an occurrence of 4.7%, KIMS showed an incidence of 4.3% during a median follow-up of 6 years. It should be taken into account that the actual duration of GH replacement and the follow-up for CO-CS are much longer because KIMS reports the period after inclusion into the database only (Table 3). Moreover, the patients in KIMS might be a selected group with a number of years before KIMS start without any clinically active cancer. Nevertheless, analysis of the CO cohorts clearly indicated that the incidence of second neoplasms was higher in CO-CS patients compared with IGHD. For a better understanding of the detailed characteristics of these studies, an overview is provided in Table 4.
| Study Reference (No.) . | 21 . | 21 . | 22 . | Present Study . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study name | GENESIS | HypoCCS | CCSS | KIMS | ||||||
| Study objective | GH replacement and incidence of second neoplasm | GH replacement and incidence of second CNS neoplasm | GH replacement and incidence of second malignant neoplasm or meningioma | |||||||
| Study population | Children with CO-GHD | Adults with CO-GHD | Children + adults with CO-GHD | Adults with CO-GHD | Adults with AO-GHD | |||||
| Study cohorts | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls |
| Main primary cancers | Medulloblastoma (33%) | Germinoma (21%) | Leukemia (30%) | Leukemia (34%) | Germinoma (32%) | None | Germinoma (34%) | None | ||
| Leukemia (15%) | Leukemia (18%) | CNS tumor (49%) | CNS tumor (12%) | Medulloblastoma (19%) | (Idiopathic or congenital GHD) | Leukemia (20%) | (NFPA) | |||
| Medulloblastoma (16%) | Astrocytoma (15%) | Astrocytoma (17%) | ||||||||
| Astrocytoma (16%) | Glioma (14%) | Glioma (10%) | ||||||||
| GH replacement | Yes | No | Yes | No | Yes | No | Yes | Yes | Yes | Yes |
| Patients (n) | 394 | 27 | 252 | 28 | 338 | 11760 | 349 | 619 | 174 | 2449 |
| Age at primary cancer (y) | 5.4 | 7.5 | 8.4 | 8.7 | 0-9 (94%) | 0-9 (61%) | 10.4 | 9.0 | 30.1 | 46.6 |
| Age at start GH replacement (y) | 10.8 | NA | NR | NA | NR | NA | 24.5 | 28.8 | 36.0 | 53.2 |
| Follow-up (y) | 2.9 | 2.1 | 2.9 | 2.6 | NR | NR | 5.9 | 5.4 | 4.9 | 5.6 |
| Number second neoplasms (%) | 15 (3.8) | 0 | 23 (9.1) | 4 (14.3) | NA | NA | 27 (7.7) | 4 (0.6) | 9 (5.2) | 190 (7.8) |
| Second neoplasm before + during GH replacement (n) | NR | NR | NR | NR | NR | NR | 11 (3.2%) + 16 (4.6%) | 1 (0.2%) + 3 (0.5%) | 6 (3.4%) + 3 (1.7%) | 44 (1.8%) + 146 (6.0%) |
| Time GH replacement to second neoplasm (y) | 2.4 | NA | NR | NA | Meningioma: accumulating >35 y Glioma: first 20 y | 2.3 | 1.6 | 3.4 | 4.6 | |
| Type second neoplasm before + during GH replacement (n) | . | . | . | . | . | . | . | |||
| Meningioma (n) | 3 (0.8%) | 8 (3.2%) | 2 (7.1%) | 10 (3.0%) | 138 (1.2%) | 3 + 0 (0.9%) | 1 + 0 (0.6%) | |||
| Glioma (n) | 2 (0.5%) | 2 (0.8%) | 1 (3.6%) | 6 (1.8%) | 49 (0.4%) | 0 | 0 | |||
| Other CNS neoplasm (n) | 2 | 0 | — | 0 | 16 (0.1%) | 0 + 6 (1.7%) | 0 | |||
| Leukemia (n) | 3 | 0 | — | NA | NA | 0 | 0 | |||
| Thyroid carcinoma (n) | 0 | 3 | — | NA | NA | 0 + 1 | 0 | |||
| Basal cell carcinoma (n) | 0 | 3 | — | NA | NA | 6 + 7 (3.7%) | 1 + 0 (0.6%) | |||
| Other malignant (n) | 3 | 3 | 1 | NA | NA | 2 + 2 | 4 + 3 (4.0%) | |||
| Other benign (n) | 2 | 4 | — | NA | NA | NA | NA | |||
| Radiotherapy (n) | — | — | — | — | — | — | — | |||
| Meningioma (n) | 3 | 7 (1 NR) | (2 NR) | 10 | 134 | 3 | 1 | |||
| Glioma (n) | 2 | 2 | 1 | 5 | 44 | 0 | 0 | |||
| Other CNS neoplasm (n) | 2 | 0 | —. | 0 | 14 | 6 | 0 | |||
| Basal cell carcinoma (n) | 0 | 2 (1 NR) | — | — | — | 13 | 1 | |||
| Study Reference (No.) . | 21 . | 21 . | 22 . | Present Study . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study name | GENESIS | HypoCCS | CCSS | KIMS | ||||||
| Study objective | GH replacement and incidence of second neoplasm | GH replacement and incidence of second CNS neoplasm | GH replacement and incidence of second malignant neoplasm or meningioma | |||||||
| Study population | Children with CO-GHD | Adults with CO-GHD | Children + adults with CO-GHD | Adults with CO-GHD | Adults with AO-GHD | |||||
| Study cohorts | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls |
| Main primary cancers | Medulloblastoma (33%) | Germinoma (21%) | Leukemia (30%) | Leukemia (34%) | Germinoma (32%) | None | Germinoma (34%) | None | ||
| Leukemia (15%) | Leukemia (18%) | CNS tumor (49%) | CNS tumor (12%) | Medulloblastoma (19%) | (Idiopathic or congenital GHD) | Leukemia (20%) | (NFPA) | |||
| Medulloblastoma (16%) | Astrocytoma (15%) | Astrocytoma (17%) | ||||||||
| Astrocytoma (16%) | Glioma (14%) | Glioma (10%) | ||||||||
| GH replacement | Yes | No | Yes | No | Yes | No | Yes | Yes | Yes | Yes |
| Patients (n) | 394 | 27 | 252 | 28 | 338 | 11760 | 349 | 619 | 174 | 2449 |
| Age at primary cancer (y) | 5.4 | 7.5 | 8.4 | 8.7 | 0-9 (94%) | 0-9 (61%) | 10.4 | 9.0 | 30.1 | 46.6 |
| Age at start GH replacement (y) | 10.8 | NA | NR | NA | NR | NA | 24.5 | 28.8 | 36.0 | 53.2 |
| Follow-up (y) | 2.9 | 2.1 | 2.9 | 2.6 | NR | NR | 5.9 | 5.4 | 4.9 | 5.6 |
| Number second neoplasms (%) | 15 (3.8) | 0 | 23 (9.1) | 4 (14.3) | NA | NA | 27 (7.7) | 4 (0.6) | 9 (5.2) | 190 (7.8) |
| Second neoplasm before + during GH replacement (n) | NR | NR | NR | NR | NR | NR | 11 (3.2%) + 16 (4.6%) | 1 (0.2%) + 3 (0.5%) | 6 (3.4%) + 3 (1.7%) | 44 (1.8%) + 146 (6.0%) |
| Time GH replacement to second neoplasm (y) | 2.4 | NA | NR | NA | Meningioma: accumulating >35 y Glioma: first 20 y | 2.3 | 1.6 | 3.4 | 4.6 | |
| Type second neoplasm before + during GH replacement (n) | . | . | . | . | . | . | . | |||
| Meningioma (n) | 3 (0.8%) | 8 (3.2%) | 2 (7.1%) | 10 (3.0%) | 138 (1.2%) | 3 + 0 (0.9%) | 1 + 0 (0.6%) | |||
| Glioma (n) | 2 (0.5%) | 2 (0.8%) | 1 (3.6%) | 6 (1.8%) | 49 (0.4%) | 0 | 0 | |||
| Other CNS neoplasm (n) | 2 | 0 | — | 0 | 16 (0.1%) | 0 + 6 (1.7%) | 0 | |||
| Leukemia (n) | 3 | 0 | — | NA | NA | 0 | 0 | |||
| Thyroid carcinoma (n) | 0 | 3 | — | NA | NA | 0 + 1 | 0 | |||
| Basal cell carcinoma (n) | 0 | 3 | — | NA | NA | 6 + 7 (3.7%) | 1 + 0 (0.6%) | |||
| Other malignant (n) | 3 | 3 | 1 | NA | NA | 2 + 2 | 4 + 3 (4.0%) | |||
| Other benign (n) | 2 | 4 | — | NA | NA | NA | NA | |||
| Radiotherapy (n) | — | — | — | — | — | — | — | |||
| Meningioma (n) | 3 | 7 (1 NR) | (2 NR) | 10 | 134 | 3 | 1 | |||
| Glioma (n) | 2 | 2 | 1 | 5 | 44 | 0 | 0 | |||
| Other CNS neoplasm (n) | 2 | 0 | —. | 0 | 14 | 6 | 0 | |||
| Basal cell carcinoma (n) | 0 | 2 (1 NR) | — | — | — | 13 | 1 | |||
Abbreviation: GENESIS, Genetics and Neuroendocrinology of Short Stature International Study.
| Study Reference (No.) . | 21 . | 21 . | 22 . | Present Study . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study name | GENESIS | HypoCCS | CCSS | KIMS | ||||||
| Study objective | GH replacement and incidence of second neoplasm | GH replacement and incidence of second CNS neoplasm | GH replacement and incidence of second malignant neoplasm or meningioma | |||||||
| Study population | Children with CO-GHD | Adults with CO-GHD | Children + adults with CO-GHD | Adults with CO-GHD | Adults with AO-GHD | |||||
| Study cohorts | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls |
| Main primary cancers | Medulloblastoma (33%) | Germinoma (21%) | Leukemia (30%) | Leukemia (34%) | Germinoma (32%) | None | Germinoma (34%) | None | ||
| Leukemia (15%) | Leukemia (18%) | CNS tumor (49%) | CNS tumor (12%) | Medulloblastoma (19%) | (Idiopathic or congenital GHD) | Leukemia (20%) | (NFPA) | |||
| Medulloblastoma (16%) | Astrocytoma (15%) | Astrocytoma (17%) | ||||||||
| Astrocytoma (16%) | Glioma (14%) | Glioma (10%) | ||||||||
| GH replacement | Yes | No | Yes | No | Yes | No | Yes | Yes | Yes | Yes |
| Patients (n) | 394 | 27 | 252 | 28 | 338 | 11760 | 349 | 619 | 174 | 2449 |
| Age at primary cancer (y) | 5.4 | 7.5 | 8.4 | 8.7 | 0-9 (94%) | 0-9 (61%) | 10.4 | 9.0 | 30.1 | 46.6 |
| Age at start GH replacement (y) | 10.8 | NA | NR | NA | NR | NA | 24.5 | 28.8 | 36.0 | 53.2 |
| Follow-up (y) | 2.9 | 2.1 | 2.9 | 2.6 | NR | NR | 5.9 | 5.4 | 4.9 | 5.6 |
| Number second neoplasms (%) | 15 (3.8) | 0 | 23 (9.1) | 4 (14.3) | NA | NA | 27 (7.7) | 4 (0.6) | 9 (5.2) | 190 (7.8) |
| Second neoplasm before + during GH replacement (n) | NR | NR | NR | NR | NR | NR | 11 (3.2%) + 16 (4.6%) | 1 (0.2%) + 3 (0.5%) | 6 (3.4%) + 3 (1.7%) | 44 (1.8%) + 146 (6.0%) |
| Time GH replacement to second neoplasm (y) | 2.4 | NA | NR | NA | Meningioma: accumulating >35 y Glioma: first 20 y | 2.3 | 1.6 | 3.4 | 4.6 | |
| Type second neoplasm before + during GH replacement (n) | . | . | . | . | . | . | . | |||
| Meningioma (n) | 3 (0.8%) | 8 (3.2%) | 2 (7.1%) | 10 (3.0%) | 138 (1.2%) | 3 + 0 (0.9%) | 1 + 0 (0.6%) | |||
| Glioma (n) | 2 (0.5%) | 2 (0.8%) | 1 (3.6%) | 6 (1.8%) | 49 (0.4%) | 0 | 0 | |||
| Other CNS neoplasm (n) | 2 | 0 | — | 0 | 16 (0.1%) | 0 + 6 (1.7%) | 0 | |||
| Leukemia (n) | 3 | 0 | — | NA | NA | 0 | 0 | |||
| Thyroid carcinoma (n) | 0 | 3 | — | NA | NA | 0 + 1 | 0 | |||
| Basal cell carcinoma (n) | 0 | 3 | — | NA | NA | 6 + 7 (3.7%) | 1 + 0 (0.6%) | |||
| Other malignant (n) | 3 | 3 | 1 | NA | NA | 2 + 2 | 4 + 3 (4.0%) | |||
| Other benign (n) | 2 | 4 | — | NA | NA | NA | NA | |||
| Radiotherapy (n) | — | — | — | — | — | — | — | |||
| Meningioma (n) | 3 | 7 (1 NR) | (2 NR) | 10 | 134 | 3 | 1 | |||
| Glioma (n) | 2 | 2 | 1 | 5 | 44 | 0 | 0 | |||
| Other CNS neoplasm (n) | 2 | 0 | —. | 0 | 14 | 6 | 0 | |||
| Basal cell carcinoma (n) | 0 | 2 (1 NR) | — | — | — | 13 | 1 | |||
| Study Reference (No.) . | 21 . | 21 . | 22 . | Present Study . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study name | GENESIS | HypoCCS | CCSS | KIMS | ||||||
| Study objective | GH replacement and incidence of second neoplasm | GH replacement and incidence of second CNS neoplasm | GH replacement and incidence of second malignant neoplasm or meningioma | |||||||
| Study population | Children with CO-GHD | Adults with CO-GHD | Children + adults with CO-GHD | Adults with CO-GHD | Adults with AO-GHD | |||||
| Study cohorts | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls |
| Main primary cancers | Medulloblastoma (33%) | Germinoma (21%) | Leukemia (30%) | Leukemia (34%) | Germinoma (32%) | None | Germinoma (34%) | None | ||
| Leukemia (15%) | Leukemia (18%) | CNS tumor (49%) | CNS tumor (12%) | Medulloblastoma (19%) | (Idiopathic or congenital GHD) | Leukemia (20%) | (NFPA) | |||
| Medulloblastoma (16%) | Astrocytoma (15%) | Astrocytoma (17%) | ||||||||
| Astrocytoma (16%) | Glioma (14%) | Glioma (10%) | ||||||||
| GH replacement | Yes | No | Yes | No | Yes | No | Yes | Yes | Yes | Yes |
| Patients (n) | 394 | 27 | 252 | 28 | 338 | 11760 | 349 | 619 | 174 | 2449 |
| Age at primary cancer (y) | 5.4 | 7.5 | 8.4 | 8.7 | 0-9 (94%) | 0-9 (61%) | 10.4 | 9.0 | 30.1 | 46.6 |
| Age at start GH replacement (y) | 10.8 | NA | NR | NA | NR | NA | 24.5 | 28.8 | 36.0 | 53.2 |
| Follow-up (y) | 2.9 | 2.1 | 2.9 | 2.6 | NR | NR | 5.9 | 5.4 | 4.9 | 5.6 |
| Number second neoplasms (%) | 15 (3.8) | 0 | 23 (9.1) | 4 (14.3) | NA | NA | 27 (7.7) | 4 (0.6) | 9 (5.2) | 190 (7.8) |
| Second neoplasm before + during GH replacement (n) | NR | NR | NR | NR | NR | NR | 11 (3.2%) + 16 (4.6%) | 1 (0.2%) + 3 (0.5%) | 6 (3.4%) + 3 (1.7%) | 44 (1.8%) + 146 (6.0%) |
| Time GH replacement to second neoplasm (y) | 2.4 | NA | NR | NA | Meningioma: accumulating >35 y Glioma: first 20 y | 2.3 | 1.6 | 3.4 | 4.6 | |
| Type second neoplasm before + during GH replacement (n) | . | . | . | . | . | . | . | |||
| Meningioma (n) | 3 (0.8%) | 8 (3.2%) | 2 (7.1%) | 10 (3.0%) | 138 (1.2%) | 3 + 0 (0.9%) | 1 + 0 (0.6%) | |||
| Glioma (n) | 2 (0.5%) | 2 (0.8%) | 1 (3.6%) | 6 (1.8%) | 49 (0.4%) | 0 | 0 | |||
| Other CNS neoplasm (n) | 2 | 0 | — | 0 | 16 (0.1%) | 0 + 6 (1.7%) | 0 | |||
| Leukemia (n) | 3 | 0 | — | NA | NA | 0 | 0 | |||
| Thyroid carcinoma (n) | 0 | 3 | — | NA | NA | 0 + 1 | 0 | |||
| Basal cell carcinoma (n) | 0 | 3 | — | NA | NA | 6 + 7 (3.7%) | 1 + 0 (0.6%) | |||
| Other malignant (n) | 3 | 3 | 1 | NA | NA | 2 + 2 | 4 + 3 (4.0%) | |||
| Other benign (n) | 2 | 4 | — | NA | NA | NA | NA | |||
| Radiotherapy (n) | — | — | — | — | — | — | — | |||
| Meningioma (n) | 3 | 7 (1 NR) | (2 NR) | 10 | 134 | 3 | 1 | |||
| Glioma (n) | 2 | 2 | 1 | 5 | 44 | 0 | 0 | |||
| Other CNS neoplasm (n) | 2 | 0 | —. | 0 | 14 | 6 | 0 | |||
| Basal cell carcinoma (n) | 0 | 2 (1 NR) | — | — | — | 13 | 1 | |||
Abbreviation: GENESIS, Genetics and Neuroendocrinology of Short Stature International Study.
The current study is limited because of the registry study design, in which one can only rely on the information provided by the investigators. Direct access to original data are not feasible in registries. Nevertheless, the results seem to be in line with the Safety and Appropriateness of Growth Hormone Treatments in Europe (SAGhE) cohort study on cancer risk in patients treated with growth hormone in childhood (30). The SAGhE study showed a lower cancer incidence in CS patients than the KIMS study presented here (SIR 7.6 vs 10.39). The follow-up of the SAGhE study was longer with 14.8 patient-years vs average 6 years. It was observed that the SIR decreases over time since treatment start. It could be hypothesized that a longer follow-up period in the KIMS analysis would bring a lower SIR more in line with SAGhE study.
Regarding the occurrence of a second neoplasm during GH replacement in the AO cohorts, the current study reports an incidence of 1.7% in the AO-CS cohort over a median follow-up period of 4.9 years, whereas the percentage was 6.2% in the NFPA cohort, which served as a control. The two groups differed significantly in age. NFPA patients were almost 17 years older than AO-CS. Age- and sex-adjusted results showed that, during GH replacement, the incidence of a second neoplasm in AO-CS was similar compared with NFPA patients.
Analysis of the type of second neoplasm disclosed the predominant occurrence of cerebral malignancies and basal cell carcinomas in both CO-CS and AO-CS before adult GH replacement and in CO-CS during adult GH replacement occurred exclusively in patients treated by radiotherapy for their primary malignancy. CO-CS patients treated with radiotherapy had a 2.1% cumulative incidence of a subsequent CNS neoplasm during KIMS, whereas the incidence was 0% in those who had no radiotherapy. This is in line with data showing that radiotherapy increased the risk for both cerebral malignancy and basal cell carcinomas (31–35). The effect of chemotherapy is hard to substantiate and other confounding factors could not be accounted for as no patients with a possible genetic risk such as neurofibromatosis were included in the current study. These second neoplasms were also responsible for the larger part of deaths, probably related to the use of radiotherapy, which may also be associated to the deaths due to cerebrovascular accidents.
In conclusion, data analysis of KIMS, the largest pharmacoepidemic database of GH replacement in adults, confirmed that an increased risk of new neoplasm in CO-CS was observed, which was not the case in AO-CS, although the follow-up period may possibly be too restricted. Radiotherapy plays a preponderant role in the occurrence of basal cell carcinomas and is also related to the development of a second malignant tumors. Although the role of GH replacement in this process is still not obvious, these findings underline the necessity for a life-long close follow-up of CSs.
Abbreviations:
- AO
adult-onset
- BMI
body mass index
- CCSS
Childhood Cancer Survival Study
- CI
confidence interval
- CNS
central nervous system
- CO
childhood-onset
- CS
cancer survivor
- GH
growth hormone
- GHD
growth hormone deficiency
- HypoCCS
Hypopituitary Control and Complications Study
- IGHD
idiopathic growth hormone deficiency
- KIMS
Pfizer International Metabolic Database
- NFPA
nonfunctioning pituitary adenoma
- RR
risk ratio
- SAGhE
Safety and Appropriateness of Growth Hormone Treatments in Europe
- SDS
standard deviation score
- SIR
standardized incidence ratio.
Acknowledgments
Disclosure Summary: A.F.M. is employed by Pfizer, Inc., and performed all statistical analyses D.M. has received honoraria for presentations and consultation from HRA, Ipsen, Novartis, Novo-Nordisk, and Pfizer. U.F.-R. has received speaker’s honoraria from Pfizer, NovoNordisk, and Novartis. C.-C.H. is employed by Pfizer, Inc. A.L. has received honoraria for presentations and/or consultations from Ipsen, Novo Nordisk, Merck, and Pfizer. R.A. is a member of the KIMS Steering Committee. K.K.-M. states no disclosures. The KIMS database is sponsored by Pfizer Inc.
References
The Food and Drug Administration. Label for Genotropin. Revised September 2014. Available at: http://www.fdaguidance.net/wp-content/uploads/2014/10/Drug-Safety-Labeling-Changes.pdf. Accessed 6 December 2017.



