-
PDF
- Split View
-
Views
-
Cite
Cite
Xin Zhang, Chen Wang, Yansong Lin, Pilot Dose Comparison of Apatinib in Chinese Patients With Progressive Radioiodine-Refractory Differentiated Thyroid Cancer, The Journal of Clinical Endocrinology & Metabolism, Volume 103, Issue 10, October 2018, Pages 3640–3646, https://doi.org/10.1210/jc.2018-00381
Close - Share Icon Share
Abstract
Apatinib has shown overwhelming efficacy in progressive radioiodine-refractory differentiated thyroid cancer (RAIR-DTC) starting at a 750-mg dosing protocol; however, a relatively high incidence of treatment-associated adverse events (TAAEs) was observed, which reduced quality of life and interrupted the treatment.
To evaluate the efficacy and safety of apatinib with two different dosing schedules [750 or 500 mg once a day (q.d.)] in RAIR-DTC.
Twenty patients were sequentially recruited to receive apatinib beginning at 750 (n = 10) or 500 (n = 10) mg q.d. Efficacy and safety were compared in each 28-day cycle at the beginning two cycles and every two cycles thereafter.
After six treatment cycles, the best disease control rates were 100% for the 750- and 500-mg schedules, respectively, and the best objective response rates were 90.0% and 70.0% (P = 0.58), respectively. The two dosing schedules did not differ regarding greatest reduction in target lesion size (−42.7% vs −40.5% for the 750- vs 500-mg schedule, P = 0.48) and thyroglobulin level (−82.5% vs −94.3% for the 750- vs 500-mg schedule, P = 0.14). All patients experienced TAAEs, and the two dosing schedules showed similar incidence in TAAEs of grade ≥3 (100% vs 70% for 750 vs 500 mg, P = 0.21). However, the frequency of TAAEs was much higher in the 750-mg schedule (26.8 ± 6.5 vs 18.1 ± 6.5 in any grades, P = 0.01; 5.2 ± 3.0 vs 1.6 ± 1.3 in grade ≥3, P < 0.01).
Within six cycles of follow-up, the 500-mg starting dose protocol might be less toxic than the 750-mg protocol, whereas the efficacy was similar between the two dosages.
The prognosis of patients with differentiated thyroid cancer (DTC), classified histologically as papillary, follicular (including Hürthle cell), or poorly differentiated, is usually favorable with surgery, selective radioactive iodine (RAI), and l-thyroxine suppressive therapy, even in patients who present with metastatic RAI-avid disease. RAI is considered a gold standard in the treatment of metastatic disease. However, around two-thirds of these patients become refractory to RAI, with a 10-year survival rate as low as 10%. Some patients die within 3 to 5 years (1, 2).
Due to the lack of effective traditional cytotoxic drugs and systemic treatment, drugs need to be developed for patients with radioactive iodine-refractory differentiated thyroid cancer (RAIR-DTC). Sorafenib and lenvatinib are the only tyrosine kinase inhibitors (TKIs) that have demonstrated efficacy in dedicated, multicenter, phase III trials and that have been authorized in America and the European Union for use in treating patients with RAIR-DTC (3–5). However, only sorafenib is available in China, and the price is too high for most of the Chinese patients with RAIR-DTC. Apatinib, a small-molecule TKI, shares the same targets with sorafenib and lenvatinib (i.e., vascular endothelial growth factor receptor 2 and platelet-derived growth factor receptor) (6–8). It has been approved by the China Food and Drug Administration for treatment of advanced gastric carcinoma after failure of at least two lines of systemic therapy (9–11). Its efficacy and safety have been proven in metastatic triple-negative breast cancer (12) and non–small cell lung cancer (13). The phase I clinical trial has shown that apatinib is well tolerated as a single agent at a daily dose ≤750 mg (7). In our previous research, we explored apatinib in progressive RAIR-DTC with a 750-mg dosing schedule. It showed rapid response and high efficacy within the first 8 weeks (14). However, a relatively high incidence of adverse events (AEs) has been observed over the study period, which would reduce the quality of life to some extent. Meanwhile, the efficacy might also be affected by either treatment interruption or dose downregulation caused by AEs. Hence, another 10 patients with progressive RAIR-DTC were enrolled to receive apatinib at a reduced initial dose of 500 mg once a day (q.d.). In this pilot study, we reported the efficacy and safety data in terms of total target lesions (TLs), serum thyroglobulin (Tg) level, and AEs of apatinib in progressive RAIR-DTC and preliminarily compared the difference between the two dosing schedules.
Methods
This study was approved by the institutional review board of the Peking Medical College Hospital Ethics Committee (reference number HS970), including the supplemental protocol of the initial dosing schedule with 500 mg q.d. apatinib, and was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. All patients were fully informed and provided written informed consent before recruitment into this study.
Study design and treatment
This was an open-label phase II trial of oral apatinib (Jiangsu Hengrui Medicine, Lianyungang, China) for the treatment of progressive RAIR-DTC. Twenty patients were sequentially recruited to receive apatinib at a starting dose of 750 or 500 mg q.d. A treatment cycle was defined as 28 days. After commencement of apatinib, patients were clinically followed for the first two cycles and every two cycles thereafter. Treatment interruption was required for those who developed AEs of grade 3 or higher. Sequential reduction in dose (with a 250-mg tapered dose every turn) was allowed. For the 500-mg group, the dose could escalate to 750 mg at the discretion of the investigators if the AEs were tolerable and if no apparent efficacy was observed. Once the dose was reduced to 250 mg q.d. due to serious AEs, it could not be escalated. Treatment interruption caused by toxicities of apatinib was allowed for no more than two times or a maximum duration of 2 weeks (either continuously or cumulatively) in one cycle. Treatment continued until the patients presented disease progression or drug intolerance or if they withdrew consent.
Patients were enrolled if they were aged 18 years or older; had metastatic RAIR-DTC; had progressed within the past 14 months according to Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1) (15); had at least one measurable lesion evaluated by CT or MRI according to RECIST 1.1; had Eastern Cooperative Oncology Group performance status 0 to 2; and had adequate bone marrow, liver, and renal function. RAIR was defined as the presence of at least one TL without RAI uptake. RAIR was also diagnosed in patients whose tumors had RAI uptake and either progressed after one RAI treatment, showed activity of at least 3.7 GBq (≥100 mCi) within the past 12 months, or progressed after two RAI treatments within 12 months of each other (with the last such treatment administered more than 12 months ago) or received cumulative RAI activity of ≥22.3 GBq (≥600 mCi). Patients who had received VEGFR-TKI, such as vandetanib, cabozantinib, and lenvatinib, within 1 month of the study were excluded.
The primary efficacy measurements of this phase II clinical trial were disease control rate (DCR) and objective response rate (ORR) based on RECIST 1.1 and change in serum Tg concentration. The secondary efficacy measurements were overall survival, progression-free survival (PFS), and duration of response. The present report described and compared the response including DCR, ORR, time to response, and changes in TLs and Tg during the first six treatment cycles. AEs were recorded to evaluate safety between the two different initial doses of apatinib (750 and 500 mg q.d.) in RAIR-DTC.
Assessment of efficacy and safety profiles
DCR included complete response, partial response (PR), and stable disease. ORR included complete response and PR according to RECIST 1.1. All treatment-related toxicities were collected and graded according to Common Terminology Criteria for Adverse Events version 4.0 (CTCAE 4.0) (16).
Statistical analysis
Quantitative values were expressed as mean ± SD or median and range when appropriate. Two-sample t test and Mann-Whitney U test were used for comparison of continuous variables, and Fisher exact test was performed for baseline and treatment discrete variables. SPSS (version 20.0; SPSS Inc., Chicago, IL) and Prism 6 (GraphPad Software, San Diego, CA) were used for statistical analysis. A P value <0.05 was considered statistically significant.
Results
Patient characteristics
A total of 20 patients with progressive metastatic RAIR-DTC were sequentially included and divided into two cohorts, in which dosage was started with apatinib at 750 mg (n = 10) or 500 mg once daily (n = 10) in 2016. The patient characteristics are shown in Table 1. There was no statistical difference between two cohorts regarding baseline demographics and disease characteristics (P > 0.05) (Table 1).
| . | Apatinib Dosing Protocol . | P Value . | |
|---|---|---|---|
| 750 mg (n = 10) . | 500 mg (n = 10) . | ||
| Age, y, mean ± SD (range) | 55.3 ± 14.42 (33–78) | 54.0 ± 13.64 (27–70) | 0.84 |
| Sex, male/female | 5/5 | 5/5 | 0.67 |
| Pathology | 0.37 | ||
| PTC | 9 | 6 | |
| FTC | 0 | 2 | |
| PoorlyPTC | 1 | 2 | |
| Median RAI accumulation, mCi (range) | 315 (200–630) | 410 (150–1150) | 0.12 |
| VEGF mutation | 1.00 | ||
| Positive | 4 | 3 | |
| Negative | 5 | 6 | |
| NA | 1 | 1 | |
| Therapy before apatinib | 1.00 | ||
| Surgery, RIT | 8 | 7 | |
| Surgery, RIT, sorafenib | 2 | 2 | |
| Surgery, RIT, sorafenib, vandetanib | 0 | 1 | |
| ECOG performance status | 0.65 | ||
| 0 | 3 | 4 | |
| 1 | 7 | 5 | |
| 2 | 0 | 1 | |
| . | Apatinib Dosing Protocol . | P Value . | |
|---|---|---|---|
| 750 mg (n = 10) . | 500 mg (n = 10) . | ||
| Age, y, mean ± SD (range) | 55.3 ± 14.42 (33–78) | 54.0 ± 13.64 (27–70) | 0.84 |
| Sex, male/female | 5/5 | 5/5 | 0.67 |
| Pathology | 0.37 | ||
| PTC | 9 | 6 | |
| FTC | 0 | 2 | |
| PoorlyPTC | 1 | 2 | |
| Median RAI accumulation, mCi (range) | 315 (200–630) | 410 (150–1150) | 0.12 |
| VEGF mutation | 1.00 | ||
| Positive | 4 | 3 | |
| Negative | 5 | 6 | |
| NA | 1 | 1 | |
| Therapy before apatinib | 1.00 | ||
| Surgery, RIT | 8 | 7 | |
| Surgery, RIT, sorafenib | 2 | 2 | |
| Surgery, RIT, sorafenib, vandetanib | 0 | 1 | |
| ECOG performance status | 0.65 | ||
| 0 | 3 | 4 | |
| 1 | 7 | 5 | |
| 2 | 0 | 1 | |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; FTC, follicular thyroid cancer; NA, not available; PTC, papillary thyroid cancer; Poorly-PTC, poorly differentiated thyroid cancer; RIT, radioactive iodine therapy; VEGF, vascular endothelial growth factor.
| . | Apatinib Dosing Protocol . | P Value . | |
|---|---|---|---|
| 750 mg (n = 10) . | 500 mg (n = 10) . | ||
| Age, y, mean ± SD (range) | 55.3 ± 14.42 (33–78) | 54.0 ± 13.64 (27–70) | 0.84 |
| Sex, male/female | 5/5 | 5/5 | 0.67 |
| Pathology | 0.37 | ||
| PTC | 9 | 6 | |
| FTC | 0 | 2 | |
| PoorlyPTC | 1 | 2 | |
| Median RAI accumulation, mCi (range) | 315 (200–630) | 410 (150–1150) | 0.12 |
| VEGF mutation | 1.00 | ||
| Positive | 4 | 3 | |
| Negative | 5 | 6 | |
| NA | 1 | 1 | |
| Therapy before apatinib | 1.00 | ||
| Surgery, RIT | 8 | 7 | |
| Surgery, RIT, sorafenib | 2 | 2 | |
| Surgery, RIT, sorafenib, vandetanib | 0 | 1 | |
| ECOG performance status | 0.65 | ||
| 0 | 3 | 4 | |
| 1 | 7 | 5 | |
| 2 | 0 | 1 | |
| . | Apatinib Dosing Protocol . | P Value . | |
|---|---|---|---|
| 750 mg (n = 10) . | 500 mg (n = 10) . | ||
| Age, y, mean ± SD (range) | 55.3 ± 14.42 (33–78) | 54.0 ± 13.64 (27–70) | 0.84 |
| Sex, male/female | 5/5 | 5/5 | 0.67 |
| Pathology | 0.37 | ||
| PTC | 9 | 6 | |
| FTC | 0 | 2 | |
| PoorlyPTC | 1 | 2 | |
| Median RAI accumulation, mCi (range) | 315 (200–630) | 410 (150–1150) | 0.12 |
| VEGF mutation | 1.00 | ||
| Positive | 4 | 3 | |
| Negative | 5 | 6 | |
| NA | 1 | 1 | |
| Therapy before apatinib | 1.00 | ||
| Surgery, RIT | 8 | 7 | |
| Surgery, RIT, sorafenib | 2 | 2 | |
| Surgery, RIT, sorafenib, vandetanib | 0 | 1 | |
| ECOG performance status | 0.65 | ||
| 0 | 3 | 4 | |
| 1 | 7 | 5 | |
| 2 | 0 | 1 | |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; FTC, follicular thyroid cancer; NA, not available; PTC, papillary thyroid cancer; Poorly-PTC, poorly differentiated thyroid cancer; RIT, radioactive iodine therapy; VEGF, vascular endothelial growth factor.
Tumor response
At the beginning of apatinib treatment, a relatively higher ORR was achieved with the 750-mg schedule (60.0%) than with the 500-mg schedule (30.0%, P = 0.37) after one cycle. ORR in both schedules increased with treatment, but the discrepancies between the two schedules gradually diminished. One patient in the 750-mg dosing schedule discontinued treatment at the end of the sixth cycle due to death caused by pulmonary infection unrelated to apatinib treatment. The best ORR (all PR) was 90.0% in the 750-mg schedule after six cycles, which was a little higher than that in the 500-mg schedule (70%; P = 0.58, not statistically significant) (Fig. 1A). The median times to first PR for patients in the 750-mg and 500-mg schedules were 0.9 and 1.8 months, respectively (P = 0.14). Stable disease occurred in one patient (10%) in the 750-mg schedule and in two patients (20%) in the 500-mg schedule; one patient from each schedule discontinued treatment of progression disease (10% in each group). After six treatment cycles, most of the patients reached substantial structural response from apatinib, with a median best reduction in tumor size of −42.7% (range, −23.9% to −60.6%) for the 750-mg schedule and −40.5% (range, −2.9% to −52.4%) for the 500-mg schedule (P = 0.48) (Fig. 1B). DCR for the 750-mg schedule was 100%, whereas that for the 500-mg schedule was 90% (P = 1.00) in terms of best response in six treatment cycles. The 6-month PFS rates were 88.9% in the 750-mg schedule and 90% in the 500-mg schedule (P = 1.00).
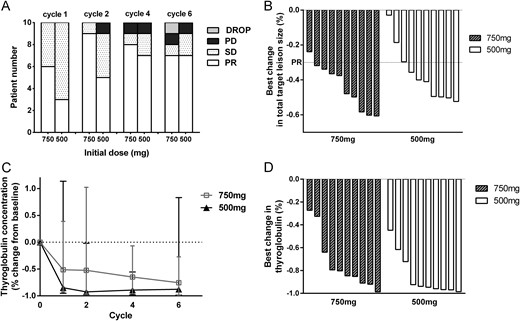
Efficacy data of apatinib. (A) Waterfall plot showing the best change in TL size for individual patients in each schedule. Best change in total TL size was defined as the difference in the sum of the longest diameter of the TLs from baseline (median, range). Negative values referred to maximum reduction. (B) Tumor response in the 750-mg schedule and the 500-mg schedule according to RECIST 1.1 in each 28-day cycle of apatinib. The numbers of patients were calculated using the patients evaluated as PR, stable disease (SD), progression disease (PD), and drop of the clinical trial (DROP). (C) Tg concentration change in each schedule. (D) Best change in Tg concentrations (defined as the difference in Tg concentration from baseline).
Tg changes
All patients achieved biochemical response to some extents. The response of Tg was similar in the two schedules without statistical difference. The concentrations of Tg dropped rapidly since the start of treatment. And the reduction gap between schedules became smaller in the treatment duration (Fig. 1C). The greatest reduction in Tg was recorded with a median reduction of −82.5% (range, −27.3% to −98.7%) in the 750-mg schedule and a median reduction of −94.3% (range, −44.8% to −98.6%, P = 0.14) in the 500-mg schedule compared with the baseline data (Fig. 1D).
Toxicity profiles and dose adjustment
Treatment-associated adverse events (TAAEs) occurred in all patients treated with apatinib. The most frequent TAAEs were hand-foot skin reaction (HFSR) (95%), proteinuria (80%), fatigue (75%), increased aspartate aminotransferase (75%), and hypertension (70%). There was only one patient in the 750-mg schedule who experienced grade 4 TAAEs (hypocalcemia). The occurrence rate of grade 3 or 4 TAAEs was similar in the two schedules (100% vs 70% for 750 mg vs 500 mg, P = 0.21) (Table 2).
| TAAEa . | 750 mg (n = 10) . | 500 mg (n = 10) . | P Value . | Total . | ||||
|---|---|---|---|---|---|---|---|---|
| All Grades . | ≥Grade 3 . | All Grades . | ≥Grade 3 . | All Grades . | ≥Grade 3 . | All Grades . | ≥Grade 3 . | |
| HFSR | 10 (100)b | 6 (60) | 9 (90) | 2 (20) | 1.00 | 0.17 | 19 (95) | 8 (40) |
| Proteinuria | 8 (80) | 1 (10) | 8 (80) | 2 (20) | 1.00 | 1.00 | 16 (80) | 3 (15) |
| Fatigue | 7 (70) | 0 (0) | 8 (80) | 0 (0) | 1.00 | NA | 15 (75) | 0 (0) |
| AST increased | 9 (90) | 0 (0) | 6 (60) | 0 (0) | 0.30 | NA | 15 (75) | 0 (0) |
| Hypertension | 8 (80) | 5 (50) | 6 (60) | 2 (20) | 0.63 | 0.35 | 14 (70) | 7 (35) |
| Diarrhea | 4 (40) | 2 (20) | 9 (90) | 1 (10) | 0.06 | 1.00 | 13 (65) | 3 (15) |
| Hypocalcemia | 9 (90) | 3 (30) | 3 (30) | 2 (20) | 0.02 | 1.00 | 12 (60) | 5 (25) |
| Mucositis oral | 4 (40) | 0 (0) | 7 (70) | 1 (10) | 0.37 | 1.00 | 11 (55) | 1 (5) |
| ALT increased | 6 (60) | 0 (0) | 3 (30) | 1 (10) | 0.37 | 1.00 | 9 (45) | 1 (5) |
| Gastric pain | 3 (30) | 2 (20) | 5 (50) | 2 (20) | 0.65 | 1.00 | 8 (40) | 4 (20) |
| Blood bilirubin increased | 4 (40) | 0 (0) | 3 (30) | 0 (0) | 1.00 | NA | 7 (35) | 0 (0) |
| Pharyngalgia | 3 (30) | 1 (10) | 3 (30) | 0 (0) | 1.00 | 1.00 | 6 (30) | 1 (5) |
| Neutrophil count decreased | 3 (30) | 1 (10) | 3 (30) | 0 (0) | 1.00 | 1.00 | 6 (30) | 1 (5) |
| Dyspepsia | 5 (50) | 0 (0) | 1 (10) | 0 (0) | 0.14 | NA | 6 (30) | 0 (0) |
| GGT increased | 3 (30) | 1 (10) | 2 (20) | 1 (10) | 1.00 | 1.00 | 5 (25) | 2 (10) |
| Platelet count decreased | 4 (40) | 1 (10) | 1 (10) | 0 (0) | 0.30 | 1.00 | 5 (25) | 1 (5) |
| Anorexia | 0 (0) | 0 (0) | 4 (40) | 1 (10) | 0.09 | 1.00 | 4 (20) | 1 (5) |
| Dysgeusia | 4 (40) | 0 (0) | 1 (10) | 0 (0) | 0.30 | NA | 5 (25) | 0 (0) |
| WBC decreased | 2 (20) | 0 (0) | 2 (20) | 0 (0) | 1.00 | NA | 4 (20) | 0 (0) |
| Headache | 1 (10) | 0 (0) | 3 (30) | 0 (0) | 0.58 | NA | 4 (20) | 0 (0) |
| Toothache | 0 (0) | 0 (0) | 3 (30) | 1 (10) | 0.21 | 1.00 | 3 (15) | 1 (5) |
| Hyperuricemia | 1 (10) | 0 (0) | 2 (20) | 0 (0) | 1.00 | NA | 3 (15) | 0 (0) |
| Hyperglycemia | 0 (0) | 0 (0) | 3 (30) | 0 (0) | 0.21 | NA | 3 (15) | 0 (0) |
| Hypokalemia | 1 (10) | 0 (0) | 1 (10) | 1 (10) | 1.00 | 1.00 | 2 (10) | 1 (5) |
| Nausea | 2 (20) | 1 (10) | 0 (0) | 0 (0) | 0.47 | 1.00 | 2 (10) | 1 (5) |
| Dysphagia | 2 (20) | 1 (10) | 0 (0) | 0 (0) | 0.47 | 1.00 | 2 (10) | 1 (5) |
| Vomiting | 2 (20) | 0 (0) | 0 (0) | 0 (0) | 0.47 | NA | 2 (10) | 0 (0) |
| Alopecia | 0 (0) | 0 (0) | 2 (20) | 0 (0) | 0.47 | NA | 2 (10) | 0 (0) |
| Dysgeusia | 4 (40) | 0 (0) | 1 (10) | 0 (0) | 0.30 | NA | 5 (25) | 0 (0) |
| TAAEa . | 750 mg (n = 10) . | 500 mg (n = 10) . | P Value . | Total . | ||||
|---|---|---|---|---|---|---|---|---|
| All Grades . | ≥Grade 3 . | All Grades . | ≥Grade 3 . | All Grades . | ≥Grade 3 . | All Grades . | ≥Grade 3 . | |
| HFSR | 10 (100)b | 6 (60) | 9 (90) | 2 (20) | 1.00 | 0.17 | 19 (95) | 8 (40) |
| Proteinuria | 8 (80) | 1 (10) | 8 (80) | 2 (20) | 1.00 | 1.00 | 16 (80) | 3 (15) |
| Fatigue | 7 (70) | 0 (0) | 8 (80) | 0 (0) | 1.00 | NA | 15 (75) | 0 (0) |
| AST increased | 9 (90) | 0 (0) | 6 (60) | 0 (0) | 0.30 | NA | 15 (75) | 0 (0) |
| Hypertension | 8 (80) | 5 (50) | 6 (60) | 2 (20) | 0.63 | 0.35 | 14 (70) | 7 (35) |
| Diarrhea | 4 (40) | 2 (20) | 9 (90) | 1 (10) | 0.06 | 1.00 | 13 (65) | 3 (15) |
| Hypocalcemia | 9 (90) | 3 (30) | 3 (30) | 2 (20) | 0.02 | 1.00 | 12 (60) | 5 (25) |
| Mucositis oral | 4 (40) | 0 (0) | 7 (70) | 1 (10) | 0.37 | 1.00 | 11 (55) | 1 (5) |
| ALT increased | 6 (60) | 0 (0) | 3 (30) | 1 (10) | 0.37 | 1.00 | 9 (45) | 1 (5) |
| Gastric pain | 3 (30) | 2 (20) | 5 (50) | 2 (20) | 0.65 | 1.00 | 8 (40) | 4 (20) |
| Blood bilirubin increased | 4 (40) | 0 (0) | 3 (30) | 0 (0) | 1.00 | NA | 7 (35) | 0 (0) |
| Pharyngalgia | 3 (30) | 1 (10) | 3 (30) | 0 (0) | 1.00 | 1.00 | 6 (30) | 1 (5) |
| Neutrophil count decreased | 3 (30) | 1 (10) | 3 (30) | 0 (0) | 1.00 | 1.00 | 6 (30) | 1 (5) |
| Dyspepsia | 5 (50) | 0 (0) | 1 (10) | 0 (0) | 0.14 | NA | 6 (30) | 0 (0) |
| GGT increased | 3 (30) | 1 (10) | 2 (20) | 1 (10) | 1.00 | 1.00 | 5 (25) | 2 (10) |
| Platelet count decreased | 4 (40) | 1 (10) | 1 (10) | 0 (0) | 0.30 | 1.00 | 5 (25) | 1 (5) |
| Anorexia | 0 (0) | 0 (0) | 4 (40) | 1 (10) | 0.09 | 1.00 | 4 (20) | 1 (5) |
| Dysgeusia | 4 (40) | 0 (0) | 1 (10) | 0 (0) | 0.30 | NA | 5 (25) | 0 (0) |
| WBC decreased | 2 (20) | 0 (0) | 2 (20) | 0 (0) | 1.00 | NA | 4 (20) | 0 (0) |
| Headache | 1 (10) | 0 (0) | 3 (30) | 0 (0) | 0.58 | NA | 4 (20) | 0 (0) |
| Toothache | 0 (0) | 0 (0) | 3 (30) | 1 (10) | 0.21 | 1.00 | 3 (15) | 1 (5) |
| Hyperuricemia | 1 (10) | 0 (0) | 2 (20) | 0 (0) | 1.00 | NA | 3 (15) | 0 (0) |
| Hyperglycemia | 0 (0) | 0 (0) | 3 (30) | 0 (0) | 0.21 | NA | 3 (15) | 0 (0) |
| Hypokalemia | 1 (10) | 0 (0) | 1 (10) | 1 (10) | 1.00 | 1.00 | 2 (10) | 1 (5) |
| Nausea | 2 (20) | 1 (10) | 0 (0) | 0 (0) | 0.47 | 1.00 | 2 (10) | 1 (5) |
| Dysphagia | 2 (20) | 1 (10) | 0 (0) | 0 (0) | 0.47 | 1.00 | 2 (10) | 1 (5) |
| Vomiting | 2 (20) | 0 (0) | 0 (0) | 0 (0) | 0.47 | NA | 2 (10) | 0 (0) |
| Alopecia | 0 (0) | 0 (0) | 2 (20) | 0 (0) | 0.47 | NA | 2 (10) | 0 (0) |
| Dysgeusia | 4 (40) | 0 (0) | 1 (10) | 0 (0) | 0.30 | NA | 5 (25) | 0 (0) |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ-glutamyl transferase; NA, not available; WBC, white blood cell.
Listed are all TAAEs occurring in ≥2 patients.
Data in parentheses are percentages.
| TAAEa . | 750 mg (n = 10) . | 500 mg (n = 10) . | P Value . | Total . | ||||
|---|---|---|---|---|---|---|---|---|
| All Grades . | ≥Grade 3 . | All Grades . | ≥Grade 3 . | All Grades . | ≥Grade 3 . | All Grades . | ≥Grade 3 . | |
| HFSR | 10 (100)b | 6 (60) | 9 (90) | 2 (20) | 1.00 | 0.17 | 19 (95) | 8 (40) |
| Proteinuria | 8 (80) | 1 (10) | 8 (80) | 2 (20) | 1.00 | 1.00 | 16 (80) | 3 (15) |
| Fatigue | 7 (70) | 0 (0) | 8 (80) | 0 (0) | 1.00 | NA | 15 (75) | 0 (0) |
| AST increased | 9 (90) | 0 (0) | 6 (60) | 0 (0) | 0.30 | NA | 15 (75) | 0 (0) |
| Hypertension | 8 (80) | 5 (50) | 6 (60) | 2 (20) | 0.63 | 0.35 | 14 (70) | 7 (35) |
| Diarrhea | 4 (40) | 2 (20) | 9 (90) | 1 (10) | 0.06 | 1.00 | 13 (65) | 3 (15) |
| Hypocalcemia | 9 (90) | 3 (30) | 3 (30) | 2 (20) | 0.02 | 1.00 | 12 (60) | 5 (25) |
| Mucositis oral | 4 (40) | 0 (0) | 7 (70) | 1 (10) | 0.37 | 1.00 | 11 (55) | 1 (5) |
| ALT increased | 6 (60) | 0 (0) | 3 (30) | 1 (10) | 0.37 | 1.00 | 9 (45) | 1 (5) |
| Gastric pain | 3 (30) | 2 (20) | 5 (50) | 2 (20) | 0.65 | 1.00 | 8 (40) | 4 (20) |
| Blood bilirubin increased | 4 (40) | 0 (0) | 3 (30) | 0 (0) | 1.00 | NA | 7 (35) | 0 (0) |
| Pharyngalgia | 3 (30) | 1 (10) | 3 (30) | 0 (0) | 1.00 | 1.00 | 6 (30) | 1 (5) |
| Neutrophil count decreased | 3 (30) | 1 (10) | 3 (30) | 0 (0) | 1.00 | 1.00 | 6 (30) | 1 (5) |
| Dyspepsia | 5 (50) | 0 (0) | 1 (10) | 0 (0) | 0.14 | NA | 6 (30) | 0 (0) |
| GGT increased | 3 (30) | 1 (10) | 2 (20) | 1 (10) | 1.00 | 1.00 | 5 (25) | 2 (10) |
| Platelet count decreased | 4 (40) | 1 (10) | 1 (10) | 0 (0) | 0.30 | 1.00 | 5 (25) | 1 (5) |
| Anorexia | 0 (0) | 0 (0) | 4 (40) | 1 (10) | 0.09 | 1.00 | 4 (20) | 1 (5) |
| Dysgeusia | 4 (40) | 0 (0) | 1 (10) | 0 (0) | 0.30 | NA | 5 (25) | 0 (0) |
| WBC decreased | 2 (20) | 0 (0) | 2 (20) | 0 (0) | 1.00 | NA | 4 (20) | 0 (0) |
| Headache | 1 (10) | 0 (0) | 3 (30) | 0 (0) | 0.58 | NA | 4 (20) | 0 (0) |
| Toothache | 0 (0) | 0 (0) | 3 (30) | 1 (10) | 0.21 | 1.00 | 3 (15) | 1 (5) |
| Hyperuricemia | 1 (10) | 0 (0) | 2 (20) | 0 (0) | 1.00 | NA | 3 (15) | 0 (0) |
| Hyperglycemia | 0 (0) | 0 (0) | 3 (30) | 0 (0) | 0.21 | NA | 3 (15) | 0 (0) |
| Hypokalemia | 1 (10) | 0 (0) | 1 (10) | 1 (10) | 1.00 | 1.00 | 2 (10) | 1 (5) |
| Nausea | 2 (20) | 1 (10) | 0 (0) | 0 (0) | 0.47 | 1.00 | 2 (10) | 1 (5) |
| Dysphagia | 2 (20) | 1 (10) | 0 (0) | 0 (0) | 0.47 | 1.00 | 2 (10) | 1 (5) |
| Vomiting | 2 (20) | 0 (0) | 0 (0) | 0 (0) | 0.47 | NA | 2 (10) | 0 (0) |
| Alopecia | 0 (0) | 0 (0) | 2 (20) | 0 (0) | 0.47 | NA | 2 (10) | 0 (0) |
| Dysgeusia | 4 (40) | 0 (0) | 1 (10) | 0 (0) | 0.30 | NA | 5 (25) | 0 (0) |
| TAAEa . | 750 mg (n = 10) . | 500 mg (n = 10) . | P Value . | Total . | ||||
|---|---|---|---|---|---|---|---|---|
| All Grades . | ≥Grade 3 . | All Grades . | ≥Grade 3 . | All Grades . | ≥Grade 3 . | All Grades . | ≥Grade 3 . | |
| HFSR | 10 (100)b | 6 (60) | 9 (90) | 2 (20) | 1.00 | 0.17 | 19 (95) | 8 (40) |
| Proteinuria | 8 (80) | 1 (10) | 8 (80) | 2 (20) | 1.00 | 1.00 | 16 (80) | 3 (15) |
| Fatigue | 7 (70) | 0 (0) | 8 (80) | 0 (0) | 1.00 | NA | 15 (75) | 0 (0) |
| AST increased | 9 (90) | 0 (0) | 6 (60) | 0 (0) | 0.30 | NA | 15 (75) | 0 (0) |
| Hypertension | 8 (80) | 5 (50) | 6 (60) | 2 (20) | 0.63 | 0.35 | 14 (70) | 7 (35) |
| Diarrhea | 4 (40) | 2 (20) | 9 (90) | 1 (10) | 0.06 | 1.00 | 13 (65) | 3 (15) |
| Hypocalcemia | 9 (90) | 3 (30) | 3 (30) | 2 (20) | 0.02 | 1.00 | 12 (60) | 5 (25) |
| Mucositis oral | 4 (40) | 0 (0) | 7 (70) | 1 (10) | 0.37 | 1.00 | 11 (55) | 1 (5) |
| ALT increased | 6 (60) | 0 (0) | 3 (30) | 1 (10) | 0.37 | 1.00 | 9 (45) | 1 (5) |
| Gastric pain | 3 (30) | 2 (20) | 5 (50) | 2 (20) | 0.65 | 1.00 | 8 (40) | 4 (20) |
| Blood bilirubin increased | 4 (40) | 0 (0) | 3 (30) | 0 (0) | 1.00 | NA | 7 (35) | 0 (0) |
| Pharyngalgia | 3 (30) | 1 (10) | 3 (30) | 0 (0) | 1.00 | 1.00 | 6 (30) | 1 (5) |
| Neutrophil count decreased | 3 (30) | 1 (10) | 3 (30) | 0 (0) | 1.00 | 1.00 | 6 (30) | 1 (5) |
| Dyspepsia | 5 (50) | 0 (0) | 1 (10) | 0 (0) | 0.14 | NA | 6 (30) | 0 (0) |
| GGT increased | 3 (30) | 1 (10) | 2 (20) | 1 (10) | 1.00 | 1.00 | 5 (25) | 2 (10) |
| Platelet count decreased | 4 (40) | 1 (10) | 1 (10) | 0 (0) | 0.30 | 1.00 | 5 (25) | 1 (5) |
| Anorexia | 0 (0) | 0 (0) | 4 (40) | 1 (10) | 0.09 | 1.00 | 4 (20) | 1 (5) |
| Dysgeusia | 4 (40) | 0 (0) | 1 (10) | 0 (0) | 0.30 | NA | 5 (25) | 0 (0) |
| WBC decreased | 2 (20) | 0 (0) | 2 (20) | 0 (0) | 1.00 | NA | 4 (20) | 0 (0) |
| Headache | 1 (10) | 0 (0) | 3 (30) | 0 (0) | 0.58 | NA | 4 (20) | 0 (0) |
| Toothache | 0 (0) | 0 (0) | 3 (30) | 1 (10) | 0.21 | 1.00 | 3 (15) | 1 (5) |
| Hyperuricemia | 1 (10) | 0 (0) | 2 (20) | 0 (0) | 1.00 | NA | 3 (15) | 0 (0) |
| Hyperglycemia | 0 (0) | 0 (0) | 3 (30) | 0 (0) | 0.21 | NA | 3 (15) | 0 (0) |
| Hypokalemia | 1 (10) | 0 (0) | 1 (10) | 1 (10) | 1.00 | 1.00 | 2 (10) | 1 (5) |
| Nausea | 2 (20) | 1 (10) | 0 (0) | 0 (0) | 0.47 | 1.00 | 2 (10) | 1 (5) |
| Dysphagia | 2 (20) | 1 (10) | 0 (0) | 0 (0) | 0.47 | 1.00 | 2 (10) | 1 (5) |
| Vomiting | 2 (20) | 0 (0) | 0 (0) | 0 (0) | 0.47 | NA | 2 (10) | 0 (0) |
| Alopecia | 0 (0) | 0 (0) | 2 (20) | 0 (0) | 0.47 | NA | 2 (10) | 0 (0) |
| Dysgeusia | 4 (40) | 0 (0) | 1 (10) | 0 (0) | 0.30 | NA | 5 (25) | 0 (0) |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ-glutamyl transferase; NA, not available; WBC, white blood cell.
Listed are all TAAEs occurring in ≥2 patients.
Data in parentheses are percentages.
All patients experienced dose interruption (32 vs 19 times in the 750-mg and the 500-mg schedule, respectively, with 30 vs 15 being apatinib related). Subsequently, 4 of 10 patients continued to receive 750 mg q.d., and 6 of 10 patients had dose reduction (4 to 500 mg q.d. and 2 to 250 mg q.d.) in the 750-mg schedule. As for the 500-mg schedule, 5 of 10 patients continued with 500 mg q.d., and 5 of 10 received 250 mg q.d. The mean daily dose of apatinib in the 750-mg schedule was higher compared with the 500-mg schedule (543.34 mg vs 405.25 mg, P = 0.02). The total dose statistically differed in each follow-up even with dose regulation (Fig. 2A). The occurrence of grade 3 or 4 TAAEs was earlier in the 750-mg schedule (median, 0.50 cycle; range, 0.25 to 1.93 cycles) than in the 500-mg schedule (median, 1.03 cycles; range, 0.46 to 4.11 cycles; P = 0.13) (Fig. 2B). TAAE frequency of all grades was significantly higher in the 750-mg schedule compared with the 500-mg schedule. Patients in the 750-mg schedule had a mean TAAE rate of 10.5 ± 5.0 times since the first cycle, compared with 5.3 ± 2.5 (P = 0.01) in the 500-mg schedule. The TAAE frequency growth rate decreased with ongoing treatment, reaching mean occurrence rates of 26.8 ± 6.5 in the 750-mg schedule and 18.1 ± 6.5 in the 500-mg schedule at the end of the sixth cycle (P = 0.01) (Fig. 2C). Statistical difference was also observed in grade 3 or 4 TAAEs in the 750-mg and the 500-mg schedule, with 5.2 ± 3.0 vs 1.6 ± 1.3 (P < 0.01) in median occurrence rates (Fig. 2D).
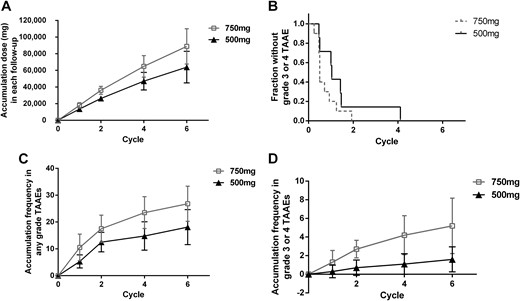
Safety data. (A) Total dose in each cycle, illustrated as mean dose ± SD. (B) First time the grade 3 TAAE occurred. (C) Accumulation frequency in any grade TAAE (mean ± SD). (D) Accumulation frequency in grade 3 or 4 TAAEs (mean ± SD).
Discussion
TKIs, many of which share the common target of the VEGF receptor (e.g., sorafenib, lenvatinib, and vandetanib), have recently emerged as highly promising therapies for metastatic RAIR-DTC, and two (sorafenib and lenvatinib) have been studied in phase III clinical trials. A substantial PFS over placebo has been observed (10.8 vs 5.8 months for sorafenib and 18.3 vs 3.6 months for lenvatinib). Sorafenib and lenvatinib have been approved for use in the treatment of patients with RAIR-DTC in the United States and in the European Union (3, 4, 17, 18). However, the options for Chinese patients with RAIR-DTC are limited because only sorafenib has been approved by the China Food and Drug Administration; lenvatinib is still under phase III clinical trial in China. In our earlier study, upregulation of angiogenesis in RAIR DTC lesions was observed with integrinαvβ3-targeted 99mTc-3PRGD2 imaging (19). Apatinib is a novel antiangiogenesis TKI explored in RAIR-DTC. One distinguishing feature of apatinib was its rapid response in target tumor size, Tg, and glucose metabolism, which was observed in the first 8 weeks starting at 750 mg q.d. in 10 patients with progressive RAIR-DTC. In addition, apatinib was associated with a median time to PR of 0.9 months in the preliminary observation, compared with 2.0 months with lenvatinib (4). A starting dose of 750 mg might help reduce tumor size at the beginning of treatment, which might offer an additional opportunity for surgeons to remove the foci or relieve compression symptoms in a short time. It was unknown whether rapid response in this short-term observation would bring effect for PFS or overall survival in long-term follow-up. With further observation, the 6-month PFS rate of apatinib was 88.9% in the 750-mg schedule, higher than that of lenvatinib (77.5%), which might indicate a promising PFS rate.
However, like other TKIs, a large number of TAAEs were recorded in the first two cycles. All patients in the 750-mg schedule experienced TAAEs. With a comparison of sorafenib and lenvatinib, apparent higher occurrence rates of apatinib were recorded in terms of HFSR, hypertension, proteinuria, increased alanine aminotransferase, fatigue, hypocalcemia, dyspepsia, and oral mucositis (12). To determine the optimal dosing protocol, another 10 patients with progressive RAIR-DTC were enrolled to receive apatinib at a starting dose of 500 mg q.d. Within the six-cycle observation, a relatively lower PR rate was observed with the 500-mg initial dosing schedule than with the 750-mg initial dosing schedule; this difference was associated with a median time to PR of 1.8 months. The discrepancy became smaller, yet no significant statistical difference was observed in median time to PR (P = 0.14), best TL (P = 0.48), and Tg (P = 0.14) reduction between the 750-mg schedule and the 500-mg schedule. The 6-month PFS rate was 90%.
Even with initial dose reduction, the TAAE profiles did not differ in type and occurrence rate among the two schedules, highlighting the inherent toxicity character of apatinib (Table 2). However, the quantitative change in TAAE showed significant statistical difference (P < 0.05) in terms of the occurrence frequency requiring more treatment interruption and dose reduction. Even though TAAEs were controllable with proper medical interventions and were rarely severe and life threatening, low-grade TAAE (e.g., HFSR, fatigue, and diarrhea) might have a huge impact on the patients’ quality of life. Besides, the cost of medical treatment is important for patients. With fewer TAAEs and lower mean daily doses, the costs of apatinib and side effect management were reduced. Considering fewer TAAEs and lower cost, the 500-mg initial dosing schedule might be safer and more economical than the 750-mg schedule. In a phase III clinical trial with a placebo group, an initial dose of 500 mg q.d. further revealed the efficacy, safety, and cost-effectiveness of apatinib (ClinicalTrials.gov NCT03048877).
This study has several limitations. Only 20 patients were selected for this study, and the selection was not blinded, which might influence the outcomes and the implications of difference between the treatment arms. Furthermore, most of the patients were still under treatment, and some key data (e.g., PFS) were not gathered in this short-term observation period; therefore, our data might not accurately reflect the efficacy and safety response of the treatment. A follow-up study of these patients is ongoing.
Conclusion
Apatinib is an oral multikinase inhibitor that seems to be a promising therapeutic option in patients with progressive RAIR-DTC. An initial dose of 500 mg q.d. is safer and more economical compared with an initial dose of 750 mg q.d. Further research is needed to address the best response in terms of survival and quality of life.
Abbreviations:
- AE
adverse event
- DCR
disease control rate
- HFSR
hand-foot skin reaction
- ORR
objective response rate
- PFS
progression-free survival
- PR
partial response
- q.d.
once a day
- RAI
radioactive iodine
- RAIR-DTC
radioiodine-refractory differentiated thyroid cancer
- TAAE
treatment-associated adverse event
- Tg
thyroglobulin
- TKI
tyrosine kinase inhibitor
- TL
target lesion
Acknowledgments
The authors thank the patients and their families, all investigators who participated in this study, and the Jiangsu Hengrui Medicine Co., Ltd for providing apatinib.
Financial Support: This work was supported by National Natural Science Foundation of China Grants 81571714 and 81771875 (to Y.L.).
Clinical Trial Information: ClinicalTrials.gov no. NCT02731352 (registered 7 April 2016).
Author Contributions: X.Z. wrote the paper. X.Z. and C.W. carried out the experiment, including patient follow-up and data collection and analysis. Y.L. designed and supervised the experiment.
Disclosure Summary: The authors have nothing to disclose.



