-
PDF
- Split View
-
Views
-
Cite
Cite
Jie Xu, Xiuyue Jia, Yang Gu, David F. Lewis, Xin Gu, Yuping Wang, Vitamin D Reduces Oxidative Stress–Induced Procaspase-3/ROCK1 Activation and MP Release by Placental Trophoblasts, The Journal of Clinical Endocrinology & Metabolism, Volume 102, Issue 6, 1 June 2017, Pages 2100–2110, https://doi.org/10.1210/jc.2016-3753
Close - Share Icon Share
Abstract
Increased microparticle (MP) shedding by placental trophoblasts contributes to maternal vascular inflammatory response and endothelial dysfunction in preeclampsia. Vitamin D has beneficial effects in pregnancy; however, its effect on trophoblast MP release has not been investigated.
To investigate if vitamin D could protect trophoblasts from oxidative stress–induced MP release.
Placental trophoblasts were isolated from uncomplicated and preeclamptic placentas. Effects of vitamin D on MP release induced by oxidative stress inducer CoCl2 were studied.
Annexin V+ MPs were assessed by flow cytometry. Expression of caveolin-1, endothelial nitric oxide synthase (eNOS), procaspase-3, cleaved caspase-3, and Rho-associated coiled-coil protein kinase 1 (ROCK1) in trophoblasts and trophoblast-derived MPs were determined by Western blot.
Trophoblasts from preeclamptic pregnancies released significantly more MPs than cells from uncomplicated pregnancies (P < 0.01). CoCl2-induced increase in MP release was associated with upregulation of caveolin-1 and downregulation of eNOS expression in trophoblasts (P < 0.05), which could be attenuated by 1,25(OH)2D3. Moreover, 1,25(OH)2D3 could also inhibit CoCl2-induced procaspase-3 cleavage and ROCK1 activation in trophoblasts. Consistently, CoCl2-induced upregulation of procaspase-3, cleaved caspase-3, and ROCK1 expression in trophoblast-derived MPs were also reduced in cells treated with 1,25(OH)2D3.
Placental trophoblasts from preeclamptic pregnancies released more MP than cells from uncomplicated pregnancies. Oxidative stress–induced increase in MP shedding is associated with upregulation of caveolin-1 and downregulation of eNOS expression in placental trophoblasts. Inhibition of caspase-3 cleavage and ROCK1 activation, together with upregulation of eNOS expression, could be the potential cellular/molecular mechanism(s) of vitamin D protective effects on placental trophoblasts.
During pregnancy, placental syncytiotrophoblasts continuously shed microvesicles, including microparticles (MPs) and exosomes, into the maternal circulation (1, 2). These microvesicles are recognized as important carriers for proteins, lipids, and nucleic acids, which play crucial roles in placental-maternal cross talk during pregnancy. Trophoblast microvesicles are able to interact with maternal vascular endothelium, coagulation components, and immune cells, and subsequently regulate vascular and immune system function during pregnancy (3). Placental MP and exosome shedding is increased in pregnancy disorders such as preeclampsia (1, 4). Trophoblast MPs released from preeclamptic placentas were found to express higher levels of endoglin and plasminogen activator inhibitors (5) and could stimulate peripheral blood mononuclear cells to produce more proinflammatory cytokines and chemokines, including interleukin (IL)–6, IL-8, IL-17, tumor necrosis factor–α, chemokine (C-C motif) ligand 2, and chemokine (C-X-C motif) ligand 1, than cells from uncomplicated placentas (6). Thus, it is believed that trophoblast MPs contribute significantly to increased maternal vascular inflammatory response in preeclampsia (7).
Recent studies have shown that vitamin D is not only an important nutrient for bone health, but also exerts beneficial effects on multiple organs and systems, including modulation of vascular function and adaptive immunity. Sufficient vitamin D levels are important for both maternal and fetal health, whereas vitamin D deficiency/insufficiency during pregnancy increases the risks of pregnancy disorders, including preeclampsia and preterm delivery (8–10). Our laboratory previously reported that vitamin D exerted anti-inflammatory and antioxidative properties in placental trophoblasts through inhibition of oxidative stress–induced increases in inflammatory cytokine tumor necrosis factor–α production and cyclooxygenase-2 expression (11). To further study beneficial effects of vitamin D on placental trophoblasts, in this study, we focused on MP release and quantified MPs released by trophoblasts from uncomplicated and preeclamptic placentas. We tested the hypothesis that vitamin D could reduce oxidative stress–induced trophoblast MP shedding. To investigate the potential mechanism(s) of vitamin D protective effects on placental trophoblasts, we determined caveolin-1 and endothelial nitric oxide synthase (eNOS) expression in trophoblasts and trophoblast-derived MPs. Apoptosis pathway signaling molecules caspase-3 and Rho-associated coiled-coil protein kinase 1 (ROCK1) expression were also examined.
Materials and Methods
Chemicals and reagents
Dulbecco’s modified Eagle medium, Hanks balanced salt solution, Percoll (P1644), 1,25(OH)2D3 (D1570), ROCK inhibitor Y27632 (Y0503), cytochrome c (C4186), superoxide dismutase (S2515), and β-actin antibody were purchased from Sigma Chemicals (St. Louis, MO). Cobalt chloride (CoCl2, 4532-02) was from Mallinckrodt Chemicals (Phillipsburg, NJ); Phorbol 12-myristate 13-acetate (PMA, P-1680) was from LC Laboratories (Woburn, MA); fetal bovine serum was from Atalantic Biologicals (Flowery Branch, GA); and APC-Annexin V (BD 550474) and annexin V binding buffer (BD 556454) were from BD Biosciences (San Jose, CA). Antibodies for caveolin-1 (N-20), eNOS (C-20, sc-654), ROCK1 (G-6, sc-17794), and procaspase-3 (E-8, sc-7272) were purchased from Santa Cruz Biotechnology (San Diego, CA); antibody for cleaved caspase-3 (Asp175, 5A1E, product 9664) was from Cell Signaling Technology (Danvers, MA); and mouse anti-human eNOS antibody (610297) used for immunofluorescent staining was from BD Transduction Laboratories (Franklin Lakes, NJ). All other chemicals were from Sigma unless otherwise noted.
Placenta collection
Placental collection was approved by the Institutional Review Board at Louisiana State University Health Sciences Center-Shreveport and collected immediately after delivery at the hospital of University Health Shreveport. A total of 31 placentas were used in the study, 25 from uncomplicated and six from preeclamptic pregnancies. Uncomplicated pregnancy is defined as pregnancy with blood pressure <140/90 mm Hg and absence of proteinuria and obstetrical and medical complications. Diagnosis of preeclampsia is defined as follows: sustained systolic blood pressure ≥140 mm Hg or a sustained diastolic blood pressure ≥90 mm Hg on two separate readings, proteinuria measurement of 1+ or more on dipstick, or 24-hour urine protein collection with ≥300 mg in the specimen. Smokers were excluded. None of the pregnant women had signs of infection. Demographic data including maternal age, racial status, gravida, body mass index, blood pressure, gestational age at delivery, and delivery mode are shown in Table 1.
| Variables . | Normal (n = 25) . | Preeclampsia (n = 6) . | P Value . |
|---|---|---|---|
| Maternal age, y | 25 ± 5 (18–35) | 21 ± 2 (19–23) | 0.029 |
| Racial status, n | |||
| White | 5 | 0 | ND |
| Black | 19 | 6 | ND |
| Other | 1 | 0 | ND |
| Body mass index, kg/m2 | 33 ± 7 (21–46) | 38 ± 8 (26–50) | 0.185 |
| Blood pressure, mm Hg | |||
| Systolic | 122 ± 14 (95–140) | 167 ± 8 (156–175) | <0.001 |
| Diastolic | 72 ± 8 (53–85) | 102 ± 8 (92–113) | <0.001 |
| Primigravida, n | 7 | 4 | ND |
| Serum creatinine, mg/dL | 0.61 ± 0.12 (0.34–0.81) | 0.82 ± 0.5 (0.46–1.8) | 0.497 |
| Gestational age at delivery, wk+days | 38+6 ± 1+1 (35+6–40+4) | 37+4 ± 2+1 (35+0–40+4) | 0.160 |
| Delivery mode, n | |||
| Vaginal delivery | 13 | 3 | ND |
| C-section | 12 | 3 | ND |
| Variables . | Normal (n = 25) . | Preeclampsia (n = 6) . | P Value . |
|---|---|---|---|
| Maternal age, y | 25 ± 5 (18–35) | 21 ± 2 (19–23) | 0.029 |
| Racial status, n | |||
| White | 5 | 0 | ND |
| Black | 19 | 6 | ND |
| Other | 1 | 0 | ND |
| Body mass index, kg/m2 | 33 ± 7 (21–46) | 38 ± 8 (26–50) | 0.185 |
| Blood pressure, mm Hg | |||
| Systolic | 122 ± 14 (95–140) | 167 ± 8 (156–175) | <0.001 |
| Diastolic | 72 ± 8 (53–85) | 102 ± 8 (92–113) | <0.001 |
| Primigravida, n | 7 | 4 | ND |
| Serum creatinine, mg/dL | 0.61 ± 0.12 (0.34–0.81) | 0.82 ± 0.5 (0.46–1.8) | 0.497 |
| Gestational age at delivery, wk+days | 38+6 ± 1+1 (35+6–40+4) | 37+4 ± 2+1 (35+0–40+4) | 0.160 |
| Delivery mode, n | |||
| Vaginal delivery | 13 | 3 | ND |
| C-section | 12 | 3 | ND |
Data are expressed as mean ± standard deviation (range) or as otherwise indicated.
Abbreviation: ND, not determined.
| Variables . | Normal (n = 25) . | Preeclampsia (n = 6) . | P Value . |
|---|---|---|---|
| Maternal age, y | 25 ± 5 (18–35) | 21 ± 2 (19–23) | 0.029 |
| Racial status, n | |||
| White | 5 | 0 | ND |
| Black | 19 | 6 | ND |
| Other | 1 | 0 | ND |
| Body mass index, kg/m2 | 33 ± 7 (21–46) | 38 ± 8 (26–50) | 0.185 |
| Blood pressure, mm Hg | |||
| Systolic | 122 ± 14 (95–140) | 167 ± 8 (156–175) | <0.001 |
| Diastolic | 72 ± 8 (53–85) | 102 ± 8 (92–113) | <0.001 |
| Primigravida, n | 7 | 4 | ND |
| Serum creatinine, mg/dL | 0.61 ± 0.12 (0.34–0.81) | 0.82 ± 0.5 (0.46–1.8) | 0.497 |
| Gestational age at delivery, wk+days | 38+6 ± 1+1 (35+6–40+4) | 37+4 ± 2+1 (35+0–40+4) | 0.160 |
| Delivery mode, n | |||
| Vaginal delivery | 13 | 3 | ND |
| C-section | 12 | 3 | ND |
| Variables . | Normal (n = 25) . | Preeclampsia (n = 6) . | P Value . |
|---|---|---|---|
| Maternal age, y | 25 ± 5 (18–35) | 21 ± 2 (19–23) | 0.029 |
| Racial status, n | |||
| White | 5 | 0 | ND |
| Black | 19 | 6 | ND |
| Other | 1 | 0 | ND |
| Body mass index, kg/m2 | 33 ± 7 (21–46) | 38 ± 8 (26–50) | 0.185 |
| Blood pressure, mm Hg | |||
| Systolic | 122 ± 14 (95–140) | 167 ± 8 (156–175) | <0.001 |
| Diastolic | 72 ± 8 (53–85) | 102 ± 8 (92–113) | <0.001 |
| Primigravida, n | 7 | 4 | ND |
| Serum creatinine, mg/dL | 0.61 ± 0.12 (0.34–0.81) | 0.82 ± 0.5 (0.46–1.8) | 0.497 |
| Gestational age at delivery, wk+days | 38+6 ± 1+1 (35+6–40+4) | 37+4 ± 2+1 (35+0–40+4) | 0.160 |
| Delivery mode, n | |||
| Vaginal delivery | 13 | 3 | ND |
| C-section | 12 | 3 | ND |
Data are expressed as mean ± standard deviation (range) or as otherwise indicated.
Abbreviation: ND, not determined.
Trophoblast isolation and culture
Trophoblasts were isolated by trypsin digestion and further purified by Percoll gradient centrifugation as previously described (12) and cultured in six-well plates (5 × 106 cells per well) with Dulbecco’s modified Eagle medium supplemented with fetal bovine serum and antibiotics. At the end of each experiment, MPs were isolated from culture supernatants. Total cellular protein was extracted and used to determine protein expression.
MP isolation and flow cytometry analysis
Trophoblast MPs were isolated from cell culture medium by a two-step centrifugation process as previously described (13). Isolated MPs were (1) labeled with fluorochrome allophycocyanin-conjugated annexin V antibody for flow cytometry analysis; (2) fixed with 2.5% glutaraldehyde for transmission electron microscope (TEM) examination; or (3) lysed for protein expression study.
For flow cytometry analysis, freshly isolated MPs were incubated with APC-Annexin V antibody and then measured by a BD LSR II flow cytometer (BD Biosciences) as previously described (14). TruCount tube from Becton Dickinson (San Diego, CA) with a known number of fluorescent beads was used as an internal control. Data were analyzed using FlowJo cell analysis software (Tree Star, Ashland, OR). MP count was normalized by total cellular protein per well.
Electron microscopy
Cultured trophoblasts and freshly isolated trophoblast MPs were fixed with 2.5% glutaraldehyde for 24 hours, postfixed in 1% osmium tetroxide mixed with 0.8% potassium ferricyanide in 0.1 M cacodylate buffer, and processed as previously described (14). Ultrathin sections were then stained with uranyl acetate–lead citrate solution and examined by a Hitachi H-7650 transmission electron microscope (Hitachi, Tokyo, Japan).
Superoxide generation assay
Trophoblast superoxide generation was determined by cytochrome C reduction assay and measured by a double-beam spectrophotometer (Ultrospec 3000; Pharmacia Biotech, Cambridge, UK) as previously described (15, 16). The amount of superoxide generation was calculated as O2– (nmol) = 47.7 × Asuperoxide, and was normalized by total cellular protein per well.
Immunofluorescent staining
Cells were cultured on glass coverslips in 24-well plates and fixed with ice-cold methanol and permeabilized with 80% acetone. Cells were incubated with primary anti-human caveolin-1 or anti-human eNOS antibody followed by matched Alexa 488- or Cy3-conjugated secondary antibody. After staining, coverslips were mounted on glass slides with Vectashield Mounting Medium with 4′,6-diamidino-2-phenylindole (Vector Laboratory Inc., Burlingame, California) and reviewed under a fluorescent microscope (Olympus IX-71; Olympus, Japan). Images were captured with a digital camera linked to a personal computer with imaging software PictureFrame (Uptronics Inc., Sunnyvale, CA).
Protein expression by Western blot
Trophoblast and MP protein expression for caveolin-1, eNOS, procaspase-3, cleaved caspase-3, and ROCK1 were examined by Western blot. An aliquot of 10 µg of total trophoblast cellular protein or whole lysate of trophoblast MPs isolated per well was subject to electrophoresis. The bound antibody was visualized with an enhanced chemiluminescent detection kit (Amersham Corp., Arlington Heights, IL). The band density was analyzed by Image J software (National Institutes of Health). β-actin expression was determined and used to normalize relative protein expression for trophoblasts.
Statistical analysis
A Mann-Whitney U test was used to compare annexin V+ MP release by trophoblasts from uncomplicated and preeclamptic placentas and demographic data from whom placenta was used in the study. One-way analysis of variance was used to compare effects of CoCl2-induced trophoblast MP release, superoxide generation, and relative protein expression by trophoblasts and MPs in cells treated with CoCl2 in the presence or absence of 1,25(OH)2D3. A Student-Newman-Keuls test was used as a post hoc test. Prism 5 (GraphPad Software, La Jolla, CA) was used for the data analysis. A probability level <0.05 was considered statistically significant. Data are plotted by box and whisker plots in Figures 1–4.
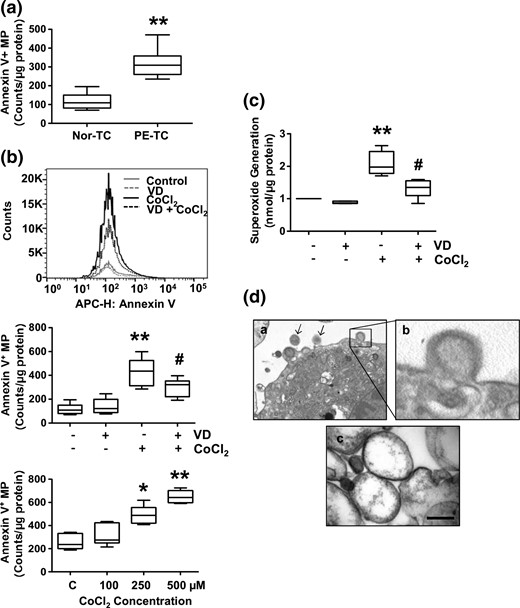
MP release and superoxide generation by placental trophoblast cells. (a) MP shedding by trophoblasts from uncomplicated and preeclamptic placentas. Annexin V+ MPs were assessed by flow cytometry. Data are expressed as counts per microgram of protein per well. Annexin V+ MP shedding was significantly more by trophoblasts from preeclamptic placentas (PE-TC) than those from uncomplicated placentas (Nor-TC). **P < 0.01. (b) CoCl2-induced increase in trophoblast MP shedding could be attenuated when cells were pretreated with 1,25(OH)2D3 (VD). Trophoblasts from uncomplicated placentas were used. The upper panel shows a representative flow cytometry measurement of annexin V+ MPs released by control cells, cells treated with VD, cells treated with CoCl2, and cells treated with VD + CoCl2. APC-H, allophycocyanin-height. The middle panel box and whisker plots show data from six independent experiments. **P < 0.01 (CoCl2 treated versus control); #P < 0.05 (VD + CoCl2 versus CoCl2). The lower panel shows a dose response effect of CoCl2 on MPs release by trophoblasts. *P < 0.05 and **P < 0.01 (CoCl2 treated versus control). Data are from trophoblasts isolated from three placentas, each cultured in duplicate. (c) Consistent with MP shedding, CoCl2-induced increase in trophoblast superoxide generation could also be attenuated when cells were pretreated with 1,25(OH)2D3. **P < 0.01 (CoCl2 treated versus control); #P < 0.05 (VD + CoCl2 versus CoCl2). (d) Trophoblasts and MPs assessed by electron microscopy. Panel a shows an image of trophoblast with membrane blebbing and MPs (magnification, 30,000×; arrows, MPs). Panel b shows and enlargement of the insert of panel a, showing membrane blebbing. Panel c shows an image of MPs, showing that isolated trophoblast MPs are intact and with sizes between 0.1 and 1.0 µm in diameters (magnification, 150,000×; bar, 200 µm).
Results
Trophoblasts from preeclamptic placentas shed more MPs than cells from uncomplicated placentas
It is generally thought that MPs shed from placental trophoblasts play a critical role in the pathogenesis of preeclampsia. Using annexin V as an indicator of shed MPs, we first determined MPs released by placental trophoblasts from uncomplicated and preeclamptic pregnancies. Our results showed that trophoblasts from preeclamptic placentas shed significantly more MPs than cells from uncomplicated placentas (P < 0.01) [Fig. 1(a)].
Vitamin D attenuates oxidative stress–induced increases in MP shedding and superoxide generation by trophoblasts
We then tested if oxidative stress could promote trophoblast MP release and if vitamin D has any effects on MP shedding. In this experiment, trophoblasts isolated from uncomplicated placentas were used. CoCl2 at a concentration of 250 µM was used to induce trophoblast oxidative stress. We choose CoCl2 because CoCl2 is a hypoxic mimetic agent that has been widely used to induce oxidative stress in various tissue and cell culture studies, including trophoblasts (12, 17–20). 1,25(OH)2D3 at a concentration of 50 nM was used as a bioactive vitamin D. We found that MP release was significantly higher by cells treated with CoCl2 than those were not (P < 0.01). In contrast, cells pretreated with 1,25(OH)2D3 plus CoCl2 released significantly less MPs than cells treated with CoCl2 alone (P < 0.05) [Fig. 1(b), upper and middle panels]. A dose response effect of CoCl2 on MP release by trophoblasts was also determined, in which cells were treated with CoCl2 at concentrations of 100, 250, and 500 µM. Our results showed that MP release was dose-dependently increased in cells treated with CoCl2 [Fig. 1(b), lower panel]. Consistent with MP shedding, vitamin D could also inhibit trophoblast superoxide generation induced by CoCl2 [Fig. 1(c)]. These data suggest that vitamin D could not only inhibit CoCl2-induced increase in superoxide generation, but also reduce CoCl2-induced MP shedding by placental trophoblasts.
Cultured trophoblasts and isolated MPs were also examined by electron microscopy. Representative images are shown in Fig. 1(d), which confirms that isolated trophoblast MPs are within the proper size limit (0.1–1.0 µm).
Effects of vitamin D on caveolin-1 and eNOS expression in trophoblasts and trophoblast-derived MPs under oxidative stress
It is known that MP formation/signaling events are associated with lipid rafts (21). Caveolae is a specific lipid raft of the cell membrane. Caveolin-1 is a major caveolae protein and eNOS is present in caveolae. To determine whether altered caveolin-1 and eNOS expression are associated with increased MP release induced by CoCl2, cells were treated with CoCl2 at concentrations of 100, 250, and 500 µM, and caveolin-1 and eNOS expression were examined in trophoblasts and trophoblast-derived MPs. As shown in Fig. 2(a) and (b), caveolin-1 expression was dose-dependently increased in both trophoblasts and trophoblast-derived MPs in cells treated with CoCl2. In contrast, eNOS expression was dose-dependently decreased in trophoblasts, but increased in trophoblast-derived MPs. These data suggest that increased trophoblast caveolin-1 expression and decreased trophoblast eNOS expression are associated with increased MP shedding induced by CoCl2. In addition, because MPs are small membrane-bound vesicles, increased MP caveolin-1 and eNOS expression also suggest that elevated MP release could be closely associated with the degree of oxidative stress insult in placental trophoblasts.
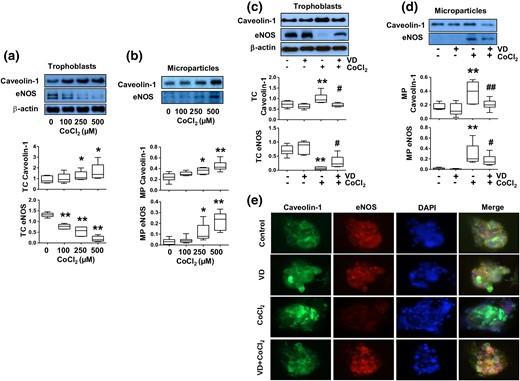
Effects of 1,25(OH)2D3 (VD) on altered caveolin-1 and eNOS expression induced by CoCl2 in trophoblasts and trophoblast-derived MPs. Trophoblasts from uncomplicated pregnancies were used. (a) Protein expression for caveolin-1 and eNOS in trophoblasts treated with different concentrations of CoCl2. The box and whisker plots show relative protein expression after being normalized against β actin from six independent experiments. CoCl2 induced a dose-dependent increase in caveolin-1 expression, but a dose-dependent decrease in eNOS expression in trophoblasts. *P < 0.05; **P < 0.01 (treated versus control cells). (b) Protein expression for caveolin-1 and eNOS in trophoblast-derived MPs from cells treated with different concentrations of CoCl2. The box and whisker plots show relative protein expression after being normalized by total cellular protein per well. MP expression of caveolin-1 and eNOS were dose-dependent increased in cells treated with CoCl2. *P < 0.05; **P < 0.01. (c) Caveolin-1 and eNOS expression in trophoblast cells treated with CoCl2 in the presence or absence of 1,25(OH)2D3. VD prevented CoCl2-induced increased caveolin-1 expression and decreased eNOS expression in trophoblasts. The box and whisker plots show relative protein expression after being normalized against β actin. **P < 0.01 (CoCl2 treated versus control); #P < 0.05 (VD + CoCl2 versus CoCl2 treated). (d) Expression of caveolin-1 and eNOS in trophoblast-derived MPs in cells treated with CoCl2 in the presence or absence of 1,25(OH)2D3. The increased MP caveolin-1 and eNOS expression induced by CoCl2 was reduced when cells were treated with VD. The box and whisker plots show relative protein expression after being adjusted by total cellular protein per well from 6 independent experiments. **P < 0.01 (CoCl2 treated versus control); #P < 0.05 and ##P < 0.01 (VD + CoCl2 versus CoCl2 treated). (e) Representative imaging of immunofluorescent staining of caveolin-1 and eNOS in trophoblasts treated with CoCl2 in the presence or absence of 1,25(OH)2D3. Consistent with Western blot results, VD could inhibit CoCl2-induced increased caveolin-1 expression and decreased eNOS expression in placental trophoblasts.
We next tested effects of vitamin D on caveolin-1 and eNOS expression in trophoblasts and trophoblast-derived MPs in cells treated with CoCl2. In this experiment, CoCl2 at a concentration of 250 µM and 1,25(OH)2D3 at a concentration of 50 nM were used. Our results showed that caveolin-1 and eNOS expression were not different in trophoblasts and trophoblast-derived MPs in control cells and in cells treated with 1,25(OH)2D3 alone. However, CoCl2-induced increased caveolin-1 expression in trophoblasts and trophoblast-derived MPs was significantly reduced when 1,25(OH)2D3 was present in culture (P < 0.05) [Fig. 2(c) and 2(d), respectively]. In contrast, CoCl2-induced decrease in eNOS expression in trophoblasts and increase in eNOS expression in MPs could also be partially inhibited in cells treated with 1,25(OH)2D3 [Fig. 2(c) and 2(d)]. These results were consistent with reduced MP release by cells that were treated with 1,25(OH)2D3 detected by flow cytometry, as shown in Fig. 1(b). Caveolin-1 and eNOS expression were also examined by immunofluorescent staining. Consistent with Western blot data, 1,25(OH)2D3 could inhibit increased caveolin-1 expression and decreased eNOS expression induced by CoCl2 [Fig. 2(e)].
Effects of vitamin D on caspase-3 and ROCK1 expression in trophoblasts and trophoblast-derived MPs under oxidative stress
The mechanism by which vitamin D reduces MP formation/release by trophoblasts under oxidative stress is unclear. It is known that one of the signaling pathways that induces MP formation is caspase-3 activation. Apoptosis-induced caspase-3 cleavage/activation could further activate ROCK1, which would then lead to membrane structure disruption and subsequently to membrane blebbing and MP formation. Because oxidative stress–induced trophoblast apoptosis (22) could be a major cause of increased MP shedding in preeclampsia, we tested if the protective effect of vitamin D on trophoblast MP release is through regulation of caspase-3 and ROCK1 activation. We first determined procaspase-3, cleaved caspase-3, and ROCK1 expression in trophoblasts and trophoblast-derived MPs in cells treated with different doses of CoCl2. As shown in Fig. 3(a) and 3(b), we found that procaspase-3 expression was decreased but cleaved caspase-3 expression was increased dose-dependently in cells treated with CoCl2, indicating that CoCl2 could induce caspase-3 apoptosis pathway activation in trophoblasts. The data on ROCK1 expression is interesting. The antibody for ROCK1 used in the study recognizes both the full length of inactive form at 160 kDa and the cleaved form at 130 kDa. Although total relative ROCK1 expression was reduced in cells treated with CoCl2 [Fig. 3(a)], the reduced inactive form of ROCK1 was correlated with increased cleaved ROCK1 expression in cells treated with CoCl2. Moreover, expression for procaspase-3, cleaved caspase-3, and ROCK1 were all dose-dependently increased in MPs when cells were treated with CoCl2. The ROCK1 band detected in MPs is at 130 kDa, showing that MPs carry cleaved ROCK1. These results suggest that CoCl2-induced oxidative stress could promote caspase-3 cleavage and ROCK1 activation associated with elevated MP release in placental trophoblasts.
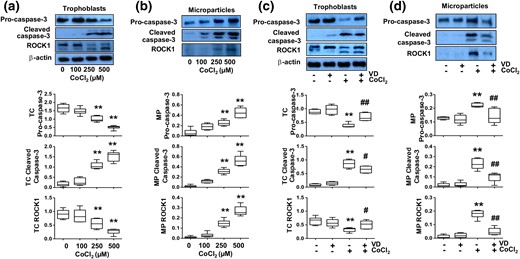
Effects of 1,25(OH)2D3 (VD) on altered caspase-3 and ROCK1 expression induced by CoCl2 in trophoblasts and trophoblast-derived MPs. Trophoblasts from uncomplicated pregnancies were used. (a) Protein expression for procaspase-3, cleaved caspase-3, and ROCK1 in trophoblasts treated with different concentrations of CoCl2. The box and whisker plots show relative expression after being normalized with β actin in each sample. CoCl2 induced a dose-dependent decrease in procaspase-3 expression, but a dose-dependent increase in cleaved caspase-3 expression. Two bands were detected for ROCK1, inactive form at 160 kDa and a cleaved form at 130 kDa. Although expression of inactive form of ROCK1 was reduced, and activated form was increased in cells treated with CoCl2, total ROCK1 expression was also dose-dependent decreased in cells treated with CoCl2. **P < 0.01. (b) Protein expression for procaspase-3, cleaved caspase-3, and ROCK1 expression in trophoblast-derived MPs from trophoblasts treated with different concentrations of CoCl2. The box and whisker plots show relative expression after being normalized with total cellular protein per well. A 130-kDa band for ROCK1 was detected in trophoblast-derived MPs. CoCl2-induced dose-dependent increases in procaspase-3, cleaved caspase-3, and ROCK1 expression in trophoblast-derived MPs. **P < 0.01. (c) Expression of procaspase-3, cleaved caspase-3, and ROCK1 in trophoblast cells treated with CoCl2 in the presence or absence of 1,25(OH)2D3. The box and whisker plots show relative expression after being normalized with β-actin expression. **P < 0.01 (CoCl2 treated versus control); #P < 0.05 and ##P < 0.01 (VD + CoCl2 versus CoCl2 treated). (d) Expression of procaspase-3, cleaved caspase-3, and ROCK1 in trophoblast-derived MPs from cells treated with CoCl2 in the presence or absence of 1,25(OH)2D3. VD inhibited CoCl2-induced increases in procaspase-3, cleaved caspase-3, and ROCK1 expression in trophoblast-derived MPs. The box and whisker plots show relative protein expression after being adjusted by total cellular protein per well. **P < 0.01 (CoCl2 treated versus control); ##P < 0.01 (VD + CoCl2 versus CoCl2 treated). Data are from six independent experiments.
We further determined effects of 1,25(OH)2D3 on CoCl2-induced caspase-3 cleavage and ROCK1 activation. As expected, we found that cells treated with 1,25(OH)2D3 showed less caspase-3 cleavage and ROCK1 activation than cells treated with CoCl2 alone [Fig. 3(c)]. Expression of procaspase-3, cleaved caspase-3, and cleaved ROCK1 were all reduced in trophoblast-derived MPs from cells treated with 1,25(OH)2D3 plus CoCl2 [Fig. 3(d)]. These data demonstrate that vitamin D could protect trophoblasts from oxidative stress–induced caspase-3 cleavage and ROCK1 activation.
Attenuation of ROCK1 activation inhibits MP release by placental trophoblasts
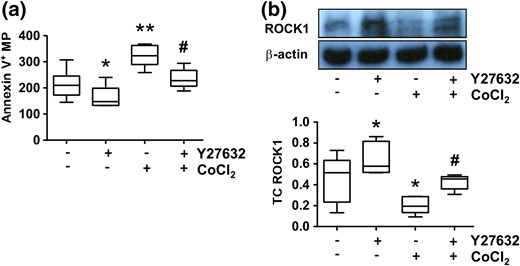
Effects of ROCK1 inhibitor on MP release and ROCK1 expression by placental trophoblasts. Trophoblasts from uncomplicated pregnancies were used. (a) Effects of ROCK1 inhibitor Y27632 on trophoblast MP shedding. Trophoblasts were treated with CoCl2 in the presence or absence of Y27632. Annexin V+ MPs were assessed by flow cytometry, and MP counts were normalized by total cellular protein. Cells treated with Y27632 released significantly less annexin V+ MPs in both control cells and in cells treated with CoCl2. *P < 0.05 and **P < 0.01 (treated versus control); #P < 0.05 (Y27632 + CoCl2 versus CoCl2 alone). (b) ROCK1 expression in trophoblasts treated with CoCl2 in the presence or absence of Y27632. The box and whisker plots show relative expression after being normalized with β-actin expression in each sample. Y27632 could preserve ROCK1 expression in trophoblasts. *P < 0.05 (treated versus control); #P < 0.05 (Y27632 + CoCl2 versus CoCl2 treated alone). Data are from six independent experiments. Increased ROCK1 expression was correlated with reduced MP release in Y27632-treated cells with or without CoCl2 treatment.
Discussion
In this study, we demonstrated that trophoblasts from preeclamptic placentas shed significantly more MPs than cells from uncomplicated placentas, which provide further evidence of placental trophoblast dysfunction in preeclampsia. It is widely accepted that trophoblast-derived MPs play substantial roles in inducing exaggerated maternal systemic inflammatory response in preeclampsia (23, 24). In contrast with syncytial knots and larger debris shed by trophoblasts, which could elicit a local inflammatory reaction by phagocytosis of endometrial endothelial cells or being trapped in the pulmonary capillary (25, 26), trophoblast MPs can pass through the lung capillary and reach the maternal peripheral circulation, where they directly act on maternal endothelial and immune cells (1) to produce systemic inflammatory response. MPs from preeclamptic placentas inducing increases in proinflammatory cytokine and chemokine generation by peripheral blood mononuclear cells has been demonstrated (6). Therefore, it is considered that a lesser amount of trophoblast MP shedding might diminish systemic inflammation response and reduce vascular damage in preeclampsia.
Because increased trophoblast oxidative stress is one of the most substantial pathophysiologic events in preeclamptic placentas and vitamin D has been considered to have beneficial effects in pregnancy, in this study we specifically investigated if vitamin D exerts protective effects on placental trophoblasts through inhibition of oxidative stress–induced MP release. Using CoCl2 as an oxidative stress inducer, we found that CoCl2 could stimulate trophoblast MP shedding and superoxide generation, and the CoCl2-induced increased MP shedding and superoxide generation could be attenuated when trophoblasts were pretreated with 1,25(OH)2D3. These results suggest that increased MP shedding is associated with increased oxidative stress in placental trophoblasts. These data also suggest that vitamin D could protect trophoblasts from oxidative stress–induced MP shedding.
MPs form when cells lose asymmetric distribution of membrane lipids between the inner and outer leaflets of a plasma membrane and shed MPs are composed of a phospholipid bilayer containing transmembrane proteins, receptors, enzymes, and messenger RNA derived from their parent cells (27). To study cell membrane components that are involved in MP formation and the potential mechanism(s) that vitamin D protects trophoblasts from oxidative stress–induced MP shedding, we specifically determined membrane-associated proteins caveolin-1 and eNOS expression in trophoblasts and trophoblast-derived MPs. We also examined potential roles of vitamin D in CoCl2-mediated caspase-3 cleavage and ROCK1 activation in trophoblasts and trophoblast-derived MPs. Several substantial findings were made.
First, we found that caveolin-1 expression was dose-dependently increased in both trophoblasts and trophoblast-derived MPs in cells treated with CoCl2. In contrast, eNOS expression was dose-dependently decreased in trophoblasts, but dose-dependently increased in trophoblast-derived MPs in cells treated with CoCl2. Caveolin-1 is the major scaffolding protein of caveolae, which is an important membrane lipid raft, and functions as the center of signal transduction and endocytosis. Both caveolin-1 and eNOS are detected in the cell membrane of trophoblasts (28, 29). Because MPs are the bud of the plasma membrane derived from their parent cells, upregulated caveolin-1 and eNOS expression in trophoblast-derived MPs, along with reduced eNOS expression in trophoblasts that were treated with CoCl2, provide considerable evidence that oxidative stress–induced increase in MP shedding is associated with altered caveolin-1 and eNOS expression in trophoblasts. Although little is known about the relationship of caveolin-1 and eNOS in trophoblasts, specific interactions between caveolin and eNOS have been reported in endothelial cells and cardiac myocytes (30). There are two cytoplasmic domains of caveolin-1 that could interact with eNOS, one at the N-terminal oligomerization domain and the other at the C-terminal tail (31, 32). A study also revealed that caveolin-1 could inhibit eNOS activity via a direct binding between a scaffolding domain in caveolin-1 and a motif in the oxygenase domain in eNOS (32). In addition, functional association of eNOS and caveolin-1 was also reported by Ju et al. (33). Using a yeast two-hybrid system, these investigators found that eNOS catalytic activity was significantly inhibited when eNOS was interacted with caveolin-1 (33), indicating that caveolin-1 could regulate eNOS functionality when their physiologic relationship was interrupted. The direct interaction of eNOS with the structural protein caveolin-1 provides a biochemical rationale for the association of eNOS/caveolin-1 in the regulation of cellular function (30). In addition, upregulation of caveolin-1 expression associated with increased apoptosis was also reported in macrophages (34). Although, we did not specifically study the interaction of caveolin-1 and eNOS in trophoblasts and trophoblast-derived MPs, our data of upregulation of caveolin-1 expression and downregulation of eNOS expression in cells challenged with CoCl2 support the concept that upregulation of caveolin-1 expression could lead to downregulation of eNOS expression in placental trophoblasts, which might be involved in trophoblast apoptosis induced by increased oxidative stress. Nonetheless, the findings of 1,25(OH)2D3 suppression of CoCl2-induced increase in trophoblast caveolin-1 expression and decrease in eNOS expression suggest that vitamin D could modulate the interaction of caveolin-1 and eNOS related to the MP shedding by trophoblasts.
Preservation of eNOS in cells treated with CoCl2 by 1,25(OH)2D3 is another important finding in our study. Similar phenomenon was also observed in endothelial cells treated with 1,25(OH)2D3 when cells were cultured under a lowered oxygen condition (14). It is known that most of the biologic actions of vitamin D are initiated by activation of its receptor, vitamin D receptor (VDR). We previously reported that VDR is strongly expressed in trophoblasts of uncomplicated placentas, but significantly reduced in trophoblasts of preeclamptic placentas (12). We also demonstrated that altered protein expression for vitamin D metabolic system molecules, including VDR, 25-hydroxylase, 1,25-hydroxylase, and 24-hydroxylase, found in trophoblasts of preeclamptic placentas could be produced by treatment of trophoblasts from uncomplicated placentas with hypoxia mimic agent CoCl2 (12). Although we did not test the relationship of VDR and eNOS in trophoblasts in the current study, upregulation of eNOS expression induced by 1,25(OH)2D3 could be caused by the presence of VDR element in eNOS promoter (35). Therefore, it is expected that modulation of eNOS by 1,25(OH)2D3 may promote eNOS function associated with inhibition of MP shedding by trophoblasts.
Vitamin D offsetting oxidative stress–induced caspase-3 cleavage and ROCK1 activation in trophoblast cells is the most important finding in our study. It is known that caspases are crucial mediators of programmed cell death (apoptosis), and caspase-3 is identified as a key indicator of cell apoptosis. ROCK1 activation could lead to disruption of membrane skeleton structure, membrane blebbing, and subsequent MP formation (36, 37). We examined procaspase-3, cleaved caspase-3, and ROCK1 expression in trophoblasts and trophoblast-derived MPs and found that CoCl2 induced a dose-dependent decrease in procaspase-3 expression and a dose-dependent increase in cleaved caspase-3 expression were paralleled with reduced inactive ROCK1 expression and increased cleaved ROCK1 expression in trophoblasts. In addition, expression of procaspase-3, cleaved caspase-3, and cleaved ROCK1 were all dose-dependently increased in trophoblast-derived MPs. These data indicate (1) oxidative stress could activate caspase-3 and ROCK1 signaling pathway molecules in placental trophoblasts, and (2) shed MPs are enriched with cleaved caspase-3 and activated ROCK1. Chang et al. (38) reported that caspase-3–induced ROCK1 activation played an essential role in cardiomyocyte apoptosis. ROCK1-mediated membrane blebbing and MP shedding were also reported in various cell types, including lymphoma cells (38, 39). Although we did not test consequences of shed MPs in the current study, there is no doubt that in an in vivo situation, such as in preeclampsia, increased shedding of caspase-3, and ROCK1-enriched trophoblast MPs would provoke endothelial cell, platelet, and leukocyte activation; promote inflammatory cytokine production; and subsequently induce vascular dysfunction. Strikingly, the changes in procaspase-3, cleaved caspase-3, and ROCK1 expression induced by CoCl2 in trophoblasts and trophoblast-derived MPs could all be partially attenuated by 1,25(OH)2D3, indicating that 1,25(OH)2D3 could prevent caspase-3 cleavage and ROCK1 activation associated with MP shedding in placental trophoblasts. This notion is further supported by the findings of repression of CoCl2-induced increased MP shedding and ROCK1 activation by ROCK1 inhibitor Y27632.
It has been well accepted that vitamin D insufficiency/deficiency during pregnancy is associated with increased risk of preeclampsia (8, 9, 40). However, reports from clinical trials and epidemiologic studies on vitamin D supplement in prevention or decrease of the incidence of preeclampsia are inconsistent. Some have found that vitamin D supplementation could reduce the incidence of preeclampsia (41, 42), but others have not (43, 44). The inconsistency could be because of heterogeneous factors, such as different gestational ages at a trial entry, different doses of vitamin D intake, and different metabolic responses to vitamin D in individuals during pregnancy. A recent study by Mirzakhani et al. (43) is interesting. Although no effect of vitamin D supplement on the incidence of preeclampsia was found, further analysis revealed that women who had sufficient vitamin D levels (at least 30 ng/mL) in both early and late pregnancy, regardless of treatment group, showed a significantly lower incidence of preeclampsia as compared with those who had insufficient levels at these time points. Moreover, these investigators also found that no occurrence of preeclampsia was noted in women with baseline serum vitamin D value > 37 ng/mL. These data suggest that baseline levels of vitamin D in early pregnancy or before pregnancy may be critical for preeclampsia development. It is known that the function of vitamin D is regulated via its receptor VDR, and people with VDR polymorphisms often have low levels of vitamin D (45, 46). In addition, VDR polymorphisms are also found to be associated with several diseases, including cardiovascular diseases, type II diabetes, and cancer (47–49). We previously reported that VDR expression is significantly reduced in placental trophoblasts from women with preeclampsia (12). Using DNA extracted from maternal blood, Zhan et al. (50) did find VDR gene variation is associated with preeclampsia. However, no information is available to show if VDR polymorphisms are present in placental trophoblasts in preeclampsia, which warrants further investigation. This may answer the question if VDR polymorphisms could predict response-to-treatment in vitamin D trials.
In summary, this study demonstrated vitamin D protective effects on oxidative stress–mediated increases in MP shedding and superoxide generation by placental trophoblasts. These findings are important because oxidative stress–induced trophoblast dysfunction and increased MP shedding are believed to play a fundamental role in placental pathology and systemic vascular dysfunction in preeclampsia. Our data also suggest that regulation of caveolin-1 and eNOS expression/function and inhibition of caspase-3 cleavage and ROCK1 activation could be a plausible cellular mechanism of vitamin D on suppression of MP shedding by placental trophoblasts. More in-depth studies of vitamin D function on placental trophoblast cells are needed to better understand the protective role of vitamin D in pregnancy.
Abbreviations:
- eNOS
endothelial nitric oxide synthase
- IL
interleukin
- MP
microparticle
- ROCK1
Rho-associated coiled-coil protein kinase 1
- VDR
vitamin D receptor.
Acknowledgments
This work was supported in part by the National Institutes of Health, National Institute of Child Health and Human Development Grant R21HD076289 (to Y.W.).
Disclosure Summary: The authors have nothing to disclose.
References
Author notes
Address all correspondence and requests for reprints to: Yuping Wang, MD, PhD, Departments of Obstetrics and Gynecology, Louisiana State University Health Sciences Center–Shreveport, Shreveport, Louisiana 71130. E-mail: [email protected].



