-
PDF
- Split View
-
Views
-
Cite
Cite
Margaret F. Lippincott, Yee-Ming Chan, Dianali Rivera Morales, Stephanie B. Seminara, Continuous Kisspeptin Administration in Postmenopausal Women: Impact of Estradiol on Luteinizing Hormone Secretion, The Journal of Clinical Endocrinology & Metabolism, Volume 102, Issue 6, 1 June 2017, Pages 2091–2099, https://doi.org/10.1210/jc.2016-3952
Close - Share Icon Share
Abstract
Kisspeptin stimulates the reproductive endocrine cascade in both men and women. Circulating sex steroids are thought to modulate the ability of kisspeptin to stimulate gonadotropin-releasing hormone (GnRH)–induced luteinizing hormone (LH) release.
To probe the effects of sex steroids on kisspeptin-stimulated GnRH-induced LH pulses.
Eight healthy postmenopausal women.
Subjects underwent every-10-minute blood sampling to measure GnRH-induced LH secretion at baseline and in response to a continuous kisspeptin infusion (12.5 µg/kg/h) over 24 hours. A subset of the participants also received kisspeptin (0.313 µg/kg) and GnRH (75 ng/kg) intravenous boluses.
Postmenopausal women are resistant to the stimulatory effect of continuous kisspeptin on LH secretion. Postmenopausal women receiving estradiol replacement therapy are also resistant to kisspeptin initially, but they demonstrate a significant increase in LH pulse amplitude in direct proportion to the circulating estradiol concentration and duration of kisspeptin administration.
Kisspeptin administration has complex effects on GnRH, and by extension, on LH secretion. The ability of kisspeptin to affect LH secretion can be modulated by the ambient sex-steroid milieu in a time- and dose-dependent manner.
Kisspeptin plays a key role in the stimulation of the reproductive endocrine cascade in both men and women. Kisspeptin stimulates gonadotropin-releasing hormone (GnRH)–induced LH secretion across mammalian species (1–5). Both adult (3, 6–9) and prepubertal animals (10–12) respond to single-bolus administration of kisspeptin; moreover, intermittent kisspeptin administration can advance the timing of sexual maturation (13).
Despite a plethora of studies utilizing kisspeptin administration, basic questions remain regarding the manner in which kisspeptin stimulates GnRH neurons and the factors that influence kisspeptin signaling. Although initial reports demonstrated that kisspeptin behaves as a powerful stimulus for GnRH secretion, subsequent studies have demonstrated that kisspeptin fails to stimulate GnRH in particular physiologic and pathophysiologic states. For example, women in the luteal and periovulatory phase of the menstrual cycle respond robustly to a single intravenous (IV) bolus of kisspeptin, but women in the early to midfollicular phase do not (14, 15). The reason for this is unclear; one possibility is that prevailing sex-steroid levels modulate the ability of kisspeptin to stimulate GnRH neurons, with higher estrogen concentrations facilitating and low estrogen levels inhibiting kisspeptin-induced LH responses (16).
To probe the effects of sex steroids on kisspeptin-stimulated GnRH-induced LH pulses, we administered kisspeptin to postmenopausal women (PMW), in whom estradiol concentrations are uniformly low at baseline and to whom we could administer exogenous estradiol in a classic replacement paradigm. We used a high-dose, 24-hour continuous kisspeptin infusion so that we could evaluate both immediate and delayed effects of the kisspeptin infusion on LH release. A subset of women received boluses of GnRH, kisspeptin, or both to determine whether estradiol exerted modulatory effects at the level of the hypothalamus or the pituitary.
Materials and Methods
Subjects and eligibility criteria
This study was approved by the Institutional Review Board of Massachusetts General Hospital (MGH)/Partners Health Care, and all subjects gave written informed consent. A total of eight healthy PMW participated in this study. The average age of the women was 57.8 ± 1.0 years (mean ± standard error of the mean), and the average body mass index was 24.2 ± 1.4 kg/m2. All subjects had normal timing of puberty, regular menses during their reproductive years, and a normal timing of menopause. All women had a normal physical examination, including normal secondary sexual characteristics and normal baseline laboratories (complete blood count, electrolytes, renal function, liver function, prolactin, and thyroid studies).
Study design
Subjects were admitted to the Harvard Catalyst Clinical Research Center of MGH for a 36-hour protocol (Fig. 1) starting at 8:00 am in the morning. During the first six hours of the study, subjects underwent every-10-minute blood sampling to assess baseline endogenous LH pulsatility in the absence of any peptide administration. Six hours into the study, a kisspeptin infusion (12.5 μg/kg/h IV) was initiated, and every-10-minute blood sampling continued. This infusion continued for the next 24 hours. At hour 30 of the study, the kisspeptin infusion was discontinued. Every-10-minute blood sampling continued for another 6 hours, so that the study concluded after 36 hours of blood sampling. To keep within safety guidelines for blood drawing, blood sampling was performed once every hour from hours 12 to 22 (Fig. 1).
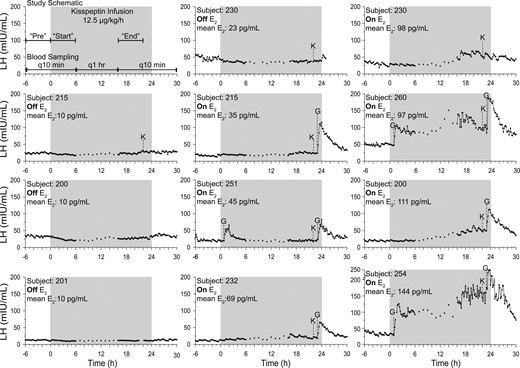
Eleven studies from eight PMW undergoing every-10-minute blood sampling and 24-hour kisspeptin infusions of 12.5 μg/kg/h (gray shaded box). G, 75 ng/kg GnRH by IV bolus; K, 0.313 μg/kg kisspeptin by IV bolus; mean E2, mean serum estradiol across study.
Four subjects (nos. 200, 201, 215, and 230) completed this protocol in the absence of any hormone treatment (−E2). Seven women (subjects no. 200, 215, 230, 232, 251, 254, and 260) performed the protocol during the 13th and 14th days of a 2-week treatment with transdermal estradiol (+E2, 50 to 100 μg/d), with three women (subjects no. 200, 215, and 230) completing both arms (−E2 and +E2).
At the beginning and/or end of the kisspeptin infusion, kisspeptin and/or GnRH IV boluses were administered to a subset of PMW with the goal of testing the functional integrity of the hypothalamic GnRH neurons and pituitary gonadotrophs (details are shown in Fig. 1).
One and 23 hours after the initiation of the infusion, a subset of women received IV GnRH (75 ng/kg); these boluses were administered to assess changes in pituitary sensitivity from the beginning to the end of the infusion. Twenty-two hours into the kisspeptin infusion, a subset of women received IV kisspeptin [0.313 µg/kg (which equals 0.24 mg/kg)]; this bolus was administered while the infusion was ongoing to test for desensitization to kisspeptin.
Kisspeptin and GnRH were synthesized by PolyPeptide Laboratories (San Diego, CA), and the 10-amino-acid isoform of kisspeptin (corresponding to amino acids 112 to 121 of the preprohormone) was used in these studies.
Laboratory assays and pulse analysis
During outpatient screening, study subjects had laboratory testing for follicle-stimulating hormone (FSH), LH, and estradiol performed at LabCorp using the Elecsys 1010/2010 system with Modular Analytics E170 (Roche Diagnostics, Indianapolis, IN). During inpatient studies, LH was measured at each time point and estradiol was measured on pools of samples collected over 2-hour windows using previously described assays (17–19). Both the MGH Core Laboratory and the University of Virginia Core Laboratory processed inpatient LH and estradiol samples. Seventy-five of these samples were sent to both laboratories for LH; r2 correlation between the two laboratories was 90% with no significant deviation from linearity. LH values in this article are reported on the basis of the MGH Core Laboratory values. The conversion between these two laboratories by linear regression over the range of values is [University of Virginia Core] = 1.103 × [MGH Core] − 4.841. A validated modification of the method of Santen and Bardin (18) was used to identify LH pulses. Three defined time points were examined to evaluate the effect of kisspeptin on LH secretion. Mean serum LH, mean LH pulse amplitude, and LH pulse frequency were calculated for all three time ranges: preinfusion (6 hours prior to the start of the kisspeptin infusion), the first 6-hour infusion at the start of the infusion, and 6 hours at the end of the infusion (hours 16 to 22 of the infusion) prior to administration of GnRH or kisspeptin boluses (hours 22 to 24 of the infusion). The last 2 hours were excluded, given that subjects received a kisspeptin or GnRH bolus that could confound the analysis.
Statistics
All values are represented as mean ± standard deviation unless otherwise noted. LH levels, LH pulse amplitude, and LH pulse frequency were calculated for the three 6-hour time periods (preinfusion, start, and end prior to bolus infusion). One-way analysis of variance was used to examine the effect of time on LH levels and LH pulse frequency across study subjects with post hoc Tukey’s multiple comparison test. For analysis of variance for LH levels, missing time points were filled in with the average value from that time period. Paired t tests were used to examine LH amplitude between time periods across study subjects. Linear regression was used to establish the relationship between LH levels, LH pulse amplitude, and LH pulse frequency and estradiol.
Results
Subject characteristics
All PMW (n = 8) had normal age at menopause, were studied within 13 years of natural menopause, had normal body mass index, and had the expected neuroendocrine signature of low estradiol and elevated gonadotropins (Table 1).
| . | No Estradiol Group (−E2; n = 4) . | Estradiol Group (+E2; n = 7) . |
|---|---|---|
| Mean ± SEM (Range) . | Mean ± SEM (Range) . | |
| Chronological age, y | 59.3 ± 0.4 (58.3–60.2) | 57.9 ± 1.2 (53.4–60.9) |
| Age since menopause, y | 9.7 ± 1.3 (6.7–12.9) | 6.6 ± 1.6 (1.2–12.7) |
| Age at menopause, y | 49.6 ± 1.3 (47.3–53) | 51.3 ± 1.1 (49–56) |
| BMI, kg/m2 | 26.3 ± 2.3 (19.4–29.3) | 23.6 ± 1.5 (19.3–29.7) |
| LH, mIU/mL | 38.8 ± 6.2 (20.9–49.2) | 55.0 ± 6.6 (35.7–80) |
| FSH,a mIU/mL | 69.2 ± 8.5 (44.6–83.5) | 97.5 ± 8.1 (73.3–130.1) |
| Estradiol, pg/mL | 7.4 ± 1.1 (6–10.5) | 9.4 ± 1.5 (6–15.2) |
| . | No Estradiol Group (−E2; n = 4) . | Estradiol Group (+E2; n = 7) . |
|---|---|---|
| Mean ± SEM (Range) . | Mean ± SEM (Range) . | |
| Chronological age, y | 59.3 ± 0.4 (58.3–60.2) | 57.9 ± 1.2 (53.4–60.9) |
| Age since menopause, y | 9.7 ± 1.3 (6.7–12.9) | 6.6 ± 1.6 (1.2–12.7) |
| Age at menopause, y | 49.6 ± 1.3 (47.3–53) | 51.3 ± 1.1 (49–56) |
| BMI, kg/m2 | 26.3 ± 2.3 (19.4–29.3) | 23.6 ± 1.5 (19.3–29.7) |
| LH, mIU/mL | 38.8 ± 6.2 (20.9–49.2) | 55.0 ± 6.6 (35.7–80) |
| FSH,a mIU/mL | 69.2 ± 8.5 (44.6–83.5) | 97.5 ± 8.1 (73.3–130.1) |
| Estradiol, pg/mL | 7.4 ± 1.1 (6–10.5) | 9.4 ± 1.5 (6–15.2) |
Abbreviations: BMI, body mass index; SEM, standard error of the mean.
FSH values in group that received estradiol (+E2) compared with those who received no estradiol (−E2); P = 0.04.
| . | No Estradiol Group (−E2; n = 4) . | Estradiol Group (+E2; n = 7) . |
|---|---|---|
| Mean ± SEM (Range) . | Mean ± SEM (Range) . | |
| Chronological age, y | 59.3 ± 0.4 (58.3–60.2) | 57.9 ± 1.2 (53.4–60.9) |
| Age since menopause, y | 9.7 ± 1.3 (6.7–12.9) | 6.6 ± 1.6 (1.2–12.7) |
| Age at menopause, y | 49.6 ± 1.3 (47.3–53) | 51.3 ± 1.1 (49–56) |
| BMI, kg/m2 | 26.3 ± 2.3 (19.4–29.3) | 23.6 ± 1.5 (19.3–29.7) |
| LH, mIU/mL | 38.8 ± 6.2 (20.9–49.2) | 55.0 ± 6.6 (35.7–80) |
| FSH,a mIU/mL | 69.2 ± 8.5 (44.6–83.5) | 97.5 ± 8.1 (73.3–130.1) |
| Estradiol, pg/mL | 7.4 ± 1.1 (6–10.5) | 9.4 ± 1.5 (6–15.2) |
| . | No Estradiol Group (−E2; n = 4) . | Estradiol Group (+E2; n = 7) . |
|---|---|---|
| Mean ± SEM (Range) . | Mean ± SEM (Range) . | |
| Chronological age, y | 59.3 ± 0.4 (58.3–60.2) | 57.9 ± 1.2 (53.4–60.9) |
| Age since menopause, y | 9.7 ± 1.3 (6.7–12.9) | 6.6 ± 1.6 (1.2–12.7) |
| Age at menopause, y | 49.6 ± 1.3 (47.3–53) | 51.3 ± 1.1 (49–56) |
| BMI, kg/m2 | 26.3 ± 2.3 (19.4–29.3) | 23.6 ± 1.5 (19.3–29.7) |
| LH, mIU/mL | 38.8 ± 6.2 (20.9–49.2) | 55.0 ± 6.6 (35.7–80) |
| FSH,a mIU/mL | 69.2 ± 8.5 (44.6–83.5) | 97.5 ± 8.1 (73.3–130.1) |
| Estradiol, pg/mL | 7.4 ± 1.1 (6–10.5) | 9.4 ± 1.5 (6–15.2) |
Abbreviations: BMI, body mass index; SEM, standard error of the mean.
FSH values in group that received estradiol (+E2) compared with those who received no estradiol (−E2); P = 0.04.
PMW −E2: response to 24-hour kisspeptin infusion
Four PMW received a kisspeptin infusion in the absence of sex-steroid addback. In response to the initiation of the kisspeptin infusion, LH concentrations failed to increase (Fig. 1; Supplemental Fig. 1). In contrast to the expected robust increase in serum LH, mean serum LH demonstrated a modest decline from preinfusion levels (mean LH: pre: 29.3 ± 14.8 mIU/mL; start: 22.9 ± 10.6 mIU/mL; P < 0.0001) with a marked decrease in mean LH pulse amplitude (pre: 5.6 mIU/mL; start: 2.5 mIU/mL; P = 0.005). There was no significant change in LH pulse frequency (mean no. of pulses in 6 hours: pre: 5.8; start 4.3; P = 0.4) (Table 2; Fig. 2).
. | −E2 (n = 4) . | +E2 <55 pg/mL (n = 2) . | +E2 >55 pg/mL (n = 3) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Pre . | Start . | End . | Pre . | Start . | End . | Pre . | Start . | End . |
| Clock time | 0800–1400, day 1 | 1400–2000, day 1 | 0600–1200, day 2 | 0800–1400, day 1 | 1400–2000, day 1 | 0600–1200, day 2 | 0800–1400, day 1 | 1400–2000, day 1 | 0600–1200, day 2 |
| Hours of infusion | −6 to 0 | 0–6 | 16–22 | −6 to 0 | 0–6 | 16–22 | −6 to 0 | 0–6 | 16–22 |
| Mean LH (mIU/mL) | 29.3 ± 14.8 | 22.9 ± 10.5 | 24.7 ± 10.7 | 18.9 ± 3.1 | — | 21.8 ± 2.9 | 20.5 ± 9.6 | 20.5 ± 6.3 | 43.3 ± 18.6 |
| LH pulse amplitude (mIU/mL) | 5.9 ± 3.5 | 2.5 ± 1.4 | 3.5 ± 1.5 | 4.9 ± 2.3 | — | 4.1 ± 1.4 | 4.9 ± 1.4 | 4.0 ± 2.0 | 11.3 ± 6.0 |
| LH pulse frequency (no. of pulses in 6 h) | 5.8 ± 1.0 | 4.3 ± 1.0 | 5.3 ± 2.2 | 4.5 ± 0.7 | — | 5.5 ± 0.7 | 4.0 ± 0.0 | 4.3 ± 0.6 | 4.0 ± 0.0 |
. | −E2 (n = 4) . | +E2 <55 pg/mL (n = 2) . | +E2 >55 pg/mL (n = 3) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Pre . | Start . | End . | Pre . | Start . | End . | Pre . | Start . | End . |
| Clock time | 0800–1400, day 1 | 1400–2000, day 1 | 0600–1200, day 2 | 0800–1400, day 1 | 1400–2000, day 1 | 0600–1200, day 2 | 0800–1400, day 1 | 1400–2000, day 1 | 0600–1200, day 2 |
| Hours of infusion | −6 to 0 | 0–6 | 16–22 | −6 to 0 | 0–6 | 16–22 | −6 to 0 | 0–6 | 16–22 |
| Mean LH (mIU/mL) | 29.3 ± 14.8 | 22.9 ± 10.5 | 24.7 ± 10.7 | 18.9 ± 3.1 | — | 21.8 ± 2.9 | 20.5 ± 9.6 | 20.5 ± 6.3 | 43.3 ± 18.6 |
| LH pulse amplitude (mIU/mL) | 5.9 ± 3.5 | 2.5 ± 1.4 | 3.5 ± 1.5 | 4.9 ± 2.3 | — | 4.1 ± 1.4 | 4.9 ± 1.4 | 4.0 ± 2.0 | 11.3 ± 6.0 |
| LH pulse frequency (no. of pulses in 6 h) | 5.8 ± 1.0 | 4.3 ± 1.0 | 5.3 ± 2.2 | 4.5 ± 0.7 | — | 5.5 ± 0.7 | 4.0 ± 0.0 | 4.3 ± 0.6 | 4.0 ± 0.0 |
Mean LH, LH pulse amplitude, and LH pulse frequency values are given as mean ± standard deviation.
. | −E2 (n = 4) . | +E2 <55 pg/mL (n = 2) . | +E2 >55 pg/mL (n = 3) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Pre . | Start . | End . | Pre . | Start . | End . | Pre . | Start . | End . |
| Clock time | 0800–1400, day 1 | 1400–2000, day 1 | 0600–1200, day 2 | 0800–1400, day 1 | 1400–2000, day 1 | 0600–1200, day 2 | 0800–1400, day 1 | 1400–2000, day 1 | 0600–1200, day 2 |
| Hours of infusion | −6 to 0 | 0–6 | 16–22 | −6 to 0 | 0–6 | 16–22 | −6 to 0 | 0–6 | 16–22 |
| Mean LH (mIU/mL) | 29.3 ± 14.8 | 22.9 ± 10.5 | 24.7 ± 10.7 | 18.9 ± 3.1 | — | 21.8 ± 2.9 | 20.5 ± 9.6 | 20.5 ± 6.3 | 43.3 ± 18.6 |
| LH pulse amplitude (mIU/mL) | 5.9 ± 3.5 | 2.5 ± 1.4 | 3.5 ± 1.5 | 4.9 ± 2.3 | — | 4.1 ± 1.4 | 4.9 ± 1.4 | 4.0 ± 2.0 | 11.3 ± 6.0 |
| LH pulse frequency (no. of pulses in 6 h) | 5.8 ± 1.0 | 4.3 ± 1.0 | 5.3 ± 2.2 | 4.5 ± 0.7 | — | 5.5 ± 0.7 | 4.0 ± 0.0 | 4.3 ± 0.6 | 4.0 ± 0.0 |
. | −E2 (n = 4) . | +E2 <55 pg/mL (n = 2) . | +E2 >55 pg/mL (n = 3) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Pre . | Start . | End . | Pre . | Start . | End . | Pre . | Start . | End . |
| Clock time | 0800–1400, day 1 | 1400–2000, day 1 | 0600–1200, day 2 | 0800–1400, day 1 | 1400–2000, day 1 | 0600–1200, day 2 | 0800–1400, day 1 | 1400–2000, day 1 | 0600–1200, day 2 |
| Hours of infusion | −6 to 0 | 0–6 | 16–22 | −6 to 0 | 0–6 | 16–22 | −6 to 0 | 0–6 | 16–22 |
| Mean LH (mIU/mL) | 29.3 ± 14.8 | 22.9 ± 10.5 | 24.7 ± 10.7 | 18.9 ± 3.1 | — | 21.8 ± 2.9 | 20.5 ± 9.6 | 20.5 ± 6.3 | 43.3 ± 18.6 |
| LH pulse amplitude (mIU/mL) | 5.9 ± 3.5 | 2.5 ± 1.4 | 3.5 ± 1.5 | 4.9 ± 2.3 | — | 4.1 ± 1.4 | 4.9 ± 1.4 | 4.0 ± 2.0 | 11.3 ± 6.0 |
| LH pulse frequency (no. of pulses in 6 h) | 5.8 ± 1.0 | 4.3 ± 1.0 | 5.3 ± 2.2 | 4.5 ± 0.7 | — | 5.5 ± 0.7 | 4.0 ± 0.0 | 4.3 ± 0.6 | 4.0 ± 0.0 |
Mean LH, LH pulse amplitude, and LH pulse frequency values are given as mean ± standard deviation.
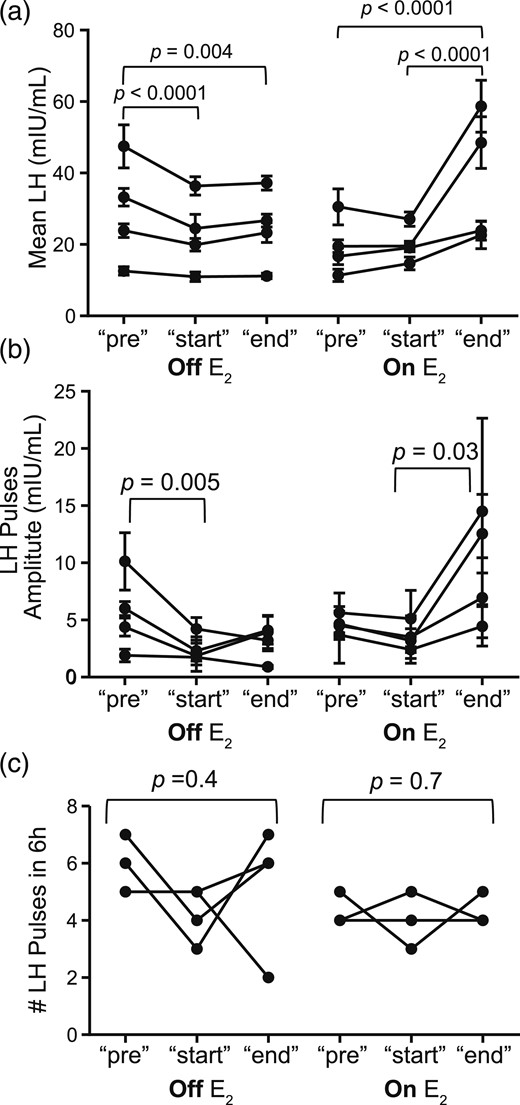
(a) Mean LH with standard deviation; (b) mean LH pulse amplitude with standard deviation; (c) number of LH pulses in 6 hours for eight kisspeptin infusion studies for the pre, start, and end time points, comparing off-estradiol (−E2) studies for subjects no. 200, 201, 215, and 230 to on-estradiol (+E2) studies for subjects no. 200, 215, 230, and 232.
As the infusion proceeded, there was little change in the pattern of LH release. The mean LH concentrations in the 6 hours at the end of the infusion were comparable to those observed in the first 6 hours (mean LH: start: 22.9 ± 10.6 mIU/mL; end: 24.7 ± 10.7 mIU/mL; P > 0.05). LH pulse amplitude at the end of the infusion remained comparable to that observed at the beginning of the infusion (mean LH amplitude: start: 2.5 mIU/mL; end: 3.0 mIU/mL; P = 0.1). LH pulse frequency remained unchanged (mean no. of pulses at start: 5.8; end: 5.3; P = 0.4) (Table 2; Fig. 2).
PMW +E2: response to 24-hour kisspeptin infusion
Seven PMW received the same kisspeptin infusion after wearing E2 patches for 2 weeks (E2 range on days of the study: 35 to 144 pg/mL). Three PMW +E2 participants (nos. 251, 254, and 260) who received GnRH 1 hour into the start of the kisspeptin infusion were excluded from the statistical analysis to prevent confounding. Four PMW +E2 participants (nos. 200, 215, 230, and 232) failed to demonstrate any initial stimulatory effect of kisspeptin on LH release (Fig. 1). There were no appreciable changes in mean LH concentrations from their preinfusion levels (mean LH: pre: 19.5 ± 8.1; start: 20.1 ± 5.2; P > 0.05). Similarly, there were no changes in LH pulse amplitude (mean LH amplitude: pre: 4.6 ± 0.8; start: 3.6 ± 1.1; P = 0.2) or LH pulse frequency (no. of pulses in 6 hours: pre: 4.3 ± 0.5; start: 4.0 ± 0.8; P = 0.7) (Fig. 2).
However, a marked change in LH secretion occurred during the later hours of the kisspeptin infusion in PMW +E2 participants. Approximately 12 hours after the infusion initiation, mean LH levels increased. The mean LH concentrations during the end of the kisspeptin infusion were significantly higher compared with those obtained at the start of the infusion (subjects no. 200, 215, 230, and 232: mean LH start: 20.1 ± 5.2; end: 38.4 ± 18.0; P = < 0.0001) (Fig. 1). This increase in mean LH concentration was due to an increase in LH pulse amplitude (LH amplitude start: 3.6 ± 1.1; end: 9.6 ± 4.7; P = 0.03) but not LH pulse frequency (pulses in 6 hours at start: 4.0 ± 0.8; end: 4.3 ± 0.5; P = 0.7) (Fig. 2). These changes in LH secretion correlated with the serum estradiol level achieved as a result of the sex-steroid addback (Fig. 3). A significant positive correlation was observed between E2 and LH pulse amplitude; however, the robust magnification in LH pulse amplitude is only seen when mean E2 concentrations surpassed 55 pg/mL (Supplemental Fig. 2). The LH pulse parameters of three PMW +E2 subjects with E2 >55 pg/mL (nos. 200, 230, and 232) and two PMW +E2 subjects with E2 <55 pg/mL (nos. 215 and 251) are displayed in Table 2. One of the women with E2 <55 pg/mL (no. 251) had received a bolus of GnRH 1 hour into the start of the kisspeptin infusion; because LH returned to baseline after the GnRH bolus, data excepting the time during the GnRH bolus were included in this analysis. These results suggest that estradiol modulates LH pulse amplitude in response to kisspeptin only after a threshold dose is achieved.

Linear regression of the relationship between LH parameters and estradiol by predetermined time points. (a) Preinfusion (pre), (b) start, and (c) end time for mean LH, mean LH pulse amplitude, and number of pulses in 6 hours. Included studies: off-estradiol (−E2) subjects no. 200, 201, 215, and 230, and on-estradiol (+E2) subjects no. 200, 215, 230, and 232. The three remaining studies in which a GnRH bolus was given during the “start” time period were excluded.
Three PMW participated in both the −E2 and +E2 arms of the study (nos. 200, 215, and 230). This paired analysis revealed the same patterns seen across the unpaired data. Kisspeptin inhibited mean LH levels and LH pulse amplitude in the hypoestrogenic state (Table 3; Supplemental Fig. 1). Kisspeptin administration with estrogen addback resulted in a significant increase in LH levels over time, again secondary to an increase in LH pulse amplitude (Table 3). Thus, the interaction between kisspeptin and estradiol that results in an amplification of LH secretion is not due to individual subject variability.
| . | −E2 (n = 3) . | +E2 (n = 3) . | ||||
|---|---|---|---|---|---|---|
| Clock time | 0800–1400, day 1 | 1400–2000, day 1 | 0600–1200, day 2 | 0800–1400, day 1 | 1400–2000, day 1 | 0600–1200, day 2 |
| Hours of infusion | −6 to 0 | 0–6 | 16–22 | −6 to 0 | 0–6 | 16–22 |
| Mean LH (mIU/mL) | 34.9 ± 11.9 | 26.9 ± 18.5 | 29.1 ± 7.2 | 22.3 ± 7.3 | 21.9 ± 4.5 | 43.7 ± 17.9 |
| LH amplitude (mIU/mL) | 6.8 ± 3.0 | 2.8 ± 1.3 | 3.5 ± 0.5 | 4.7 ± 1.0 | 3.6 ± 1.4 | 10.5 ± 5.3 |
| LH frequency (no. of pulses in 6 h) | 6.0 ± 1.0 | 4.0 ± 1.0 | 6.3 ± 0.6 | 4.3 ± 0.6 | 4.0 ± 1.0 | 4.3 ± 0.6 |
| . | −E2 (n = 3) . | +E2 (n = 3) . | ||||
|---|---|---|---|---|---|---|
| Clock time | 0800–1400, day 1 | 1400–2000, day 1 | 0600–1200, day 2 | 0800–1400, day 1 | 1400–2000, day 1 | 0600–1200, day 2 |
| Hours of infusion | −6 to 0 | 0–6 | 16–22 | −6 to 0 | 0–6 | 16–22 |
| Mean LH (mIU/mL) | 34.9 ± 11.9 | 26.9 ± 18.5 | 29.1 ± 7.2 | 22.3 ± 7.3 | 21.9 ± 4.5 | 43.7 ± 17.9 |
| LH amplitude (mIU/mL) | 6.8 ± 3.0 | 2.8 ± 1.3 | 3.5 ± 0.5 | 4.7 ± 1.0 | 3.6 ± 1.4 | 10.5 ± 5.3 |
| LH frequency (no. of pulses in 6 h) | 6.0 ± 1.0 | 4.0 ± 1.0 | 6.3 ± 0.6 | 4.3 ± 0.6 | 4.0 ± 1.0 | 4.3 ± 0.6 |
Mean LH, LH pulse amplitude, and LH pulse frequency values are given as mean ± standard deviation.
| . | −E2 (n = 3) . | +E2 (n = 3) . | ||||
|---|---|---|---|---|---|---|
| Clock time | 0800–1400, day 1 | 1400–2000, day 1 | 0600–1200, day 2 | 0800–1400, day 1 | 1400–2000, day 1 | 0600–1200, day 2 |
| Hours of infusion | −6 to 0 | 0–6 | 16–22 | −6 to 0 | 0–6 | 16–22 |
| Mean LH (mIU/mL) | 34.9 ± 11.9 | 26.9 ± 18.5 | 29.1 ± 7.2 | 22.3 ± 7.3 | 21.9 ± 4.5 | 43.7 ± 17.9 |
| LH amplitude (mIU/mL) | 6.8 ± 3.0 | 2.8 ± 1.3 | 3.5 ± 0.5 | 4.7 ± 1.0 | 3.6 ± 1.4 | 10.5 ± 5.3 |
| LH frequency (no. of pulses in 6 h) | 6.0 ± 1.0 | 4.0 ± 1.0 | 6.3 ± 0.6 | 4.3 ± 0.6 | 4.0 ± 1.0 | 4.3 ± 0.6 |
| . | −E2 (n = 3) . | +E2 (n = 3) . | ||||
|---|---|---|---|---|---|---|
| Clock time | 0800–1400, day 1 | 1400–2000, day 1 | 0600–1200, day 2 | 0800–1400, day 1 | 1400–2000, day 1 | 0600–1200, day 2 |
| Hours of infusion | −6 to 0 | 0–6 | 16–22 | −6 to 0 | 0–6 | 16–22 |
| Mean LH (mIU/mL) | 34.9 ± 11.9 | 26.9 ± 18.5 | 29.1 ± 7.2 | 22.3 ± 7.3 | 21.9 ± 4.5 | 43.7 ± 17.9 |
| LH amplitude (mIU/mL) | 6.8 ± 3.0 | 2.8 ± 1.3 | 3.5 ± 0.5 | 4.7 ± 1.0 | 3.6 ± 1.4 | 10.5 ± 5.3 |
| LH frequency (no. of pulses in 6 h) | 6.0 ± 1.0 | 4.0 ± 1.0 | 6.3 ± 0.6 | 4.3 ± 0.6 | 4.0 ± 1.0 | 4.3 ± 0.6 |
Mean LH, LH pulse amplitude, and LH pulse frequency values are given as mean ± standard deviation.
Hypothalamic vs pituitary effect of kisspeptin
In women not receiving GnRH or kisspeptin boluses, the average LH pulse amplitude at the end of the kisspeptin infusion was greater than that at the start of the kisspeptin infusion by 164%. To determine whether the increase in LH amplitude observed at the end of kisspeptin infusions in the PMW +E2 arm was due to enhanced pituitary responsiveness to GnRH, three PMW +E2 subjects received GnRH IVB at 1 and 23 hours after initiation of the kisspeptin infusion (nos. 251, 254, and 260). The amplitude of GnRH-induced LH pulses at the end of the kisspeptin infusion was modestly increased compared with the amplitude of GnRH-induced LH pulses at the start of the kisspeptin infusion by 47%. This indirect assessment suggests that the increase in LH pulse amplitude induced by a prolonged kisspeptin infusion in the setting of physiologic estradiol levels is partially but not wholly due to increases in pituitary sensitivity in GnRH, and suggests that there are contributions from additional factors, such as increased GnRH neuronal sensitivity to kisspeptin.
Discussion
The role of kisspeptin as a robust stimulator of GnRH-induced LH release has been demonstrated across several mammalian species, including the human. Continuous kisspeptin has been shown to induce an immediate LH increase in healthy men (20), and bolus administration has been reported to stimulate LH release in PMW (21). Although these prior reports framed our expectations for the current study, we observed, to our surprise, that PMW are resistant to the stimulatory effect of continuous kisspeptin on LH secretion. PMW receiving estradiol replacement are also initially resistant to kisspeptin, but they demonstrate a significant increase in LH pulse amplitude in direct proportion to the circulating estradiol concentration and duration of kisspeptin administration. Thus, the ability of kisspeptin to influence LH secretion can be modulated by the ambient sex-steroid milieu in a time- and dose-dependent manner.
Two seminal observations regarding GnRH biology first described in the nonhuman primate provide an important context for considering how kisspeptin might affect GnRH, and by extension, LH release. Bolus or repetitive administration of GnRH stimulates LH and FSH release; continuous administration causes desensitization (22). In the healthy cycling women in the follicular phase, a continuous GnRH infusion over 72 hours stimulates gonadotropin secretion, and then causes pituitary and gonadal desensitization (23). Similar to follicular-phase women, administration of a continuous GnRH infusion in PMW results in an initial stimulation of LH and FSH secretion, followed by decreases in both gonadotropins (24).
After the discovery of the role of kisspeptin as a gatekeeper of sexual maturation, the initial effects of exogenous kisspeptin administration appeared to parallel those of GnRH—although not completely. Single or repetitive bolus administration of kisspeptin stimulates GnRH/LH release in eugonadal men (4, 25) and in women in the luteal and periovulatory phases of the menstrual cycle (14, 15). However, kisspeptin does not stimulate GnRH/LH secretion in follicular-phase women (14, 15, 26); this observation holds true over a wide range of kisspeptin doses (14, 26). In contrast to the administration of continuous GnRH, which stimulates gonadotropin release in PMW, our data demonstrate that continuous kisspeptin administration fails to stimulate LH release in reproductive senescence. Given that pituitary gonadotropes retain their sensitivity to exogenous GnRH during menopause, the resistance to kisspeptin appears to be localized to the level of GnRH neurons and not the pituitary gonadotropes (27). The finding that kisspeptin does not acutely stimulate LH secretion in PMW is consonant with multiple studies in follicular-phase women (14, 15, 26) but at variance with one study showing an increase in the LH area under the curve in response to a single kisspeptin IV bolus in follicular-phase and menopausal women (21), potentially due to differences in how the LH response was analyzed. This could be a result of the difference in administration between kisspeptin via IV bolus and kisspeptin via infusion, given that the infusion delivers the same amount of kisspeptin as is found in a bolus over a one- to two-minute period. Future studies directly comparing bolus administration to a continuous infusion of kisspeptin are needed to determine if these modes of administration have different effects in menopausal women. In men, both IV bolus and infusions of kisspeptin result in an immediate GnRH-induced LH increase (20).
In fact, by utilizing every-10-minute blood sampling, we found that, rather than having stimulatory effects, continuous kisspeptin dampens LH release. Although continuous administration of kisspeptin at the dose and duration used in this study did not achieve the complete desensitization of the hypothalamic-pituitary-gonadal cascade that has been demonstrated with administration of kisspeptin analogs in healthy men over several days (28), we observed a 40% reduction in LH pulse amplitude at the start of the kisspeptin infusion, suggesting a reduced quantity of GnRH released per secretory event.
Because healthy cycling women are more responsive to exogenous kisspeptin during the periovulatory phase of the menstrual cycle compared with the early follicular phase, we tested the possibility that the presence of estradiol might modify the response to kisspeptin. Previous studies of the developmental changes across puberty in the response to kisspeptin in the nonhuman primate demonstrate the importance of estradiol in kisspeptin-induced GnRH secretion and a “switch” from ovarian steroid-independent to -dependent mechanisms (11). In addition, estrogen itself has long been understood to change GnRH and gonadotropin secretion through negative and positive feedback; kisspeptin is hypothesized to serve as a mediator of these effects (29). These feedback mechanisms, negative and positive, are preserved in menopausal women (30, 31). Furthermore, estradiol replacement therapy administered to PMW at a dose and duration similar to that used in this study resulted in an acute twofold increase in LH secretory-burst mass in response to an exogenous GnRH bolus of 75 ng/kg (32).
In the setting of estradiol replacement, continuous kisspeptin failed to stimulate LH secretion when initially administered to PMW; this was observed over a range of estradiol concentrations. At the start of the infusion, LH pulse amplitude and frequency were maintained, unlike the 40% suppression of LH pulse amplitude in estrogen-deficient PMW. Although estrogen can increase LH secretion through positive feedback, the dose and duration of estrogen are important factors for these effects (33, 34). Whereas the duration of estradiol treatment in this study is sufficient to induce positive feedback, the serum estradiol levels are below the levels of 200 to 270 pg/mL that are associated with positive feedback in PMW (35). Notably, however, LH pulse amplitudes begin to increase after several hours of kisspeptin administration. This pattern is reminiscent of the findings of Narayanaswamy et al. (16), who observed an LH response to kisspeptin in follicular-phase women, but only after approximately 4 hours of peptide administration. In PMW, we observed this marked and delayed increase in LH in subjects who achieved an estradiol level of 55 pg/mL or greater, suggesting that a threshold dose is required to observe this effect. A similar correlation between ambient estradiol values and the kisspeptin-induced LH response has been observed in follicular-phase women (16). It is unlikely that traditional positive feedback mechanisms could explain this delayed amplification of LH levels as they were observed (1) only after 12 hours of kisspeptin administration, and (2) at modest ambient estradiol levels. Rather, it appears that the increase in LH was driven by the combination of the presence of estradiol and prolonged exposure to kisspeptin.
Although indirect, comparisons between GnRH and kisspeptin boluses suggest that the increases in LH secretion in response to kisspeptin in the estrogen-replete state occur at both the level of the hypothalamus and the level of the pituitary. The mechanisms underlying this delayed increase are uncertain and may occur either directly or indirectly. In the presence of estradiol, kisspeptin increases glutamatergic and GABAergic signaling in GnRH neurons via presynaptic neurons (36–38). Stimulation of kisspeptin-responsive elements within the GnRH gene increases transcription of Gnrh1 mRNA, and increases GnRH protein levels in an estradiol-dependent manner (39–42). Estradiol itself may modify kisspeptin receptor affinity or recycling dynamics, although the directionality of these changes requires further study (43, 44). At the level of the pituitary, estrogen regulates both the expression of kisspeptin receptor and the ability of kisspeptin-responsive elements to increase pituitary LH production (45, 46).
There are factors that may have influenced the findings of this study. None of the PMW studied had been receiving continuous hormone replacement since menopause, and chronic estrogen deficiency may have effects on the ability to respond to kisspeptin. In support of this possibility, we have observed that individuals with congenital hypogonadotropic hypogonadism are nonresponsive to kisspeptin (47). Administration of exogenous GnRH is known to increase sensitivity to subsequent GnRH boluses given 2 hours later in PMW receiving estradiol; although this study separated GnRH boluses by 16 hours, the first exogenous bolus of GnRH could itself have increased the capacity of the pituitary to respond to the second exogenous GnRH bolus (48).
Thus, the use of exogenous kisspeptin as a probe of GnRH and LH secretory dynamics continues to reveal tremendous complexity in the neuroendocrine signaling pathways within the reproductive cascade. Although continuous kisspeptin administration does not immediately stimulate LH release in the postmenopausal state, estradiol appears to affect responsiveness to kisspeptin in a time- and dose-dependent manner. The mechanisms that underlie that modulation, and which neuronal populations are modulating the effects of estradiol, await further study.
Abbreviations:
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- IV
intravenous
- LH
luteinizing hormone
- MGH
Massachusetts General Hospital
- PMW
postmenopausal women.
Acknowledgments
We thank the research subjects, members of the Massachusetts General Hospital Reproductive Endocrine Unit for discussions and reading of the manuscript, staff of the Harvard Catalyst Clinical Research Center for assistance with the frequent sampling studies, the Massachusetts General Hospital Clinical Laboratory Research Core and Patrick Sluss, as well as the Ligand Assay and Analysis Core of the Center for Research in Reproduction at the University of Virginia, for assistance with assays.
This work was supported by Grant R01 HD043341 from the Eunice K. Shriver National Institute for Child Health and Human Development (NICHD) of the National Institutes of Health and the Harvard Catalyst/Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health Grants UL1 RR 025758 and UL1 TR000170, and financial contributions from Harvard University and its affiliated academic health care centers). S.B.S. is a Robert and Laura Reynolds Research Scholar and was also supported by NICHD Grant K24 HD067388. Y.-M.C. was supported by a Doris Duke Clinical Scientist Development Award (Grant 2013110). M.F.L. was supported by NICHD Grant F32 HD078083, a Postdoctoral Fellowship Award for Clinical Research from the Massachusetts General Hospital Executive Committee on Research Fund for Medical Discovery, and a Catalyst Medical Research Investigator Training Award from Harvard Catalyst. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health.
Clinical trial registry: ClinicalTrials.gov no. NCT01438073 (registered 24 August 2011).
Disclosure Summary: The authors have nothing to disclose.
References
Author notes
Address all correspondence and requests for reprints to: Stephanie B. Seminara, MD, 55 Fruit Street, BHX 5, Boston, Massachusetts 02114; E-mail: [email protected].



