-
PDF
- Split View
-
Views
-
Cite
Cite
Lindsey A. Sjaarda, Rose G. Radin, Robert M. Silver, Emily Mitchell, Sunni L. Mumford, Brian Wilcox, Noya Galai, Neil J. Perkins, Jean Wactawski-Wende, Joseph B. Stanford, Enrique F. Schisterman, Preconception Low-Dose Aspirin Restores Diminished Pregnancy and Live Birth Rates in Women With Low-Grade Inflammation: A Secondary Analysis of a Randomized Trial, The Journal of Clinical Endocrinology & Metabolism, Volume 102, Issue 5, 1 May 2017, Pages 1495–1504, https://doi.org/10.1210/jc.2016-2917
Close - Share Icon Share
Abstract
Inflammation is linked to causes of infertility. Low-dose aspirin (LDA) may improve reproductive success in women with chronic, low-grade inflammation.
To investigate the effect of preconception-initiated LDA on pregnancy rate, pregnancy loss, live birth rate, and inflammation during pregnancy.
Stratified secondary analysis of a multicenter, block-randomized, double-blind, placebo-controlled trial.
Four US academic medical centers, 2007 to 2012.
Healthy women aged 18 to 40 years (N = 1228) with one to two prior pregnancy losses actively attempting to conceive.
Preconception-initiated, daily LDA (81 mg) or matching placebo taken up to six menstrual cycles attempting pregnancy and through 36 weeks’ gestation in women who conceived.
Confirmed pregnancy, live birth, and pregnancy loss were compared between LDA and placebo, stratified by tertile of preconception, preintervention serum high-sensitivity C-reactive protein (hsCRP) (low, <0.70 mg/L; middle, 0.70 to <1.95 mg/L; high, ≥1.95 mg/L).
Live birth occurred in 55% of women overall. The lowest pregnancy and live birth rates occurred among the highest hsCRP tertile receiving placebo (44% live birth). LDA increased live birth among high-hsCRP women to 59% (relative risk, 1.35; 95% confidence interval, 1.08 to 1.67), similar to rates in the lower and mid-CRP tertiles. LDA did not affect clinical pregnancy or live birth in the low (live birth: 59% LDA, 54% placebo) or midlevel hsCRP tertiles (live birth: 59% LDA, 59% placebo).
In women attempting conception with elevated hsCRP and prior pregnancy loss, LDA may increase clinical pregnancy and live birth rates compared with women without inflammation and reduce hsCRP elevation during pregnancy.
Inflammation is an increasingly recognized factor contributing to reproductive dysfunction, including several common causes of infertility such as pelvic inflammatory disease (1), polycystic ovary syndrome (2), endometriosis (3), and recurrent pregnancy loss (4). Aspirin, an anti-inflammatory agent (5), is generally regarded as safe, widely available, and inexpensive and is used routinely for prevention of other health complications linked to inflammation. Specifically, low-dose aspirin (LDA; i.e., “baby aspirin,” 81 mg/d) is used to reduce cardiovascular risk mortality in patients with elevated risk (6, 7), and a recent report by the 2014 US Preventive Services Task Force recommended prophylactic use of LDA beginning at 12 weeks’ gestation for women at high risk of preeclampsia (8). Prior to conception in infertile women, aspirin therapy showed mixed efficacy in improving endometrial vascularization, placentation, and pregnancy rates resulting from assisted reproductive technologies (9–12). Lastly, preconception-initiated LDA resulted in a nonstatistically significant increase in the live birth rate (58% vs 53% in placebo) among 1228 women with a history of pregnancy loss participating in the Effects of Aspirin in Gestation and Reproduction (EAGeR) randomized trial.
High-sensitivity C-reactive protein (hsCRP) is commonly used as a biomarker of systemic, chronic inflammation, and moderately elevated hsCRP (≥2.11 mg/L) strongly predicted individuals most likely to exhibit a statistically significant reduction in myocardial infarction risk from daily LDA therapy (13). Thus, it is logical to hypothesize that hsCRP may indicate a systemic, low-grade inflammatory milieu amenable to daily LDA therapy to also improve reproductive health outcomes. Furthermore, in contrast to the paucity of data examining preconception inflammation and successful reproduction, a growing body of evidence supports a link between C-reactive protein measured during pregnancy and risk of pregnancy complications, including gestational diabetes, preeclampsia, and low birthweight (14–16). Therefore, the aim of this analysis was to determine the effect of LDA, according to preconception inflammatory status, on pregnancy rate, pregnancy loss, live birth rate, and hsCRP elevation during pregnancy in women attempting pregnancy as part of the EAGeR randomized trial with a history of one to two pregnancy losses.
Materials and Methods
The EAGeR trial was a multicenter, block-randomized, double-blind, placebo-controlled trial of 1228 women recruited from four medical centers in the United States from 2007 to 2012. Institutional review board approval was obtained at each study site and the data coordinating center. All participants provided written informed consent. A data safety and monitoring board optimized patient safety. Adverse events were monitored and reported to the data safety and monitoring board throughout the trial by a committee blinded to treatment status. The trial was registered with ClinicalTrials.gov as NCT00467363. Full details of the study design, methods, and participant characteristics have been previously described (17).
Study design and population
Women trying to conceive who were aged 18 to 40 years with regular menstrual cycles of 21 to 42 days in length, no known history of infertility, and confirmation of one or two prior pregnancy losses were eligible for the EAGeR trial. In the parent trial, participants were block-randomized by study center and eligibility stratum, which was defined based on specific eligibility qualifications (17): (1) original stratum, women with exactly one documented pregnancy loss <20 weeks’ gestation during the preceding 12 months and (2) expanded stratum, women not meeting all criteria for the original stratum and having one or two prior pregnancy losses of any gestational age at any time in the past. Exclusion criteria for all women included but were not limited to clinical indication for use of anticoagulant therapy or chronic use of nonsteroidal anti-inflammatory drugs, major medical disorders (e.g., diabetes, hypertension), and any self-reported history of physician-diagnosed infertility or subfertility including, but not limited to, related conditions such as polycystic ovary syndrome, endometriosis, or pelvic inflammatory disease (17). However, no specific testing was conducted to rule out such conditions at study entry. The overall and eligibility stratum-specific effects of LDA on live birth, the primary outcome of the EAGeR trial, have been reported (18).
Recruitment occurred primarily by physician/nurse referral within clinical sites in participating medical centers and was supplemented with household mailings, local health promotion events, posters, social media, brochures, and local media (19). Eligible women were scheduled for a baseline (prerandomization) visit to coincide with days 2 to 4 of their menstrual cycle.
Treatment and study procedures
Participants were block-randomized 1:1 using a computerized algorithm by study center and eligibility stratum (original/expanded) to receive either the intervention [81 mg LDA plus 400 μg folic acid daily (n = 615; 275 original, 340 expanded)] or placebo [plus 400 μg folic acid (n = 613; 274 original, 339 expanded)]. Treatment assignment was implemented by the data coordinating center; participants, clinicians, and investigators were blinded to treatment status. Study pills were taken daily until completion of six cycles of follow-up while attempting pregnancy or until week 36 of gestation for those who became pregnant.
Participants attended two study visits per menstrual cycle for the first two cycles attempting pregnancy and once per cycle thereafter. Visits were timed to occur around ovulation and days 2 to 4 of the expected next cycle (postcycle visit). Fertility monitors were used to assist with timing of intercourse to optimize conception and scheduling study visits (Clearblue Easy Fertility Monitor; Swiss Precision Diagnostics, Petit-Lancy, Switzerland). In women becoming pregnant, blood was collected at study visits scheduled approximately monthly through 36 weeks’ gestation.
Outcome measures
Primary outcomes for the present analysis were clinically confirmed pregnancy and live birth. Clinically confirmed pregnancies were identified by intrauterine gestational sac on ultrasound at 6 to 7 weeks’ gestation, clinical recording of fetal heart tones, or clinical confirmation of pregnancy at a later stage. Ectopic pregnancies (n = 6) were categorized as clinical pregnancy losses but not clinically confirmed pregnancies. Live birth was documented via abstraction of medical records. For women with no clinically confirmed pregnancy, follow-up ended after six menstrual cycles or after two pregnancy losses, whichever occurred first.
Pregnancy losses were also examined as a secondary outcome. Clinically confirmed losses were defined as losses occurring after clinical confirmation of pregnancy. Human chorionic gonadotropin (hCG)–detected losses were pregnancies detected by hCG testing but not confirmed by ultrasound (n = 55). An hCG-detected pregnancy was determined from a positive result on a “real-time” urine pregnancy test (Quidel Quickvue; Quidel Corporation, San Diego, CA), which was sensitive to 25 mIU/mL hCG, conducted each time participants reported missing menses on any postcycle visit (n = 34), or from batched urine hCG testing performed after study completion on stored samples from the last 10 days of each woman’s first and second cycle of study participation (using daily first-morning urine collected at home) and on spot urine samples collected at all postcycle visits (n = 21) (17, 18).
Biochemical analysis
C-reactive protein was measured in serum samples collected at the baseline (prerandomization) study visit (days 2 to 4 of menses), in addition to samples collected at 8, 20, and 36 weeks’ gestation. An immunoturbidimetric assay using a Roche COBAS 6000 autoanalyzer (Roche Diagnostics, Indianapolis, IN) was used to measure hsCRP to a limit of detection of 0.15 mg/L. Interassay coefficients of variation were 5.1% at 1.05 mg/L and 6.7% at 3.12 mg/L.
Statistical analysis
All analyses followed intent-to-treat principles in that analyses were completed according to assigned treatment, and no exclusions were made based on treatment compliance. As such, all women were included in analyses, except for 140 women who withdrew prior to observing the primary outcome (i.e., live birth) and an additional 19 women who did not have hsCRP assessed at baseline (Fig. 1). Baseline characteristics of women with missing hsCRP were similar to those with measured hsCRP, except that women with missing hsCRP were less likely to have greater than high school education (P = 0.007).
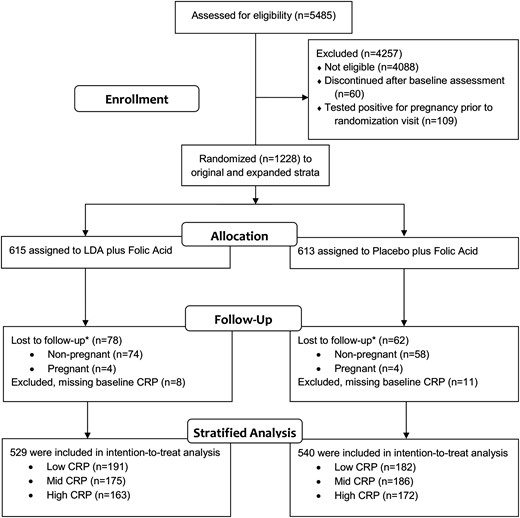
Participant flow of the EAGeR trial and analysis of LDA effect on live birth stratified by baseline hsCRP. *Lost to follow-up defined as women who withdrew without achieving pregnancy in six cycles of preconception follow-up (nonpregnant) or withdrew during pregnancy prior to observing birth outcome (pregnant). CRP, C-reactive protein.
Log binomial models with robust variance were used to estimate the risk ratio of LDA vs placebo for outcomes of clinically confirmed pregnancy, live birth, and pregnancy loss in the total group, stratified by baseline hsCRP tertile (low, <0.73 mg/L; middle, 0.73 to <2.24 mg/L; high, ≥2.24 mg/L). Tertiles of hsCRP were applied to ensure equivalent sample size to enhance statistical power to detect an effect within each level of hsCRP. Models were repeated, and all subsequent analysis was conducted after excluding 55 (28 LDA, 27 placebo) women with hsCRP ≥10 mg/L, a level noted in previous literature to be consistent with recent or ongoing acute causes of hsCRP elevation (e.g., infection, injury) as opposed to chronic low-grade immune activation (low, <0.70 mg/L; middle, 0.70 to <1.95 mg/L; high, ≥1.95 mg/L after excluding hsCRP ≥10 mg/L) (20, 21). The proportion of hsCRP ≥10 mg/L in our study population of ∼5% was expected in this relatively healthy group of women (22). Furthermore, using available diary entries from 1 day prior to 2 days after the hsCRP measurement at the randomization visit, report of any illness or side effects was 2.5 times as common among women with hsCRP ≥10 mg/L than those with hsCRP <10 mg/L (25% vs 11%, P = 0.002). Unfortunately, data on illness prior to 1 day before the blood draw were not available, limiting a full characterization of potentially relevant acute illness. Tertiles of hsCRP were used to evenly divide the study population; however, the resulting cut-point defining the top tertile (1.95 mg/L) was similar to definitions of high hsCRP used by others (23, 24).
To examine the effect of LDA on pregnancy and live birth among women with low-grade inflammation (third tertile, hsCRP ≥1.95 mg/L) separately by adiposity status, analysis was stratified by above and below the median waist-to-hip ratio as a proxy of central adiposity, as well as by normal [body mass index (BMI) <25 kg/m2] and overweight/obese (BMI ≥25 kg/m2) body mass.
Lastly, generalized estimating equations models were used to evaluate treatment group differences in log-transformed hsCRP longitudinally across pregnancy, stratified by baseline hsCRP tertile. For this analysis to correctly estimate the effect of LDA on log(hsCRP) across pregnancy, inverse probability weights were implemented to control for potential bias introduced by restricting the analysis postrandomization to only women becoming pregnant (25, 26).
Because treatment allocation was randomized, all models evaluating the effect of LDA are reported without adjustment for any covariates. Consistent with analysis of a randomized trial, adjustment for age, BMI, marital status, and income did not appreciably change estimates, and findings remained unchanged (data not shown). All analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC). The number needed to treat with confidence intervals (CIs) was calculated using a standard calculator (http://www.graphpad.com/quickcalcs/NNT1; GraphPad Software, La Jolla, CA).
Results
A total of 1228 women were recruited and randomly assigned to LDA or placebo between 15 June 2007 and 15 July 2011 and followed up until August 2012. Participants were predominantly white (95%), married or living with a partner (98%), educated (86% more than high school education), and employed (76%) (Table 1). With increasing hsCRP tertile, BMI increased (P < 0.001), waist-to-hip ratio increased (P < 0.001), education level decreased (P = 0.01), and time since the last pregnancy loss increased (P = 0.01).
| Characteristic . | Overall Trial (N = 1228) . | Low hsCRP, <0.73 mg/L . | Middle hsCRP, 0.73 to <2.24 mg/L . | High hsCRP, ≥2.24 mg/L . | |||
|---|---|---|---|---|---|---|---|
| LDA (n = 209) . | Placebo (n = 196) . | LDA (n = 201) . | Placebo (n = 198) . | LDA (n = 196) . | Placebo (n = 206) . | ||
| Age, y | 28.7 (4.8) | 29.3 (4.7) | 27.7 (4.3) | 28.1 (4.8) | 29.1 (4.8) | 29.1 (5.1) | 29.1 (4.9) |
| BMI | |||||||
| kg/m2 | 26.3 (6.6) | 22.4 (3.9) | 22.5 (3.5) | 25.5 (5.3) | 25.2 (4.4) | 30.9 (7.4) | 31.2 (6.8) |
| UW/NW/OW/OB, % | 3/49/24/24 | 5/81/9/5 | 10/69/18/3 | 1/52/29/18 | 3/51/29/17 | 1/25/23/51 | 1/14/35/50 |
| Waist-to-hip ratio | 0.811 (0.072) | 0.782 (0.54) | 0.790 (0.059) | 0.805 (0.069) | 0.808 (0.068) | 0.841 (0.080) | 0.839 (0.075) |
| White (vs nonwhite) racea | 1162 (94.6) | 194 (92.8) | 184 (93.9) | 190 (94.5) | 190 (96) | 185 (94.4) | 199 (96.6) |
| Marital status: married or living with partner (vs other) | 1198 (98) | 206 (98.6) | 189 (96.4) | 198 (98.5) | 197 (99.5) | 193 (98.5) | 194 (94.2) |
| Education: more than high school | 1057 (86.1) | 187 (89.9) | 179 (91.3) | 172 (85.6) | 169 (85.4) | 162 (82.7) | 174 (84.5) |
| Income, per year | |||||||
| ≤$19,999 | 94 (7.7) | 18 (8.6) | 17 (8.7) | 13 (6.5) | 13 (6.6) | 18 (9.2) | 13 (6.3) |
| $20,000–$39,999 | 312 (25.4) | 35 (16.7) | 52 (26.5) | 47 (23.5) | 51 (25.8) | 61 (31.1) | 60 (29.1) |
| $40,000–$74,999 | 181 (14.8) | 27 (12.9) | 27 (13.8) | 32 (16) | 29 (14.6) | 32 (16.3) | 31 (15) |
| $75,000–$99,999 | 149 (12.1) | 44 (21.1) | 16 (8.2) | 19 (9.5) | 26 (13.1) | 21 (10.7) | 22 (10.7) |
| ≥$100,000 | 491 (40) | 85 (40.7) | 84 (42.9) | 89 (44.5) | 79 (39.9) | 64 (32.7) | 80 (38.8) |
| Employed (vs not) | 895 (75.6) | 155 (76) | 140 (74.5) | 146 (77.2) | 138 (71.1) | 145 (75.5) | 157 (79.7) |
| Prior live births | |||||||
| 0 | 571 (46.5) | 94 (45) | 91 (46.4) | 106 (52.7) | 98 (49.5) | 80 (40.8) | 90 (43.7) |
| 1 | 443 (36.1) | 84 (40.2) | 70 (35.7) | 63 (31.3) | 67 (33.8) | 71 (36.2) | 82 (39.8) |
| 2 | 214 (17.4) | 31 (14.8) | 35 (17.9) | 32 (15.9) | 33 (16.7) | 45 (23) | 34 (16.5) |
| Number of previous pregnancy losses | |||||||
| 1 | 825 (67.2) | 152 (72.7) | 133 (67.9) | 133 (66.2) | 136 (68.7) | 130 (66.3) | 124 (60.2) |
| 2 | 403 (32.8) | 57 (27.3) | 63 (32.1) | 68 (33.8) | 62 (31.3) | 66 (33.7) | 82 (39.8) |
| Time from last loss to randomization | |||||||
| ≤4 mo | 651 (53.8) | 123 (60) | 105 (54.1) | 106 (54.1) | 116 (59.5) | 96 (49.7) | 93 (45.6) |
| 5–8 mo | 222 (18.4) | 31 (15.1) | 45 (23.2) | 31 (15.8) | 36 (18.5) | 40 (20.7) | 34 (16.7) |
| 9–12 mo | 99 (8.2) | 12 (5.9) | 9 (4.6) | 26 (13.3) | 15 (7.7) | 12 (6.2) | 24 (11.8) |
| >12 mo | 237 (19.6) | 39 (19) | 35 (18) | 33 (16.8) | 28 (14.4) | 45 (23.3) | 53 (26) |
| Characteristic . | Overall Trial (N = 1228) . | Low hsCRP, <0.73 mg/L . | Middle hsCRP, 0.73 to <2.24 mg/L . | High hsCRP, ≥2.24 mg/L . | |||
|---|---|---|---|---|---|---|---|
| LDA (n = 209) . | Placebo (n = 196) . | LDA (n = 201) . | Placebo (n = 198) . | LDA (n = 196) . | Placebo (n = 206) . | ||
| Age, y | 28.7 (4.8) | 29.3 (4.7) | 27.7 (4.3) | 28.1 (4.8) | 29.1 (4.8) | 29.1 (5.1) | 29.1 (4.9) |
| BMI | |||||||
| kg/m2 | 26.3 (6.6) | 22.4 (3.9) | 22.5 (3.5) | 25.5 (5.3) | 25.2 (4.4) | 30.9 (7.4) | 31.2 (6.8) |
| UW/NW/OW/OB, % | 3/49/24/24 | 5/81/9/5 | 10/69/18/3 | 1/52/29/18 | 3/51/29/17 | 1/25/23/51 | 1/14/35/50 |
| Waist-to-hip ratio | 0.811 (0.072) | 0.782 (0.54) | 0.790 (0.059) | 0.805 (0.069) | 0.808 (0.068) | 0.841 (0.080) | 0.839 (0.075) |
| White (vs nonwhite) racea | 1162 (94.6) | 194 (92.8) | 184 (93.9) | 190 (94.5) | 190 (96) | 185 (94.4) | 199 (96.6) |
| Marital status: married or living with partner (vs other) | 1198 (98) | 206 (98.6) | 189 (96.4) | 198 (98.5) | 197 (99.5) | 193 (98.5) | 194 (94.2) |
| Education: more than high school | 1057 (86.1) | 187 (89.9) | 179 (91.3) | 172 (85.6) | 169 (85.4) | 162 (82.7) | 174 (84.5) |
| Income, per year | |||||||
| ≤$19,999 | 94 (7.7) | 18 (8.6) | 17 (8.7) | 13 (6.5) | 13 (6.6) | 18 (9.2) | 13 (6.3) |
| $20,000–$39,999 | 312 (25.4) | 35 (16.7) | 52 (26.5) | 47 (23.5) | 51 (25.8) | 61 (31.1) | 60 (29.1) |
| $40,000–$74,999 | 181 (14.8) | 27 (12.9) | 27 (13.8) | 32 (16) | 29 (14.6) | 32 (16.3) | 31 (15) |
| $75,000–$99,999 | 149 (12.1) | 44 (21.1) | 16 (8.2) | 19 (9.5) | 26 (13.1) | 21 (10.7) | 22 (10.7) |
| ≥$100,000 | 491 (40) | 85 (40.7) | 84 (42.9) | 89 (44.5) | 79 (39.9) | 64 (32.7) | 80 (38.8) |
| Employed (vs not) | 895 (75.6) | 155 (76) | 140 (74.5) | 146 (77.2) | 138 (71.1) | 145 (75.5) | 157 (79.7) |
| Prior live births | |||||||
| 0 | 571 (46.5) | 94 (45) | 91 (46.4) | 106 (52.7) | 98 (49.5) | 80 (40.8) | 90 (43.7) |
| 1 | 443 (36.1) | 84 (40.2) | 70 (35.7) | 63 (31.3) | 67 (33.8) | 71 (36.2) | 82 (39.8) |
| 2 | 214 (17.4) | 31 (14.8) | 35 (17.9) | 32 (15.9) | 33 (16.7) | 45 (23) | 34 (16.5) |
| Number of previous pregnancy losses | |||||||
| 1 | 825 (67.2) | 152 (72.7) | 133 (67.9) | 133 (66.2) | 136 (68.7) | 130 (66.3) | 124 (60.2) |
| 2 | 403 (32.8) | 57 (27.3) | 63 (32.1) | 68 (33.8) | 62 (31.3) | 66 (33.7) | 82 (39.8) |
| Time from last loss to randomization | |||||||
| ≤4 mo | 651 (53.8) | 123 (60) | 105 (54.1) | 106 (54.1) | 116 (59.5) | 96 (49.7) | 93 (45.6) |
| 5–8 mo | 222 (18.4) | 31 (15.1) | 45 (23.2) | 31 (15.8) | 36 (18.5) | 40 (20.7) | 34 (16.7) |
| 9–12 mo | 99 (8.2) | 12 (5.9) | 9 (4.6) | 26 (13.3) | 15 (7.7) | 12 (6.2) | 24 (11.8) |
| >12 mo | 237 (19.6) | 39 (19) | 35 (18) | 33 (16.8) | 28 (14.4) | 45 (23.3) | 53 (26) |
Data are mean ± standard deviation or n (%). Information was missing for waist-to-hip ratio (n = 10), income (n = 1), education (n = 1), employment (n = 44), and time from last loss to randomization (n = 19).
Abbreviations: NW, normal weight (BMI 18.5 to <25 kg/m2); OB, obese (BMI ≥30 kg/m2); OW, overweight (BMI 25 to <30 kg/m2); UW, underweight (BMI <18.5 kg/m2).
Race/ethnicity was based on self-report on study questionnaires.
| Characteristic . | Overall Trial (N = 1228) . | Low hsCRP, <0.73 mg/L . | Middle hsCRP, 0.73 to <2.24 mg/L . | High hsCRP, ≥2.24 mg/L . | |||
|---|---|---|---|---|---|---|---|
| LDA (n = 209) . | Placebo (n = 196) . | LDA (n = 201) . | Placebo (n = 198) . | LDA (n = 196) . | Placebo (n = 206) . | ||
| Age, y | 28.7 (4.8) | 29.3 (4.7) | 27.7 (4.3) | 28.1 (4.8) | 29.1 (4.8) | 29.1 (5.1) | 29.1 (4.9) |
| BMI | |||||||
| kg/m2 | 26.3 (6.6) | 22.4 (3.9) | 22.5 (3.5) | 25.5 (5.3) | 25.2 (4.4) | 30.9 (7.4) | 31.2 (6.8) |
| UW/NW/OW/OB, % | 3/49/24/24 | 5/81/9/5 | 10/69/18/3 | 1/52/29/18 | 3/51/29/17 | 1/25/23/51 | 1/14/35/50 |
| Waist-to-hip ratio | 0.811 (0.072) | 0.782 (0.54) | 0.790 (0.059) | 0.805 (0.069) | 0.808 (0.068) | 0.841 (0.080) | 0.839 (0.075) |
| White (vs nonwhite) racea | 1162 (94.6) | 194 (92.8) | 184 (93.9) | 190 (94.5) | 190 (96) | 185 (94.4) | 199 (96.6) |
| Marital status: married or living with partner (vs other) | 1198 (98) | 206 (98.6) | 189 (96.4) | 198 (98.5) | 197 (99.5) | 193 (98.5) | 194 (94.2) |
| Education: more than high school | 1057 (86.1) | 187 (89.9) | 179 (91.3) | 172 (85.6) | 169 (85.4) | 162 (82.7) | 174 (84.5) |
| Income, per year | |||||||
| ≤$19,999 | 94 (7.7) | 18 (8.6) | 17 (8.7) | 13 (6.5) | 13 (6.6) | 18 (9.2) | 13 (6.3) |
| $20,000–$39,999 | 312 (25.4) | 35 (16.7) | 52 (26.5) | 47 (23.5) | 51 (25.8) | 61 (31.1) | 60 (29.1) |
| $40,000–$74,999 | 181 (14.8) | 27 (12.9) | 27 (13.8) | 32 (16) | 29 (14.6) | 32 (16.3) | 31 (15) |
| $75,000–$99,999 | 149 (12.1) | 44 (21.1) | 16 (8.2) | 19 (9.5) | 26 (13.1) | 21 (10.7) | 22 (10.7) |
| ≥$100,000 | 491 (40) | 85 (40.7) | 84 (42.9) | 89 (44.5) | 79 (39.9) | 64 (32.7) | 80 (38.8) |
| Employed (vs not) | 895 (75.6) | 155 (76) | 140 (74.5) | 146 (77.2) | 138 (71.1) | 145 (75.5) | 157 (79.7) |
| Prior live births | |||||||
| 0 | 571 (46.5) | 94 (45) | 91 (46.4) | 106 (52.7) | 98 (49.5) | 80 (40.8) | 90 (43.7) |
| 1 | 443 (36.1) | 84 (40.2) | 70 (35.7) | 63 (31.3) | 67 (33.8) | 71 (36.2) | 82 (39.8) |
| 2 | 214 (17.4) | 31 (14.8) | 35 (17.9) | 32 (15.9) | 33 (16.7) | 45 (23) | 34 (16.5) |
| Number of previous pregnancy losses | |||||||
| 1 | 825 (67.2) | 152 (72.7) | 133 (67.9) | 133 (66.2) | 136 (68.7) | 130 (66.3) | 124 (60.2) |
| 2 | 403 (32.8) | 57 (27.3) | 63 (32.1) | 68 (33.8) | 62 (31.3) | 66 (33.7) | 82 (39.8) |
| Time from last loss to randomization | |||||||
| ≤4 mo | 651 (53.8) | 123 (60) | 105 (54.1) | 106 (54.1) | 116 (59.5) | 96 (49.7) | 93 (45.6) |
| 5–8 mo | 222 (18.4) | 31 (15.1) | 45 (23.2) | 31 (15.8) | 36 (18.5) | 40 (20.7) | 34 (16.7) |
| 9–12 mo | 99 (8.2) | 12 (5.9) | 9 (4.6) | 26 (13.3) | 15 (7.7) | 12 (6.2) | 24 (11.8) |
| >12 mo | 237 (19.6) | 39 (19) | 35 (18) | 33 (16.8) | 28 (14.4) | 45 (23.3) | 53 (26) |
| Characteristic . | Overall Trial (N = 1228) . | Low hsCRP, <0.73 mg/L . | Middle hsCRP, 0.73 to <2.24 mg/L . | High hsCRP, ≥2.24 mg/L . | |||
|---|---|---|---|---|---|---|---|
| LDA (n = 209) . | Placebo (n = 196) . | LDA (n = 201) . | Placebo (n = 198) . | LDA (n = 196) . | Placebo (n = 206) . | ||
| Age, y | 28.7 (4.8) | 29.3 (4.7) | 27.7 (4.3) | 28.1 (4.8) | 29.1 (4.8) | 29.1 (5.1) | 29.1 (4.9) |
| BMI | |||||||
| kg/m2 | 26.3 (6.6) | 22.4 (3.9) | 22.5 (3.5) | 25.5 (5.3) | 25.2 (4.4) | 30.9 (7.4) | 31.2 (6.8) |
| UW/NW/OW/OB, % | 3/49/24/24 | 5/81/9/5 | 10/69/18/3 | 1/52/29/18 | 3/51/29/17 | 1/25/23/51 | 1/14/35/50 |
| Waist-to-hip ratio | 0.811 (0.072) | 0.782 (0.54) | 0.790 (0.059) | 0.805 (0.069) | 0.808 (0.068) | 0.841 (0.080) | 0.839 (0.075) |
| White (vs nonwhite) racea | 1162 (94.6) | 194 (92.8) | 184 (93.9) | 190 (94.5) | 190 (96) | 185 (94.4) | 199 (96.6) |
| Marital status: married or living with partner (vs other) | 1198 (98) | 206 (98.6) | 189 (96.4) | 198 (98.5) | 197 (99.5) | 193 (98.5) | 194 (94.2) |
| Education: more than high school | 1057 (86.1) | 187 (89.9) | 179 (91.3) | 172 (85.6) | 169 (85.4) | 162 (82.7) | 174 (84.5) |
| Income, per year | |||||||
| ≤$19,999 | 94 (7.7) | 18 (8.6) | 17 (8.7) | 13 (6.5) | 13 (6.6) | 18 (9.2) | 13 (6.3) |
| $20,000–$39,999 | 312 (25.4) | 35 (16.7) | 52 (26.5) | 47 (23.5) | 51 (25.8) | 61 (31.1) | 60 (29.1) |
| $40,000–$74,999 | 181 (14.8) | 27 (12.9) | 27 (13.8) | 32 (16) | 29 (14.6) | 32 (16.3) | 31 (15) |
| $75,000–$99,999 | 149 (12.1) | 44 (21.1) | 16 (8.2) | 19 (9.5) | 26 (13.1) | 21 (10.7) | 22 (10.7) |
| ≥$100,000 | 491 (40) | 85 (40.7) | 84 (42.9) | 89 (44.5) | 79 (39.9) | 64 (32.7) | 80 (38.8) |
| Employed (vs not) | 895 (75.6) | 155 (76) | 140 (74.5) | 146 (77.2) | 138 (71.1) | 145 (75.5) | 157 (79.7) |
| Prior live births | |||||||
| 0 | 571 (46.5) | 94 (45) | 91 (46.4) | 106 (52.7) | 98 (49.5) | 80 (40.8) | 90 (43.7) |
| 1 | 443 (36.1) | 84 (40.2) | 70 (35.7) | 63 (31.3) | 67 (33.8) | 71 (36.2) | 82 (39.8) |
| 2 | 214 (17.4) | 31 (14.8) | 35 (17.9) | 32 (15.9) | 33 (16.7) | 45 (23) | 34 (16.5) |
| Number of previous pregnancy losses | |||||||
| 1 | 825 (67.2) | 152 (72.7) | 133 (67.9) | 133 (66.2) | 136 (68.7) | 130 (66.3) | 124 (60.2) |
| 2 | 403 (32.8) | 57 (27.3) | 63 (32.1) | 68 (33.8) | 62 (31.3) | 66 (33.7) | 82 (39.8) |
| Time from last loss to randomization | |||||||
| ≤4 mo | 651 (53.8) | 123 (60) | 105 (54.1) | 106 (54.1) | 116 (59.5) | 96 (49.7) | 93 (45.6) |
| 5–8 mo | 222 (18.4) | 31 (15.1) | 45 (23.2) | 31 (15.8) | 36 (18.5) | 40 (20.7) | 34 (16.7) |
| 9–12 mo | 99 (8.2) | 12 (5.9) | 9 (4.6) | 26 (13.3) | 15 (7.7) | 12 (6.2) | 24 (11.8) |
| >12 mo | 237 (19.6) | 39 (19) | 35 (18) | 33 (16.8) | 28 (14.4) | 45 (23.3) | 53 (26) |
Data are mean ± standard deviation or n (%). Information was missing for waist-to-hip ratio (n = 10), income (n = 1), education (n = 1), employment (n = 44), and time from last loss to randomization (n = 19).
Abbreviations: NW, normal weight (BMI 18.5 to <25 kg/m2); OB, obese (BMI ≥30 kg/m2); OW, overweight (BMI 25 to <30 kg/m2); UW, underweight (BMI <18.5 kg/m2).
Race/ethnicity was based on self-report on study questionnaires.
The overall pregnancy and live birth rates of the study were 67% (n = 732) and 55% (n = 597), respectively. The mean (standard deviation) concentration of hsCRP was 2.79 (5.16) mg/L, and median (interquartile range) was 1.19 (0.55 to 3.20) mg/L, representing the expected right-skewed distribution of hsCRP concentrations.
Among women in the lowest and middle hsCRP tertiles, clinically confirmed pregnancies did not differ by treatment (lowest hsCRP, 70% LDA, 68% placebo; middle hsCRP, 72% LDA, 67% placebo). However, among those with the highest hsCRP, clinical pregnancy rates were 71% (LDA) and 54% (placebo) (Fig. 2). As such, LDA treatment was associated with a 31% increase in clinically confirmed pregnancy incidence among women in the highest hsCRP tertile (Table 2). Similarly, live birth did not differ by treatment in the lower hsCRP tertiles (lowest hsCRP, 59% LDA, 54% placebo; middle hsCRP, 59% LDA, 59% placebo), but live birth was lower (44%) among women receiving placebo in the high-hsCRP group compared with 59% receiving LDA (Fig. 2). As such, LDA was associated with a 35% increase in live birth among women in the highest hsCRP tertile (Table 2). Results summarized previously reflect the population of women with hsCRP <10 mg/L as this level of hsCRP likely reflects chronic low-grade inflammation, as opposed to acute immune activation attributable to recent or ongoing injury or infection (20, 21). However, results both including and excluding women with hsCRP ≥10 mg/L are presented (Table 2).
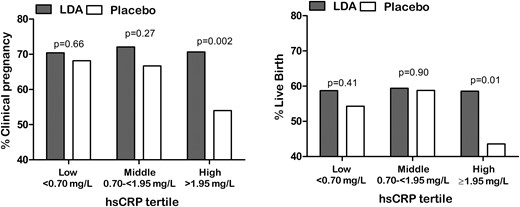
Proportion of women with clinically confirmed pregnancy and live birth by baseline hsCRP concentrations and treatment group. Data indicate percentage of pregnancy or live birth in women having hsCRP <10 mg/L.
Effect of LDA Treatment vs Placebo on Pregnancy and Live Birth Incidence Stratified by Baseline hsCRP Tertile
| Characteristic . | Clinically Confirmed Pregnancya . | Live Birth . | ||
|---|---|---|---|---|
| LDA . | Placebo . | LDA . | Placebo . | |
| All women, No. | 529 | 540 | 529 | 540 |
| Low hsCRP, <0.73 mg/L | ||||
| No. of participants | 191 | 182 | 191 | 182 |
| Achieved outcome, No. (%) | 135 (70.7) | 122 (67.0) | 114 (59.7) | 98 (53.9) |
| Risk ratio (95% CI) | 1.05 (0.92–1.21) | 1.11 (0.93–1.32) | ||
| Middle hsCRP, 0.73 to <2.24 mg/L | ||||
| No. of participants | 175 | 186 | 175 | 186 |
| Achieved outcome, No. (%) | 124 (70.9) | 125 (67.2) | 102 (58.3) | 109 (58.6) |
| Risk ratio (95% CI) | 1.05 (0.92–1.21) | 0.99 (0.84–1.18) | ||
| High hsCRP, ≥2.24 mg/L | ||||
| No. of participants | 163 | 172 | 163 | 172 |
| Achieved outcome, No. (%) | 111 (68.1) | 96 (55.8) | 89 (54.6) | 76 (44.2) |
| Risk ratio (95% CI) | 1.22 (1.03–1.45)c | 1.24 (0.99–1.54)d | ||
| Women with hsCRP <10 mg/L,b No. | 501 | 513 | 501 | 513 |
| Low hsCRP, <0.70 mg/L | ||||
| No. of participants | 179 | 173 | 179 | 173 |
| Achieved outcome, No. (%) | 126 (70.4) | 118 (68.2) | 105 (58.7) | 94 (54.3) |
| Risk ratio (95% CI) | 1.03 (0.90–1.19) | 1.08 (0.90–1.30) | ||
| Middle hsCRP, 0.70 to <1.95 mg/L | ||||
| No. of participants | 165 | 177 | 165 | 177 |
| Achieved outcome, No. (%) | 119 (72.1) | 118 (66.7) | 98 (59.4) | 104 (58.8) |
| Risk ratio (95% CI) | 1.08 (0.94–1.25) | 1.01 (0.85–1.21) | ||
| High hsCRP, ≥1.95 mg/L | ||||
| No. of participants | 157 | 163 | 157 | 163 |
| Achieved outcome, No. (%) | 111 (70.7) | 88 (54.0) | 92 (58.6) | 71 (43.6) |
| Risk ratio (95% CI) | 1.31 (1.10–1.56)e | 1.35 (1.08–1.67)e | ||
| Characteristic . | Clinically Confirmed Pregnancya . | Live Birth . | ||
|---|---|---|---|---|
| LDA . | Placebo . | LDA . | Placebo . | |
| All women, No. | 529 | 540 | 529 | 540 |
| Low hsCRP, <0.73 mg/L | ||||
| No. of participants | 191 | 182 | 191 | 182 |
| Achieved outcome, No. (%) | 135 (70.7) | 122 (67.0) | 114 (59.7) | 98 (53.9) |
| Risk ratio (95% CI) | 1.05 (0.92–1.21) | 1.11 (0.93–1.32) | ||
| Middle hsCRP, 0.73 to <2.24 mg/L | ||||
| No. of participants | 175 | 186 | 175 | 186 |
| Achieved outcome, No. (%) | 124 (70.9) | 125 (67.2) | 102 (58.3) | 109 (58.6) |
| Risk ratio (95% CI) | 1.05 (0.92–1.21) | 0.99 (0.84–1.18) | ||
| High hsCRP, ≥2.24 mg/L | ||||
| No. of participants | 163 | 172 | 163 | 172 |
| Achieved outcome, No. (%) | 111 (68.1) | 96 (55.8) | 89 (54.6) | 76 (44.2) |
| Risk ratio (95% CI) | 1.22 (1.03–1.45)c | 1.24 (0.99–1.54)d | ||
| Women with hsCRP <10 mg/L,b No. | 501 | 513 | 501 | 513 |
| Low hsCRP, <0.70 mg/L | ||||
| No. of participants | 179 | 173 | 179 | 173 |
| Achieved outcome, No. (%) | 126 (70.4) | 118 (68.2) | 105 (58.7) | 94 (54.3) |
| Risk ratio (95% CI) | 1.03 (0.90–1.19) | 1.08 (0.90–1.30) | ||
| Middle hsCRP, 0.70 to <1.95 mg/L | ||||
| No. of participants | 165 | 177 | 165 | 177 |
| Achieved outcome, No. (%) | 119 (72.1) | 118 (66.7) | 98 (59.4) | 104 (58.8) |
| Risk ratio (95% CI) | 1.08 (0.94–1.25) | 1.01 (0.85–1.21) | ||
| High hsCRP, ≥1.95 mg/L | ||||
| No. of participants | 157 | 163 | 157 | 163 |
| Achieved outcome, No. (%) | 111 (70.7) | 88 (54.0) | 92 (58.6) | 71 (43.6) |
| Risk ratio (95% CI) | 1.31 (1.10–1.56)e | 1.35 (1.08–1.67)e | ||
Pregnancy identified by 6- to 7-week ultrasound.
Baseline hsCRP concentrations ≥10 mg/L, which are indicative of acute inflammatory processes such as infection or injury, were excluded (n = 55) (20, 21).
P < 0.05.
P < 0.06.
P ≤ 0.01.
Effect of LDA Treatment vs Placebo on Pregnancy and Live Birth Incidence Stratified by Baseline hsCRP Tertile
| Characteristic . | Clinically Confirmed Pregnancya . | Live Birth . | ||
|---|---|---|---|---|
| LDA . | Placebo . | LDA . | Placebo . | |
| All women, No. | 529 | 540 | 529 | 540 |
| Low hsCRP, <0.73 mg/L | ||||
| No. of participants | 191 | 182 | 191 | 182 |
| Achieved outcome, No. (%) | 135 (70.7) | 122 (67.0) | 114 (59.7) | 98 (53.9) |
| Risk ratio (95% CI) | 1.05 (0.92–1.21) | 1.11 (0.93–1.32) | ||
| Middle hsCRP, 0.73 to <2.24 mg/L | ||||
| No. of participants | 175 | 186 | 175 | 186 |
| Achieved outcome, No. (%) | 124 (70.9) | 125 (67.2) | 102 (58.3) | 109 (58.6) |
| Risk ratio (95% CI) | 1.05 (0.92–1.21) | 0.99 (0.84–1.18) | ||
| High hsCRP, ≥2.24 mg/L | ||||
| No. of participants | 163 | 172 | 163 | 172 |
| Achieved outcome, No. (%) | 111 (68.1) | 96 (55.8) | 89 (54.6) | 76 (44.2) |
| Risk ratio (95% CI) | 1.22 (1.03–1.45)c | 1.24 (0.99–1.54)d | ||
| Women with hsCRP <10 mg/L,b No. | 501 | 513 | 501 | 513 |
| Low hsCRP, <0.70 mg/L | ||||
| No. of participants | 179 | 173 | 179 | 173 |
| Achieved outcome, No. (%) | 126 (70.4) | 118 (68.2) | 105 (58.7) | 94 (54.3) |
| Risk ratio (95% CI) | 1.03 (0.90–1.19) | 1.08 (0.90–1.30) | ||
| Middle hsCRP, 0.70 to <1.95 mg/L | ||||
| No. of participants | 165 | 177 | 165 | 177 |
| Achieved outcome, No. (%) | 119 (72.1) | 118 (66.7) | 98 (59.4) | 104 (58.8) |
| Risk ratio (95% CI) | 1.08 (0.94–1.25) | 1.01 (0.85–1.21) | ||
| High hsCRP, ≥1.95 mg/L | ||||
| No. of participants | 157 | 163 | 157 | 163 |
| Achieved outcome, No. (%) | 111 (70.7) | 88 (54.0) | 92 (58.6) | 71 (43.6) |
| Risk ratio (95% CI) | 1.31 (1.10–1.56)e | 1.35 (1.08–1.67)e | ||
| Characteristic . | Clinically Confirmed Pregnancya . | Live Birth . | ||
|---|---|---|---|---|
| LDA . | Placebo . | LDA . | Placebo . | |
| All women, No. | 529 | 540 | 529 | 540 |
| Low hsCRP, <0.73 mg/L | ||||
| No. of participants | 191 | 182 | 191 | 182 |
| Achieved outcome, No. (%) | 135 (70.7) | 122 (67.0) | 114 (59.7) | 98 (53.9) |
| Risk ratio (95% CI) | 1.05 (0.92–1.21) | 1.11 (0.93–1.32) | ||
| Middle hsCRP, 0.73 to <2.24 mg/L | ||||
| No. of participants | 175 | 186 | 175 | 186 |
| Achieved outcome, No. (%) | 124 (70.9) | 125 (67.2) | 102 (58.3) | 109 (58.6) |
| Risk ratio (95% CI) | 1.05 (0.92–1.21) | 0.99 (0.84–1.18) | ||
| High hsCRP, ≥2.24 mg/L | ||||
| No. of participants | 163 | 172 | 163 | 172 |
| Achieved outcome, No. (%) | 111 (68.1) | 96 (55.8) | 89 (54.6) | 76 (44.2) |
| Risk ratio (95% CI) | 1.22 (1.03–1.45)c | 1.24 (0.99–1.54)d | ||
| Women with hsCRP <10 mg/L,b No. | 501 | 513 | 501 | 513 |
| Low hsCRP, <0.70 mg/L | ||||
| No. of participants | 179 | 173 | 179 | 173 |
| Achieved outcome, No. (%) | 126 (70.4) | 118 (68.2) | 105 (58.7) | 94 (54.3) |
| Risk ratio (95% CI) | 1.03 (0.90–1.19) | 1.08 (0.90–1.30) | ||
| Middle hsCRP, 0.70 to <1.95 mg/L | ||||
| No. of participants | 165 | 177 | 165 | 177 |
| Achieved outcome, No. (%) | 119 (72.1) | 118 (66.7) | 98 (59.4) | 104 (58.8) |
| Risk ratio (95% CI) | 1.08 (0.94–1.25) | 1.01 (0.85–1.21) | ||
| High hsCRP, ≥1.95 mg/L | ||||
| No. of participants | 157 | 163 | 157 | 163 |
| Achieved outcome, No. (%) | 111 (70.7) | 88 (54.0) | 92 (58.6) | 71 (43.6) |
| Risk ratio (95% CI) | 1.31 (1.10–1.56)e | 1.35 (1.08–1.67)e | ||
Pregnancy identified by 6- to 7-week ultrasound.
Baseline hsCRP concentrations ≥10 mg/L, which are indicative of acute inflammatory processes such as infection or injury, were excluded (n = 55) (20, 21).
P < 0.05.
P < 0.06.
P ≤ 0.01.
Overall, the number needed to treat was seven women (95% CI, 3.9 to 23.8) within the highest hsCRP tertile for every additional live birth observed, whereas the effect of LDA among all women (i.e., without hsCRP screening) was not statistically significant (number needed to treat = 19; 95% CI, 8.9 if helpful to 158.3 if harmful).
Further examination of live birth restricted to women with high hsCRP (hsCRP ≥ 1.95 mg/L), stratified by waist-to-hip ratio as a measure of central adiposity, showed a positive effect of LDA on live birth among women with a waist-to-hip below the median [relative risk (RR), 1.60; 95% CI, 1.11 to 2.30) and a weaker effect among women above the waist-to-hip median (RR, 1.18; 95% CI, 0.89 to 1.56). Repeating this analysis, stratifying instead on normal BMI (n = 81; median hsCRP, 3.27 mg/L; interquartile range, 2.26 to 4.63) vs overweight/obese BMI (n = 293; median hsCRP, 3.99; interquartile range, 2.89 to 5.35), produced similar results with an increase in live birth among normal-weight women (LDA 76% vs placebo 50%; RR, 1.53; 95% CI, 1.00 to 2.35) but no statistically significant increase among overweight/obese women (LDA 49% vs placebo 42%; RR, 1.16; 95% CI, 0.88 to 1.53).
LDA had no effect on clinical pregnancy losses. However, LDA was associated with a slight increase in the overall risk of pregnancy loss among the mid-hsCRP group before excluding hsCRP ≥10 mg/L. When only women with hsCRP <10 mg/L were considered, all associations between LDA treatment and pregnancy loss were null (Supplemental Table 1).
Last, similar to the pregnancy and live birth findings, longitudinal hsCRP concentrations across pregnancy (8, 20, and 36 weeks’ gestation) were lower among women assigned to LDA compared with placebo in the highest baseline hsCRP tertile (P = 0.04), but no treatment differences were observed in the middle and lower hsCRP tertiles (Fig. 3).
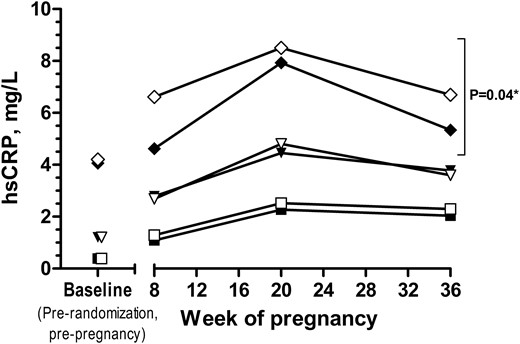
Difference in hsCRP concentrations in LDA vs placebo groups during pregnancy. Symbols indicate geometric means of log-transformed hsCRP at baseline (prerandomization) and throughout pregnancy. Squares indicate the lower hsCRP tertile (closed, LDA; open, placebo), triangles indicate the middle hsCRP tertile (closed, LDA; open, placebo), and diamonds indicate the higher hsCRP tertile (closed, LDA; open, placebo). *Notation of significance indicates treatment group (LDA vs placebo) difference across pregnancy (excluding baseline) from generalized estimating equations models within each hsCRP tertile (assigned at baseline).
Discussion
These findings elucidate two concepts: (1) systemic, chronic, low-grade inflammation may harm women’s ability to become pregnant and their inflammation changes through pregnancy, and (2) this detriment may be restored to expected levels using preconception-initiated LDA therapy. Specifically, among women with relatively elevated baseline hsCRP having a history of pregnancy loss, LDA restored clinically confirmed pregnancy and live birth rates and lowered hsCRP throughout pregnancy. Moreover, LDA did not reduce pregnancy loss but increased pregnancy rates by 31% among women entering the study with hsCRP concentrations among the top one-third of the study population. Such an effect was most predominant among lean women with higher hsCRP (women with normal BMI or below median waist-to-hip ratio), in whom the live birth rate increased by 60% with LDA treatment. Furthermore, preconception-initiated LDA therapy lowered hsCRP concentrations throughout pregnancy, with ∼20% lower hsCRP concentrations at 36 weeks’ gestation, in women with high hsCRP at baseline.
Inflammation in reproduction has been studied primarily in the context of pregnancy and its complications, assisted reproductive technologies, and gynecologic pathology (e.g., polycystic ovary syndrome). The current findings among healthy women with mildly elevated hsCRP, however, are consistent with randomized trials of trace mineral treatment interventions in women with polycystic ovary syndrome reporting increased pregnancy rates concomitant with lowering hsCRP (27, 28). Notably, women in the current study were excluded if polycystic ovary syndrome (or other such conditions) were self-reported to be previously diagnosed by a physician, although the presence of undiagnosed or unreported disease cannot be ruled out. Also, the differential impact of inflammatory status on reproductive success observed here may help explain prior findings of baseline hsCRP being unrelated to pregnancy rates following assisted reproductive technologies in a study of UK women selected to be nonobese having relatively lower hsCRP overall (29), compared with a report of higher baseline hsCRP being implicated in lower pregnancy success in another assisted reproductive technologies population with overall wider range of hsCRP in study subjects (30). Certainly, inflammation impeding reproductive processes is plausible given the delicately controlled inflammatory mechanisms involving interleukins, tumor necrosis factor–α, and other inflammatory mediators that are necessary in the complex stages leading to ovulation (31) and in endometrial receptivity and embryo implantation (32). Indeed, previous data describing the sex ratio of offspring in the EAGeR trial suggested that higher inflammation discordantly affected viability of male compared with female conceptions (33). Clearly, mild inflammation may have more substantial population-level effects on reproduction than previously thought.
Because cyclooxygenase-2 function, which is directly inhibited by aspirin, is interrelated with many inflammatory mediators involved in reproductive processes, aspirin may inhibit downstream effects of chronically upregulated inflammatory pathways (34, 35). In addition, it is plausible that processes of repair, scarring, and embryo receptivity in women with a history of pregnancy loss, which affects 20% to 30% of all conceptions (36, 37), may be linked to systemic, chronic inflammation that is improved with LDA. Importantly, when we excluded hsCRP values ≥10 mg/L, a level that prompts retesting in clinical cardiovascular risk assessment and is consistent with acute phase inflammatory events (e.g., infection) (20, 21), the effect of LDA increased considerably. Of note, a previous study reported that 80% of observed hsCRP concentrations ≥10 mg/L were associated with a self-report of a cold or flu episode in the preceding 90 days (20), explaining why such values are considered uninformative in characterizing chronic low-grade inflammation.
In addition to its effects on attaining pregnancy, LDA suppressed hsCRP elevation among women in the highest hsCRP tertile during pregnancy, a time when inflammation is known to increase (38, 39), but with no effect on hsCRP changes in women with a lower preconception hsCRP level. This finding is consistent with prior reports indicating that aspirin treatment lowered hsCRP concentrations in patients with inflammatory burden (e.g., cardiovascular disease, metabolic syndrome) (40, 41) but did not affect inflammation or health outcomes among participants who were healthy or without inflammation-related pathology (42, 43). Prepregnancy hsCRP and impacts on the subsequent hsCRP trajectory in pregnancy could have important clinical and population heath implications given the breadth of evidence indicating a link between preconception and late-pregnancy hsCRP, in addition to the link between hsCRP measured in pregnancy predicting risk of important and prevalent pregnancy complications, including gestational diabetes, preeclampsia, and low birthweight (14–16). Larger studies are now required to determine whether screening hsCRP and using risk-based LDA therapy prior to conception would reduce the prevalence of such complications, an effect that would have tremendous public health impacts.
Like pregnancy, obesity is a state of increased inflammatory burden, and as expected, we observed increasing mean BMI across the tertiles of hsCRP concentrations. However, although average differences in BMI across hsCRP tertiles reflect this relationship, 14% to 21% of women in the lowest hsCRP tertile were overweight or obese, and conversely, 15% to 26% of women in the highest hsCRP tertile were not overweight or obese. Thus, BMI or central adiposity status was not necessarily consistent with hsCRP status. Furthermore, a statistically significant effect of LDA was identified among normal weight, but a lesser, nonsignificant effect was observed in overweight/obese women with higher hsCRP, indicating that LDA may affect pathways of inflammation independent of those linked to adiposity. Alternatively, a higher aspirin dose may be required to elicit positive effects in women with larger body mass.
Regarding risks of using LDA for improvement of reproductive outcomes, comparison with its utilization in cardiovascular health is useful. For example, in parallel to the current study, middle-aged men in the highest quartile of hsCRP experienced a 56% reduction in myocardial infarction events with aspirin treatment, with attenuated effects in patients with lower hsCRP (13). However, 118 men must be treated with LDA to prevent one myocardial infarction event over 5 years (7), whereas the current study indicates hsCRP screening could reduce the number needed to treat with LDA to only seven women for every one live birth gained in 6 months of attempting pregnancy if such practice were to be adopted in the future. Moreover, use of LDA in reproductive-age women for purposes of achieving pregnancy and live birth would represent a time-limited treatment period compared with indefinite use for cardiovascular event protection in older adults. As such, hsCRP screening to inform targeted therapy to reduce unnecessary burden and/or risk may be considered in future evaluation of using LDA in healthy women. Among EAGeR participants, LDA therapy was well tolerated (44). Also, given the notably lower rate of pregnancy loss in the mid-hsCRP placebo group, rather than higher/increased losses in the LDA group, we believe our finding of increased pregnancy loss in this group, when women with >10 mg/L were included, was a chance finding. Currently, LDA is recommended after 12 weeks’ gestation for reducing risk of preeclampsia in high-risk pregnant women (8).
The size of this study, which allowed for stratification by hsCRP level, and the block-randomized, double-blind, placebo-controlled design with prospective monitoring of conception and clinical pregnancy confirmation were notable strengths. One drawback was the trial limiting observation to six cycles of attempted conception; thus, our results cannot be extrapolated to women (or couples) with conception delay and infertility. Furthermore, our population only included women with a history of pregnancy loss; however, up to 20% to 30% of all conceptions end in loss (36, 37), making this population relevant to a large proportion of women trying to conceive. Furthermore, pregnancy loss may lead to greater anxiety about becoming pregnant again; thus, interventions to help women in this situation may be of particular value. In addition, the study population was predominantly (95%) white, educated, and economically advantaged, limiting the generalizability of these findings. Lastly, although women were advised not to use aspirin while participating in this study, as with any study of a low-cost, widely available intervention, some women may have used aspirin over-the-counter. However, given the double-blind, randomized nature of the study, we would expect that aspirin was not differentially used between treatment groups; thus, our findings are unlikely to be affected by bias stemming from over-the-counter availability of aspirin.
In summary, LDA increased the chances of clinically recognized pregnancy and live birth in women with moderately elevated hsCRP and prior pregnancy loss. hsCRP is attractive as a screening test because it is an established biomarker, for which standardized, relatively inexpensive clinical assays are readily available. In addition to replicating the present findings, next steps should include further data collection to examine inflammation as related both to fertility and pregnancy outcomes. These collective findings should inform further discussion of any expanded use of aspirin in reproductive medicine, as well as the potential use of hsCRP as an informative screening test to guide its clinical application once appropriate clinical cut-points in this setting are also determined.
Abbreviations:
- BMI
body mass index
- CI
confidence interval
- EAGeR
Effects of Aspirin in Gestation and Reproduction
- hCG
human chorionic gonadotropin
- hsCRP
high-sensitivity C-reactive protein
- LDA
low-dose aspirin
- RR
relative risk.
Acknowledgments
We thank the EAGeR participants for their extraordinary commitment to the study, all of the EAGeR investigators and staff who devoted their time and energy to the success of this trial, and the data safety and monitoring board members for ongoing oversight, constant support, and advice throughout the trial.
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland (contract Nos. HHSN267200603423, HHSN267200603424, and HHSN267200603426).
Clinical trial registry: ClinicalTrials.gov no. NCT00467363 (registered 27 April 2007).
Disclosure Summary: The authors have nothing to disclose.
References
Escobar-Morreale HF, Luque-Ramirez M, Gonzalez F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril. 2011;95:1048–1058.e1–2.
Author notes
Address all correspondence and requests for reprints to: Lindsey A. Sjaarda, PhD, 6710B Rockledge Dr, MSC 7004, Bethesda, Maryland 20892. E-mail: [email protected].



