-
PDF
- Split View
-
Views
-
Cite
Cite
Alfredo Berruti, Salvatore Grisanti, Alina Pulzer, Mélanie Claps, Fulvia Daffara, Paola Loli, Massimo Mannelli, Marco Boscaro, Emanuela Arvat, Guido Tiberio, Stefanie Hahner, Barbara Zaggia, Francesco Porpiglia, Marco Volante, Martin Fassnacht, Massimo Terzolo, Long-Term Outcomes of Adjuvant Mitotane Therapy in Patients With Radically Resected Adrenocortical Carcinoma, The Journal of Clinical Endocrinology & Metabolism, Volume 102, Issue 4, 1 April 2017, Pages 1358–1365, https://doi.org/10.1210/jc.2016-2894
Close - Share Icon Share
Abstract
In 2007, a retrospective case-control study provided evidence that adjuvant mitotane prolongs recurrence-free survival (RFS) in patients with radically resected adrenocortical carcinoma (ACC).
We aimed to confirm the prognostic role of adjuvant mitotane in the same series after 9 additional years of follow-up.
One hundred sixty-two ACC patients who did not recur or die after a landmark period of 3 months were considered. Forty-seven patients were enrolled in four Italian centers where adjuvant mitotane was routinely recommended (mitotane group), 45 patients in four Italian centers where no adjuvant strategy was undertaken (control group 1), and 70 German patients left untreated after surgery (control group 2).
The primary aim was RFS, the secondary was overall survival.
An increased risk of recurrence was found in both control cohorts [group 1: hazard ratio (HR) = 2.98; 95% confidence interval (CI), 1.75 to 5.09; P < 0.0001; group 2: HR = 2.61; 95% CI, 1.56 to 4.36; P < 0.0001] compared with the mitotane group. The risk of death was higher in control group 1 (HR = 2.03; 95% CI, 1.17 to 3.51; P = 0.011) but not in control group 2 (HR = 1.60; 95% CI, 0.94 to 2.74; P = 0.083), which had better prognostic factors and more aggressive treatment of recurrences than control group 1. The benefit of adjuvant mitotane on RFS was observed regardless of the hormone secretory status.
Adjuvant mitotane is associated with prolonged RFS, without any apparent influence by the tumor secretory status. The retrospective nature of the study is a major limitation.
Radical surgery can potentially cure patients with adrenocortical carcinoma (ACC), a rare and aggressive endocrine malignancy (1). However, ACC has a high propensity to recur and tumor recurrence affects significantly life expectancy of ACC patients (2–4). Although complete tumor removal is an important prognostic factor, achieving the surgical radicality status does not prevent disease recurrence (1–4). This provides a rationale for adjuvant therapy and mitotane, an adrenolytic drug with an established role in the treatment of patients with advanced ACC, has been used in the adjuvant setting (1).
In 2007, we reported the results of a multicenter, retrospective, case-control study comparing the outcome of ACC patients managed at some Italian centers, where adjuvant mitotane treatment was used following radical surgery, with that of patients managed at other Italian centers, where adjuvant strategies were not adopted. To further control for potential biases, a cohort of German patients who were treated with surgery only was added as a second control group (5). The main strength of this study was that it included patients whose treatment assignment was not related to their characteristics but to the center policy. Recurrence-free survival (RFS) was significantly prolonged in the mitotane group when compared with both control groups. Therefore, the study provided evidence that adjuvant mitotane may be of benefit to patients with radically resected ACC. The retrospective nature of the study, however, does not allow to definitively support adjuvant mitotane treatment of all patients. The study renewed interest in adjuvant mitotane treatment but attracted criticisms (1, 6). A major concern was the duration of follow-up, which was considered not long enough due to a relatively low number of recurrences and deaths in patients receiving adjuvant mitotane.
In the current study, we report the long-term outcomes of the original patient series with 9 additional years of follow-up. The primary aim was to confirm the prognostic role of adjuvant mitotane therapy on RFS; secondary aims were to assess the effects of mitotane on overall survival (OS) and the predictive role of cortisol hypersecretion on mitotane efficacy.
Patients and Methods
The study details were reported previously (5). Briefly, we reviewed the outcome of 102 consecutive patients with ACC who had undergone radical surgery at eight tertiary referral centers in Italy from 1985 through 2003. Inclusion criteria were age greater than 18 years, pathological diagnosis of ACC, and the availability of preoperative and postoperative computed tomographic or magnetic resonance imaging scans. Exclusion criteria were macroscopically incomplete resection, incomplete tumor staging, concomitant cancers within the previous 5 years, clinically significant concomitant diseases, and adjuvant therapies other than mitotane. Forty-seven patients were enrolled in four centers where adjuvant mitotane was recommended irrespective of patient and tumor characteristics (mitotane group), whereas 55 patients were enrolled in four centers where no adjuvant strategy was undertaken after surgery (control group 1). The German control group comprised 75 ACC patients from the German Adrenocortical Carcinoma Registry with available information on diagnostic procedures, surgical outcomes, and follow-up similar to those used to evaluate the Italian study population (control group 2). The institutional ethics committee at each clinical center approved the study.
Complete resection was defined as no evidence of macroscopic residual disease on the basis of surgical reports, histopathological analysis, and postoperative imaging. All histologic diagnoses were confirmed by experienced pathologists and reviewed centrally in more than 75% of cases (6). Staging was reported according to the McFarlane–Sullivan criteria (4). Follow-up visits, including images, were performed every 6 months. Disease recurrence was defined as radiologic evidence of a new lesion during follow-up. Follow-up for this study was closed in December 2013.
The primary aim was to compare RFS, defined as the time elapsing from the date of surgery to the first documentation of recurrence, in patients who received adjuvant mitotane therapy with that of patients who did not. Secondary aims were OS, defined as the time elapsing from the date of surgery to the date of death. For both RFS and OS, patients who did not experience the event (recurrence or death, respectively) were censored at the time of last follow-up examination.
Data analysis was done using SPSS version 17 (SPSS Inc., IBM). RFS and OS were estimated according to the Kaplan–Meier method; the respective comparisons between groups were performed using the log-rank test. The Cox regression model was used to assess in univariate and multivariate analyses the predictive role of mitotane treatment, clinical, and pathological variables on RFS and OS. The likelihood ratio was used to assess the significance of covariates included in each model. All P values are two-sided, and results were considered significant at P ≤ 0.05. The Cox analysis was also used to assess the presence of heterogeneity in the prognostic effect of the cortisol excess in patients stratified according to hormone secretion. In these subgroups, any modification of the prognostic effect was assessed by including the appropriate covariate interaction terms in the model. To reduce the inherent bias of patients with early progression and death, all survival analyses were performed with the landmark method, using a fixed landmark point at month 3. Patients who experienced the event (recurrence or death) before the landmark point were excluded from the analysis.
Results
Of the 177 patients included in the original study (5), 15 recurred or died within the first 3 months (landmark point), 10 patients in control group 1 and five patients in control group 2, and were therefore excluded from the present analysis. Among the fully assessable 162 patients, 47 patients received adjuvant mitotane (mitotane group), 45 patients formed control group 1, and 70 patients formed control group 2. Patient characteristics are detailed in Table 1.
| Characteristic . | Mitotane Group (N = 47) . | Control Group 1 (N = 45) . | P Value . | Control Group 2 (N = 70) . | P Value . |
|---|---|---|---|---|---|
| Age, y | |||||
| Median | 42.0 | 44.0 | 0.26 | 49.4 | 0.01 |
| Range | 18–67 | 21–73 | 19–83 | ||
| Sex, n (%) | |||||
| Male | 11 (23) | 18 (40) | 0.09 | 23 (33) | 0.27 |
| Female | 36 (77) | 27 (60) | 47 (67) | ||
| Tumor stage, n (%) | |||||
| I | 3 (6) | 4 (9) | 0.83 | 9 (13) | 0.02 |
| II | 27 (57) | 25 (57) | 49 (70) | ||
| III | 11 (24) | 11 (25.0) | 11 (16) | ||
| IV | 6 (13) | 4 (9) | 1 (1) | ||
| Functional status, n (%) | |||||
| Evaluated patients, n | 47 | 45 | 45 | ||
| Secreting tumors | 24 (51) | 17 (38) | 0.20 | 27 (60) | 0.39 |
| Glucocorticoids with or without androgens | 22 (47) | 12 (27) | 22 (49) | ||
| Androgens | 2 (4) | 3 (7) | 4 (9) | ||
| Aldosterone | 0 | 1 (2) | 1 (2) | ||
| Estradiol | 0 | 1 (2) | 0 | ||
| Nonsecreting tumors | 23 (49) | 28 (62) | 18 (40) | ||
| Weiss score | 0.1 | ||||
| Total patients evaluated, n | 45 | 39 | 41 | ||
| Median (range) | 6 (3–9) | 5 (3–7) | 5 (3–9) |
| Characteristic . | Mitotane Group (N = 47) . | Control Group 1 (N = 45) . | P Value . | Control Group 2 (N = 70) . | P Value . |
|---|---|---|---|---|---|
| Age, y | |||||
| Median | 42.0 | 44.0 | 0.26 | 49.4 | 0.01 |
| Range | 18–67 | 21–73 | 19–83 | ||
| Sex, n (%) | |||||
| Male | 11 (23) | 18 (40) | 0.09 | 23 (33) | 0.27 |
| Female | 36 (77) | 27 (60) | 47 (67) | ||
| Tumor stage, n (%) | |||||
| I | 3 (6) | 4 (9) | 0.83 | 9 (13) | 0.02 |
| II | 27 (57) | 25 (57) | 49 (70) | ||
| III | 11 (24) | 11 (25.0) | 11 (16) | ||
| IV | 6 (13) | 4 (9) | 1 (1) | ||
| Functional status, n (%) | |||||
| Evaluated patients, n | 47 | 45 | 45 | ||
| Secreting tumors | 24 (51) | 17 (38) | 0.20 | 27 (60) | 0.39 |
| Glucocorticoids with or without androgens | 22 (47) | 12 (27) | 22 (49) | ||
| Androgens | 2 (4) | 3 (7) | 4 (9) | ||
| Aldosterone | 0 | 1 (2) | 1 (2) | ||
| Estradiol | 0 | 1 (2) | 0 | ||
| Nonsecreting tumors | 23 (49) | 28 (62) | 18 (40) | ||
| Weiss score | 0.1 | ||||
| Total patients evaluated, n | 45 | 39 | 41 | ||
| Median (range) | 6 (3–9) | 5 (3–7) | 5 (3–9) |
All P values are for comparisons between each control group and the mitotane group. Patients with stage IV ACC had infiltration of adjacent organs; none had distant metastases.
| Characteristic . | Mitotane Group (N = 47) . | Control Group 1 (N = 45) . | P Value . | Control Group 2 (N = 70) . | P Value . |
|---|---|---|---|---|---|
| Age, y | |||||
| Median | 42.0 | 44.0 | 0.26 | 49.4 | 0.01 |
| Range | 18–67 | 21–73 | 19–83 | ||
| Sex, n (%) | |||||
| Male | 11 (23) | 18 (40) | 0.09 | 23 (33) | 0.27 |
| Female | 36 (77) | 27 (60) | 47 (67) | ||
| Tumor stage, n (%) | |||||
| I | 3 (6) | 4 (9) | 0.83 | 9 (13) | 0.02 |
| II | 27 (57) | 25 (57) | 49 (70) | ||
| III | 11 (24) | 11 (25.0) | 11 (16) | ||
| IV | 6 (13) | 4 (9) | 1 (1) | ||
| Functional status, n (%) | |||||
| Evaluated patients, n | 47 | 45 | 45 | ||
| Secreting tumors | 24 (51) | 17 (38) | 0.20 | 27 (60) | 0.39 |
| Glucocorticoids with or without androgens | 22 (47) | 12 (27) | 22 (49) | ||
| Androgens | 2 (4) | 3 (7) | 4 (9) | ||
| Aldosterone | 0 | 1 (2) | 1 (2) | ||
| Estradiol | 0 | 1 (2) | 0 | ||
| Nonsecreting tumors | 23 (49) | 28 (62) | 18 (40) | ||
| Weiss score | 0.1 | ||||
| Total patients evaluated, n | 45 | 39 | 41 | ||
| Median (range) | 6 (3–9) | 5 (3–7) | 5 (3–9) |
| Characteristic . | Mitotane Group (N = 47) . | Control Group 1 (N = 45) . | P Value . | Control Group 2 (N = 70) . | P Value . |
|---|---|---|---|---|---|
| Age, y | |||||
| Median | 42.0 | 44.0 | 0.26 | 49.4 | 0.01 |
| Range | 18–67 | 21–73 | 19–83 | ||
| Sex, n (%) | |||||
| Male | 11 (23) | 18 (40) | 0.09 | 23 (33) | 0.27 |
| Female | 36 (77) | 27 (60) | 47 (67) | ||
| Tumor stage, n (%) | |||||
| I | 3 (6) | 4 (9) | 0.83 | 9 (13) | 0.02 |
| II | 27 (57) | 25 (57) | 49 (70) | ||
| III | 11 (24) | 11 (25.0) | 11 (16) | ||
| IV | 6 (13) | 4 (9) | 1 (1) | ||
| Functional status, n (%) | |||||
| Evaluated patients, n | 47 | 45 | 45 | ||
| Secreting tumors | 24 (51) | 17 (38) | 0.20 | 27 (60) | 0.39 |
| Glucocorticoids with or without androgens | 22 (47) | 12 (27) | 22 (49) | ||
| Androgens | 2 (4) | 3 (7) | 4 (9) | ||
| Aldosterone | 0 | 1 (2) | 1 (2) | ||
| Estradiol | 0 | 1 (2) | 0 | ||
| Nonsecreting tumors | 23 (49) | 28 (62) | 18 (40) | ||
| Weiss score | 0.1 | ||||
| Total patients evaluated, n | 45 | 39 | 41 | ||
| Median (range) | 6 (3–9) | 5 (3–7) | 5 (3–9) |
All P values are for comparisons between each control group and the mitotane group. Patients with stage IV ACC had infiltration of adjacent organs; none had distant metastases.
The mitotane group and control group 1 were well balanced with respect to tumor stage, hormone secretory status, and Weiss score. Control group 2 had a lower proportion of patients with stage III to IV ACC than the mitotane group. In addition, patients in control group 2 were significantly older than patients in the mitotane group. There were no major differences in the surgical approach among the various groups as most patients of all groups underwent open surgery compared with laparoscopic surgery (mitotane group, 94%; control group 1, 96%; and control group 2, 89%).
The median follow-up period after surgery of surviving patients at the last follow-up examination was 141 months (range, 42 to 199) in the mitotane group, 142 months (range, 25 to 219) in control group 1, and 128 months (range, 38 to 323) in control group 2. Recurrence was documented in 121 patients (75%): 25 in the mitotane group (53%), 40 in control group 1 (89%), and 56 in control group 2 (80%). The pattern of recurrence between the three groups was comparable and is detailed in Table 2. Treatment of recurrence included more frequently surgery in both the mitotane group and control group 2 than control group 1 (64%, 66%, and 35%, respectively). Death from ACC was reported in 100 patients (62%): 23 in the mitotane group (49%), 36 (90%) in control group 1, and 41 (59%) in control group 2.
| Patient Group (Recurrences, N) . | Site of Recurrence . | Treatment of Recurrence . | |||||
|---|---|---|---|---|---|---|---|
| Local, N (%) . | Liver, N (%) . | Lung, N (%) . | Multiple, N (%) . | Surgery, N (%) . | Systemic Therapy, N (%) . | Other, N (%) . | |
| Group 1 (25) | 12 (48) | 4 (16) | 5 (20) | 4 (16) | 16 (64) | 8 (32) | 1 (4) |
| Group 2 (40) | 20 (50) | 4 (10) | 7 (17) | 9 (23) | 14 (35) | 23 (58) | 3 (7) |
| Group 3 (56) | 29 (52) | 5 (9) | 6 (11) | 16 (28) | 37 (66) | 16 (29) | 3 (5) |
| Patient Group (Recurrences, N) . | Site of Recurrence . | Treatment of Recurrence . | |||||
|---|---|---|---|---|---|---|---|
| Local, N (%) . | Liver, N (%) . | Lung, N (%) . | Multiple, N (%) . | Surgery, N (%) . | Systemic Therapy, N (%) . | Other, N (%) . | |
| Group 1 (25) | 12 (48) | 4 (16) | 5 (20) | 4 (16) | 16 (64) | 8 (32) | 1 (4) |
| Group 2 (40) | 20 (50) | 4 (10) | 7 (17) | 9 (23) | 14 (35) | 23 (58) | 3 (7) |
| Group 3 (56) | 29 (52) | 5 (9) | 6 (11) | 16 (28) | 37 (66) | 16 (29) | 3 (5) |
The category surgery includes also patients treated with surgery and other modalities.
| Patient Group (Recurrences, N) . | Site of Recurrence . | Treatment of Recurrence . | |||||
|---|---|---|---|---|---|---|---|
| Local, N (%) . | Liver, N (%) . | Lung, N (%) . | Multiple, N (%) . | Surgery, N (%) . | Systemic Therapy, N (%) . | Other, N (%) . | |
| Group 1 (25) | 12 (48) | 4 (16) | 5 (20) | 4 (16) | 16 (64) | 8 (32) | 1 (4) |
| Group 2 (40) | 20 (50) | 4 (10) | 7 (17) | 9 (23) | 14 (35) | 23 (58) | 3 (7) |
| Group 3 (56) | 29 (52) | 5 (9) | 6 (11) | 16 (28) | 37 (66) | 16 (29) | 3 (5) |
| Patient Group (Recurrences, N) . | Site of Recurrence . | Treatment of Recurrence . | |||||
|---|---|---|---|---|---|---|---|
| Local, N (%) . | Liver, N (%) . | Lung, N (%) . | Multiple, N (%) . | Surgery, N (%) . | Systemic Therapy, N (%) . | Other, N (%) . | |
| Group 1 (25) | 12 (48) | 4 (16) | 5 (20) | 4 (16) | 16 (64) | 8 (32) | 1 (4) |
| Group 2 (40) | 20 (50) | 4 (10) | 7 (17) | 9 (23) | 14 (35) | 23 (58) | 3 (7) |
| Group 3 (56) | 29 (52) | 5 (9) | 6 (11) | 16 (28) | 37 (66) | 16 (29) | 3 (5) |
The category surgery includes also patients treated with surgery and other modalities.
Mitotane was given following a low-dose regimen (7), and median duration of adjuvant treatment was 42 months (range, 4 to 162). Of the mitotane-treated patients, 11 patients suspended treatment while being free of disease as a decision of the treating physicians (end of the planned course of therapy), five patients discontinued definitively mitotane for toxicity (one for leucopenia, one for elevation in liver enzymes, two for general toxicity, and one for neurologic toxicity), and two discontinued mitotane at the time of disease recurrence. Mitotane was continued in the other patients with recurrent ACC. Adverse events of mitotane treatment are given in Table 3.
| Event . | Grade (Patients, N) . | |||
|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | |
| Anorexia | 20 | 7 | 0 | 0 |
| Asthenia or fatigue | 17 | 6 | 2 | 0 |
| Ataxia | 2 | 2 | 4 | 0 |
| Confusion | 5 | 6 | 2 | 0 |
| Diarrhea | 10 | 5 | 0 | 0 |
| Elevated GGT | 20 | 13 | 7 | 0 |
| Elevated AST/ALT | 19 | 4 | 1 | 0 |
| Gynecomastia | 2 | 2 | 0 | 0 |
| Leukopenia | 4 | 1 | 1 | 0 |
| Nausea or vomiting | 14 | 11 | 3 | 0 |
| Vertigo | 4 | 5 | 4 | 0 |
| Event . | Grade (Patients, N) . | |||
|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | |
| Anorexia | 20 | 7 | 0 | 0 |
| Asthenia or fatigue | 17 | 6 | 2 | 0 |
| Ataxia | 2 | 2 | 4 | 0 |
| Confusion | 5 | 6 | 2 | 0 |
| Diarrhea | 10 | 5 | 0 | 0 |
| Elevated GGT | 20 | 13 | 7 | 0 |
| Elevated AST/ALT | 19 | 4 | 1 | 0 |
| Gynecomastia | 2 | 2 | 0 | 0 |
| Leukopenia | 4 | 1 | 1 | 0 |
| Nausea or vomiting | 14 | 11 | 3 | 0 |
| Vertigo | 4 | 5 | 4 | 0 |
Adverse events were graded accorded to the National Cancer Institute–Common Terminology Criteria for Adverse Events. Most patients had multiple adverse events. Because of the retrospective nature of the study, underreporting of side effects should be considered a possibility.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; GGT, gamma-glutamyl transferase.
| Event . | Grade (Patients, N) . | |||
|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | |
| Anorexia | 20 | 7 | 0 | 0 |
| Asthenia or fatigue | 17 | 6 | 2 | 0 |
| Ataxia | 2 | 2 | 4 | 0 |
| Confusion | 5 | 6 | 2 | 0 |
| Diarrhea | 10 | 5 | 0 | 0 |
| Elevated GGT | 20 | 13 | 7 | 0 |
| Elevated AST/ALT | 19 | 4 | 1 | 0 |
| Gynecomastia | 2 | 2 | 0 | 0 |
| Leukopenia | 4 | 1 | 1 | 0 |
| Nausea or vomiting | 14 | 11 | 3 | 0 |
| Vertigo | 4 | 5 | 4 | 0 |
| Event . | Grade (Patients, N) . | |||
|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | |
| Anorexia | 20 | 7 | 0 | 0 |
| Asthenia or fatigue | 17 | 6 | 2 | 0 |
| Ataxia | 2 | 2 | 4 | 0 |
| Confusion | 5 | 6 | 2 | 0 |
| Diarrhea | 10 | 5 | 0 | 0 |
| Elevated GGT | 20 | 13 | 7 | 0 |
| Elevated AST/ALT | 19 | 4 | 1 | 0 |
| Gynecomastia | 2 | 2 | 0 | 0 |
| Leukopenia | 4 | 1 | 1 | 0 |
| Nausea or vomiting | 14 | 11 | 3 | 0 |
| Vertigo | 4 | 5 | 4 | 0 |
Adverse events were graded accorded to the National Cancer Institute–Common Terminology Criteria for Adverse Events. Most patients had multiple adverse events. Because of the retrospective nature of the study, underreporting of side effects should be considered a possibility.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; GGT, gamma-glutamyl transferase.
Mitotane treatment was associated with longer RFS compared with both control groups [Fig. 1(A)]. The median RFS was 42 months in the mitotane group, 17 months in control group 1 (P < 0.001), and 26 months in control group 2 (P = 0.005). The median OS was 161 months in the mitotane group, compared with 65 months in control group 1 (P = 0.007) and 92 months in control group 2 [P = 0.28; Fig. 1(B)].
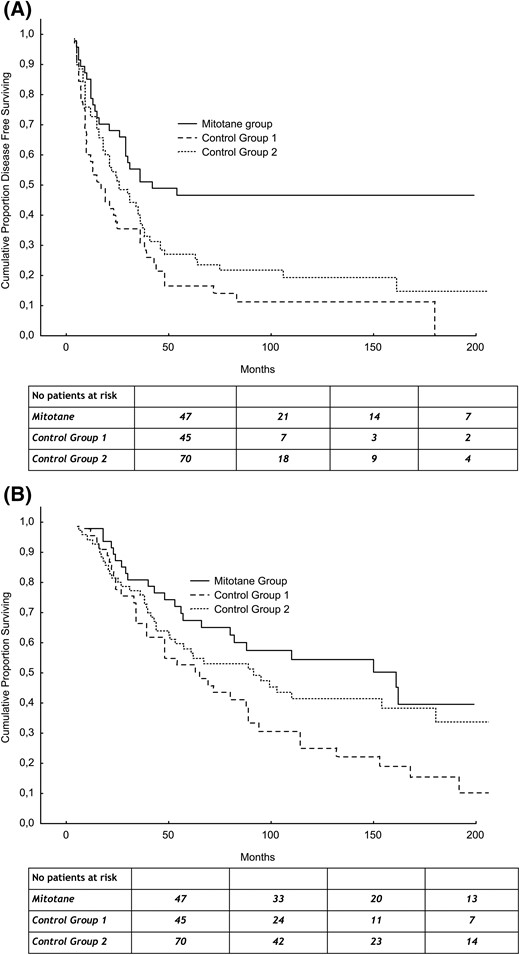
(A) RFS of adjuvant mitotane group and control groups. (B) OS of adjuvant mitotane group and control groups.
Table 4 reports the results of the univariate and multivariate Cox analyses. Adjuvant mitotane treatment was an independent predictive factor for RFS in multivariate analysis after adjusting for age, sex, and tumor stage. The risk of recurrence was significantly higher either in control group 1 [hazard ratio (HR) = 2.98; 95% confidence interval (CI), 1.75 to 5.09; P < 0.0001] or in control group 2 (HR = 2.61; 95% CI, 1.56 to 4.36; P < 0.0001) when compared with the mitotane group.
Effect of Mitotane on RFS and OS According to Univariate and Multivariate Analyses
| . | Univariate Analysis . | Multivariate Analysisa . | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| RFS | ||||||
| Adjuvant mitotane | 1 | 1 | ||||
| Control group 1 | 2.55 | 1.54–4.21 | <0.0001 | 2.98 | 1.75–5.09 | <0.0001 |
| Control group 2 | 1.87 | 1.17–3.00 | 0.009 | 2.61 | 1.56–4.36 | <0.0001 |
| Sex | 1.33 | 0.92–1.94 | 0.13 | 1.27 | 0.86–1.88 | 0.22 |
| Age | 0.98 | 0.97–0.99 | 0.011 | 0.98 | 0.96–0.99 | 0.002 |
| Stage | ||||||
| I | 1 | 1 | ||||
| II | 1.62 | 0.81–3.25 | 0.17 | 1.96 | 0.97–3.94 | 0.060 |
| III | 1.88 | 0.88–4.02 | 0.10 | 2.36 | 1.09–5.12 | 0.029 |
| IV | 2.08 | 0.80–5.39 | 0.13 | 4.45 | 1.62–12.24 | 0.004 |
| OS | ||||||
| Adjuvant mitotane | 1 | 1 | ||||
| Control group 1 | 2.007 | 1.19–3.39 | 0.009 | 2.03 | 1.17–3.51 | 0.011 |
| Control group 2 | 1.337 | 0.80–2.23 | 0.266 | 1.60 | 0.94–2.74 | <0.083 |
| Sex | 1.18 | 0.78–1.77 | 0.44 | 1.09 | 0.71–1.69 | 0.69 |
| Age | 0.99 | 0.97–1.00 | 0.21 | 0.99 | 0.97–1.00 | 0.17 |
| Stage | ||||||
| I | 1 | 1 | ||||
| II | 1.81 | 0.78–4.20 | 0.17 | 1.85 | 0.79–4.32 | 0.16 |
| III | 2.41 | 0.98–5.95 | 0.06 | 2.37 | 0.95–5.93 | 0.065 |
| IV | 2.75 | 0.98–7.75 | 0.055 | 3.20 | 1.11–9.28 | 0.032 |
| . | Univariate Analysis . | Multivariate Analysisa . | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| RFS | ||||||
| Adjuvant mitotane | 1 | 1 | ||||
| Control group 1 | 2.55 | 1.54–4.21 | <0.0001 | 2.98 | 1.75–5.09 | <0.0001 |
| Control group 2 | 1.87 | 1.17–3.00 | 0.009 | 2.61 | 1.56–4.36 | <0.0001 |
| Sex | 1.33 | 0.92–1.94 | 0.13 | 1.27 | 0.86–1.88 | 0.22 |
| Age | 0.98 | 0.97–0.99 | 0.011 | 0.98 | 0.96–0.99 | 0.002 |
| Stage | ||||||
| I | 1 | 1 | ||||
| II | 1.62 | 0.81–3.25 | 0.17 | 1.96 | 0.97–3.94 | 0.060 |
| III | 1.88 | 0.88–4.02 | 0.10 | 2.36 | 1.09–5.12 | 0.029 |
| IV | 2.08 | 0.80–5.39 | 0.13 | 4.45 | 1.62–12.24 | 0.004 |
| OS | ||||||
| Adjuvant mitotane | 1 | 1 | ||||
| Control group 1 | 2.007 | 1.19–3.39 | 0.009 | 2.03 | 1.17–3.51 | 0.011 |
| Control group 2 | 1.337 | 0.80–2.23 | 0.266 | 1.60 | 0.94–2.74 | <0.083 |
| Sex | 1.18 | 0.78–1.77 | 0.44 | 1.09 | 0.71–1.69 | 0.69 |
| Age | 0.99 | 0.97–1.00 | 0.21 | 0.99 | 0.97–1.00 | 0.17 |
| Stage | ||||||
| I | 1 | 1 | ||||
| II | 1.81 | 0.78–4.20 | 0.17 | 1.85 | 0.79–4.32 | 0.16 |
| III | 2.41 | 0.98–5.95 | 0.06 | 2.37 | 0.95–5.93 | 0.065 |
| IV | 2.75 | 0.98–7.75 | 0.055 | 3.20 | 1.11–9.28 | 0.032 |
Adjustment for age, sex, and stage.
Effect of Mitotane on RFS and OS According to Univariate and Multivariate Analyses
| . | Univariate Analysis . | Multivariate Analysisa . | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| RFS | ||||||
| Adjuvant mitotane | 1 | 1 | ||||
| Control group 1 | 2.55 | 1.54–4.21 | <0.0001 | 2.98 | 1.75–5.09 | <0.0001 |
| Control group 2 | 1.87 | 1.17–3.00 | 0.009 | 2.61 | 1.56–4.36 | <0.0001 |
| Sex | 1.33 | 0.92–1.94 | 0.13 | 1.27 | 0.86–1.88 | 0.22 |
| Age | 0.98 | 0.97–0.99 | 0.011 | 0.98 | 0.96–0.99 | 0.002 |
| Stage | ||||||
| I | 1 | 1 | ||||
| II | 1.62 | 0.81–3.25 | 0.17 | 1.96 | 0.97–3.94 | 0.060 |
| III | 1.88 | 0.88–4.02 | 0.10 | 2.36 | 1.09–5.12 | 0.029 |
| IV | 2.08 | 0.80–5.39 | 0.13 | 4.45 | 1.62–12.24 | 0.004 |
| OS | ||||||
| Adjuvant mitotane | 1 | 1 | ||||
| Control group 1 | 2.007 | 1.19–3.39 | 0.009 | 2.03 | 1.17–3.51 | 0.011 |
| Control group 2 | 1.337 | 0.80–2.23 | 0.266 | 1.60 | 0.94–2.74 | <0.083 |
| Sex | 1.18 | 0.78–1.77 | 0.44 | 1.09 | 0.71–1.69 | 0.69 |
| Age | 0.99 | 0.97–1.00 | 0.21 | 0.99 | 0.97–1.00 | 0.17 |
| Stage | ||||||
| I | 1 | 1 | ||||
| II | 1.81 | 0.78–4.20 | 0.17 | 1.85 | 0.79–4.32 | 0.16 |
| III | 2.41 | 0.98–5.95 | 0.06 | 2.37 | 0.95–5.93 | 0.065 |
| IV | 2.75 | 0.98–7.75 | 0.055 | 3.20 | 1.11–9.28 | 0.032 |
| . | Univariate Analysis . | Multivariate Analysisa . | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| RFS | ||||||
| Adjuvant mitotane | 1 | 1 | ||||
| Control group 1 | 2.55 | 1.54–4.21 | <0.0001 | 2.98 | 1.75–5.09 | <0.0001 |
| Control group 2 | 1.87 | 1.17–3.00 | 0.009 | 2.61 | 1.56–4.36 | <0.0001 |
| Sex | 1.33 | 0.92–1.94 | 0.13 | 1.27 | 0.86–1.88 | 0.22 |
| Age | 0.98 | 0.97–0.99 | 0.011 | 0.98 | 0.96–0.99 | 0.002 |
| Stage | ||||||
| I | 1 | 1 | ||||
| II | 1.62 | 0.81–3.25 | 0.17 | 1.96 | 0.97–3.94 | 0.060 |
| III | 1.88 | 0.88–4.02 | 0.10 | 2.36 | 1.09–5.12 | 0.029 |
| IV | 2.08 | 0.80–5.39 | 0.13 | 4.45 | 1.62–12.24 | 0.004 |
| OS | ||||||
| Adjuvant mitotane | 1 | 1 | ||||
| Control group 1 | 2.007 | 1.19–3.39 | 0.009 | 2.03 | 1.17–3.51 | 0.011 |
| Control group 2 | 1.337 | 0.80–2.23 | 0.266 | 1.60 | 0.94–2.74 | <0.083 |
| Sex | 1.18 | 0.78–1.77 | 0.44 | 1.09 | 0.71–1.69 | 0.69 |
| Age | 0.99 | 0.97–1.00 | 0.21 | 0.99 | 0.97–1.00 | 0.17 |
| Stage | ||||||
| I | 1 | 1 | ||||
| II | 1.81 | 0.78–4.20 | 0.17 | 1.85 | 0.79–4.32 | 0.16 |
| III | 2.41 | 0.98–5.95 | 0.06 | 2.37 | 0.95–5.93 | 0.065 |
| IV | 2.75 | 0.98–7.75 | 0.055 | 3.20 | 1.11–9.28 | 0.032 |
Adjustment for age, sex, and stage.
Adjuvant mitotane treatment was also an independent predictive factor for OS in multivariate analysis after adjusting for age, sex, and tumor stage. In comparison with the mitotane group, the risk of death was significantly higher in control group 1 (HR = 2.03; 95% CI, 1.17 to 3.51; P = 0.011) but not in control group 2 (HR = 1.60; 95% CI, 0.94 to 2.74; P = 0.083).
The efficacy of adjuvant mitotane was also explored stratifying patients according to cortisol secretion by ACC in 137 evaluable patients. Mitotane effect on RFS did not differ according to the presence of overt cortisol excess. The risk of recurrence was higher in control groups 1 and 2 than in the mitotane group, both in patients with nonsecreting tumors (control group 1, HR = 2.16; 95% CI, 1.12 to 4.17; P = 0.022, and control group 2, HR = 2.00; 95% CI, 0.93 to 4.34; P = 0.077) and in patients with cortisol-secreting ACC (control group 1, HR = 4.51; 95% CI, 1.92 to 10.60; P = 0.001, and control group 2, HR = 1.79; 95% CI, 0.86 to 3.73; P = 0.12). When assessing HR values in control group 2, it has to be considered that only 45 out of 70 patients (64%) were evaluable for ACC secretion.
Discussion
The evidence on adjuvant mitotane therapy after radical resection of ACC is sparse and flawed by several limitations, such as limited statistical power, absence of a matched control group, RFS not uniformly defined, and response duration unclearly reported. Moreover, all studies but one were retrospective and used different formulations of mitotane at variable doses, ranging daily from 3 to 20 g (Table 5). In this scenario, the most informative data were provided by our retrospective case-control study involving a large cohort of ACC patients who were assigned to adjuvant mitotane or no treatment independently on patient’s characteristics (5). The study included a group of mitotane-treated patients and two contemporary control groups of untreated patients matched for the major prognostic factors (actually, control group 2 had better prognostic features). This is strength of the study, as in other studies, the presence of unfavorable characteristics was likely a factor supporting the decision to prescribe adjuvant mitotane and this have introduced biased data (Table 3). Very recently, Postlewait et al. (23) reported a retrospective analysis of the outcomes of 207 ACC patients who underwent resection at 13 centers in the United States. They found that adjuvant mitotane was associated with decreased RFS and OS. The difference with our results may be readily explained by the selection of patients at unfavorable prognosis for mitotane treatment. The patients who were treated with mitotane had a higher frequency of stage IV metastatic tumors and, indeed, chemotherapy was frequently associated to mitotane therapy. Also, the frequency of cortisol excess, another negative prognostic factor, was higher in the mitotane group. Because 42% of the 88 patients treated with mitotane had stage IV ACC, this series is not comparable to ours, with only 13% stage IV ACC.
| Study (Reference) . | Patient Characteristics . | Study Outcome . |
|---|---|---|
| (8) | Mitotane group (n = 4) | OS of 74 ± 33 mo in patients who received adjuvant mitotane. No data on RFS. |
| No control group | ||
| (9) | Mitotane group (n = 7) | After 1 to 4 y from surgery, 6/7 patients treated with adjuvant mitotane are still alive. No data on RFS. |
| No control group | ||
| (10) | Mitotane group (n = 21) | No difference in OS between group 1 vs group 2 patients. No data on RFS. |
| Control group (n = 25) | ||
| (11) | Mitotane group (n = 7) | Mean RFS of 2.4 y in group 1 vs 2.5 y in group 2 patients. |
| Control group (n = 43) | ||
| (12) | Mitotane group (n = 8) | Median RFS of 10 mo in group 1 vs 23 mo for group 2 patients (P < 0.01). |
| Control group (n = 6) | ||
| (13) | Mitotane group (n = 11) | Median OS of the group 1 was 51 mo vs 61 mo for group 2 patients (n = 15) (P = NS). No data on RFS. |
| Control group (n = 15) | ||
| (14) | Mitotane group (n = 7) | Median RFS of 8 mo in group 1 vs 13 mo in group 2 patients (P = NS). |
| Control group (n = 11) | ||
| (15) | Mitotane group (n = 4) | RFS was of 18 to 68 mo. |
| No control group | ||
| (16) | Mitotane group (n = 32) | At the last follow-up, 56% of group 1 patients are alive vs 12% of group 2. No data on RFS. |
| Control group (n = 8) | ||
| (3) | Mitotane group (n = 83) | No difference in OS between group 1 and group 2 patients. No data on RFS. |
| Control group (n = 74) | ||
| (17) | Mitotane group (n = 11) | Recurrence developed within 1 y in 8 patients followed prospectively. No data on RFS. |
| No control group | ||
| (18) | Mitotane group (n = 86) | No difference in RFS between group 1 and group 2 patients (P = 0.34). |
| Control group (n = 80) | ||
| (19) | Mitotane group (n = 22) | Increased risk of recurrence in group 2 patients (HR = 1.95; 95% CI, 1.06–3.59); P = 0.03 at multivariable analysis. |
| Control group (n = 196) | ||
| (20) | Mitotane group (n = 35) | Reduced risk of death in group 1 (HR = 0.38; 95% CI, 0.12–1.28); P = 0.11 at multivariable analysis. |
| Control group (n = 114) | ||
| (21) | High-level mitotane group (n = 24) | Reduced risk of death in group 1 (HR = 0.25; 95% CI, 0.06–1.00); P = 0.049 at Poisson regression. |
| Low-level mitotane group (n = 13) | ||
| (22) | Mitotane group (n = 105) | Reduced risk of recurrence in group 1 (HR = 0.72; 95% CI, 0.53–0.98); P = 0.037 at multivariable analysis. |
| Control group (n = 159) |
| Study (Reference) . | Patient Characteristics . | Study Outcome . |
|---|---|---|
| (8) | Mitotane group (n = 4) | OS of 74 ± 33 mo in patients who received adjuvant mitotane. No data on RFS. |
| No control group | ||
| (9) | Mitotane group (n = 7) | After 1 to 4 y from surgery, 6/7 patients treated with adjuvant mitotane are still alive. No data on RFS. |
| No control group | ||
| (10) | Mitotane group (n = 21) | No difference in OS between group 1 vs group 2 patients. No data on RFS. |
| Control group (n = 25) | ||
| (11) | Mitotane group (n = 7) | Mean RFS of 2.4 y in group 1 vs 2.5 y in group 2 patients. |
| Control group (n = 43) | ||
| (12) | Mitotane group (n = 8) | Median RFS of 10 mo in group 1 vs 23 mo for group 2 patients (P < 0.01). |
| Control group (n = 6) | ||
| (13) | Mitotane group (n = 11) | Median OS of the group 1 was 51 mo vs 61 mo for group 2 patients (n = 15) (P = NS). No data on RFS. |
| Control group (n = 15) | ||
| (14) | Mitotane group (n = 7) | Median RFS of 8 mo in group 1 vs 13 mo in group 2 patients (P = NS). |
| Control group (n = 11) | ||
| (15) | Mitotane group (n = 4) | RFS was of 18 to 68 mo. |
| No control group | ||
| (16) | Mitotane group (n = 32) | At the last follow-up, 56% of group 1 patients are alive vs 12% of group 2. No data on RFS. |
| Control group (n = 8) | ||
| (3) | Mitotane group (n = 83) | No difference in OS between group 1 and group 2 patients. No data on RFS. |
| Control group (n = 74) | ||
| (17) | Mitotane group (n = 11) | Recurrence developed within 1 y in 8 patients followed prospectively. No data on RFS. |
| No control group | ||
| (18) | Mitotane group (n = 86) | No difference in RFS between group 1 and group 2 patients (P = 0.34). |
| Control group (n = 80) | ||
| (19) | Mitotane group (n = 22) | Increased risk of recurrence in group 2 patients (HR = 1.95; 95% CI, 1.06–3.59); P = 0.03 at multivariable analysis. |
| Control group (n = 196) | ||
| (20) | Mitotane group (n = 35) | Reduced risk of death in group 1 (HR = 0.38; 95% CI, 0.12–1.28); P = 0.11 at multivariable analysis. |
| Control group (n = 114) | ||
| (21) | High-level mitotane group (n = 24) | Reduced risk of death in group 1 (HR = 0.25; 95% CI, 0.06–1.00); P = 0.049 at Poisson regression. |
| Low-level mitotane group (n = 13) | ||
| (22) | Mitotane group (n = 105) | Reduced risk of recurrence in group 1 (HR = 0.72; 95% CI, 0.53–0.98); P = 0.037 at multivariable analysis. |
| Control group (n = 159) |
Results of our previous article (9) are not included.
Abbreviation: NS, not significant.
| Study (Reference) . | Patient Characteristics . | Study Outcome . |
|---|---|---|
| (8) | Mitotane group (n = 4) | OS of 74 ± 33 mo in patients who received adjuvant mitotane. No data on RFS. |
| No control group | ||
| (9) | Mitotane group (n = 7) | After 1 to 4 y from surgery, 6/7 patients treated with adjuvant mitotane are still alive. No data on RFS. |
| No control group | ||
| (10) | Mitotane group (n = 21) | No difference in OS between group 1 vs group 2 patients. No data on RFS. |
| Control group (n = 25) | ||
| (11) | Mitotane group (n = 7) | Mean RFS of 2.4 y in group 1 vs 2.5 y in group 2 patients. |
| Control group (n = 43) | ||
| (12) | Mitotane group (n = 8) | Median RFS of 10 mo in group 1 vs 23 mo for group 2 patients (P < 0.01). |
| Control group (n = 6) | ||
| (13) | Mitotane group (n = 11) | Median OS of the group 1 was 51 mo vs 61 mo for group 2 patients (n = 15) (P = NS). No data on RFS. |
| Control group (n = 15) | ||
| (14) | Mitotane group (n = 7) | Median RFS of 8 mo in group 1 vs 13 mo in group 2 patients (P = NS). |
| Control group (n = 11) | ||
| (15) | Mitotane group (n = 4) | RFS was of 18 to 68 mo. |
| No control group | ||
| (16) | Mitotane group (n = 32) | At the last follow-up, 56% of group 1 patients are alive vs 12% of group 2. No data on RFS. |
| Control group (n = 8) | ||
| (3) | Mitotane group (n = 83) | No difference in OS between group 1 and group 2 patients. No data on RFS. |
| Control group (n = 74) | ||
| (17) | Mitotane group (n = 11) | Recurrence developed within 1 y in 8 patients followed prospectively. No data on RFS. |
| No control group | ||
| (18) | Mitotane group (n = 86) | No difference in RFS between group 1 and group 2 patients (P = 0.34). |
| Control group (n = 80) | ||
| (19) | Mitotane group (n = 22) | Increased risk of recurrence in group 2 patients (HR = 1.95; 95% CI, 1.06–3.59); P = 0.03 at multivariable analysis. |
| Control group (n = 196) | ||
| (20) | Mitotane group (n = 35) | Reduced risk of death in group 1 (HR = 0.38; 95% CI, 0.12–1.28); P = 0.11 at multivariable analysis. |
| Control group (n = 114) | ||
| (21) | High-level mitotane group (n = 24) | Reduced risk of death in group 1 (HR = 0.25; 95% CI, 0.06–1.00); P = 0.049 at Poisson regression. |
| Low-level mitotane group (n = 13) | ||
| (22) | Mitotane group (n = 105) | Reduced risk of recurrence in group 1 (HR = 0.72; 95% CI, 0.53–0.98); P = 0.037 at multivariable analysis. |
| Control group (n = 159) |
| Study (Reference) . | Patient Characteristics . | Study Outcome . |
|---|---|---|
| (8) | Mitotane group (n = 4) | OS of 74 ± 33 mo in patients who received adjuvant mitotane. No data on RFS. |
| No control group | ||
| (9) | Mitotane group (n = 7) | After 1 to 4 y from surgery, 6/7 patients treated with adjuvant mitotane are still alive. No data on RFS. |
| No control group | ||
| (10) | Mitotane group (n = 21) | No difference in OS between group 1 vs group 2 patients. No data on RFS. |
| Control group (n = 25) | ||
| (11) | Mitotane group (n = 7) | Mean RFS of 2.4 y in group 1 vs 2.5 y in group 2 patients. |
| Control group (n = 43) | ||
| (12) | Mitotane group (n = 8) | Median RFS of 10 mo in group 1 vs 23 mo for group 2 patients (P < 0.01). |
| Control group (n = 6) | ||
| (13) | Mitotane group (n = 11) | Median OS of the group 1 was 51 mo vs 61 mo for group 2 patients (n = 15) (P = NS). No data on RFS. |
| Control group (n = 15) | ||
| (14) | Mitotane group (n = 7) | Median RFS of 8 mo in group 1 vs 13 mo in group 2 patients (P = NS). |
| Control group (n = 11) | ||
| (15) | Mitotane group (n = 4) | RFS was of 18 to 68 mo. |
| No control group | ||
| (16) | Mitotane group (n = 32) | At the last follow-up, 56% of group 1 patients are alive vs 12% of group 2. No data on RFS. |
| Control group (n = 8) | ||
| (3) | Mitotane group (n = 83) | No difference in OS between group 1 and group 2 patients. No data on RFS. |
| Control group (n = 74) | ||
| (17) | Mitotane group (n = 11) | Recurrence developed within 1 y in 8 patients followed prospectively. No data on RFS. |
| No control group | ||
| (18) | Mitotane group (n = 86) | No difference in RFS between group 1 and group 2 patients (P = 0.34). |
| Control group (n = 80) | ||
| (19) | Mitotane group (n = 22) | Increased risk of recurrence in group 2 patients (HR = 1.95; 95% CI, 1.06–3.59); P = 0.03 at multivariable analysis. |
| Control group (n = 196) | ||
| (20) | Mitotane group (n = 35) | Reduced risk of death in group 1 (HR = 0.38; 95% CI, 0.12–1.28); P = 0.11 at multivariable analysis. |
| Control group (n = 114) | ||
| (21) | High-level mitotane group (n = 24) | Reduced risk of death in group 1 (HR = 0.25; 95% CI, 0.06–1.00); P = 0.049 at Poisson regression. |
| Low-level mitotane group (n = 13) | ||
| (22) | Mitotane group (n = 105) | Reduced risk of recurrence in group 1 (HR = 0.72; 95% CI, 0.53–0.98); P = 0.037 at multivariable analysis. |
| Control group (n = 159) |
Results of our previous article (9) are not included.
Abbreviation: NS, not significant.
Though the retrospective nature of our study is a major limitation, the rarity of ACC makes challenging the organization of a prospective randomized clinical trial. As a matter of fact, we are currently undertaking the first, to our knowledge, randomized controlled trial in patients with ACC following radical extirpation—the ADIUVO study (www.epiclin.it/adiuvo)—but recruitment is difficult and results are not expected for several years. Meanwhile, physicians who are treating ACC are left with the dilemma of prescribing or not postoperative mitotane treatment (6). To provide guidance in this controversial area, a panel of experts recommended adjuvant mitotane in radically resected ACC patients at high risk of recurrence (24), and this recommendation has been incorporated in currently available guidelines (1, 25). Therefore, we reanalyzed the outcome data of the original patient cohorts after a longer follow-up, more than 9 years after the end of the original study.
To reduce the “immortal-time” bias, in the updated analysis we calculated RFS and OS estimates from the time point of 3 months (landmark method), thus excluding from the analysis the patients with early progression and death. We set the landmark point at 3 months because at that time, mitotane treatment has been started in all patients. With the introduction of the landmark analysis the median RFS of patients in control group 1 increased consistently in this study as opposed to the original one (17 vs 10 months), whereas the difference was marginal in control group 2 (26 vs 25 months). Even with the introduction of a landmark analysis and after a median follow-up of more than 10 years, the substantial advantage in terms of reduction of the risk of recurrence of patients who underwent adjuvant mitotane compared with patients of both control groups was confirmed both in univariate and multivariate analyses. Mitotane treated patients had also a significantly longer survival rate than patients included in control group 1, whereas the survival advantage over control group 2 just failed to attain the statistical significance. However, control group 2 had a better risk profile, with a significantly higher percentage of stage I to II tumors, portending improved outcome. Moreover, treatment of recurrences was more aggressive in control group 2 than in control group 1. Although the pattern of recurrence between the three groups was comparable, with local recurrences found in about 50% of cases, surgery was most frequently used in both the mitotane group and control group 2 than in control group 1 (64%, 66%, and 35%, respectively). Because surgical approaches are generally regarded as superior to treatment regimens consisting only of medical therapy to manage recurrent ACC (2, 26, 27), we may argue that this difference in the management contributed to the better OS observed in control group 2 than control group 1.
The recently published experience of the University of Michigan with adjuvant therapies for ACC shows that adjuvant mitotane treatment was associated with a significantly prolonged RFS in multivariate analysis (HR = 0.723; 95% CI, 0.533 to 0.981; P = 0.037), although the effect on OS did not reach levels of significance (HR = 0.887; 95% CI, 0.621 to 1.268; P = 0.511) (22).
Another point of controversy is whether mitotane could be better suited to treat secreting ACC, due to its inhibitory effects on adrenal steroidogenesis. In a French series of 166 patients, mitotane was not effective in improving RFS in the overall cohort, although in the subgroup of patients with cortisol excess a tendency toward a beneficial effect was seen (18). This finding raised the issue that the efficacy of adjuvant mitotane therapy may be limited to patients with cortisol-secreting tumors. However, cortisol was not a predictor of adjuvant mitotane efficacy in a large, multicenter, dataset recently published (28). In the current study, the efficacy of mitotane on RFS did not differ when patients were stratified by presence of cortisol excess. These data further support the antineoplastic activity of mitotane irrespective of the cortisol secretory status.
In conclusion, this updated analysis with longer follow-up and landmark analysis confirms that among patients with macroscopically complete removal of ACC, the use of adjuvant mitotane compared with observation is associated with prolonged RFS independently of hormone activity. We think that the present results strengthen the recommendation of adjuvant mitotane as part of the postoperative management of ACC patients. Another important finding is that an aggressive use of surgery to treat ACC recurrences may be associated with prolonged survival. Repeat surgery for recurrent ACC may provide a benefit in OS independently from mitotane use. These data are of particular interest for surgeons operating on ACC patients, who should refer these patients to centers with specific expertise on surgical treatment of ACC and management of mitotane therapy. Interestingly, early referral of stage II ACC patients to specialized centers has been associated with an improved outcome (20). The retrospective nature of the study does not allow us to establish definitively the value of adjuvant mitotane treatment. There might be subsets of patients (i.e., patients with small, low-grade tumors) who may not benefit from a strategy of adjuvant mitotane treatment. A currently ongoing randomized trial (ADIUVO study) is specifically aiming to establish mitotane efficacy in patients at low/intermediate risk of recurrence.
Abbreviations:
- ACC
adrenocortical carcinoma
- CI
confidence interval
- HR
hazard ratio
- OS
overall survival
- RFS
recurrence-free survival.
Acknowledgments
The following persons actively participated and are coauthors of the study: Letizia Canu (Department Experimental and Clinical Biomedical Sciences “Mario Serio,” University of Florence, Florence, Italy); Filippo Ceccato and Carla Scaroni (Endocrinology Unit, Department of Medicine, Padova University Hospital, Padova, Italy); Erika Grossrubatscher (Endocrine Unit, Department of Medical Specialties, Ospedale Niguarda Cà Granda, Milano, Italy); and Matthias Kroiss, Michaela Haaf, Cristina Ronchi, and Timo Deutschbein (Department of Internal Medicine I, Division of Endocrinology and Diabetology, University Hospital, University of Würzburg, Würzburg, Germany).
This work was supported by a research grant from Associazione Italiana per la Ricerca sul Cancro (grant number 14411), the Deutsche Forschungsgemeinschaft (grant number FA 466/4-1), the Italian Ministry of University and Scientific Research (grant number FIRB RBAP1153LS_005), and the University of Turin (grant number TERMATEN 12). This work was also supported by a private grant from Mrs. Serena Ambrogini and family in memory of her son Guido Cioni.
Disclosure Summary: The authors have nothing to disclose.
References
Author notes
Address all correspondence and requests for reprints to: Alfredo Berruti, MD, Medical Oncology, Department of Medical and Surgical Specialties, Radiological Sciences, and Public Health, University of Brescia, Spedali Civili Hospital, Piazzale Spedali Civili 1, 25123 Brescia, Italy. E-mail: [email protected].



