-
PDF
- Split View
-
Views
-
Cite
Cite
Ilkka Vuorimies, Mervi K. Mäyränpää, Helena Valta, Heikki Kröger, Sanna Toiviainen-Salo, Outi Mäkitie, Bisphosphonate Treatment and the Characteristics of Femoral Fractures in Children With Osteogenesis Imperfecta, The Journal of Clinical Endocrinology & Metabolism, Volume 102, Issue 4, 1 April 2017, Pages 1333–1339, https://doi.org/10.1210/jc.2016-3745
Close - Share Icon Share
Abstract
The short-term benefits of bisphosphonates (BPs) are evident in the treatment of children with osteogenesis imperfecta (OI), but some concerns related to long-term effects remain.
To elucidate the effect of BPs on characteristics of femoral fractures in children with OI.
Retrospective cohort study at a university hospital.
The study included 93 patients with OI. We recorded fracture histories and analyzed all femoral fractures for location and fracture type using radiographs obtained at fracture diagnosis. Effects of BPs were evaluated by comparing fracture characteristics in three groups: patients (1) naive to BPs, (2) receiving ongoing BP treatment, and (3) whose treatment was discontinued.
In total, 127 femoral fractures occurred in 24 patients. Of the fractures, 63 (50%) occurred in patients naive to BPs, 44 (35%) during BP treatment, and 20 (16%) after treatment discontinuation. Mid or distal shaft fractures were most common (41%), followed by subtrochanteric (33%) and distal (20%) fractures. Almost all fractures were transverse (65%) or oblique (28%). The pattern of femoral fractures was similar in all three BP treatment groups (P = 0.78 for location; P = 0.35 for fracture type) and was not related to cumulative BP dose. Instead, OI type correlated with fracture characteristics, and distal location and transverse configuration were more common in the more severe types III and IV compared with type I OI.
Characteristics of femoral fractures in children with OI are affected by OI type but not by BP exposure.
Osteogenesis imperfecta (OI) is an inherited disorder that is characterized by bone fragility, which often leads to multiple fractures and bone deformities (1). Up to 90% of cases are dominantly inherited and result from heterozygous mutations in the genes COL1A1 or COL1A2 that encode type 1 collagen; mutations alter either the quantity or structure of type 1 collagen. The remaining 10% of patients with OI have defects in other proteins that are responsible for posttranslational modification, folding, or secretion of type 1 collagen. Several such genetic forms of OI have been described (2, 3). OI can be divided into five subtypes, as suggested by Van Dijk and Sillence (4), reflecting its widely variable clinical severity.
Bisphosphonates (BPs) are commonly used in the treatment of pediatric patients with OI. Since the first reports more than 15 years ago, several studies have shown the beneficial effects of BPs on bone density, fracture rates, and overall well-being (5–8). Some concerns still remain regarding long-term effects and safety of BPs in growing children. One of these involves atypical femoral fractures, which in adults with primary osteoporosis have been associated with prolonged use of BPs (9). Atypical femoral fractures are defined as subtrochanteric femoral fractures characterized by: (1) location in the femoral shaft, (2) a substantially transverse and minimally comminuted configuration, (3) flaring of the lateral cortex at the fracture site, and (4) minimal or no trauma energy (10). The major pathogenetic mechanism has been suggested to be the suppressive effect of BPs on bone remodeling, which leads to nonhealing microcracks and ultimately to full-scale fractures. The localized cortical thickening, also typical for stress fractures, supports this hypothesis, although evidence remains insufficient (10).
A few case reports have described atypical femoral fractures in adult patients with OI after BP treatment (11–14), and recently, Vasanwala et al. (15) reported on recurrent femoral fractures with atypical features in a BP-treated teenager with OI. Despite the wide use of BPs in children with OI, only one larger study has evaluated atypical femoral fractures in BP-treated pediatric patients with OI. The result of the study suggested that BP treatment could be associated with a change in features of femoral fractures (16). Given that metabolic characteristics of the growing bone significantly differ from those of the adult skeleton, we chose to perform a systematic evaluation of femur fractures in our pediatric OI population to determine whether BP treatment affects characteristics of femoral fractures in children with OI.
Patients and Methods
Study population
The study population comprised children and adolescents who were followed at the Metabolic Bone Clinic, Children's Hospital, Helsinki University Hospital, Finland. The clinic is responsible for coordination of patient care in moderate to severe OI, and for planning and follow-up of medical treatment for all pediatric patients with OI in Finland. We estimate that approximately 90% of the Finnish pediatric patients with OI are or have been under follow-up at the clinic. These patients were eligible for this study if they were born between 1990 and 2012, and had the diagnosis of OI. In total, 93 patients (54 boys and 39 girls; age range, 1.0 to 22.0 years at the end of data collection) met these criteria. The study was a retrospective analysis of patient records, and the protocol was approved by the Research Ethics Committee, Children’s Hospital, Helsinki University Hospital.
BP treatment
BP treatment for each patient was individualized on the basis of clinical features and symptoms, including sustained fractures and areal bone mineral density. Most of the patients received intravenous pamidronate (9 mg/kg/y divided into three to 12, 1- to 3-day infusion cycles per year, depending on age and disease severity) or zoledronic acid (0.1 mg/kg/y divided into two to four infusions per year). A few patients with mild OI received orally administered risedronate (2.5 mg/d for patients weighing 10 to 30 kg and 5 mg/d for patients weighing more than 30 kg). Treatment was paused (i.e., patients were given a drug holiday) for at least 1 year after 2 to 3 years of BP treatment. Symptoms, BMD, and fracture incidence were monitored yearly, and BP was restarted if BMD deteriorated or pain or new fractures occurred. The cumulative BP dose per body weight was expressed as milligrams per kilogram. Because three BPs were used, we also used BP coefficients to enable comparison between various treatments. These coefficients, introduced by Shaw and Bishop (17), are based on relative potency of the molecules to inhibit bone resorption in vitro. The applied coefficients were 100 for pamidronate, 10,000 for zoledronic acid, and 2000 for risedronate. The low bioavailability of orally administered risedronate (0.63%) was also taken into account. For example, 2 years of standard zoledronic acid treatment equals a cumulative dose of 2000 BP units/kg (2 × 0.1 mg/kg × 10,000 BP units/mg), and 2 years of standard pamidronate treatment equals a cumulative dose of 1800 BP units/kg (2 × 9 mg/kg × 100 BP units/mg).
Data collection
In this retrospective study, we performed a detailed analysis of femoral fractures that occurred in the study population before 31 December 2012, using medical records and radiographs. Femoral fractures occurring before age 1.0 year were excluded from this study because of generally different pattern and trauma mechanisms of these fractures. Complete records were available for 89 (96%) of the 93 eligible patients (51 boys and 38 girls). All radiographs obtained at the time of fracture were collected and re-evaluated by authors I.V. and M.K.M. to assess fracture location and type. The patients' basic demographic characteristics at the time of trauma, the length and status of BP treatment, and the BP preparations used were collected from patient records. The clinical type of OI, as classified according to Van Dijk and Sillence (4), presence of intramedullary rods, and the trauma energy that led to the fracture were also recorded. Of all fractures, 17 (11%) were regarded as refractures and analyzed separately. We defined refractures as fractures occurring at least partially along the previous fracture line within a year or before its full ossification.
Characterization of fractures
The location of fractures was categorized into four groups: (1) proximal fractures, including femoral neck fractures, intertrochanteric fractures, and slipped capital femoral epiphysis; (2) subtrochanteric fractures comprising the proximal third of the femoral shaft (diaphysis); (3) femoral shaft fractures comprising the mid and distal thirds of the femoral shaft; and (4) distal fractures of femur comprising the fractures of distal metaphysis, physis, and epiphysis. The type of fracture, representing the shape of fracture line, was categorized into one of three groups: (1) transverse fractures, (2) oblique fractures, and (3) other fractures, which included fractures not fitting into these categories (e.g., spiral, comminuted, or torus fractures) (Fig. 1).
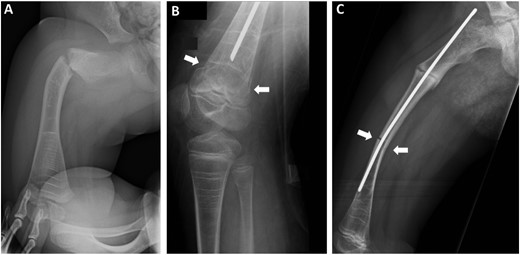
Examples of different fracture types. Three separate femoral fractures occurred in a girl with type III OI. She has been treated with BP since the age of 1 month. (A) A transverse fracture in the subtrochanteric region of the femur occurred at 1.9 years of age. (B) A transverse fracture of the distal femur, shown by white arrows, occurred at 7.4 years of age. (C) An oblique fracture of the femoral shaft, shown by white arrows, occurred at 8.3 years of age (a partially ossified fracture in the subtrochanteric region is also visible). Note the dense lines (Harris lines) caused by cyclic intravenous BP treatment in all three radiographs.
To assess the effect of BP treatment on fracture characteristics, the femoral fractures were divided into three groups on the basis of treatment status at the time of trauma: (1) patient was naive to BP treatment; (2) BP treatment was ongoing; and (3) the patient had been receiving BP treatment, but the treatment was at least temporarily discontinued (drug holiday). Treatment was regarded as ongoing if the time elapsed after the last infusion was less than the duration of an infusion cycle. Furthermore, to assess the effect of cumulative BP dose, we divided fractures according to the patient’s total BP dose expressed in coefficients (mg/kg × relative potency of specific BPs) (17). When comparing effects of high vs low cumulative BP dose, we used 2000 BP units/kg, which is equivalent to 2 years of standard zoledronic acid treatment, as a cut point.
Statistical analyses
The distributions of fracture location and fracture type were compared between three groups (grouped by BP treatment and OI type) with Pearson’s χ2 test or, if the number of observations was <10, with Fisher’s exact test including Monte Carlo approximation. Continuous background characteristics were compared between the three treatment groups with analysis of variance. P values less than 0.05 were considered statistically significant. Statistical calculations were performed using SPSS software for Windows (version 22; SPSS, Chicago, IL).
Results
Cohort characteristics
The initial patient cohort included 93 patients with OI (54 boys, 58%) born between 1990 and 2012. Altogether, they had sustained 149 femoral fractures. Fractures had occurred in 25 patients with OI during the study period; 68 of the patients (73%) had not sustained any femoral fractures. For 127 fractures (85%) in 24 patients (14 boys and 10 girls), sufficient medical records and radiographs were available, and these fractures comprised the final research material. The distribution of OI types among the 24 patients was: type I, 11 patients; type III, six patients, including one with an autosomal recessive form; type IV, seven patients, including one with an autosomal recessive form.
Fracture characteristics
The distribution of femoral fractures (n = 127) in the different OI types was: type I, 26 fractures (20%); type III, 28 fractures (22%); type IV, 73 fractures (57%). The median number of femoral fractures per patient was two (range, one to 22). At the time of fracture, the median height was −2.7 standard deviations (−11.5 to 1.5 standard deviations; available for 86% of the patients), and the median age was 7.8 years (1.0 to 18.5 years) (Table 1).
Characteristics of 127 Femoral Fractures in the Cohort of 24 Children With OI
| . | BP Treatment . | Total . | P Value . | ||
|---|---|---|---|---|---|
| . | Naive . | Ongoing . | Discontinued . | ||
| No. of fractures | 63 | 44 | 20 | 127 | |
| Male, % (boys/girls) | 48 (30/33) | 52 (23/21) | 65 (13/7) | 52 (66/61) | 0.40 |
| Median age (range), y | 7.0 (1.0 to 13.0) | 8.4 (1.0 to 18.5) | 9.7 (4.9 to 16.8) | 7.8 (1.0 to 18.5) | <0.01 |
| OI type, I/III/IV | 16/0/47 | 8/20/16 | 2/8/10 | 26/28/73 | <0.001 |
| Median height (range), SD | −2.7 (−5.2 to 0) | −2.2 (−11.5 to 1.0) | −3.0 (−6.8 to 0) | −2.7 (−11.5 to 1.0) | 0.29 |
| Intramedullary rod (%) | 32 (51) | 30 (68) | 17 (85) | 79 (62) | 0.014 |
| Refractures (%) | 13 (21) | 2 (5) | 2 (10) | 17 (13) | 0.049 |
| . | BP Treatment . | Total . | P Value . | ||
|---|---|---|---|---|---|
| . | Naive . | Ongoing . | Discontinued . | ||
| No. of fractures | 63 | 44 | 20 | 127 | |
| Male, % (boys/girls) | 48 (30/33) | 52 (23/21) | 65 (13/7) | 52 (66/61) | 0.40 |
| Median age (range), y | 7.0 (1.0 to 13.0) | 8.4 (1.0 to 18.5) | 9.7 (4.9 to 16.8) | 7.8 (1.0 to 18.5) | <0.01 |
| OI type, I/III/IV | 16/0/47 | 8/20/16 | 2/8/10 | 26/28/73 | <0.001 |
| Median height (range), SD | −2.7 (−5.2 to 0) | −2.2 (−11.5 to 1.0) | −3.0 (−6.8 to 0) | −2.7 (−11.5 to 1.0) | 0.29 |
| Intramedullary rod (%) | 32 (51) | 30 (68) | 17 (85) | 79 (62) | 0.014 |
| Refractures (%) | 13 (21) | 2 (5) | 2 (10) | 17 (13) | 0.049 |
The fractures are divided into three groups according to patients’ BP treatment status at time of trauma. Naive: patient was naive to BP treatment; ongoing: BP treatment was ongoing; discontinued: the patient had been receiving BP treatment, but treatment was at least temporarily discontinued (drug holiday). P values refer to differences between the three treatment groups. SD, standard deviation.
Characteristics of 127 Femoral Fractures in the Cohort of 24 Children With OI
| . | BP Treatment . | Total . | P Value . | ||
|---|---|---|---|---|---|
| . | Naive . | Ongoing . | Discontinued . | ||
| No. of fractures | 63 | 44 | 20 | 127 | |
| Male, % (boys/girls) | 48 (30/33) | 52 (23/21) | 65 (13/7) | 52 (66/61) | 0.40 |
| Median age (range), y | 7.0 (1.0 to 13.0) | 8.4 (1.0 to 18.5) | 9.7 (4.9 to 16.8) | 7.8 (1.0 to 18.5) | <0.01 |
| OI type, I/III/IV | 16/0/47 | 8/20/16 | 2/8/10 | 26/28/73 | <0.001 |
| Median height (range), SD | −2.7 (−5.2 to 0) | −2.2 (−11.5 to 1.0) | −3.0 (−6.8 to 0) | −2.7 (−11.5 to 1.0) | 0.29 |
| Intramedullary rod (%) | 32 (51) | 30 (68) | 17 (85) | 79 (62) | 0.014 |
| Refractures (%) | 13 (21) | 2 (5) | 2 (10) | 17 (13) | 0.049 |
| . | BP Treatment . | Total . | P Value . | ||
|---|---|---|---|---|---|
| . | Naive . | Ongoing . | Discontinued . | ||
| No. of fractures | 63 | 44 | 20 | 127 | |
| Male, % (boys/girls) | 48 (30/33) | 52 (23/21) | 65 (13/7) | 52 (66/61) | 0.40 |
| Median age (range), y | 7.0 (1.0 to 13.0) | 8.4 (1.0 to 18.5) | 9.7 (4.9 to 16.8) | 7.8 (1.0 to 18.5) | <0.01 |
| OI type, I/III/IV | 16/0/47 | 8/20/16 | 2/8/10 | 26/28/73 | <0.001 |
| Median height (range), SD | −2.7 (−5.2 to 0) | −2.2 (−11.5 to 1.0) | −3.0 (−6.8 to 0) | −2.7 (−11.5 to 1.0) | 0.29 |
| Intramedullary rod (%) | 32 (51) | 30 (68) | 17 (85) | 79 (62) | 0.014 |
| Refractures (%) | 13 (21) | 2 (5) | 2 (10) | 17 (13) | 0.049 |
The fractures are divided into three groups according to patients’ BP treatment status at time of trauma. Naive: patient was naive to BP treatment; ongoing: BP treatment was ongoing; discontinued: the patient had been receiving BP treatment, but treatment was at least temporarily discontinued (drug holiday). P values refer to differences between the three treatment groups. SD, standard deviation.
With respect to femoral fracture location, mid or distal shaft fractures were most common (41% of all fractures), followed by subtrochanteric and distal fractures, respectively. Regarding the fracture type, transverse fractures comprised about two-thirds of all femoral fractures. Most of the remaining fractures were oblique. Other fracture types were rare (7%) (Table 2).
| . | OI Type . | Total . | P Value . | ||
|---|---|---|---|---|---|
| I . | III . | IV . | |||
| No. of fractures | 26 | 28 | 73 | 127 | |
| Fracture location, No. (%) | 0.02 | ||||
| Proximal | 3 (12) | 2 (7) | 2 (3) | 7 (6) | 0.13 |
| Subtrochanteric | 7 (27) | 10 (36) | 25 (34) | 42 (33) | 0.75 |
| Mid or distal shaft | 16 (62) | 7 (25) | 29 (40) | 52 (41) | 0.02 |
| Distal | 0 (0) | 9 (32) | 17 (23) | 26 (20) | 0.004 |
| Fracture type, No. (%) | 0.11 | ||||
| Transverse | 12 (46) | 18 (64) | 53 (73) | 83 (65) | 0.051 |
| Oblique | 10 (38) | 9 (32) | 16 (20) | 35 (28) | 0.22 |
| Other | 4 (15) | 1 (4) | 4 (5) | 9 (7) | 0.23 |
| . | OI Type . | Total . | P Value . | ||
|---|---|---|---|---|---|
| I . | III . | IV . | |||
| No. of fractures | 26 | 28 | 73 | 127 | |
| Fracture location, No. (%) | 0.02 | ||||
| Proximal | 3 (12) | 2 (7) | 2 (3) | 7 (6) | 0.13 |
| Subtrochanteric | 7 (27) | 10 (36) | 25 (34) | 42 (33) | 0.75 |
| Mid or distal shaft | 16 (62) | 7 (25) | 29 (40) | 52 (41) | 0.02 |
| Distal | 0 (0) | 9 (32) | 17 (23) | 26 (20) | 0.004 |
| Fracture type, No. (%) | 0.11 | ||||
| Transverse | 12 (46) | 18 (64) | 53 (73) | 83 (65) | 0.051 |
| Oblique | 10 (38) | 9 (32) | 16 (20) | 35 (28) | 0.22 |
| Other | 4 (15) | 1 (4) | 4 (5) | 9 (7) | 0.23 |
P values refer to differences between the three treatment groups.
| . | OI Type . | Total . | P Value . | ||
|---|---|---|---|---|---|
| I . | III . | IV . | |||
| No. of fractures | 26 | 28 | 73 | 127 | |
| Fracture location, No. (%) | 0.02 | ||||
| Proximal | 3 (12) | 2 (7) | 2 (3) | 7 (6) | 0.13 |
| Subtrochanteric | 7 (27) | 10 (36) | 25 (34) | 42 (33) | 0.75 |
| Mid or distal shaft | 16 (62) | 7 (25) | 29 (40) | 52 (41) | 0.02 |
| Distal | 0 (0) | 9 (32) | 17 (23) | 26 (20) | 0.004 |
| Fracture type, No. (%) | 0.11 | ||||
| Transverse | 12 (46) | 18 (64) | 53 (73) | 83 (65) | 0.051 |
| Oblique | 10 (38) | 9 (32) | 16 (20) | 35 (28) | 0.22 |
| Other | 4 (15) | 1 (4) | 4 (5) | 9 (7) | 0.23 |
| . | OI Type . | Total . | P Value . | ||
|---|---|---|---|---|---|
| I . | III . | IV . | |||
| No. of fractures | 26 | 28 | 73 | 127 | |
| Fracture location, No. (%) | 0.02 | ||||
| Proximal | 3 (12) | 2 (7) | 2 (3) | 7 (6) | 0.13 |
| Subtrochanteric | 7 (27) | 10 (36) | 25 (34) | 42 (33) | 0.75 |
| Mid or distal shaft | 16 (62) | 7 (25) | 29 (40) | 52 (41) | 0.02 |
| Distal | 0 (0) | 9 (32) | 17 (23) | 26 (20) | 0.004 |
| Fracture type, No. (%) | 0.11 | ||||
| Transverse | 12 (46) | 18 (64) | 53 (73) | 83 (65) | 0.051 |
| Oblique | 10 (38) | 9 (32) | 16 (20) | 35 (28) | 0.22 |
| Other | 4 (15) | 1 (4) | 4 (5) | 9 (7) | 0.23 |
P values refer to differences between the three treatment groups.
Some statistically significant differences were observed between various OI groups (P = 0.02) with regard to fracture location. Distal fractures were much more frequent in the more severe types III and IV OI than in type I, whereas femoral shaft fractures were more common in the milder type I OI compared with types III and IV (Table 2; Fig. 2). When the more severe types III and IV, which were much more frequently treated with BPs, were grouped together and compared with type I OI, the differences were even more evident (P < 0.01). With respect to the fracture types, no differences occurred in the distribution when the three OI types were compared with each other (P = 0.11) (Table 2; Fig. 2), but when types III and IV OI were grouped together and compared with type I, transverse fractures were found to be more common in types III and IV OI (P = 0.04).
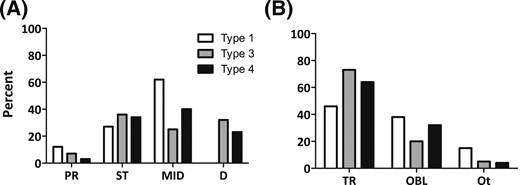
Characteristics of femoral fractures according to the patients’ OI type. (A) Fracture location. (B) Fracture type. D, distal; MID, femoral shaft; OBL, oblique; Ot, other; PR, proximal; ST, subtrochanteric; TR, transverse.
Fractures and BP treatment
Altogether, 44 (35%) of the femoral fractures occurred during BP treatment, 20 (16%) during a drug holiday, and 63 (50%) in patients naïve to BPs (Table 1). The median time period from first BP treatment exposure to fracture was 4.1 years (range, 0.1 to 11.0 years). In patients with ongoing treatment, the median duration of BP treatment preceding the fracture was 1.0 year (range, 0.1 to 3.2 years).
The distributions of fracture location and fracture type were similar in all three BP groups (patients naïve to BPs, patients with ongoing BP treatment, and patients with discontinued BP treatment), and no statistically significant associations were found (P = 0.78 and P = 0.35, respectively) (Table 3; Supplemental Fig. 1). Exclusion of refractures did not alter these results (P = 0.89 and P = 0.26, respectively). Furthermore, the pattern of fractures was similar in all treatment groups when the fractures were analyzed separately in the groups according to the patients’ type of OI (Supplemental Fig. 2). The pattern of fractures was also similar in those with high (≥2000 BP units/kg) and low (<2000 BP units/kg) cumulative BP dose (P = 0.075 and P = 0.82, respectively).
Location and Type of Femoral Fractures According to Patients' BP Treatment Status at Time of Trauma
| . | BP Treatment . | Total . | P Value . | ||
|---|---|---|---|---|---|
| Naive . | Ongoing . | Discontinued . | |||
| No. of fractures | 63 | 44 | 20 | 127 | |
| Fracture location, No. (%) | 0.78 | ||||
| Proximal | 3 (5) | 4 (9) | 0 (0) | 7 (6) | 0.44 |
| Subtrochanteric | 20 (32) | 14 (32) | 8 (40) | 42 (33) | 0.77 |
| Mid or distal shaft | 28 (44) | 17 (39) | 7 (35) | 52 (41) | 0.70 |
| Distal | 12 (19) | 9 (21) | 5 (25) | 26 (20) | 0.85 |
| Fracture type, No. (%) | 0.35 | ||||
| Transverse | 42 (67) | 26 (59) | 15 (75) | 83 (65) | 0.44 |
| Oblique | 18 (29) | 12 (27) | 5 (25) | 35 (28) | 0.95 |
| Other | 3 (5) | 6 (14) | 0 (0) | 9 (7) | 0.11 |
| . | BP Treatment . | Total . | P Value . | ||
|---|---|---|---|---|---|
| Naive . | Ongoing . | Discontinued . | |||
| No. of fractures | 63 | 44 | 20 | 127 | |
| Fracture location, No. (%) | 0.78 | ||||
| Proximal | 3 (5) | 4 (9) | 0 (0) | 7 (6) | 0.44 |
| Subtrochanteric | 20 (32) | 14 (32) | 8 (40) | 42 (33) | 0.77 |
| Mid or distal shaft | 28 (44) | 17 (39) | 7 (35) | 52 (41) | 0.70 |
| Distal | 12 (19) | 9 (21) | 5 (25) | 26 (20) | 0.85 |
| Fracture type, No. (%) | 0.35 | ||||
| Transverse | 42 (67) | 26 (59) | 15 (75) | 83 (65) | 0.44 |
| Oblique | 18 (29) | 12 (27) | 5 (25) | 35 (28) | 0.95 |
| Other | 3 (5) | 6 (14) | 0 (0) | 9 (7) | 0.11 |
P values refer to differences between the three treatment groups.
Location and Type of Femoral Fractures According to Patients' BP Treatment Status at Time of Trauma
| . | BP Treatment . | Total . | P Value . | ||
|---|---|---|---|---|---|
| Naive . | Ongoing . | Discontinued . | |||
| No. of fractures | 63 | 44 | 20 | 127 | |
| Fracture location, No. (%) | 0.78 | ||||
| Proximal | 3 (5) | 4 (9) | 0 (0) | 7 (6) | 0.44 |
| Subtrochanteric | 20 (32) | 14 (32) | 8 (40) | 42 (33) | 0.77 |
| Mid or distal shaft | 28 (44) | 17 (39) | 7 (35) | 52 (41) | 0.70 |
| Distal | 12 (19) | 9 (21) | 5 (25) | 26 (20) | 0.85 |
| Fracture type, No. (%) | 0.35 | ||||
| Transverse | 42 (67) | 26 (59) | 15 (75) | 83 (65) | 0.44 |
| Oblique | 18 (29) | 12 (27) | 5 (25) | 35 (28) | 0.95 |
| Other | 3 (5) | 6 (14) | 0 (0) | 9 (7) | 0.11 |
| . | BP Treatment . | Total . | P Value . | ||
|---|---|---|---|---|---|
| Naive . | Ongoing . | Discontinued . | |||
| No. of fractures | 63 | 44 | 20 | 127 | |
| Fracture location, No. (%) | 0.78 | ||||
| Proximal | 3 (5) | 4 (9) | 0 (0) | 7 (6) | 0.44 |
| Subtrochanteric | 20 (32) | 14 (32) | 8 (40) | 42 (33) | 0.77 |
| Mid or distal shaft | 28 (44) | 17 (39) | 7 (35) | 52 (41) | 0.70 |
| Distal | 12 (19) | 9 (21) | 5 (25) | 26 (20) | 0.85 |
| Fracture type, No. (%) | 0.35 | ||||
| Transverse | 42 (67) | 26 (59) | 15 (75) | 83 (65) | 0.44 |
| Oblique | 18 (29) | 12 (27) | 5 (25) | 35 (28) | 0.95 |
| Other | 3 (5) | 6 (14) | 0 (0) | 9 (7) | 0.11 |
P values refer to differences between the three treatment groups.
Intramedullary rods in the fractured femur were present in 51% of the fractures occurring in patients naïve to BPs, 68% of the fractures occurring in patients receiving ongoing BP treatment, and 85% of the fractures occurring in patients with discontinued treatment. The intramedullary rodding was neither associated with fracture location (P = 0.74) nor fracture type (P = 0.23). The refractures were instead significantly more frequent (21%) in patients naïve to BPs when compared with patients with ongoing (5%) and discontinued BP treatment (10%; P = 0.049) (Table 1).
More than half (61%) of all fractures occurred during daily activities, regardless of the status of BP treatment. The distribution of the trauma mechanism did not differ significantly between the groups of patients with ongoing and discontinued BP treatment. Traumas leading to fractures tended to occur more commonly during exercise in patients naive to BPs (P = 0.056) (Supplemental Table 1; Fig. 3).
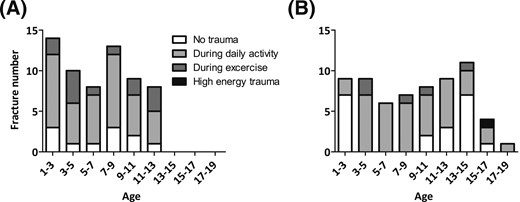
Distribution of trauma mechanisms according to patients' age (years) at the time of fracture. (A) Patients naive to BPs; (B) patients treated with BPs.
Discussion
We performed a detailed analysis of 127 femoral fractures in our pediatric OI population to assess whether BP treatment affects features of these fractures. Contrary to general concerns, there was no difference in characteristics of femoral fractures, with respect to their location or fracture type, between patients treated and not treated with BPs. Instead, the clinical OI type was associated with fracture characteristics, and transverse configuration and distal location were more common features in femoral fractures occurring in patients with type III or IV OI compared with patients with the milder type I OI.
Atypical femoral fractures and their association with BP exposure have been significant concerns in adults treated for osteoporosis, and there have been fears that BP treatment would alter the characteristics of femoral fractures in children with OI as well. However, only one previous study evaluated this issue in the pediatric OI population (16). The study showed a remarkable shift toward subtrochanteric localization of femoral fractures in association with BP treatment; BP-naïve patients mostly suffered fractures in the middiaphyseal region of the femur, whereas after BP treatment, almost all femoral fractures (14 of 16) were subtrochanteric.
Our study does not support these findings. We carefully evaluated the radiographs that were obtained at the time of fracture and combined the data with detailed information on BP exposure, as collected from hospital records. When a total of 127 femoral fractures were reviewed, 42 were classified as subtrochanteric fractures. Altogether, 48% of these occurred in subjects naïve to BPs, and 52% in patients with ongoing or preceding BP exposure. The pattern of femoral fractures was found to be similar in other respects in all three BP treatment groups.
We observed that, instead of BP treatment, the clinical subtype of OI was associated with different characteristics of femoral fractures. Fractures in the distal femur and transverse configuration were more common in the more severe types III and IV compared with the milder type I OI. Transverse configuration has been found to be a frequent feature of long-bone fractures that occur in children with OI when compared with healthy children (18). This can be explained by impaired bone material properties, which prevent bones from resisting bending forces, and by an insufficient amount of bone. It is less clear why patients with more severe OI had a propensity to suffer distal femoral fractures. One of the factors modifying the location of femoral fractures in OI is intramedullary rodding, especially when the rod has become short in relation to bone length. However, we could not find a significant association between intramedullary rodding and the fracture site. Another factor potentially affecting the location of factures is deformities, which are more commonly present in patients with more severe OI. However, femoral bending is usually located in the proximal third of the diaphysis and should shift the location of the fractures to the subtrochanteric region, not to the distal femur. Distal femur fractures also occur frequently in children with disuse-associated osteoporosis (19), and particularly in severe OI, disuse may be an important additional risk factor for fractures.
Several factors should be considered when comparing skeletal effects of BPs in adults with postmenopausal or secondary osteoporosis and in children with OI. The mechanical properties and structure of bone in patients with OI differ markedly from normal bone. In contrast with postmenopausal osteoporosis, the bone matrix is in fact hypermineralized in OI (20), making it stiffer and harder (21). Together with impaired bone mass, hypermineralization leads to bone fragility, the main clinical characteristic of OI. Because the antiresorptive agents in BPs mainly affect osteoclasts, the ability of BPs to increase bone mass in children with OI is thought to be mainly due to effects on modeling, leading to increased cortical thickness (22) and reduced cortical porosity (23). The trabecular number is also increased, but the trabecular thickness and architecture remain unchanged (22). Reports concerning the effects of BP treatment on intrinsic material properties are contradictory (24–26). However, results from human studies suggest that pamidronate treatment would not compromise these properties in children with OI (21, 27). A reason for inconsistent results may be the varying lengths of the treatments: in human studies, treatment length has been up to 5 years, whereas harmful effects have been seen in mouse models after 12 weeks of treatment, a duration which corresponds to treatment from toddler age to young adulthood in humans (25). The femoral fractures with atypical features reported by Vasanwala et al. (15) in a teenager with OI occurred late, after 5.1 years of continuous and relatively high-dose intravenous pamidronate exposure. The length of BP treatment, in addition to the younger patient cohort and the inclusion of patients with type I OI, is also a major difference between the study populations in the current study and in the study by Nicolaou et al. (16). The average length of BP treatment preceding fractures was 6.5 years without standard drug holidays in the study by Nicolaou et al.16), whereas in our study, the total duration was 4.1 years, on average, and drug holidays were used. In addition, the pamidronate and risedronate doses used in our clinic were considerably smaller than those used by Nicolaou et al. (16). BPs also decrease remodeling activity, and a common concern has been that BP treatment could decrease this activity to a level at which microcracks could accumulate because of impaired healing. However, remodeling activity is characteristically elevated in OI (28) and is possibly the reason for bone pain, and a slight decrease in remodeling activity during BP treatment may even be beneficial for the material properties of bone and the well-being of the patients.
Our data were collected during a time when a drug holiday of at least 1 year after 2 to 3 years of BP treatment was a common practice in our clinic. Hence, these results cannot be generalized to current clinical practice in most centers, where BPs are used for much longer periods and with lower doses in the maintenance phase of the treatment. There is no consensus regarding drug holidays in pediatric BP treatment. It has been speculated that discontinuation of BP treatment could increase interface fractures between the BP-treated and untreated areas of bone. Because the appearance of BP-induced bands differs among patients and treatment modalities, and because previous fractures and surgeries also affect the appearance of bone, we did not separately evaluate this issue. However, such fractures would be distal fractures in the femur, and these were equally common in all treatment groups, which suggests that interface fractures are not a major concern. This study suggests that relatively short periods of BP treatment do not alter the pattern of femoral fractures. We also observed that there were no significant alterations in fracture patterns after the discontinuation of BPs, and on the basis of this finding, drug holidays should be considered at least in some patients.
We recognize some limitations of our study. The cohort was relatively small, and the data were collected retrospectively, which potentially introduced some bias into the results. However, we reviewed the original radiographs of each fracture for fracture characteristics. We included material multiple fractures that occurred in the same patients. Using fractures that are possibly related to each other should, however, overestimate the effects of BP treatment, and therefore does not challenge the reliability of our results. We also performed a separate analysis omitting refractures, and this did not change our results. Furthermore, the treatment groups were not identical with respect to the OI type distribution. To evaluate the effect of this dissimilarity, the analyses were also performed in groups according to OI type, which did not alter the results. Unfortunately, several patients had received more than one type of BP; therefore, we were not able to assess the effects of different BPs separately. Despite these limitations, which are inherent to clinical studies of rare diseases, we believe that our findings provide valuable information regarding the effects of BPs on femoral fractures in children with OI.
In conclusion, the current study showed no evidence of any alterations in the pattern of femoral fractures in children with OI treated with BPs, and thus did not support the previous findings of increasing trends in subtrochanteric region fractures (16). During the study, regularly arranged drug holidays were a common practice in our clinic, which may at least partially have protected the patients from the potential harmful long-term effects associated with BPs. However, further studies are needed to elucidate optimal BP treatment protocols in children with OI.
Abbreviations:
Acknowledgments
This work was supported by the Sigrid Juselius Foundation, the Folkhälsan Research Foundation, the Academy of Finland, the Foundation for Pediatric Research, the Helsinki University Research Funds, the Swedish Research Council, the Novo Nordisk Foundation, the European Society for Paediatric Endocrinology Research Unit, the Swedish Childhood Cancer Foundation, and the Stockholm County Council (ALF project).
Disclosure Summary: The authors have nothing to disclose.
References
Author notes
Address all correspondence and requests for reprints to: Outi Mäkitie, MD, PhD, Folkhälsan Institute of Genetics, PO Box 63, FIN-00014, University of Helsinki, Helsinki, Finland. E-mail: [email protected].



