-
PDF
- Split View
-
Views
-
Cite
Cite
Marie Lindhardt Johansen, Casper P. Hagen, Mikkel G. Mieritz, Ole D. Wolthers, Carsten Heuck, Jørgen Holm Petersen, Anders Juul, Pubertal Progression and Reproductive Hormones in Healthy Girls With Transient Thelarche, The Journal of Clinical Endocrinology & Metabolism, Volume 102, Issue 3, 1 March 2017, Pages 1001–1008, https://doi.org/10.1210/jc.2016-2871
Close - Share Icon Share
Abstract
Detailed evaluation of pubertal progression in girls from longitudinal studies is sparse, and the phenomenon of transient thelarche (TT), defined as the appearance, regression, and subsequent reappearance of breast buds, in healthy girls remains undescribed.
To describe TT in terms of pubertal progression, growth, genotypes, and reproductive hormones and to apply new puberty nomograms for breast stages, pubic hair, and menarche.
A prospective, longitudinal population-based study.
Ninety-eight healthy Danish schoolchildren (Caucasian girls) followed longitudinally as part of the COPENHAGEN Puberty Study were included in the evaluation of TT. A total of 1466 girls from 2 cross-sectional studies were included in the creation of the puberty nomograms.
None.
Pubertal progression, specifically thelarche, reproductive hormones, genotype, and growth.
Twelve of 98 (12%) girls experienced TT. A larger proportion of girls with TT entered puberty by the pubarche pathway (50%) compared with girls with normal progression (15.4%), P = 0.014. Girls with TT progressed through puberty normally when evaluated using puberty nomograms. Reproductive hormones and growth velocity were lower at the first (transient) thelarche than the second (permanent) thelarche.
TT is a frequent phenomenon that appears to be a peripheral occurrence independent of central puberty. It does not appear to affect subsequent pubertal progression as evaluated by our new puberty nomograms.
Age at onset of puberty has declined in industrialized countries, including the United States, United Kingdom, and Denmark (1–4). In Denmark, the average age at breast development has changed markedly from 11 to 10 years of age between 1991 and 2006, whereas the average age at menarche only moderately changed (4 months earlier) (1). These cross-sectional findings from the COPENHAGEN Puberty Study were recently confirmed in a subsequent longitudinal study from the same Copenhagen area (5). Importantly, younger age at breast development in the 2006 cohort was not associated with higher follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels compared with blood samples from the 1991 cohort analyzed in the same laboratory using the same hormone assays. Altogether, the findings suggest that the duration of puberty from breast budding to menarche has increased, and that the earlier breast budding appears to be gonadotropin independent.
Although the median age at breast budding is declining, there is a large interindividual variation in age at onset of puberty. Genetic variation of FSH action seems to affect age at pubertal onset (6) as well as FSH levels and ovarian follicle maturation (7) in healthy girls. Whether FSH action affects the subsequent progression through puberty is unknown.
Detailed evaluation of pubertal progression requires longitudinal studies with frequent pubertal examinations. In our study of healthy Danish girls, we noticed a subgroup of girls with transient breast budding [hereafter transient thelarche (TT)]. Many pediatric endocrinologists are familiar with this phenomenon when examining girls of prepubertal age referred to their clinics due to breast budding that cannot be found upon examination. Thus, TT is well-known and has previously been described in very young girls (premature thelarche) (8–10). However, the frequency as well as the phenotypic, genotypic, and biochemical characteristics of girls with TT over the age of 8 years remains undescribed to date.
In our longitudinal cohort of healthy, Danish girls, we assessed the progression of puberty using our new puberty nomograms. In detail, we evaluated girls with TT concerning pubertal progression, growth pattern, genotypes, and reproductive hormone levels.
Materials and Methods
Subjects
The COPENHAGEN Puberty Study is a population-based study of healthy children carried out in 2005–2006 in public schools in the Copenhagen area. A subsequent longitudinal follow-up study included a total of 208 children (1, 2). Of the 208 children, 117 were girls. Nineteen girls were excluded owing to ethnic backgrounds other than Caucasian or serious health issues. In total, 98 healthy, Caucasian girls were included in this current substudy of TT. Genotypes, clinical features including Tanner staging, and biochemistry have previously been reported in these girls in association with other endpoints (1, 5, 6, 11, 12), however, never with TT as an outcome.
The puberty nomograms were based on 2 cross-sectional studies. A total of 995 girls from the cross-sectional part of COPENHAGEN Puberty Study (aged 5.8 to 20.2 years) and 471 girls (age 5.8 to 19.9 years) from 4 public schools in the town of Randers was included (13–16).
Clinical examination
Blood sampling and clinical examinations were scheduled every 6 months, and all were carried out between 2006 and 2014. The pubertal staging was performed by 6 trained physicians. The actual staging of breast budding and pubic hair was done according to Tanner’s classification (17). The larger breast was the determinant of a girl’s breast stage. Pubertal onset was defined as the first sign of puberty by pubarche (pubic hair stage PH2 or more) and/or thelarche (breast stage B2 or more). TT was defined as a girl entering breast stage B2 or larger unilaterally or bilaterally, followed by regression to breast stage B1 bilaterally, and then followed by a subsequent redevelopment to B2 or larger unilaterally or bilaterally (i.e., B2-B1-B2). For the girls with TT, the first breast development was not regarded as pubertal onset; the pubertal stage thereby had to be a permanent development to be rendered as pubertal onset. Height velocity was defined as growth in centimeters per year. Variables such as height, weight, and Tanner stages were not available at all time points for all of the girls. Thus, only girls with available data are part of the subsequent analyses.
Statistical analyses
The puberty nomograms for breast development, pubic hair development, and menarche were based on a total of 1466 healthy Caucasian girls from the cross-sectional data using the methodology of Van Buuren and Ooms (stage-line diagrams) (18, 19). Based on the nomograms, age-specific standard deviation (SD) scores were calculated for all 98 girls from the longitudinal part of the COPENHAGEN Puberty Study. The SD scores were used to validate the nomograms and to evaluate pubertal progression in the girls with TT. The puberty nomograms were calculated in R (R Foundation for Statistical Computing).
Wilcoxon’s signed-rank test was used to compare LH, FSH, estradiol, testosterone, androstenedione, anti-Müllerian hormone (AMH), inhibin B, insulin-like growth factor 1 (IGF-1), and insulin-like growth factor-binding protein 3 (IGFBP-3) concentrations at the initial breast budding compared with the second breast budding.
The Mann-Whitney U test was used to compare the age at initial B2; the time of progression from B2 to B4; height velocity; height; weight; body mass index prepubertally, at pubertal onset, and postpubertally; and reproductive hormone levels in girls with and without TT.
Fisher’s exact test was used to compare the frequency of pubertal onset by pubarche vs thelarche or a combination of pubarche and thelarche between the group of girls with TT and the rest of the girls from the longitudinal cohort. The same test was applied to determine whether the distribution of FSHB/FSHR minor alleles differed between girls with TT vs without and girls with TT vs Caucasian females from 1000 Genomes (www.1000genomes.org).
Hormone assays
Blood was drawn from an antecubital vein in the morning between 8 and 10 am. Immediately after blood sampling, the samples were centrifuged and aliquoted into cryotubes that were stored at −20°C until hormone analyses were performed. We analyzed all blood samples for all reproductive hormones in the same laboratory blinded for the technician for pubertal staging. Serum concentrations of FSH and LH were determined using the time-resolved immunofluorometric assays (Delfia; PerkinElmer, Boston, MA). The limits of detection were 0.06 and 0.05 IU/L, respectively, with intra- and interassay coefficients of variation (CVs) of <5%, respectively, in both assays. The serum estradiol concentration was determined using radioimmunoassay (Pantex, Santa Monica, CA), and with a detection limit of 18 pmol/L and intra- and interassay CVs of <8% and 13%, respectively. Inhibin B levels were measured using the Beckman Coulter GenII assay. Limit of detection was 3 pg/mL, and inter- and intra-assay CVs were <11%. Serum AMH was quantified using the Beckman Coulter enzyme immunometric assay generation I (IOT; Immunotech, Beckman Coulter, Copenhagen, Denmark) with a detection limit of 2.0 pmol/L, and the intra-assay CV was 9.2%. IGF-1 and IGFBP-3 levels were measured using Immulite 2000 IGF-1/IGFBP-3 (Siemens Health Care Diagnostics, Tarrytown, NY) on the automated Immulite 2000 (Siemens Health Care Diagnostics) with intra- and interassay CVs of <4% and 9%, respectively. Testosterone was measured using the DPC Coat-A-Count RIA kit (Diagnostic Products, Los Angeles, CA) with a detection limit of 0.23 nmol/L and intra- and interassay CVs of 7.6% and 8.6%, respectively. Concentrations of androstenedione were quantitated by specific solid-phase, competitive chemiluminescent enzyme immunoassays (Immulite 2000; Siemens Health Care Diagnostics) with a detection limit of 1.04 nmol/L. The intra- and interassay CVs were 7.1% to 10.8% and 11.0% to 14.9%, respectively.
Genotyping
Genomic DNA was isolated from peripheral blood (0.2 ml EDTA preserved) using the QuickGene-810 Nucleic Acid Isolation System (Fujifilm; Life Science Products, Tokyo, Japan). It was quantified on a NanoDrop ND-1000 spectrophotometer (Saveen Werner, Limhamn, Sweden).
All single-nucleotide polymorphisms were analyzed using the KASP single-nucleotide polymorphism genotyping assays. This assay enables biallelic discrimination via a competitive polymerase chain reaction and integration of a fluorescent resonance energy transfer quencher cassette. This took place at LGC Genomics (Hoddesdon, UK) or at the genetic laboratory in our department. The KASP genotyping assays were designed to target the following sequences: FSHB c.-211G>T (rs10835638), 5′-TATCAAATTTAATTT[G/T]TACAAAATCATCAT-3′; FSHR c.-29G>A (rs1394205), 5′-TCTCTGCAAATGCAG[A/G]AAGAAATCAGGTGG-3′; and FSHR c.2039A>G (rs6166), 5′-ATGTAAGTGGAACCA[C/T]TGGTGACTCTGGGA-3′. FSHB c.-211G>T, FSHR c.-29G>A, and FSHR c.2039A>G were available from the 98 girls from the longitudinal part of the COPENHAGEN Puberty Study, all of Caucasian origin.
Ethical considerations
The study (COPENHAGEN Puberty Study) was approved by the Danish Data Protection Agency (2015-41-4494) and by the ethics committee (KF 01 282214 and V200.1996/90). The cohort from Randers was approved by the ethics committee (1998/4524) and by the Danish Data Protection Agency (2010-41-4384)0.01 282214 and V200.1996/90). All participants and parents gave informed consent.
Results
The puberty nomograms for breast development, pubic hair, and menarche can be seen in Fig. 1. In Supplemental Fig. 1, the nomogram based on breast stages was validated by data from the longitudinal study. These girls were additionally grouped according to their FSHB and FSHR genotypes. In Supplemental Fig. 2, the nomograms for pubic hair and menarche were validated using the same girls. It can be seen from the validated nomograms that the vast majority of these healthy girls stay and end within ±2 SD in the gray-shaded area.
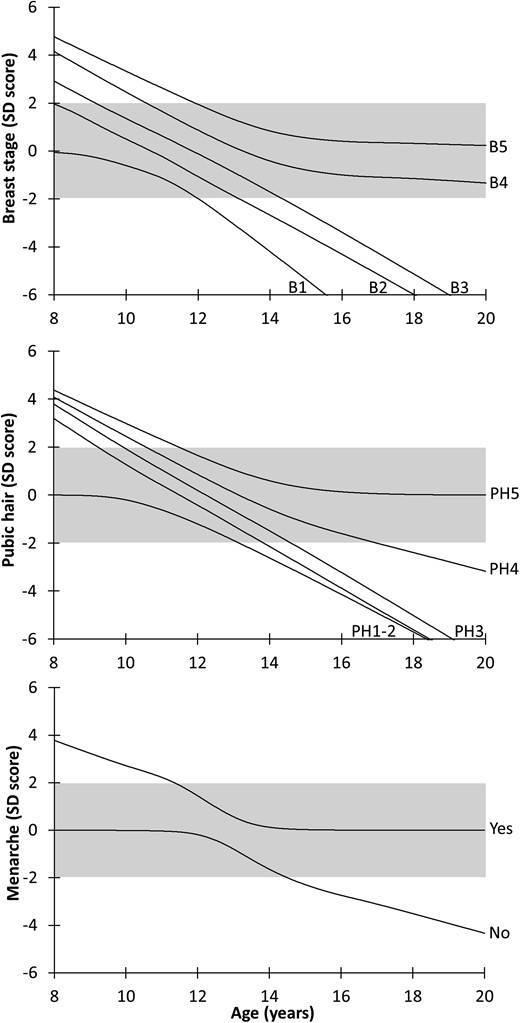
Nomograms for breast stages, pubic hair, and menarche: SD scores for Tanner stages for breast tissue (stage BI to B5) and pubic hair (stage PH1 to PH5) and menarche (yes/no) according to age. The gray-shaded area represents ±2 SD.
Thirteen girls experienced regression of their initial breast buds; however, only 12 also experienced recurrence of the buds during the study period (i.e., TT). No girls experienced regression and reappearance of breast tissue more than once. In girls with this phenomenon, puberty was initiated by pubarche in 6 of 12 girls, by combined pubarche and thelarche in 3 of 12 girls, and by thelarche in 3 of 12 girls. In the remaining 86 girls (without TT), 65 experienced pubertal onset and 21 girls did not enter puberty during follow-up. Puberty was initiated by pubarche in 10 of 65 girls, by thelarche in 53 of 65, or by combined pubarche and thelarche in 2 of 65. A higher proportion of girls with TT entered puberty by the pubarche pathway compared with girls with normal progression, P = 0.014.
In girls with TT, the time from the first to the second breast development was 1.47 years [0.88 to 4.09; median (range)]. However, after this variable period of time, pubertal development progressed normally in all girls (Fig. 2). Median age at first B2 in girls with TT was 9.8 years (7.95 to 12.88), and 10.69 years (7.18 to 12.88) in girls without, P = 0.039. One girl with TT and one girl without experienced the initial breast budding at ages earlier than 8 years (7.95 and 7.18 years, respectively).
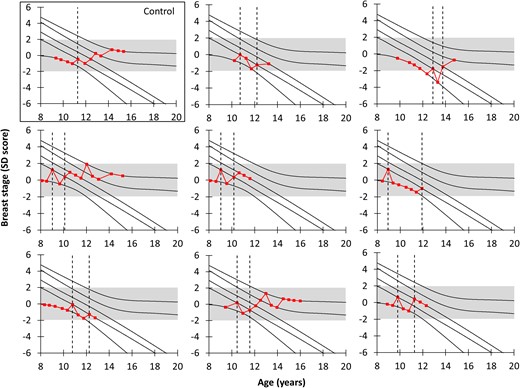
TT and subsequent breast development (nomogram). Eight randomly selected and representative girls with TT and 1 girl without (for comparison) plotted on the nomogram for breast stages. The dotted lines represent the time point(s) with onset(s) of breast development. The gray-shaded area represents ±2 SD; all girls with TT end in the gray-shaded area.
The time it took for girls to progress from B2 (the first permanent B2 in girls with TT) to B4 did not differ between girls who experienced TT and girls who did not: 2.05 years (1.86 to 3.01) vs 2.05 years (0.94 to 3.15), P = 0.647.
The median values and ranges for the hormone concentrations appear in Table 1. When comparing concentrations at the initial appearance of breast tissue compared with the reappearance, LH, FSH, IGF-1, and IGFBP-3 were significantly higher at the reappearance (Table 1). Figure 3 illustrates LH, FSH, and estradiol concentrations at the initial (and transient for girls with TT) breast budding in all girls. There were no significant differences between serum concentrations of LH, FSH, testosterone, estradiol, androstenedione, AMH, IGF-1, or IGFBP-3 at the time of the permanent breast development (B2) in girls with and without TT (all P > 0.05). Furthermore, when comparing prepubertal hormone levels (from the blood sampling closest to 3 years prior to onset), no differences were found in estradiol levels in girls with and without TT. LH and inhibin B levels were lower in girls with TT (LH, P = 0.015 and inhibin B, P = 0.018, respectively).
Hormone Concentrations During the First and Second Appearances of Breast Buds in Girls With the Phenomenon of TT
| Hormone . | 1st Breast Bud . | 2nd Breast Bud . | . | ||
|---|---|---|---|---|---|
| Median . | Range . | Median . | Range . | P Value . | |
| LH, IU/L | 0.06 | 0.03–0.15 | 0.32 | 0.12–2.48 | 0.043a |
| FSH, IU/L | 2.25 | 0.83–2.73 | 2.97 | 2.37–5.05 | 0.043a |
| AMH, pmol/L | 18 | 11–57 | 26 | 21–41 | 0.686 |
| Estradiol, pmol/L | 21 | <18–62 | 50 | 20–54 | 0.345 |
| Testosterone, nmol/L | <0.23 | <0.23–0.49 | <0.23 | <0.23–1.27 | 0.180 |
| Inhibin B, pg/mL | 22.00 | 4.00–33.00 | 39.00 | 27.00–55.00 | 0.104 |
| Androstenedione, nmol/L | 0.01 | 0.01–3.56 | 2.78 | 1.46–4.50 | 0.225 |
| IGF-1, ng/mL | 191 | 133–387 | 282 | 165–452 | 0.025a |
| IGFBP-3, ng/mL | 3900 | 3220–5360 | 4365 | 3590–6840 | 0.05a |
| Hormone . | 1st Breast Bud . | 2nd Breast Bud . | . | ||
|---|---|---|---|---|---|
| Median . | Range . | Median . | Range . | P Value . | |
| LH, IU/L | 0.06 | 0.03–0.15 | 0.32 | 0.12–2.48 | 0.043a |
| FSH, IU/L | 2.25 | 0.83–2.73 | 2.97 | 2.37–5.05 | 0.043a |
| AMH, pmol/L | 18 | 11–57 | 26 | 21–41 | 0.686 |
| Estradiol, pmol/L | 21 | <18–62 | 50 | 20–54 | 0.345 |
| Testosterone, nmol/L | <0.23 | <0.23–0.49 | <0.23 | <0.23–1.27 | 0.180 |
| Inhibin B, pg/mL | 22.00 | 4.00–33.00 | 39.00 | 27.00–55.00 | 0.104 |
| Androstenedione, nmol/L | 0.01 | 0.01–3.56 | 2.78 | 1.46–4.50 | 0.225 |
| IGF-1, ng/mL | 191 | 133–387 | 282 | 165–452 | 0.025a |
| IGFBP-3, ng/mL | 3900 | 3220–5360 | 4365 | 3590–6840 | 0.05a |
P values are calculated using Wilcoxon signed-rank tests.
Indicates significance by a 0.05 level.
Hormone Concentrations During the First and Second Appearances of Breast Buds in Girls With the Phenomenon of TT
| Hormone . | 1st Breast Bud . | 2nd Breast Bud . | . | ||
|---|---|---|---|---|---|
| Median . | Range . | Median . | Range . | P Value . | |
| LH, IU/L | 0.06 | 0.03–0.15 | 0.32 | 0.12–2.48 | 0.043a |
| FSH, IU/L | 2.25 | 0.83–2.73 | 2.97 | 2.37–5.05 | 0.043a |
| AMH, pmol/L | 18 | 11–57 | 26 | 21–41 | 0.686 |
| Estradiol, pmol/L | 21 | <18–62 | 50 | 20–54 | 0.345 |
| Testosterone, nmol/L | <0.23 | <0.23–0.49 | <0.23 | <0.23–1.27 | 0.180 |
| Inhibin B, pg/mL | 22.00 | 4.00–33.00 | 39.00 | 27.00–55.00 | 0.104 |
| Androstenedione, nmol/L | 0.01 | 0.01–3.56 | 2.78 | 1.46–4.50 | 0.225 |
| IGF-1, ng/mL | 191 | 133–387 | 282 | 165–452 | 0.025a |
| IGFBP-3, ng/mL | 3900 | 3220–5360 | 4365 | 3590–6840 | 0.05a |
| Hormone . | 1st Breast Bud . | 2nd Breast Bud . | . | ||
|---|---|---|---|---|---|
| Median . | Range . | Median . | Range . | P Value . | |
| LH, IU/L | 0.06 | 0.03–0.15 | 0.32 | 0.12–2.48 | 0.043a |
| FSH, IU/L | 2.25 | 0.83–2.73 | 2.97 | 2.37–5.05 | 0.043a |
| AMH, pmol/L | 18 | 11–57 | 26 | 21–41 | 0.686 |
| Estradiol, pmol/L | 21 | <18–62 | 50 | 20–54 | 0.345 |
| Testosterone, nmol/L | <0.23 | <0.23–0.49 | <0.23 | <0.23–1.27 | 0.180 |
| Inhibin B, pg/mL | 22.00 | 4.00–33.00 | 39.00 | 27.00–55.00 | 0.104 |
| Androstenedione, nmol/L | 0.01 | 0.01–3.56 | 2.78 | 1.46–4.50 | 0.225 |
| IGF-1, ng/mL | 191 | 133–387 | 282 | 165–452 | 0.025a |
| IGFBP-3, ng/mL | 3900 | 3220–5360 | 4365 | 3590–6840 | 0.05a |
P values are calculated using Wilcoxon signed-rank tests.
Indicates significance by a 0.05 level.
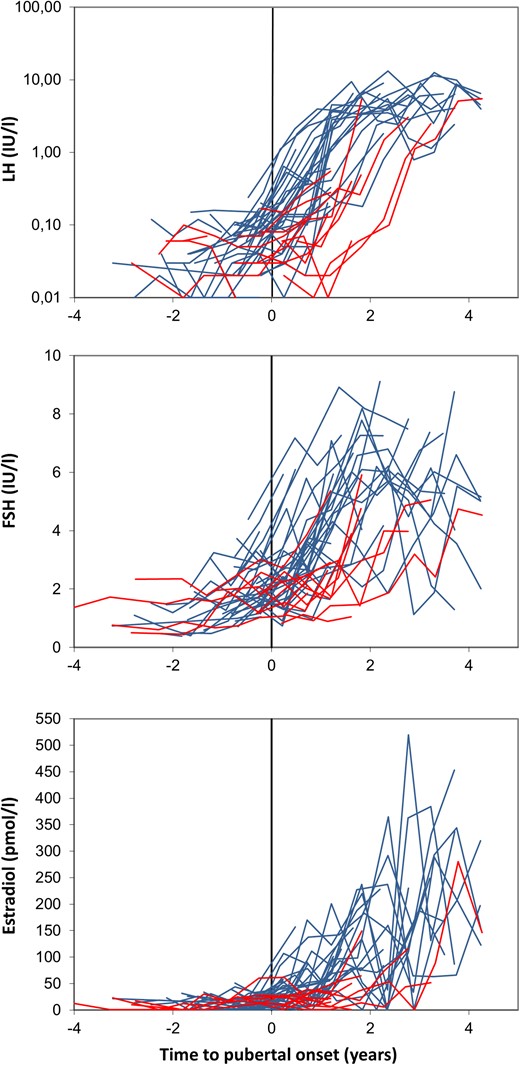
Longitudinal changes in reproductive hormones before, during, and after pubertal onset in healthy girls with or without TT. LH, FSH, and estradiol concentrations are graphed according to age at onset (initial breast development for all girls) and to whether the girls experienced TT (red) or not (blue).
No significant difference was found between the distribution of FSHB or FSHR genotypes in the group of girls with TT compared with girls without as well as in Caucasian females from 1000 Genomes regardless of grouping of all minor allele carriers (all P > 0.05) (Supplemental Table 1). Furthermore, we had the opportunity to examine whether genetic variation of FSH action appeared to affect progression of pubertal development in all girls, which it did not.
There were no differences in weight or body mass index between girls with or without TT prepubertally, at the time of transient and permanent thelarche or postpubertally (all P > 0.05). Heights likewise did not differ prepubertally or postpubertally (all P > 0.05). However, when comparing heights at the initial breast development in girls with and without TT, we found that girls with TT were significantly shorter (medians: 138.4 cm vs 144.6 cm, P = 0.010). Moreover, girls with TT were significantly taller than girls without TT when compared at the point of permanent breast development (i.e., the second breast development for girls with TT) (medians: 147.7 cm vs 144.3 cm, P = 0.012).
Lastly was a difference in growth (height velocity, centimeters per year) for girls with and without TT when comparing the velocity approximately 2 years after the initial (and impermanent in girls with TT) breast budding in all girls (P = 0.030). However, there was no difference in height velocity approximately 2 years after onset, when centered around the first permanent breast development for all girls (i.e., the second breast development for girls with TT) (P = 0.708) (Fig. 4).
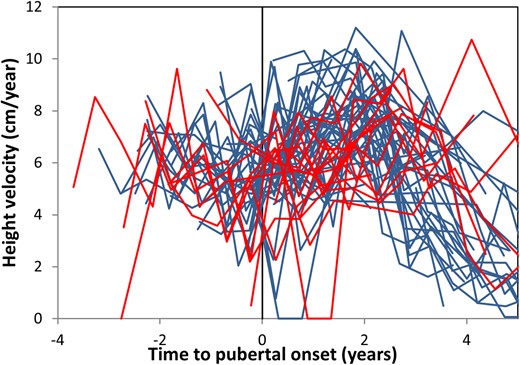
Growth velocity according to pubertal onset. Growth velocity graphed according to age at onset (initial breast development for all girls) and to whether the girls did (red) or did not (blue) experience TT.
Discussion
In this longitudinal cohort of healthy girls, we report TT to be a frequent (12%) and benign phenomenon among healthy girls based on newly developed and validated puberty nomograms.
To our knowledge, TT has never previously been described comprehensively in a longitudinal cohort of healthy individuals of girls older than 8 years of age, although the phenomenon has been observed (3) and mentioned in reviews and textbooks (20, 21). Thus, TT seems to be a relevant variant of pubertal development and thereby important to consider in clinical settings.
First, we examined reproductive hormones in girls with and without TT as well as comparing the first and the second breast development in girls with the phenomenon. We found that hormone levels did not differ when comparing the first permanent breast development in girls with and without TT. Contrarily, we found that girls with TT showed significantly lower concentrations of the gonadotropins at the initial, temporary breast development compared with the second, permanent onset. This indicates that the initial breast development in girls with TT is not a centrally regulated phenomenon. The fact that IGF-1 and IGFBP-3 are also significantly higher at the second onset further supports the notion that the growth hormone/IGF-1 axis is involved in permanent breast gland development. In accordance, we have recently demonstrated higher IGF-1 levels in boys who develop gynecomastia (22).
Additionally, we examined growth velocity to ensure that girls with TT at the initial breast budding did not display growth patterns similar to girls with a central onset of puberty. It was evident that if all girls were centered around the first permanent breast development, the growth patterns were similar. The first and transient breast buds did not appear to be associated with growth acceleration, which was observed at the second and permanent breast budding. This finding is also supported by the fact that girls with TT are significantly shorter than girls without when comparing at the time of the initial breast buds in all girls (i.e., transient breast buds in girls with TT and permanent buds in girls without TT).
To further study TT, we also looked at the progression through puberty using the statistical approach by Van Buuren and Ooms (stage-line diagrams) (18, 19), adapted to and validated by our Danish cohorts, thus creating new tools for pubertal evaluation. The corresponding puberty nomograms for Danish boys (23) were used to evaluate pubertal progression in boys with delayed puberty and highlight the clinical potential of the nomograms in boys as well as girls.
We applied this tool to the girls with TT. The nomograms revealed that the duration as well as the progression of pubertal development was comparable with that of other healthy girls. We furthermore examined the duration of puberty in these girls and found that there was no difference in the median time it took to progress from B2 to B4 (first permanent B2) in girls with and without TT. The lack of difference in duration and progression of puberty between the 2 groups supports the notion that the central onset of puberty appeared to begin with the second breast budding and indicated that the initial breast buds in girls with TT may not represent a centrally regulated event.
We therefore conclude that TT is most likely a peripheral phenomenon and not a sign of central pubertal onset. Thus, it may in fact be similar to, if not the same as, premature thelarche (PT). PT is by definition a benign occurrence of breast budding in girls <8 years of age in whom central precocious puberty has been ruled out. PT has been thoroughly studied (8, 9, 24–26), and whether it always is self-limiting or in some cases progresses to central precocious puberty is still debated (9, 24, 27).
To further explore TT, we examined the possible etiologies. First, we scrutinized the possibility that adrenal androgens are converted by aromatase action, thereby creating local estrogenization of breast tissue, as previously suggested by others (10, 26). We did so by examining the pathways of pubertal onset. Fifty percent of the girls with TT had pubarche as their first sign of puberty. This was in stark contrast to the rest of the girls (without TT), in which only 15% had pubarche as the first sign of puberty. The difference between the 2 groups was, despite a rather small group of girls with transient breast buds, highly significant. The frequency of girls entering puberty via the 3 different pathways has previously been described in the same cohort (numbers differ slightly owing to the continuation of the cohort) (5, 28). Mouritsen et al. (5) furthermore noted that androstenedione is measurable in peripheral blood samples at the initial onset of pubarche, although with interindividual variability. Likewise, Biro et al. (29) in another study noted that dehydroepiandrosterone sulfate levels were higher in girls who entered puberty through the pubarche pathway. Pubertal levels of androgens at the initial onset of pubarche would also explain why we do not find that the androgens (testosterone and androstenedione) are lower at the first compared with the second breast budding. This could indicate that, at least in a subgroup of girls, TT is associated with early adrenal maturation.
In further support of the theory of peripheral estrogenization of the breast tissue is the fact that girls with TT are taller at the time of the permanent breast development. This could indicate that the estrogen affecting the breast tissue is also affecting bone growth, although in low enough concentrations that it does not affect final height in girls with TT. Furthermore, the finding that inhibin B and LH concentrations prepubertally are lower in girls with TT not only supports the notion of TT being a peripheral event, but also possibly indicates that estrogen exerts a negative feedback on the hypothalamic-pituitary-gonadal (HPG) axis. This is in line with data previously published showing that the HPG axis is not completely silenced in childhood (30). It is, however, important to note that these are speculative but biologically plausible hypotheses.
This is also highlighted by the fact that we do not find higher levels of estrogen in girls with TT, although this could be due to the assay being too insensitive. In fact, 2 studies investigating girls with PT found that estrogen levels were significantly higher in girls with PT than in girls without (25, 26). Neither of the studies measured gonadotropins. Furthermore, Pereira et al. (26) also discuss the possibility that the higher levels of estrogens are not signs of an activation of the HPG axis, but rather reflective of a peripheral occurrence, as we also believe to be the case with TT.
Inappropriate application of maternal topical estrogen-containing creams has been reported to cause premature breast development. Likewise, exposure to household factors such as lavender or tea tree oil has been associated with gynecomastia in boys (31). Finally, exposure to endocrine-disrupting chemicals with estrogenic or antiandrogenic activity (phthalates and bisphenol A) has been associated with premature breast development. All of these factors could potentially be involved in the observed phenomenon of TT, but this remains speculative.
Lastly, we looked at whether the differences in FSHR and FSHB genotypes and thereby genetic variation in FSH action were associated with TT. We did not find any association between the FSHB and FSHR genotype distributions and TT, pubertal duration, or progression through puberty. We used data from 1000 Genomes to ensure large groups. It is important to note that our group of girls with TT is extremely small, introducing a risk of type II errors.
This study is based on the clinical Tanner staging of breast development, which some may argue is not an accurate (enough) measure of puberty. However, we sought to limit our interobserver bias by only having 6 physicians trained specifically for pubertal staging in this cohort as examiners from 2006 to 2014 when the study was concluded. A recent study from our group furthermore concludes that Tanner staging by thorough palpation is a very good measurement of actual size of breast tissue when compared with a golden standard of magnetic resonance imaging (32). Lastly, this study is one of the first to look at TT in such detail. The longitudinal design of the cohort allows for various perspectives on the phenomenon.
Following our in-depth description of TT in our healthy cohort, we therefore conclude that it is a frequent phenomenon that appears to be a peripheral occurrence independent of central puberty, thereby resembling the similarly benign phenomenon of premature thelarche. Moreover, TT does not appear to affect subsequent pubertal duration or progression, as evaluated by our new puberty nomograms.
Abbreviations:
- AMH
anti-Müllerian hormone
- CV
coefficient of variation
- FSH
follicle-stimulating hormone
- HPG
hypothalamic-pituitary-gonadal
- IGF-1
insulin-like growth factor 1
- IGFBP-3
insulin-like growth factor-binding protein 3
- LH
luteinizing hormone
- PT
premature thelarche
- SD
standard deviation
- TT
transient thelarche
Acknowledgments
M.L.J. is supported by Copenhagen University Hospital’s Research Foundation (Rigshospitalets Forskningsudvalg) through a 3-year stipend. Research by A.J. is partly supported by grants from the Capital Region of Denmark (R129-A3966) and the Ministry of Higher Education and Science (DFF-1331-00113).
Clinical trial registry: COPENHAGEN Puberty Study ClinicalTrials.gov no. NCT01411527 (registered 3 August 2011).
Disclosure Summary: The authors have nothing to disclose.
References
Neinstein LS, Gordon CM, Katzman DK, Rosen DS, Woods ER. Adolescent Health Care: A Practical Guide. 5th ed. Baltimore, MD: Williams & Wilkins; 2008.
Author notes
Address all correspondence and requests for reprints to: Anders Juul, MD, DMSc, PhD, Department of Growth and Reproduction, GR, 5064, Rigshospitalet, Blegdamsvej 9, DK-2100 Copenhagen Ø, Denmark. E-mail: [email protected].



