-
PDF
- Split View
-
Views
-
Cite
Cite
Madhu Chauhan, Ancizar Betancourt, Meena Balakrishnan, Uma Yallampalli, Yuanlin Dong, Karin Fox, Michael Belfort, Chandra Yallampalli, Impaired Vasodilatory Responses of Omental Arteries to CGRP Family Peptides in Pregnancies Complicated by Fetal Growth Restriction, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 8, 1 August 2016, Pages 2984–2993, https://doi.org/10.1210/jc.2016-1798
Close - Share Icon Share
Calcitonin gene-related peptide (CGRP), adrenomedullin (ADM), and adrenomedullin2 (ADM2)/intermedin are potent vasorelaxant peptides considered to play a role in the adaptive mechanisms in rat pregnancy through increased vasodilation in mesenteric and uterine artery.
This study was designed to demonstrate the response of omental arteries (OA) to vasoactive peptides CGRP, ADM, and ADM2 in pregnancy complications such as fetal growth restriction (FGR), and assess the changes in the expression of their receptor components in segments of OA from FGR pregnancy compared to the control.
The findings for this study are: 1) relaxation responses of OA were higher for bradykinin (78.55 ± 3.91 vs 52.67 ± 2.19; P < .05) in pregnancy with FGR compared to the normal, 2) relaxation response of OA segments to CGRP was similar with no change in the expression of G-protein couple receptor-calcitonin receptor-like receptor complex in normal healthy pregnancy and pregnancy complicated by FGR, 3) maximal relaxation response of OA were significantly (P < .05) lower for both ADM (18.2 ± 6.7 vs 38 ± 2.5) and ADM2 (26.9 ± 6.7 vs 48 ± 2.6) along with decreases in their respective ligand-receptor complex in FGR compared to the normal pregnancies, 4) expression of calcitonin receptor-like receptor mRNA was higher but its immunoreactivity was lower in OA from FGR pregnancy compared to the normal, and 5) mRNA and protein levels of RAMP1, RAMP2, and RAMP3 were lower in OA isolated from FGR pregnancies compared to the normal.
The current study demonstrates that FGR is associated with an increase in the sensitivity of OA to bradykinin and decreased sensitivity for ADM and ADM2 ligand-receptor system with no change in the response for CGRP compared to the normal healthy pregnancy, and suggests a potential role for ADM and ADM2 in the pathophysiology of maternal vasculature in FGR pregnancy.
In pregnant women with Fetal Growth Restriction, sensitivity of omental artery for Bradykinin is increased, for adrenomedullin and adrenomedullin2 is decreased with no change in the response for calcitonin gene related peptide.
Fetal growth restriction (FGR) is associated with increased risk for long-term development of cardiovascular disease in mother and the baby. It is commonly accepted that FGR is associated with impaired invasion of spiral arteries that are not transformed to low-resistance vessels resulting in placental ischemia and release of antiangiogenic factors leading to development of hypertension resulting from associated placental insufficiency, reduced fetal activity, poor maternal weight gain, and small-for-gestational age babies (1, 2). There is no difference reported in the structure between omental small arteries isolated from women with FGR and normal pregnancies (3). However, it is not known if the function of such arteries, specifically, the vascular responses of resistance vessels to vasoactive peptides such as calcitonin gene-related peptide (CGRP), adrenomedullin (ADM), and intermedin (IMD)/adrenomedullin2 (ADM2) are altered in FGR. Therefore, this study was conducted to assess if the sensitivity of maternal omental arteries to CGRP, ADM, and ADM2 are altered in FGR pregnancies compared to the normal state of pregnancy.
CGRP, ADM, and ADM2 are potent vasodilators belonging to a unique group of calcitonin/CGRP family peptide hormones important for homeostasis in diverse tissues (4–9). All three peptides exert their functional effects through a single G-protein couple receptor, calcitonin receptor-like receptor (CRLR) whose ligand binding is dictated by the presence of one of the three receptor activity modifying proteins (RAMP1, 2, or 3). CRLR in combination with RAMP1 gives rise to a CGRP receptor, whereas CRLR in combination with RAMP2 or RAMP3 forms ADM receptors (10, 11). However, ADM2 is shown to be a nonselective agonist for RAMPs but exhibits greater potency with CRLR/RAMP1 and CRLR/RAMP3 (11). CGRP, ADM, and ADM2 are shown to facilitate pregnancy-induced vascular adaptation and exhibit increased sensitivity to rat mesenteric as well as uterine artery relaxation in an endothelium-dependent and/or endothelium-independent manner (9). Pregnancy-induced increases and altered levels of CGRP (5, 12), ADM (13, 14), and ADM2 (4, 15) are reportedly involved with pregnancy complications in both human and rodent models. Fetal growth and placental development are significantly compromised by a modest decrease in expression of ADM in ADM heterozygous mice characterized by FGR (16). ADM2 has 28% structural homology to ADM and 20% to CGRP. Decreases in the plasma and placental ADM2 levels are associated with spontaneous abortion (15). Because of its recent discovery in 2004 (11), not much is known about the function and mechanism of ADM2 action in reproduction.
We have previously reported increased sensitivity of mesenteric and uterine arteries to CGRP, ADM, and ADM2 along with elevated levels of CRLR and RAMPs in pregnant compared to the nonpregnant rat (4, 8, 9, 17–19). We showed that inhibition of the endogenous CGRP, ADM, and ADM2 actions by their respective antagonists resulted in impaired placental vasculature and FGR (20–23). These pathologic findings in rats suggest that CGRP, ADM, and ADM2 may play an important role in pregnancy across species. We recently reported that relaxation responses of omental arteries (OA) to CGRP and ADM2 are greater in pregnant compared to the nonpregnant women (24). Adaptation of maternal peripheral and uterine blood vasculature to the rising needs of the fetus occurs through vasodilation of peripheral and uterine arteries. Disturbances in the uterine blood supply are associated with pregnancy complications such as FGR (25, 26). Plasma levels of ADM positively correlate with uterine arterial pulsatility index and plasma levels of ADM2 are lower in women with first trimester spontaneous abortions (15, 27). This data in human models support involvement of CGRP, ADM, and ADM2 in the regulation of vascular adaptation and feto-placental growth. The correlation of altered levels of these peptides in poor pregnancy outcomes (15, 27) suggests a possible role for these peptides in the pathogenesis of FGR, yet their functional role in the maternal vasculature associated with FGR is not known.
We hypothesize that the sensitivity to CGRP, ADM, and ADM2 is decreased in the maternal vasculature in the setting of FGR, and that the maternal expression of CGRP, ADM, and ADM2 receptor components is concomitantly decreased. Specifically, this study was designed to assess if the vascular effects of CGRP, ADM, and ADM2 are altered in segments of OA from pregnant women with FGR compared to those from women with normal pregnancies.
Materials and Methods
Human subjects
The protocol for this study was approved by the Baylor College of Medicine Institutional Review Board and was conducted according to Declaration of Helsinki Principles. Patients undergoing elective procedures for benign gynecologic indications and patients undergoing cesarean section for obstetric indications were enrolled. All patients gave informed written consent. Omental arteries were collected during laparotomy from pregnant women with healthy pregnancies (n = 15) and pregnancies complicated by FGR (n = 6). Pregnant patients were excluded from the study if they had any of the following: diabetes, fetal anomalies, multifetal pregnancy, hypertension, preeclampsia, or clinical evidence of maternal or fetal infection. OA were either snap frozen and transferred to –80° C until used for RNA extraction, embedded in optimum cutting temperature (OCT) medium to make tissue blocks for immune-histological studies or used for myograph studies. All mothers had undergone cesarean section delivery at the Texas Children's Hospital Pavilion for Women and had the same range of age and gestational age. FGR refers to a fetus that has failed to achieve its genetically determined growth potential and was defined as estimated fetal weight less than the tenth percentile with respect to the reference value for current pregnancy age. The mean gestational age for the FGR patients in this study was (36.2 ± 1.2) weeks and control was (38.6 ± 0.3) weeks and FGR was assessed by estimated fetal weight and confirmed by the birth weights (FGR = 2633 ± 352; control = 3923 ± 392).
OA segment preparation and wire myograph studies
Omental fat biopsies containing a representative vessel were immediately placed in cold oxygenated physiological salt solution, consisting of 118.2 mM NaCl, 24.8 mM NaHCO3, 4.6 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 2.5 mM CaCl2, and 10.0 mM dextrose. A small artery (200 μm normalized internal diameter) was cleaned of fat, cut, and dissected into 2-mm rings, which were mounted on a Multi Wire Myograph System (Model 620M, Danish Myo Technology) for isometric tension recording, as previously described (24). The arterial rings were precontracted with thromboxane agonist U46619 (1 μM). Cumulative dose-response curves were constructed for CGRP, ADM, and ADM2 (0.1–10 nM). The relaxation responses were calculated as percent inhibition of the U46619 initially induced contraction and the maximum possible effect for the reagent (maximal relaxation response [Emax]) was calculated accordingly.
Proximity ligation assay
Proximity ligation assay (in situ PLA) is a modification of traditional immunoassays, which is capable of detecting protein-protein interactions with high specificity and sensitivity (28). PLA probes facilitate the binding of the primary antibodies to the secondary antibody only when PLA probes are in close proximity. Each interaction between the two proteins results in bright fluorescent dots. We used PLA to demonstrate protein-protein interaction suggestive of a complex formation between CGRP, ADM, or ADM2 and CRLR using a Duolink II Fluorescence kit (Olink Biosciences) according to the manufacturer's instructions. The dilution factor for all the antibodies used was 250. Briefly, the OCT compound-embedded sections were fixed using 4% paraformaldehyde and treated with 1% sodium-dodecyl sulfate for 5 minutes for antigen retrieval. The sections were then incubated with primary antibodies (one mono and one polyclonal) followed by incubation with PLA probes. Images are observed under fluorescence microscope (Olympus, U-TV1 X) and red spots were counted using Image-Pro Plus software (Media Cybernetics) in 10 randomly selected images per replicate. Negative controls included no primary antibody or IgG isotype of primary antibody.
Immunofluorescent staining
OCT compound-embedded frozen tissue sections were fixed with methanol and acetone mixture (1:1) for 15 minutes and washed twice for 5 minutes in PBS containing Tween-20 washing solution and processed for immune-fluorescent staining and analyzed as reported earlier (29). Primary antibody staining was done with mouse anti-CD31, rabbit, anti-CRLR, anti-RAMP1, anti-RAMP2, or anti-RAMP3 antibody (Alpha Diagnostics) followed by incubation with donkey antirabbit IgG Alexa 488 or Texas red. Absence of antibody or rabbit IgG (Ready-to-Use) served as a negative control. Slides were counterstained with DAPI (Vector Laboratories, Inc.) and viewed by DP70 Digital Camera (Olympus Optical Co., Ltd.).
Quantitative real-time PCR
Real-time quantitative RT-PCR was performed by using TaqMan probes for CRLR, and RAMP1, 2, and 3 (Life Technologies). Amplification of housekeeping glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (forward primer: 5′-GGTCTCCTCTGACTTCAACA-3′, and reverse primer: 5′-GCCAAATTCGTTGTCATAC-3′) served as an endogenous control to standardize the amount of sample RNA added to a reaction. PCR conditions for TaqMan Gene Expression Assay were 2 minutes at 50° C and 10 minutes at 95° C for one cycle, then 15 seconds at 95° C, and 1 minute at 60° C for 40 cycles. All experiments were performed in triplicate. Negative controls were performed by replacing RNA templates with nuclease-free water. For no-RT control, nuclease-free water was used in place of the RT. Results were calculated using the 2–δδ cycle threshold method and expressed in folds pf increase/decrease of the gene of interest.
Drugs
CGRP and ADM were purchased from Phoenix Pharmaceutical Inc, ADM2 from Alpha Diagnostics, and U46619 and bradykinin were purchased from Sigma Chemical.
Statistical analysis
All data were presented as mean ± SEM. Relaxation responses to CGRP family peptides were expressed as percent relaxation of the initial U46619-induced contraction. Vasodilator concentration response curves were fitted to a log-logistic sigmoid relation, and Emax (maximal relaxation effect) were calculated by using GraphPad Prism. Repeated measures ANOVA (treatment and time as factors) with a Bonferroni post hoc were used for comparisons of dose response curves between groups. Expression levels of mRNA were compared between control and treatment groups using unpaired Student t test. Statistical significance was defined as P < .05.
Results
Bradykinin and CGRP induced relaxation and expression of ligand-receptor complex of CGRP in OA from FGR and normal pregnancies
Segments of OA were precontracted with thromboxane agonist U46619 (1 μM) before treating with bradykinin (BK) and CGRP. Representative traces in Figure 1A shows that BK-induced relaxation responses are increased in OA from pregnant women with FGR (n = 3) compared to the normal pregnant women (n = 9). Dose-response curves indicate that Emax for BK were (78.55 ± 3.91 vs 52.67 ± 2.19; P < .05) compared to the normal pregnancies. There was no difference in the sensitivity of the OA to CGRP (Figure 1B; P > .05). Further, similar to the relaxation responses of OA to CGRP in FGR, the presence of ligand-receptor complex for CGRP was also similar in FGR compared to the controls (Figure 1C; magnification 200×)).
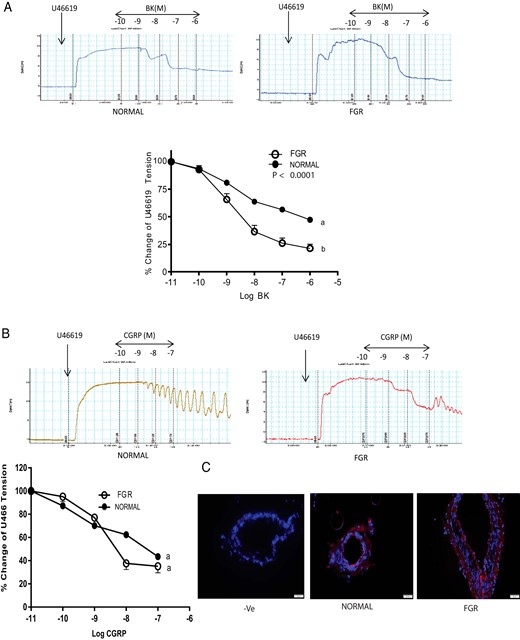
Vascular effects of BK and CGRP on segments of OA precontracted with thromboxane agonist U46619 (1 μM) in FGR pregnancy.
Representative tracings and dose response curves showing omental artery relaxation induced by (A) BK and (B) CGRP in human omental artery from pregnant women with FGR (n = 3) and without FGR (normal, n = 9), and (C) PLA assay in the omental arteries showing no differences in the red staining pertaining to CGRP and CRLR complex between FGR and normal pregnancy (magnification, 200×, n = 3). DAPI was used for blue nuclear staining. Data in (A) and (B) are presented as means ± SEM. Different letters on the curves and asterisk indicate a significant difference (P < .05) between the groups.
ADM- and ADM2-induced relaxation and expression of their respective receptor-ligand complex in OA in FGR and normal pregnancy
Segments of OA were precontracted with thromboxane agonist U46619 (1 μM) before treating with ADM (Figure 2A) and ADM2 (Figure 2C). Representative traces show that ADM and ADM2 induced relaxation responses are decreased in OA from FGR pregnancies (n = 3) compared to the normal (n = 9). As shown in Figure 2A, omental artery relaxation responses (Emax) of OA were significantly lower in FGR for ADM (18.2 ± 6.7 vs 38 ± 2.5); P > .05) and for ADM2 (Figure 2C; 26.9 ± 6.7 vs 48 ±2.6) in FGR compared to the controls. Similar to the decreases in relaxation responses, the presence of ligand–receptor complex of ADM (Figure 2B; magnification 200×) and ADM2 (Figure 2D; magnification 200×) was also lower in OA segments from FGR compared to the normal pregnancy.
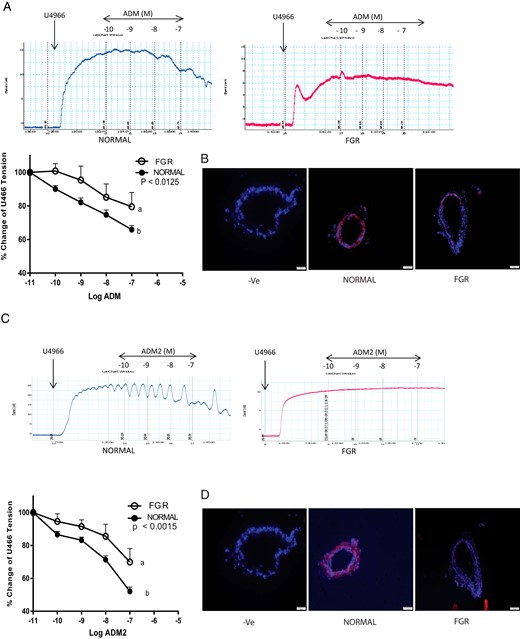
Vascular effects of ADM and ADM2 on segments of OA precontracted with thromboxane agonist U46619 (1 μM) in FGR pregnancy.
Representative tracings and dose response curves showing omental artery relaxation response induced by (A) ADM and (C) ADM2 in human omental artery from pregnant women with FGR (n = 3) and without FGR (normal, n = 9). Data are presented as means ± SEM. Different letters on the curves and the asterisk indicate a significant difference (P < .05) between the groups. Proximity ligation assay in the omental arteries showing decreases in red staining pertaining to (B) ADM-CRLR and (D) ADM2-CRLR complex in FGR compared to the normal pregnancy. DAPI was used for blue nuclear staining (magnification, 200×, n = 3).
Association of FGR with changes in the expression of CRLR in OA
RT-PCR analysis in Figure 3 demonstrates decreased immunoreactive CRLR (Figure 3B) throughout the tissue in OA from FGR compared to the normal pregnancy (n = 3; magnification 200×), although mRNA expression appeared to be elevated (Figure 3A, P < .05).
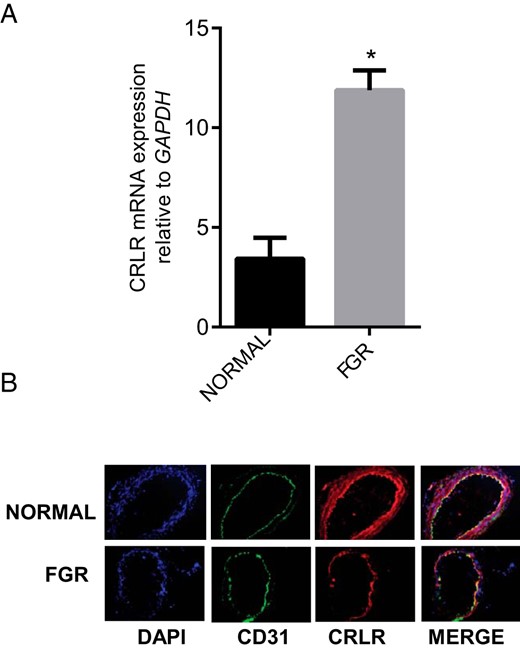
Expression of CRLR in OA from normal pregnancy and FGR pregnancy.
(A) The mRNA expression of CRLR in the omental arteries from normal pregnancy (n = 15) and FGR pregnancy (n = 6). Expression of CRLR mRNA was normalized to the expression of GAPDH mRNA. Data are presented as mean ± SEM. *P < .05 vs. normal. (B) Immunofluorescent localization of CRLR (red) in sections of the OA from normal and FGR pregnancy. CD31 (green) was used for endothelial staining, IgG for negative control (not shown) and DAPI (blue) was used for nuclear staining (n = 3; magnification, 200×).
Association of FGR with changes in the expression of RAMP1 mRNA and protein in OA
RT-PCR analysis in Figure 4 shows that FGR is associated with decreases in the expression of RAMP1 mRNA (Figure 4A; P < .05) and immunoreactivity (figure 4B) in OA compared to the normal pregnancy (n = 3; Magnification 200×).
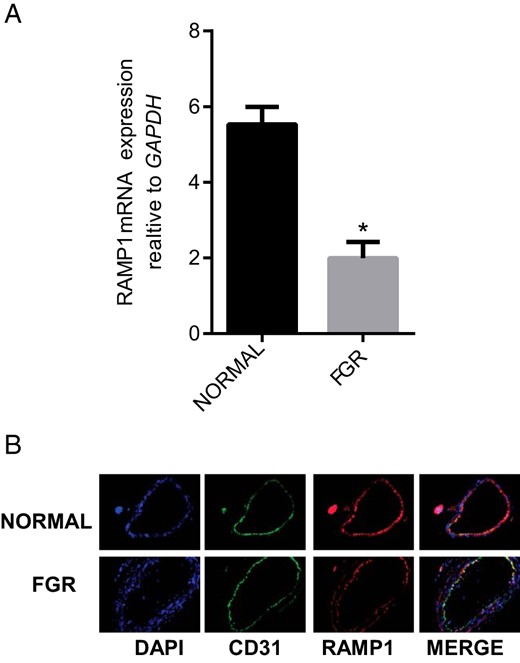
Expression of RAMP1 in OA from normal pregnancy and FGR pregnancy.
(A) The mRNA expression of RAMP1 in the omental arteries from normal pregnant (n = 15) and FGR pregnancy (n = 6). Expression of RAMP1 mRNA was normalized to the expression of GAPDH mRNA. Data are presented as mean ± SEM. *P < .05 vs. normal. (B) Immunofluorescent localization of RAMP1 (red) in sections of the OA from normal and FGR pregnancy. CD31 (green) was used for endothelial staining, IgG for negative control (not shown) and DAPI (blue) was used for nuclear staining (n = 3; magnification, 200×).
Association of FGR with changes in the expression of RAMP2 mRNA and protein in OA
RT-PCR analysis in Figure 5 shows that FGR is associated with decreases in the expression of RAMP2 mRNA (Figure 5A; P < .05) and immunoreactivity (Figure 5B) in OA compared to that in normal pregnancy (n = 3; magnification 200×).
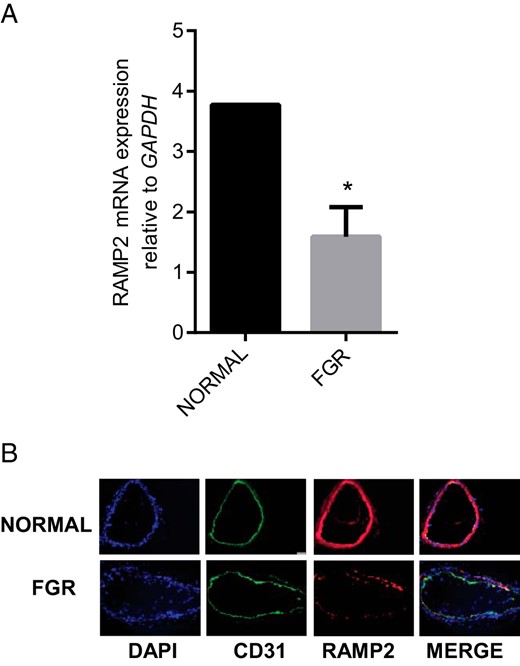
Expression of RAMP2 in OA from normal pregnancy and FGR pregnancy.
(A) The mRNA expression of RAMP2 in the omental arteries from normal pregnancy (n = 15) and FGR pregnancy (n = 6). Expression of RAMP2 mRNA was normalized to the expression of GAPDH mRNA. Data are presented as mean ± SEM. *P < .05 vs. normal. (B) Immunofluorescent localization of RAMP2 (red) in sections of the OA from normal and FGR pregnancy. CD31 (green) was used for endothelial staining, IgG for negative control (not shown). and DAPI (blue) was used for nuclear staining (n = 3; magnification, 200×).
Association of FGR with changes in the expression of RAMP3 mRNA and protein in OA
RT-PCR analysis in Figure 6 shows that FGR is associated with decreases in the expression of RAMP3 mRNA (Figure 6A; P < .05) and immunoreactivity (figure 6B) in OA compared to the normal pregnancy (n = 3; magnification 200×).
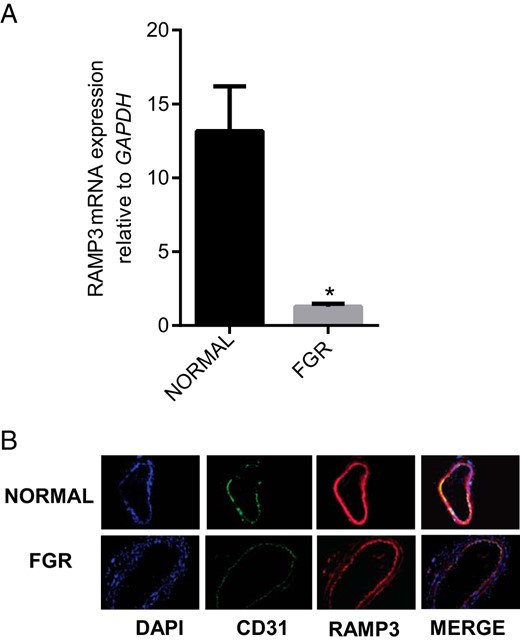
Expression of RAMP3 in OA from normal pregnancy and FGR pregnancy.
(A) The mRNA expression of RAMP3 in the omental arteries from normal pregnancy (n = 15) and FGR pregnancy (n = 6). Expression of RAMP3 mRNA was normalized to the expression of GAPDH mRNA. Data are presented as mean ± SEM. *P < .05 vs. normal. (B) Immunofluorescent localization of RAMP3 (red), in sections of the OA from normal and FGR pregnancy. CD31 (green) was used for endothelial staining, IgG for negative control (not shown) and DAPI (blue) was used for nuclear staining (n = 3; magnification, 200×).
Discussion
Our current study demonstrates that FGR is associated with increased vasodilatory responses of human maternal OA to BK and decreased vascular response to ADM and ADM2 along with decreased expression of their respective ligand-receptor complexes. There was no change in the response of OA to CGRP or in the expression of CGRP-CRLR complex in FGR compared to the control. The immunoreactivity for CRLR was lower, although CRLR mRNA was higher in FGR, whereas, expression of all three RAMPs mRNA and immunoreactivity was lower in OA from FGR compared to the control. Therefore, impaired sensitivity of the OA to ADM and ADM2, in addition to decreased expression of their respective ligand-receptor complex and receptor components may underlie pathophysiology of FGR.
Endothelial function is an integrative marker of the net effects of damage from the circulating risk factors on the arterial wall and its intrinsic capacity for repair. This study is the first to demonstrate an increased sensitivity of endothelium to BK in maternal OA from FGR pregnancies (Figure 1A) compared to normal controls. Increases in NO levels are reported to decrease systolic/end-diastolic velocity ratio (S/D ratio) in Doppler blood flow study along with improved neonatal outcome and lower incidence of complications associated with FGR, suggesting that deficiency in NO may play an important role in the causation of FGR (30). The non-NO and nonprostaglandin mediated endothelium-dependent vasodilator responses has been attributed to endothelium-derived hyperpolarizing factor/s (EDHF) and a compensatory up-regulation of hyperpolarization occurs as a response to reduce NO availability (31, 32). Figure 1A demonstrates FGR-induced compensatory increase in the vascular effects of BK in segments of OA compared to normal pregnancy. However the molecular mechanism involved remains to be assessed.
Sensitivity of rat mesenteric artery as well as uterine artery to CGRP, ADM and ADM2 is enhanced in pregnancy compared to the nonpregnant state, suggesting a functional role for these peptides in pregnancy induced peripheral and feto-maternal vascular adaptations (4;8;9;17–19;33). Similar to the studies in rat, we showed that CGRP, ADM, and ADM2 dose-dependently relaxed precontracted human OA, and that sensitivity of OA to CGRP and ADM2, but not to ADM are enhanced in pregnant compared to the nonpregnant women (24) suggesting a role for these peptides in vascular adaptations during normal human pregnancy.
Our current study shows that, there is no change in the sensitivity of OA to CGRP (Figure 1B) or its ligand-receptor complex (Figure 1C) in FGR, but the sensitivity of OA for ADM and ADM2 (Figure 2) is decreased compared to the normal pregnancy. In addition, decreases in the sensitivities of OA to ADM and ADM2 in FGR are accompanied with decreases in the expression of their respective ligand-receptor complex (Figure 2,B and D). Further, although the expression of CRLR mRNA was higher in OA from FGR (Figure 3A), there was an overall decrease in the immunoreactivity of CRLR protein (Figure 3B) accompanied with decreases in the expression of mRNA and protein for RAMP1 (Figure 4), RAMP2 (Figure 5), and RAMP3 (Figure 6) in OA from FGR compared to that from normal pregnancy. Interestingly, despite the decreases in the expression of all the three RAMPs, there was no change in either the vascular response of OA to CGRP or in the expression of CGRP-CRLR heterodimer in OA from FGR compared to that from normal pregnancy suggesting a complex interplay within the CGRP family peptides and their receptor components. This allows us to conclude that the physiological pregnancy-induced increase in sensitivity of OA to CGRP as reported earlier (11, 24), is maintained in FGR pregnancies (Figure 1B). However, the vascular responses of OA to ADM and ADM2 are impaired and the expression of their respective receptor-ligand complex is decreased in FGR compared to control normal pregnancy. Expression of CRLR and RAMPs mRNA and protein are also decreased in OA from FGR compared to that from the control normal pregnancy (Figures 345–6). Despite an impairment of OA relaxation sensitivity to these peptides, there is no measurable hypertension in these FGR patients at the time of delivery. Therefore, impaired sensitivity of OA for these peptides in patients with isolated FGR pathology without any clinical symptoms of hypertension, allows us to speculate that, although there are no clinical signs of hypertension in isolated FGR, maternal vasculature may be impaired that may predispose the patient for developing hypertension. Because these FGR patients delivered 2 weeks (36.2 ± 1.2) earlier than the normal controls (38.6 ± 0.3, the possibility remains that the FGR patients might not yet have demonstrated the clinical hypertension.
Our current results support the complexity of the ligand-receptor interactions of the CGRP peptide family as demonstrated previously in rat vascular smooth muscle cells (9), whereby knocking down of RAMP2 or RAMP3 in vascular smooth muscle cells was not only ineffective in blocking CGRP-induced cAMP generation, but instead caused an increase in cAMP production. The absence of one isotype of RAMP seemed to allow for enhanced recruitment of the other RAMP isotypes and for these isotypes to associate with CRLR, resulting in a shift of ligand-binding specificity and thus CGRP function (9, 38). The stoichiometry of CGRP, ADM, and ADM2 receptor components has been a topic of debate with an evidence for 1:1, 2:2, and 2:1 for CRLR:RAMPs (1, 2, or 3) (39, 40). Heather et al demonstrated that CGRP receptor functions at 1:1 concentration in contrast to ADM receptor which may function as a 2:2 dimer of the heterodimer (39). Although not yet studied, because of the structural similarities with ADM, ADM2 may also require a high stoichiometry of the receptor components to exhibit its vascular function. Thus, we speculate that, although the levels of receptor components that mediate the effects of these peptides are low in OA from FGR, they are low enough to hamper the vascular effects of ADM and ADM2 but not CGRP. However, further studies utilizing crystal structures of ligand-receptor complexes are necessary to fully understand RAMPs and CRLR:RAMP stoichiometry.
Further, we previously reported that ADM and ADM2 induced increases in the relaxation response of OA are mediated through nitric oxide (NO); endothelium derived hyperpolarizing factors (EDHF) and prostaglandins (24). It is likely that impaired prostaglandin and/ or NO system in FGR vasculature underlies FGR associated decreases in OA responses for ADM (Figure 2,A and B) and ADM2 (Figure 2,C and D) observed in OA segments from FGR pregnancy. This suggests that although the endothelial layer is functional, the vascular responses of ADM and ADM2 are impaired due to the decreases in their receptor system. We suspect that the absence of intact ADM and ADM2 system in resistance vasculature in women with FGR pregnancy may have resulted in the differential compensatory response of OA to BK in the endothelium in FGR compared to the normal pregnancy (Figure 1A). However, the molecular pathway leading to the impairment of ADM and ADM2 function and elevated effects of BK in FGR remains to be elucidated.
In summary, compensatory increases in relaxation responses of OA to BK and reduced vasodilatory responses to ADM and ADM2 in addition to impaired expression of their receptor system in OA segments from FGR pregnancies may implicate prevalence of similar mechanisms in other vascular beds such as uterine artery and placenta that may cause reduced blood flow to fetus in FGR pregnancy. Further studies are needed to: 1) assess if these alterations are reversed postpartum, 2) clarify whether impaired ADM and ADM2 responses associated with FGR confer long-term cardiovascular risk, and 3) assess if decreases in the ADM and ADM2 system are the cause or consequence of FGR pathology in human pregnancy.
Acknowledgments
The authors thank Ms. Sandra Garcia Dale for her incredible administrative assistance.
This work was supported by National Institutes of Health through grant HL102866 (to C.Y.).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- ADM
adrenomedullin
- ADM2
adrenomedullin2
- BK
bradykinin
- CGRP
calcitonin gene-related peptide
- CRLR
calcitonin receptor-like receptor
- Emax
maximal relaxation response
- FGR
fetal growth restriction
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- OA
omental artery
- OCT
optimum cutting temperature
- PLA
proximity ligation assay
- RAMP
receptor activity modifying protein.



