-
PDF
- Split View
-
Views
-
Cite
Cite
Henry B. Burch, Kenneth D. Burman, David S. Cooper, James V. Hennessey, Nicole O. Vietor, A 2015 Survey of Clinical Practice Patterns in the Management of Thyroid Nodules, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 7, 1 July 2016, Pages 2853–2862, https://doi.org/10.1210/jc.2016-1155
Close - Share Icon Share
The management of thyroid nodules has changed dramatically over the past two decades. In the interim, technological advances including high-resolution ultrasound and molecular testing of thyroid nodules have been introduced.
We sought to document current practices in the management thyroid nodules and assess the extent to which technological advances have been incorporated into current practice. We further sought to compare current practice to recommendations made in a recently updated American Thyroid Association (ATA) clinical practice guideline (CPG) and examine differences in thyroid nodule management among international members of U.S.-based endocrine societies.
Members of The Endocrine Society, ATA, and American Association of Clinical Endocrinologists were invited to participate in a Web-based survey dealing with testing, treatment preference, and modulating factors in patients with thyroid nodules.
A total of 897 respondents participated in the survey, including 661 members of The Endocrine Society, 454 American Association of Clinical Endocrinologists members, and 365 ATA members. Thyroid fine-needle aspiration (FNA) in 2015 is generally performed by endocrinologists (56.6%) and radiologists (31.9%), most frequently using ultrasound guidance (83.3%). Respondents in general have a lower threshold for FNA of thyroid nodules than that recommended in the updated ATA CPG. Management depends on the FNA result, with follicular lesion of undetermined significance/atypia of undetermined significance resulting in molecular testing (38.8% of respondents), repeat FNA cytology (31.5%), or immediate referral for thyroid surgery (24.4%). Nodules showing follicular neoplasm by FNA are referred for thyroid surgery by 61.2% of respondents (46.6 % lobectomy, 14.6 % total thyroidectomy) or molecular testing (29.0 %). Nodules found suspicious but not conclusive for malignancy (Bethesda category V), are referred for thyroid surgery (86.0%) and rarely undergo molecular testing (9.5%). During pregnancy, only 47.6% of respondents would perform FNA in the absence of nodular growth, with most respondents deferring FNA until after pregnancy. Endocrinologists are 64.2% less likely to perform FNA in an octogenarian than a younger patient with a comparable thyroid nodule. Striking international differences were identified in the routine measurement of calcitonin and in the use of molecular testing of thyroid nodules.
In summary, our survey of clinical endocrinologists on the management of thyroid nodules documents current practice patterns and demonstrates both concordance and focal discordance with recently updated CPGs. Both international differences and a change in practice patterns during the past two decades are demonstrated.
This survey of clinical endocrinologists on the management of thyroid nodules documents current practice patterns and demonstrates both concordance and focal discordance with recently updated CPGs.
Most women and a large proportion of men in the United States will develop a thyroid nodule during their lifetime, according to autopsy and population studies using thyroid ultrasound (US) (1, 2). The increased use of sensitive imaging techniques such as magnetic resonance imaging (MRI), computed tomography, and carotid US in an aging population has led to a surge in referrals for evaluation of incidentally discovered thyroid nodules (3, 4). Concurrent technological advances have affected clinical practice, including the increased availability of portable ultrasensitive US for the detection and sampling of even small thyroid nodules (5), the recognition of sonographic features portending a higher risk of malignancy (6), and the application of molecular profiling to cytologically indeterminate thyroid nodules (7, 8).
Approximately 90–95% of thyroid nodules are benign. An aggressive diagnostic approach to generally benign nodules or indolent microcarcinomas has led to an application of the terms “overdiagnosis” and “overtreatment” to describe the management of thyroid nodules (9). In response to this changing clinical landscape, the American Thyroid Association (ATA) recently published updated comprehensive clinical practice guidelines (CPGs) relating to the management of thyroid nodules and differentiated thyroid cancer (10). Given that the last surveys of clinical practice patterns for thyroid nodule management in North America and Europe were published 10 and 16 years ago, respectively (11, 12), it is not clear to what extent actual clinical practice differs from current professional guidelines.
The current survey was performed just prior to the release of the updated 2015 ATA guidelines (10) with the following objectives: 1) to define current clinical practice patterns in the management of thyroid nodules, 2) to compare contemporary practice to that recommended in current practice guidelines, 3) to examine changes to clinical practice during the past 1–2 decades since the last published surveys in this area, and 4) to explore international differences in the management of thyroid nodules.
Materials and Methods
Survey design
A Web-based, commercial survey management service (SurveyMonkey) was used to administer the survey. The survey included questions pertaining to diagnostic evaluation, choice of therapy, and followup of an index case of a solitary thyroid nodule (Table 1). See entire survey in Supplemental Material.
| Index Case . |
|---|
| A 52-year-old woman is found on cervical spine MRI to have an incidental 1.5-cm right thyroid nodule. The patient has no known history of thyroid disease and has otherwise been in good health. She takes no medications. She is a nonsmoker and has no history of radiation exposure. Family history is negative for thyroid disease. On examination the nodule is not palpable and there is no cervical lymphadenopathy. |
| Index Case . |
|---|
| A 52-year-old woman is found on cervical spine MRI to have an incidental 1.5-cm right thyroid nodule. The patient has no known history of thyroid disease and has otherwise been in good health. She takes no medications. She is a nonsmoker and has no history of radiation exposure. Family history is negative for thyroid disease. On examination the nodule is not palpable and there is no cervical lymphadenopathy. |
| Index Case . |
|---|
| A 52-year-old woman is found on cervical spine MRI to have an incidental 1.5-cm right thyroid nodule. The patient has no known history of thyroid disease and has otherwise been in good health. She takes no medications. She is a nonsmoker and has no history of radiation exposure. Family history is negative for thyroid disease. On examination the nodule is not palpable and there is no cervical lymphadenopathy. |
| Index Case . |
|---|
| A 52-year-old woman is found on cervical spine MRI to have an incidental 1.5-cm right thyroid nodule. The patient has no known history of thyroid disease and has otherwise been in good health. She takes no medications. She is a nonsmoker and has no history of radiation exposure. Family history is negative for thyroid disease. On examination the nodule is not palpable and there is no cervical lymphadenopathy. |
Strategy for question design
Most questions required a single best response to be selected from multiple choices. Several questions allowed additional free text comments. To limit bias, questions were constructed to omit phrasing that could influence respondents' answers, and a broad range of choices were given, arranged alphabetically, numerically, or in random order (13, 14). The survey was designed and tested to allow a completion time of approximately 10–15 minutes. Before general release, the survey was vetted through council members of the survey's target societies and the research committee of the ATA.
Contact of potential respondents
The target groups for the survey were clinically active members of The Endocrine Society (TES), the ATA, and the American Association of Clinical Endocrinologists (AACE). Respondents were contacted according to the current bylaws, regulations, and philosophies of the individual society.
Collection and summary of responses
Survey responses were anonymously collected and stored electronically by the survey service, accessible in a password-protected manner. Repeat submissions from the same Internet Protocol address were automatically blocked by the survey service. The survey Web site was open to respondents from 14 January through 30 April, 2015.
Geographical region of respondents
Respondents were grouped according to the United Nations country grouping of the following geographical regions, with the exception that Middle Eastern countries were distinguished from the general United Nations designations of Southern and Western Asia. The resultant groupings included Africa (AF); Asia (AS); Europe (EU); Latin America (LA), including Central America, South America, and the Caribbean; the Middle East (ME); North America (NA), including United States and Canada; and Oceania (OC), including primarily Australia and New Zealand. Responses from AS and OC were pooled (AO), as were responses from AF and ME (AF-ME).
Statistical analysis
Summary statistics were prepared for responses to each question. Because not every participant answered all questions, the percentage of respondents providing a given answer was calculated individually for each question, using the number of respondents to that question as the denominator. Age categories were ranked from ‘1’ (ages 26–30 y) to ‘11’ (ages 76–80 y). Logistic regression was used to explore the association of age, sex, and region on each of the preferred treatment strategies, and P-values from these multivariate analyses are presented. To allow for multiple comparisons, a Bonferroni corrected P-value of .005 (eg, an experiment-wise P-value of .05 divided by up to 10 comparisons) was considered significant. Data were analyzed using IBM SPSS Statistics for Windows, v. 21.0.
Results
Professional society membership
A total of 897 respondents participated in the survey. The 821 respondents providing society membership consisted of 661 TES members, 464 AACE members, and 365 ATA members. Multiple society membership was commonly reported.
Survey response rates
For TES members, 11 193 were sent the e-mail, and 3917 (35.0%) opened this correspondence. For ATA members, an initial email was sent to 1560 members, of whom 606 (38.9%) opened the email. A second email was sent to ATA members 2 months after the first with an opening rate of 38.2% (609/1594). Among AACE members, approximately 5400 active members were sent several newsletters that included an invitation to participate. The percentage of clinically active society members represented by survey respondents was 661/11 193 (5.9%) for TES, 365/1594 (22.9%) for the ATA, and 464/7130 (6.5%) for AACE members.
Respondent demographics
The type of medical practice reported by respondents was adult endocrinology (87.7%, 716/816 respondents), either alone (83.0%) or combined as a dual specialty such as endocrinology/internal medicine (2.8%), or endocrinology/nuclear medicine (1.0%). Additional respondents identified themselves as endocrine surgeons (5.9%), head and neck surgeons (3.7%), pediatric endocrinologists (1.7%), nuclear medicine (solo specialty) physicians (0.7%), and nonendocrine general internists (0.2%). The geographical regions of the respondents' practices included NA 63.2% (United States, 60.2%; Canada, 3.0%), EU (12.2%), LA (10.8%), AO (8.0%), and ME-AF (5.8%). Gender was reported by 809 respondents, and included 60.8% (492/809) men and 39.2% women. Among 821 respondents providing age data, 14.4% were 35 years or younger, 70.6% were 36–65 years old, and 15.0% were older than age 65 years.
Diagnostic evaluation of the index case
Laboratory testing
Figure 1A shows the percentage of respondents ordering the listed laboratory testing for most their patients similar to the index case. In addition to TSH testing, 41.5% of respondents would obtain a free T4 level and 24.3% would order thyroid peroxidase antibodies. Factors prompting respondents to measure serum calcitonin included a family history of medullary thyroid cancer by 95.0% (836/880), a family member with an unknown type of thyroid cancer by 37.3%, a patient history of pheochromocytoma by 80.0% or hyperparathyroidism by 56.3%, and the presence of coarse calcifications on thyroid US by 13.1% of respondents. Regional differences in routine calcitonin measurement are discussed below.
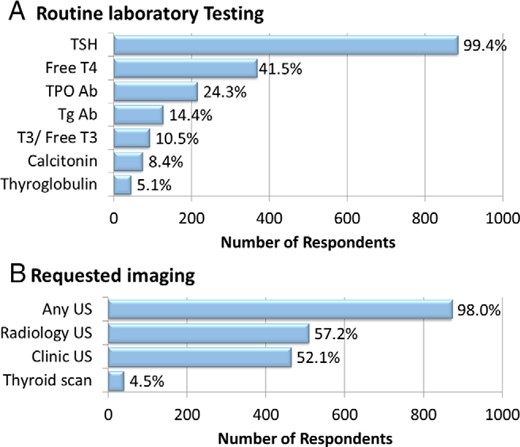
Diagnostic evaluation of the index case thyroid nodule.
Number of respondents and corresponding percentages who would request various testing modalities in the index case. Respondents were allowed to select multiple items concurrently.
Imaging
Thyroid US would be obtained at baseline in the index patient by 98.0% (872/ 890) of respondents, with the study performed by radiology alone in 45.8%, in the respondent's clinic 40.8%, or in both locations in 11.3% (Figure 1B). An assessment of cervical lymph nodes is performed routinely at the time of thyroid US at 68.5% (610/890) of respondents' centers. A radionuclide thyroid scan would be obtained by 4.5% of respondents, most of whom would also obtain US. Additional studies such as MRI or computed tomography of the neck or 2-fluoro-2-deoxy-D-glucose positron emission tomography (18FDG-PET) would be obtained by less than 1% of respondents.
Fine-needle aspiration technique
See respondent practices related to the fine-needle aspiration (FNA) technique, preparation, and interpretation in Supplemental Results.
Selection of thyroid nodules for sampling
Thyroid nodule variants
Six nodules with various sonographic features were listed and respondents were asked which nodules they would select for FNA (Table 2). Among 861 respondents, 93.8% (808/861) would sample a 1.5-cm hypoechoic nodule, in accordance with current ATA guidelines (10). Additional nodules not fulfilling criteria for FNA by these guidelines were also sampled at a relatively high rate including a 0.7-cm hypoechoic nodule with microcalcifications, which was sampled by 67.0%; a 1.4-cm complex-cystic nodule by 55.5%; a 1.2-cm isoechoic nodule, which would be aspirated by 41.6%; a 1.8-cm spongiform nodule aspirated by 37.4%; and a 2.5-cm purely cystic nodule, which would be sampled by 24.5% of respondents.
| US Features . | Maximal Diameter, cm . | FNA Requested, % Respondents . | Meets 2015 ATA Criteria for FNA . |
|---|---|---|---|
| Hypoechoic with microcalcifications | 0.7 | 67.0 | No |
| Solid, isoechoic | 1.2 | 41.6 | No |
| Complex cystic | 1.4 | 55.5 | No |
| Solid, hypoechoic | 1.5 | 93.8 | Yes |
| Spongiform | 1.8 | 37.4 | No |
| Pure cyst | 2.5 | 24.5 | No |
| US Features . | Maximal Diameter, cm . | FNA Requested, % Respondents . | Meets 2015 ATA Criteria for FNA . |
|---|---|---|---|
| Hypoechoic with microcalcifications | 0.7 | 67.0 | No |
| Solid, isoechoic | 1.2 | 41.6 | No |
| Complex cystic | 1.4 | 55.5 | No |
| Solid, hypoechoic | 1.5 | 93.8 | Yes |
| Spongiform | 1.8 | 37.4 | No |
| Pure cyst | 2.5 | 24.5 | No |
| US Features . | Maximal Diameter, cm . | FNA Requested, % Respondents . | Meets 2015 ATA Criteria for FNA . |
|---|---|---|---|
| Hypoechoic with microcalcifications | 0.7 | 67.0 | No |
| Solid, isoechoic | 1.2 | 41.6 | No |
| Complex cystic | 1.4 | 55.5 | No |
| Solid, hypoechoic | 1.5 | 93.8 | Yes |
| Spongiform | 1.8 | 37.4 | No |
| Pure cyst | 2.5 | 24.5 | No |
| US Features . | Maximal Diameter, cm . | FNA Requested, % Respondents . | Meets 2015 ATA Criteria for FNA . |
|---|---|---|---|
| Hypoechoic with microcalcifications | 0.7 | 67.0 | No |
| Solid, isoechoic | 1.2 | 41.6 | No |
| Complex cystic | 1.4 | 55.5 | No |
| Solid, hypoechoic | 1.5 | 93.8 | Yes |
| Spongiform | 1.8 | 37.4 | No |
| Pure cyst | 2.5 | 24.5 | No |
Elderly patients
In comparison with the index case of a younger patient with a 1.5-cm hypoechoic nodule, an 82-year-old with a similar nodule, having no suspicious clinical or sonographic features, and a normal TSH would less likely undergo FNA by 64.2% (553/862) of respondents, equally likely among 29.7%, and more likely to undergo FNA by 6.2% of respondents.
Multinodular thyroid glands
We next presented a 52-year-old woman with a multinodular thyroid containing at least five solid hypoechoic nodules greater than 1 cm in maximal diameter. The patient had no suspicious history, physical examination, or sonographic features, and serum TSH level was 1.5 mU/L. Among 786 respondents, 46.4% (365/786) would perform sampling of two to three of the largest nodules, 19.3% would sample the single largest nodule, 15.1% would sample all nodules greater than 1 cm in maximal diameter, 10.7% would not perform FNA at all, and 8.4% would obtain a nuclear thyroid scan to identify “cold” nodules for sampling. Free text comments by several respondents included the use of a larger size threshold (eg, 1.5 cm) for FNA in multinodular patients.
Pregnancy
Respondents were presented a 22-year-old woman with a 1.5-cm thyroid nodule discovered during the eighth week of pregnancy. Serum TSH was normal and there were no suspicious clinical or sonographic features. Among 821 respondents, 36.3% would sample the nodule during the first trimester, 11.3% would perform FNA during the second trimester, 18.3% would sample the nodule only if it grew during pregnancy, and 34.1% would postpone the FNA until after delivery.
Response to various thyroid FNA results
For the Bethesda class subcategories III–VI (follicular lesion of undetermined significance/atypia of undetermined significance [FLUS/AUS], follicular neoplasm/suspicious for follicular neoplasm, suspicious for malignancy, and malignant) (15, 16), we asked respondents to select among responses ranging from observation to additional diagnostics (molecular testing or thyroid radionuclide scanning), or referral for thyroid surgery. Figure 2 shows the distribution of responses to each of these scenarios.
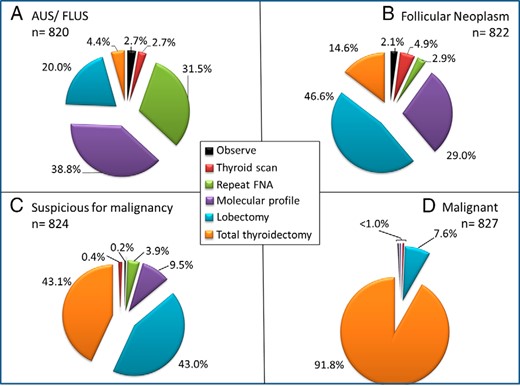
Management of thyroid nodules according to FNA result.
Management of patients found to have an indeterminate or malignant result on initial FNA. Shown is a progressive use of thyroid surgery in going from Bethesda Classification System class III (AUS/FLUS) to class VI (malignant) and a low frequency of observation alone in this setting.
AUS/FLUS
Among 820 respondents obtaining an AUS/FLUS result at initial FNA, 38.8% (318/820) would obtain molecular testing, 31.5% would repeat the FNA for cytological examination, 20.0% would refer the patient for a diagnostic lobectomy, 4.4% would recommend a total thyroidectomy, 2.7% would obtain a thyroid scan, and 2.7% would only observe the patient.
Follicular neoplasm/suspicious for follicular neoplasm
Among 822 respondents with an initial FNA showing a follicular neoplasm, the most common response was referral for diagnostic lobectomy (46.6%, 383/822), followed by molecular testing (29.0%), total thyroidectomy (14.6%), thyroid scan (4.9%), repeat FNA for cytology (2.9%), and observation alone (2.1%).
Suspicious for malignancy
Among 824 respondents with an initial FNA result interpreted as suspicious but not diagnostic for malignancy, most would refer the patient for diagnostic surgery, including lobectomy in 43.0% (354/824), or total thyroidectomy in 43.1%; 9.5% would obtain molecular testing; and only 3.9% would repeat the FNA for cytology alone. Several respondents noted the use of frozen section to determine the extent of surgery.
Malignant
Among 827 respondents obtaining a malignant FNA result, 91.8% (759/827) would refer for total thyroidectomy; 7.6% lobectomy; and <1% would pursue the remaining options including molecular testing, repeat FNA, or thyroid scan.
Followup of index case after a benign FNA
We assessed the response and followup after obtaining a benign FNA result in patients such as the index case with a 1.5-cm solitary hypoechoic solid nodule. Most respondents (65.6%, 556/848) would follow with serial thyroid US imaging at various intervals (discussed below), whereas others would repeat the US on only one occasion (19.7%), or refer back to primary care (6.7%). Routine repeat of a previously benign FNA within 3–12 months would be performed by 5.7% of respondents. The interval for a repeat US varied, with 58.9% (499/847) obtaining a repeat study in 1 year, 26.9% by 6 months, and 4.8% at 2 years. No routine repeat US would be obtained by 7.7% of respondents unless the nodule grew by palpation.
Response to solitary nodule growth
If the index case's thyroid nodule volume increased by more than 50%, most respondents would perform a repeat FNA (87.3%, 736/843), or send for a lobectomy (5.7%). Fewer than 5% would observe the patient with a repeat US in 6–12 months (4.5%), and 1.2% would give a trial of levothyroxine therapy.
Followup of multinodular thyroid glands after benign FNA
Followup for multinodular thyroid gland patients after a benign FNA would be with serial US by 72.2% (590/817) of respondents, a single repeat US by 15.8%, return to primary care by 5.5%, a routine repeat FNA to exclude a false negative by 3.7%, or follow using palpation alone by 2.8% of respondents.
Molecular testing of thyroid nodules
Methods available for molecular testing of thyroid nodules at the time of the survey included a gene expression classifier (17), and a panel consisting of a screen for mutations or rearrangements in BRAF, NRAS, HRAS, KRAS, RET/PTC1, RET/PTC2, RET/PTC3, and PAX8/PPARγ (18). Among 826 respondents, 41.4% did not use molecular testing in their practice; 22.5% used a gene expression classifier; 14.5% used a specific mutation panel; 9.8% used either method, depending on patient and physician preference; and 4.6% used both methods simultaneously. The percentage of respondents obtaining molecular testing in response to the different types of indeterminate or malignant FNA results is discussed above, in the section, Response to various thyroid FNA results.
Assessment of concordance with 2015 ATA guidelines
Table 3 summarizes the degree of concordance between survey respondents and selected recommendations from the 2015 ATA thyroid nodule and cancer CPG (10). It is important to emphasize that the survey was purposely conducted before the e-publication of these updated guidelines to assess baseline practices, although an earlier draft version of these guidelines was made available to ATA members online in 2014.
Comparison of Clinical Practice Guideline Recommendations to Survey Responses
| Record No. . | CPG Recommendation (Abbreviated) . | Survey Concordance, % . |
|---|---|---|
| R2 | Measure TSH in all thyroid nodule patients | 99.4 |
| R3 | Do not measure thyroglobulin in the assessment of thyroid nodules | 94.9 |
| R6 | Include lymph node assessment in thyroid US | 68.5 |
| R8a | Use a 1.0-cm FNA threshold for thyroid nodules with suspicious US features | 33.0 |
| R8b | Use 1.0-cm FNA threshold for thyroid nodules with intermediate suspicion US features (hypoechoic, solid, regular margins) | 93.8 |
| R8c | Use 1.5-cm FNA threshold for thyroid nodules with low suspicion US features (isoechoic or hyperechoic nodules, partially cystic with eccentric solid portion) | 58.4 |
| R8d | Use a 2.0-cm FNA threshold for thyroid nodules with very low suspicion US features (spongiform, complex cystic with no suspicious US features) | 62.6 |
| R8e | Do not use diagnostic FNA for purely cystic thyroid nodules | 75.5 |
| R9 | Use the Bethesda System for Reporting Thyroid Cytopathology | 75.0 |
| R12 | Refer patients to surgery after a malignant FNA result | 99.4 |
| R15 | For thyroid nodules with AUS/FLUS cytology results, molecular testing may be performed | 38.8 |
| R16 | Use diagnostic surgery or molecular testing for thyroid nodules with follicular neoplasm cytology results | 90.2 |
| R17 | Use diagnostic surgery for most patients with suspicious for malignancy cytology results | 86.1 |
| R18 | Do not usually use 18FDG-PET to evaluate malignancy risk in thyroid nodules | 99.7 |
| R19 | Patients with FNA results of AUS/FLUS or follicular neoplasm who are selected for surgery should undergo a thyroid lobectomy | 47.0–82.0 |
| R20 | Use total thyroidectomy for thyroid nodules with suspicious FNA results, or positive molecular testing, or clinical features that increase the suspicion for malignancy | 50.0a |
| R21a | Evaluation of thyroid nodules in multinodular thyroid patients should generally be similar to patients with a solitary nodule | 15.1b |
| R23b | For patients with a prior benign FNA and low to intermediate suspicion features on US, repeat US in 12–24 months and repeat FNA only for nodule growth or new suspicious US features | 85.3 |
| R30a | Perform FNA of new thyroid nodules during pregnancy if the TSH is normal or elevated | 47.6 |
| Record No. . | CPG Recommendation (Abbreviated) . | Survey Concordance, % . |
|---|---|---|
| R2 | Measure TSH in all thyroid nodule patients | 99.4 |
| R3 | Do not measure thyroglobulin in the assessment of thyroid nodules | 94.9 |
| R6 | Include lymph node assessment in thyroid US | 68.5 |
| R8a | Use a 1.0-cm FNA threshold for thyroid nodules with suspicious US features | 33.0 |
| R8b | Use 1.0-cm FNA threshold for thyroid nodules with intermediate suspicion US features (hypoechoic, solid, regular margins) | 93.8 |
| R8c | Use 1.5-cm FNA threshold for thyroid nodules with low suspicion US features (isoechoic or hyperechoic nodules, partially cystic with eccentric solid portion) | 58.4 |
| R8d | Use a 2.0-cm FNA threshold for thyroid nodules with very low suspicion US features (spongiform, complex cystic with no suspicious US features) | 62.6 |
| R8e | Do not use diagnostic FNA for purely cystic thyroid nodules | 75.5 |
| R9 | Use the Bethesda System for Reporting Thyroid Cytopathology | 75.0 |
| R12 | Refer patients to surgery after a malignant FNA result | 99.4 |
| R15 | For thyroid nodules with AUS/FLUS cytology results, molecular testing may be performed | 38.8 |
| R16 | Use diagnostic surgery or molecular testing for thyroid nodules with follicular neoplasm cytology results | 90.2 |
| R17 | Use diagnostic surgery for most patients with suspicious for malignancy cytology results | 86.1 |
| R18 | Do not usually use 18FDG-PET to evaluate malignancy risk in thyroid nodules | 99.7 |
| R19 | Patients with FNA results of AUS/FLUS or follicular neoplasm who are selected for surgery should undergo a thyroid lobectomy | 47.0–82.0 |
| R20 | Use total thyroidectomy for thyroid nodules with suspicious FNA results, or positive molecular testing, or clinical features that increase the suspicion for malignancy | 50.0a |
| R21a | Evaluation of thyroid nodules in multinodular thyroid patients should generally be similar to patients with a solitary nodule | 15.1b |
| R23b | For patients with a prior benign FNA and low to intermediate suspicion features on US, repeat US in 12–24 months and repeat FNA only for nodule growth or new suspicious US features | 85.3 |
| R30a | Perform FNA of new thyroid nodules during pregnancy if the TSH is normal or elevated | 47.6 |
Represents the percentage patients referred for thyroid surgery in whom total thyroidectomy would be requested.
Represents the percentage of respondents who would sample all nodules >1 cm in a patient with a multinodular goiter.
Comparison of Clinical Practice Guideline Recommendations to Survey Responses
| Record No. . | CPG Recommendation (Abbreviated) . | Survey Concordance, % . |
|---|---|---|
| R2 | Measure TSH in all thyroid nodule patients | 99.4 |
| R3 | Do not measure thyroglobulin in the assessment of thyroid nodules | 94.9 |
| R6 | Include lymph node assessment in thyroid US | 68.5 |
| R8a | Use a 1.0-cm FNA threshold for thyroid nodules with suspicious US features | 33.0 |
| R8b | Use 1.0-cm FNA threshold for thyroid nodules with intermediate suspicion US features (hypoechoic, solid, regular margins) | 93.8 |
| R8c | Use 1.5-cm FNA threshold for thyroid nodules with low suspicion US features (isoechoic or hyperechoic nodules, partially cystic with eccentric solid portion) | 58.4 |
| R8d | Use a 2.0-cm FNA threshold for thyroid nodules with very low suspicion US features (spongiform, complex cystic with no suspicious US features) | 62.6 |
| R8e | Do not use diagnostic FNA for purely cystic thyroid nodules | 75.5 |
| R9 | Use the Bethesda System for Reporting Thyroid Cytopathology | 75.0 |
| R12 | Refer patients to surgery after a malignant FNA result | 99.4 |
| R15 | For thyroid nodules with AUS/FLUS cytology results, molecular testing may be performed | 38.8 |
| R16 | Use diagnostic surgery or molecular testing for thyroid nodules with follicular neoplasm cytology results | 90.2 |
| R17 | Use diagnostic surgery for most patients with suspicious for malignancy cytology results | 86.1 |
| R18 | Do not usually use 18FDG-PET to evaluate malignancy risk in thyroid nodules | 99.7 |
| R19 | Patients with FNA results of AUS/FLUS or follicular neoplasm who are selected for surgery should undergo a thyroid lobectomy | 47.0–82.0 |
| R20 | Use total thyroidectomy for thyroid nodules with suspicious FNA results, or positive molecular testing, or clinical features that increase the suspicion for malignancy | 50.0a |
| R21a | Evaluation of thyroid nodules in multinodular thyroid patients should generally be similar to patients with a solitary nodule | 15.1b |
| R23b | For patients with a prior benign FNA and low to intermediate suspicion features on US, repeat US in 12–24 months and repeat FNA only for nodule growth or new suspicious US features | 85.3 |
| R30a | Perform FNA of new thyroid nodules during pregnancy if the TSH is normal or elevated | 47.6 |
| Record No. . | CPG Recommendation (Abbreviated) . | Survey Concordance, % . |
|---|---|---|
| R2 | Measure TSH in all thyroid nodule patients | 99.4 |
| R3 | Do not measure thyroglobulin in the assessment of thyroid nodules | 94.9 |
| R6 | Include lymph node assessment in thyroid US | 68.5 |
| R8a | Use a 1.0-cm FNA threshold for thyroid nodules with suspicious US features | 33.0 |
| R8b | Use 1.0-cm FNA threshold for thyroid nodules with intermediate suspicion US features (hypoechoic, solid, regular margins) | 93.8 |
| R8c | Use 1.5-cm FNA threshold for thyroid nodules with low suspicion US features (isoechoic or hyperechoic nodules, partially cystic with eccentric solid portion) | 58.4 |
| R8d | Use a 2.0-cm FNA threshold for thyroid nodules with very low suspicion US features (spongiform, complex cystic with no suspicious US features) | 62.6 |
| R8e | Do not use diagnostic FNA for purely cystic thyroid nodules | 75.5 |
| R9 | Use the Bethesda System for Reporting Thyroid Cytopathology | 75.0 |
| R12 | Refer patients to surgery after a malignant FNA result | 99.4 |
| R15 | For thyroid nodules with AUS/FLUS cytology results, molecular testing may be performed | 38.8 |
| R16 | Use diagnostic surgery or molecular testing for thyroid nodules with follicular neoplasm cytology results | 90.2 |
| R17 | Use diagnostic surgery for most patients with suspicious for malignancy cytology results | 86.1 |
| R18 | Do not usually use 18FDG-PET to evaluate malignancy risk in thyroid nodules | 99.7 |
| R19 | Patients with FNA results of AUS/FLUS or follicular neoplasm who are selected for surgery should undergo a thyroid lobectomy | 47.0–82.0 |
| R20 | Use total thyroidectomy for thyroid nodules with suspicious FNA results, or positive molecular testing, or clinical features that increase the suspicion for malignancy | 50.0a |
| R21a | Evaluation of thyroid nodules in multinodular thyroid patients should generally be similar to patients with a solitary nodule | 15.1b |
| R23b | For patients with a prior benign FNA and low to intermediate suspicion features on US, repeat US in 12–24 months and repeat FNA only for nodule growth or new suspicious US features | 85.3 |
| R30a | Perform FNA of new thyroid nodules during pregnancy if the TSH is normal or elevated | 47.6 |
Represents the percentage patients referred for thyroid surgery in whom total thyroidectomy would be requested.
Represents the percentage of respondents who would sample all nodules >1 cm in a patient with a multinodular goiter.
Regional differences in thyroid nodule management
Striking differences were noted in the routine measurement of serum calcitonin, with highest usage in EU (32.1%) compared with other regions including AO (16.7%), LA (6.2%), ME-AF (15.4%), and NA (2.5%) (P < .001) (Figure 3A). The specialty performing the FNA procedure also varied regionally, with 64.1% of North American endocrinologists performing the procedure themselves, compared with 50.5% in EU, 50.0% in AF-ME respondents, 38.2% in AO respondents, and 36.6% among LA respondents (P < .001). Routine use of US guidance for most thyroid FNA procedures was more common among NA respondents (88.4%) compared with LA (80.6%), AO (75.4%), EU (70.8%), and AF-ME (70.0%) respondents (P < .001). NA (31.8%) and EU (29.7%) endocrinologists were less likely to postpone FNA until after pregnancy for a nodule discovered during the first trimester compared with LA (45.3%), AO (41.3%), and AF-ME (39.1%), but this difference did not reach statistical significance (P = .074). The most striking international difference involved molecular testing (Figure 3B), with higher usage in NA (70.9%) compared with EU (34.4%), AO (25.0%), LA (22.8%), and AF-ME (19.5%) with P < .001.
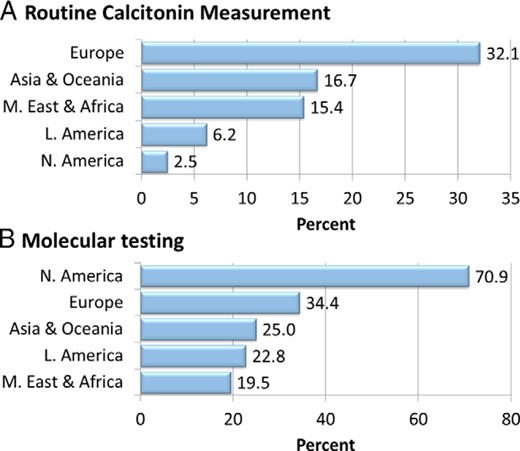
Regional differences in the diagnostic management of thyroid nodules.
Large regional differences were found in the routine measurement of calcitonin as well as in the degree of incorporation of molecular testing into respondents' practice.
Age and sex differences in thyroid nodule management
Controlling for region, age, and sex were not significant factors affecting the choice of treatment options.
Discussion
The current report provides results from a large contemporary international survey of clinical practices in the management of thyroid nodules. Respondents comprised a demographically diverse group of clinical endocrinologists, representing members from three major endocrine societies and 73 countries. Key findings include: 1) extensive assimilation of thyroid US into risk assessment and sampling of thyroid nodules; 2) selective use of molecular profiling for cytologically-indeterminate nodules, primarily in North America; 3) lower size thresholds for FNA than those recommended in current CPGs; 4) wide variation in technical aspects of FNA performance and interpretation (see Supplemental Material); and 5) a low level of comfort with observation alone in patients with cytologically indeterminate thyroid nodules.
Use of imaging
The percentage of respondents obtaining baseline US and utilizing US guidance for FNA has increased dramatically in comparison with earlier surveys. Diagnostic thyroid US would now be obtained at baseline by nearly all respondents, and US guidance would be used during FNA by more than 80% of respondents, whereas a 2000 survey of North American thyroidologists found that only 33.8% of respondents would order a diagnostic sonogram, and only 13.8% would use US to perform FNA. These changes likely reflect improvements in the quality, cost, and availability of US devices used in clinical practice, as well as recommendations for routine US use in previous and current CPGs. It is clear from our study that US guidance is the norm for most thyroid nodule aspirations performed in clinical practice. The updated ATA guidelines also recommend cervical lymph node assessment at the time of initial evaluation, but only two thirds of respondents noted routine inclusion of cervical lymph nodes in thyroid US procedures performed at their center.
Fewer than 5% of respondents would obtain a radionuclide thyroid scan, either at baseline or in response to FNA result consistent with follicular neoplasm. These findings are in contrast with previous surveys, which found that 23.2% of ATA respondents and 66% of European Thyroid Association respondents would obtain a radionuclide scan for a clinically euthyroid patient with a 3-cm thyroid nodule (11, 12).
Selection of thyroid nodules for sampling
The recently updated ATA guidelines suggest a more restrained approach to thyroid nodule sampling (10), relative to the 2009 guidelines (19). It is apparent from our survey results that a less-selective approach is being used in current clinical practice (Table 2), with two thirds of respondents preferring to sample a subcentimeter hypoechoic nodule with microcalcifications, more than half sampling a small complex-cystic nodule, and nearly one quarter selecting a pure cyst for aspiration. As these can be considered baseline results, it will be important to determine the extent to which future clinical practice changes in response to these updated guidelines.
For patients with multinodular thyroid glands containing several sonographically similar solid hypoechoic nodules, respondents favored a selective approach rather than sampling all nodules according to size criteria alone. We did not query respondents about their approach to multinodular glands with sonographically diverse nodules, which might have led to a greater attempt to apply selection criteria to each unique nodule.
Thyroid nodules in pregnancy
In the current survey, <50% of respondents would sample a new thyroid nodule during pregnancy, with the majority either postponing FNA until after pregnancy or only sampling if the nodule grows under observation during pregnancy. This finding is in contrast with both the 2009 and 2015 ATA guidelines, which recommend performance of FNA during pregnancy in biochemically euthyroid women. Given that both versions suggest that it is acceptable to wait until after pregnancy to perform surgery for low-risk papillary thyroid cancer, it is possible that many respondents prefer to avoid rendering an indeterminate or cancer diagnosis without intervention during pregnancy.
Elderly patients
The 2015 ATA Thyroid Nodule Guidelines suggest the possibility of active surveillance rather than nodule sampling in patients at high surgical risk or with a limited life expectancy. Approximately two thirds of our respondents indicated that they would be less likely to perform an FNA in an octogenarian than in a younger patient with a similar thyroid nodule. This likely reflects the indolent nature of most thyroid tumors, making it unlikely that a missed thyroid cancer will affect morbidity or mortality rates in an elderly patient. Most thyroid cancer cohort studies have shown that patient greater than age 45–55 years is a poor prognostic indicator (20, 21), whereas, in the case of papillary microcarcinomas, older age seems to be associated with a lower rate of progression (22). An earlier NA survey found that fewer respondents would recommend surgery for a thyroid nodule in an elderly patient (2 vs 20% for a younger patient) (11).
Followup after a benign FNA
After obtaining a benign FNA in a thyroid nodule, the likelihood of later finding that nodule to be malignant is 1–10% in various series (23–25), with particularly low false-negative rates in patients with benign features on thyroid US (25–27). Consequently, the 2015 ATA guidelines suggest that a repeat FNA in this setting be based on the presence or absence of suspicious sonographic features, and the rate of nodule growth under observation. This CPG recommends that nodules with highly suspicious sonographic features routinely undergo repeat US and FNA at 1 year, whereas nodules with low-to-intermediate risk sonographic features should only undergo repeat FNA if nodular growth is evident on US performed at 12–24 months (10). The present survey results are concordant with this guidance, with fewer than 10% of respondents routinely repeating FNA in the first year to exclude false-negative results at the original sampling. The response of participants in our survey to nodule volume growth of greater than 50% overwhelmingly favored a repeat FNA, although this growth is not specific for malignant thyroid nodules (28).
Molecular testing
The benefits and limitations of molecular testing of cytologically indeterminate thyroid nodules are currently being assessed in clinical practice (29–31). The 2015 ATA guidelines suggest that molecular testing or repeat FNA can be considered in lieu of either diagnostic surgery or continued surveillance for patients with AUS/FLUS cytological results and worrisome clinical or radiographic features (10). For nodules with follicular neoplasia at FNA these guidelines suggest that although diagnostic surgery is the “long-established standard of care,” an alternative is to perform molecular testing. Respondents to the current survey showed the highest usage rate for molecular testing in patients with AUS/FLUS or follicular neoplasia at FNA, with rates of approximately 50% overall. Forty percent of all respondents stated that molecular testing is not used in their practice.
Additional approaches to cytologically indeterminate thyroid nodules were assessed, and most notable is the low rate of observation alone in this setting, ranging from 0.4% in patients with cytology suspicious for malignancy to 2.7% in patients with AUS/FLUS lesions. This suggests that most respondents are uncomfortable observing a patient with an undefined, albeit low, risk of malignancy after an indeterminate FNA, with surgical referral rates ranging from 24–86% in this setting (Figure 2).
International differences in management
The observed international differences in thyroid nodule management among survey participants are of interest. In terms of routine laboratory testing, the most striking difference is the use of routine measurement of serum calcitonin by nearly one in three EU respondents compared with only 1 in 40 NA respondents. This difference is similar to that noted in earlier surveys in which 43% of European Thyroid Association members routinely measured calcitonin, compared with 4.9% of ATA members (11, 12). Although the reasons for these international differences are not known, possible explanations include the historically greater availability of pentagastrin in Europe, which is no longer available in NA (and becoming less available in Europe), for measurement of stimulated calcitonin, and a persistence of ingrained practices in these locations. Although much of the debate about routine calcitonin measurement has centered on cost-effectiveness (32), it was recently demonstrated that detection of medullary thyroid cancer through routine calcitonin screening leads to diagnosis at an earlier stage of disease (33).
Molecular testing was two to five times more likely to be performed for indeterminate thyroid nodules in North America compared with other regions of the world. This is likely related to the approval for cost reimbursement from Medicare and other insurers and extensive marketing of testing platforms in the United States.
Strengths and limitations
Our study has both strengths and limitations. The number of respondents was large, and represented a diverse international group of clinicians. The electronic mail invitation to participate provided an opportunity for more than 13 000 potential respondents to learn about the survey. A limitation of the current study is the relatively low percentage of active society membership participating, accounting for 6–29% of clinically active members of the three societies. Another limitation is an underrepresentation of non-U.S.-based endocrinologists and potential selection bias when including international members of U.S.-based endocrine societies. Therefore, the international differences noted in management practices may actually underestimate those obtained using independent surveys in those regions. In addition, it is possible that respondents to management surveys are more likely to be aware of CPGs and potentially adhere to their recommendations, although the completion of this survey just prior to the e-publication of the current ATA thyroid nodule guidelines makes this less relevant.
Conclusions
In summary, our survey of clinical endocrinologists on the management of thyroid nodules documents current practice patterns and demonstrates both concurrence and focal discordance with recently updated CPGs. Reassessment of practice patterns after adequate time for adoption of the most recent guidelines is appropriate to reassess these gaps. Both international differences and a change in practice patterns during the past two decades are demonstrated.
Acknowledgments
We thank Ms Robin Howard for assistance with the statistical analysis, and the American Thyroid Association (Dr John C. Morris, Secretary; Ms Barbara Smith, Executive Director), American Association of Clinical Endocrinologists (Dr Jeffrey Garber, liaison to ATA), and The Endocrine Society (Ms Stephanie Kutler, Director, Government Affairs) for their expert assistance in reviewing and vetting the survey and forwarding it to society membership. We also thank the many national and international colleagues who took the time to participate in this survey.
The views expressed in this manuscript are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense or the United States Government. One or more authors are military service members (or employees of the U.S. Government). This work was prepared as part of our official duties. Title 17 U.S.C. 105 provides the “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. 101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person's official duties. We certify that all individuals who qualify as authors have been listed; each has participated in the conception and design of this work, the analysis of data (when applicable), the writing of the document, and/or the approval of the submission of this version; that the document represents valid work; that if we used information derived from another source, we obtained all necessary approvals to use it and made appropriate acknowledgments in the document; and that each takes public responsibility for it.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- 18FDG-PET
2-fluoro-2-deoxy-D-glucose positron emission tomography
- AACE
American Association of Clinical Endocrinologists
- AF
Africa
- AO
pooled responses from Asia and Oceana
- AS
Asia
- ATA
American Thyroid Association
- AUS
atypia of undetermined significance
- CPG
clinical practice guideline
- EU
Europe
- FLUS
follicular lesion of undetermined significance
- FNA
fine-needle aspiration
- LA
Latin America
- ME
Middle East
- MRI
magnetic resonance imaging
- NA
North America
- OC
Oceania
- TES
The Endocrine Society
- US
ultrasound.



