-
PDF
- Split View
-
Views
-
Cite
Cite
Adriana Ricciuti, Thomas G. Travison, Giulia Di Dalmazi, Monica V. Talor, Ludovica DeVincentiis, Robert W. Manley, Shalender Bhasin, Patrizio Caturegli, Shehzad Basaria, A Subset of Men With Age-Related Decline in Testosterone Have Gonadotroph Autoantibodies, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 4, 1 April 2016, Pages 1535–1541, https://doi.org/10.1210/jc.2016-1016
Close - Share Icon Share
Abstract
Age-related decline in serum testosterone (T) is being increasingly diagnosed. In most men, it associates with low or inappropriately normal gonadotropin levels, which suggests a hypothalamic-pituitary etiology. Autoantibodies against adenohypophyseal cells have been associated with pituitary dysfunction; however, the prevalence of pituitary autoimmunity in this age-related T decline has not been assessed.
This is a proof-of-concept study with the objective of determining the prevalence of antibodies to gonadotrophs in older men with age-related low T and compare it with healthy young and older eugonadal men.
This is a cross-sectional case-control study of 182 men. Cases included 100 older men (≥65 years) with age-related low T levels; the control groups were composed of 50 young and 32 older healthy eugonadal men. Serum antibodies against the anterior pituitary gland were measured using a two-step approach: 1) single indirect immunofluorescence (ie, participant serum only) to determine the pattern of cytosolic staining; and 2) double indirect immunofluorescence (ie, participant serum plus a commercial adenohypophyseal hormone antibody) to identify the anterior pituitary cell type recognized by the patient's antibodies).
In participants with positive antipituitary antibodies, the granular cytosolic pattern (highly predictive of pituitary autoimmunity) was only seen in older men with age-related low T (4%) and none in control groups (0%, P = .001). Double indirect immunofluorescence confirmed that pituitary antibodies were exclusively directed against the gonadotrophs.
A subset of older men with age-related low T levels have specific antibodies against the gonadotrophs. Whether these antibodies are pathogenic and contributory to the age-related decline in T remains to be established.
Testosterone (T), the main sex steroid in men, plays a major role in the maintenance of male phenotype and psychosexual behavior. Classic androgen deficiency, which occurs as a result of hypothalamic-pituitary or testicular disease, has been recognized for decades. Although the prevalence of this classic (and unequivocal) androgen deficiency has not changed (1), the prescription sales of T across continents have increased exponentially over the past decade (2, 3). This observation suggests that a sizeable proportion of T therapy being prescribed is for low T levels attributed to aging (4), a condition commonly referred to as late-onset hypogonadism (5). Testosterone levels, after peaking in the second and third decade of life, gradually decline with advancing age (6, 7). Alterations at all levels of the hypothalamic–pituitary–testicular axis have been implicated in the pathophysiology of this age-related decline in T (5, 7), including Leydig cell attrition (8, 9) and neuroendocrine changes in GNRH pulsatility and responsiveness to negative feedback by androgens (10, 11).
Over the past few decades, autoimmunity has been implicated in pituitary dysfunction. Antibodies against all adenohypophyseal cells have been reported, including the lactotrophs (12), corticotrophs (13), somatotrophs (14), gonadotrophs (15), and thyrotrophs (16). However, only two studies have investigated the presence of antibodies to gonadotrophs in patients with idiopathic adult-onset androgen deficiency, with both studies focusing on relatively young adults aged 19–44 years (17, 18). In 2007, De Bellis et al reported gonadotroph antibodies in the serum in eight of 21 (38%) men with isolated hypogonadotropic hypogonadism; among the remaining 13 men, five had hypogonadism accompanied by other pituitary hormone deficiencies (17). In a subsequent report, the same group reported 19 additional patients with idiopathic hypogonadotropic hypogonadism, five of whom (26%) had positive gonadotroph antibodies (18). Interestingly, the prevalence of gonadotroph antibodies in older men with age-related decline in T levels has not been investigated even though most of these men have gonadotropins that are inappropriately normal.
We hypothesized that pituitary autoimmunity might be an additional mechanism that leads to age-related decline in serum T levels. As pituitary antibodies have been shown to be predictive of subsequent deficiencies in pituitary hormones (19), the finding of gonadotroph antibodies in aging men with low T has therapeutic implications because treatment with immunomodulatory agents could potentially prevent this age-related decline in gonadal hormones.
Materials and Methods
Study participants
This cross-sectional, proof-of-concept, case-control study analyzed baseline (ie, before any intervention) sera from 182 men from three studies. For cases, we randomly selected 100 men from the published Testosterone in Older Men with Mobility Limitation (TOM) trial (20). The sampling population comprised men 65 years and older (mean age, 74 ± 6 years) with mobility limitation and low serum T (total T <350 ng/dl or free T <50 pg/ml) (20). Low T levels in this cohort were a consequence of aging as organic causes of T deficiency (pituitary or testicular disease) were carefully excluded. These men were subsequently randomized to T or placebo. Control groups comprised both young and older eugonadal men. We randomly selected 50 young (age, 18–50 years) healthy eugonadal men from the 5α-reductase (5-AR) trial (21). The 5-AR study was designed to determine the role of dihydrotestosterone in adult male physiology. Men in this trial underwent suppression of endogenous T by GnRH agonists and were given graded doses of T along with dutasteride or placebo (21). For older controls, we randomly selected 32 older (mean age, 66 years) healthy eugonadal men from an ongoing observational study evaluating quality of life in prostate cancer (PCa) survivors who had organ-confined PCa and were cured after prostatectomy (PCa Study). All studies were approved by the Institution Review Board of Brigham and Women's Hospital.
Detection of anterior pituitary antibodies by single indirect immunofluorescence
The detection of serum antibodies against the anterior pituitary was performed by “single indirect immunofluorescence” (the primary incubation only contained the serum of the participants diluted at 1:10) on a human pituitary gland substrate using a recently published protocol (22, 23). Sections were read independently by two of the authors (A.R. and P.C.) and considered positive for the presence of anterior pituitary antibodies when stained cells were present in at least four of 20 microscopic fields (22).
Detection of gonadotroph antibodies by double indirect immunofluorescence
Sera that tested positive for anterior pituitary antibodies were analyzed by “double indirect immunofluorescence” (both the participant serum [diluted 1:10] and a commercial antigonadotropin antibody [diluted 1:5] were added during the primary incubation) on a human pituitary gland substrate. We used a rabbit polyclonal antihuman FSHβ (National Hormone & Pituitary Program, catalog no. AFPHFSH6) and a rabbit polyclonal antihuman LHβ (AFP571292393). Antibodies against the other four pituitary cell types (somatotrophs, lactotrophs, thyrotrophs, and corticotrophs) were also purchased from the National Hormone & Pituitary Program. After the primary incubation, sections were washed and costained with a fluorescein isothiocyanate–conjugated goat F(ab)2 antihuman IgG (diluted 1:400) to reveal the participant antibody binding in the green channel, and a DyLight 649-conjugated goat antirabbit IgG (Jackson Immunoresearch Laboratory) diluted 1:1000 in phosphate-buffered saline with Tween 20 to reveal the antihormone binding in the red channel. The stain 4′,6-diamidino-2-phenylindole was included in the counterstaining step to reveal nuclei in the blue channel. To determine whether the FSH and LH are cosecreted by the same gonadotroph or by distinct populations of gonadotrophs, we also costained human pituitary sections with a rabbit antihuman FSHβ and a goat antihuman LH (54091, Santa Cruz Biotechnology). Individual antibody binding was revealed by the addition of affinity-purified secondary antibodies recognizing either the rabbit or the goat immunoglobulins that conjugated with green or red fluorochromes.
Statistical analysis
A sample size of 182 participants (TOM, 100; 5-AR, 50; PCa, 32) was chosen to have a statistical power of 0.9 in detecting a between-group difference (delta) of 0.21 using a two-sided test with a significance level of 0.01. The effect size of 0.21 (0.26 − 0.05 = 0.21) was based on anterior pituitary antibody prevalence values of 0.26 in young men with idiopathic hypogonadotropic hypogonadism (18) and 0.05 in healthy controls (22). The primary outcome of the study was the prevalence of anterior pituitary antibodies, expressed dichotomously as present or absent. Differences in prevalence among the three cohorts were assessed using χ2 statistics and quantified as odds ratios with 95% confidence intervals. Secondary outcome included the levels of antibodies specific to gonadotropins. Differences among the means or medians of the groups were assessed by one-way ANOVA or Wilcoxon rank-sum test. Test generating P values <.05 (two-sided) were considered statistically significant. All analyses were performed using Stata software (Stata Corporation).
Results
Characteristics of the participants
Baseline characteristics of the participants in the three cohorts are summarized in Table 1. Men participating in the TOM trial were older, had a higher body mass index and lower hematocrit than men in the control cohorts. Per design of the three studies, serum total and free T levels were normal in the 5-AR and PCa cohorts and reduced in the TOM trial participants.
Baseline Characteristics of the Participants in the Three Cohorts (Mean±sd)
| . | TOM (n = 100) . | 5-AR (n = 50) . | PCa (n = 32) . |
|---|---|---|---|
| Age, y | 74 (5) | 38 (8) | 66 (6) |
| Height, cm | 171.0 (7) | 177.0 (7) | 174.0 (7) |
| Weight, kg | 87.0 (14) | 83.0 (11) | 84.0 (12) |
| BMI, kg/m2 | 29.9 (4.3) | 25.6 (3.2) | 28.0 (3.4) |
| Total T, ng/dl | 241 (69) | 639 (162) | 529 (192) |
| Free T, pg/ml | 44 (13) | 116 (35) | 84 (24) |
| LH, IU/liter | 7.1 (8.2) | 3.2 (1.5) | 5.2 (2.3) |
| SHBG, nmol/liter | 37.0 (17.7) | 45.2 (17.2) | 47.0 (16.8) |
| Hemoglobin, g/dl | 13.8 (1.3) | 14.9 (1.0) | 14.7 (0.8) |
| Hematocrit, % | 40.6 (3.6) | 43.4 (3.0) | 45.0 (2.7) |
| PSA ng/ml | 1.15 (0.76) | 0.69 (0.37) | 0.10 (0.05) |
| . | TOM (n = 100) . | 5-AR (n = 50) . | PCa (n = 32) . |
|---|---|---|---|
| Age, y | 74 (5) | 38 (8) | 66 (6) |
| Height, cm | 171.0 (7) | 177.0 (7) | 174.0 (7) |
| Weight, kg | 87.0 (14) | 83.0 (11) | 84.0 (12) |
| BMI, kg/m2 | 29.9 (4.3) | 25.6 (3.2) | 28.0 (3.4) |
| Total T, ng/dl | 241 (69) | 639 (162) | 529 (192) |
| Free T, pg/ml | 44 (13) | 116 (35) | 84 (24) |
| LH, IU/liter | 7.1 (8.2) | 3.2 (1.5) | 5.2 (2.3) |
| SHBG, nmol/liter | 37.0 (17.7) | 45.2 (17.2) | 47.0 (16.8) |
| Hemoglobin, g/dl | 13.8 (1.3) | 14.9 (1.0) | 14.7 (0.8) |
| Hematocrit, % | 40.6 (3.6) | 43.4 (3.0) | 45.0 (2.7) |
| PSA ng/ml | 1.15 (0.76) | 0.69 (0.37) | 0.10 (0.05) |
Abbreviations: BMI, body mass index; PSA, prostate-specific antigen.
Baseline Characteristics of the Participants in the Three Cohorts (Mean±sd)
| . | TOM (n = 100) . | 5-AR (n = 50) . | PCa (n = 32) . |
|---|---|---|---|
| Age, y | 74 (5) | 38 (8) | 66 (6) |
| Height, cm | 171.0 (7) | 177.0 (7) | 174.0 (7) |
| Weight, kg | 87.0 (14) | 83.0 (11) | 84.0 (12) |
| BMI, kg/m2 | 29.9 (4.3) | 25.6 (3.2) | 28.0 (3.4) |
| Total T, ng/dl | 241 (69) | 639 (162) | 529 (192) |
| Free T, pg/ml | 44 (13) | 116 (35) | 84 (24) |
| LH, IU/liter | 7.1 (8.2) | 3.2 (1.5) | 5.2 (2.3) |
| SHBG, nmol/liter | 37.0 (17.7) | 45.2 (17.2) | 47.0 (16.8) |
| Hemoglobin, g/dl | 13.8 (1.3) | 14.9 (1.0) | 14.7 (0.8) |
| Hematocrit, % | 40.6 (3.6) | 43.4 (3.0) | 45.0 (2.7) |
| PSA ng/ml | 1.15 (0.76) | 0.69 (0.37) | 0.10 (0.05) |
| . | TOM (n = 100) . | 5-AR (n = 50) . | PCa (n = 32) . |
|---|---|---|---|
| Age, y | 74 (5) | 38 (8) | 66 (6) |
| Height, cm | 171.0 (7) | 177.0 (7) | 174.0 (7) |
| Weight, kg | 87.0 (14) | 83.0 (11) | 84.0 (12) |
| BMI, kg/m2 | 29.9 (4.3) | 25.6 (3.2) | 28.0 (3.4) |
| Total T, ng/dl | 241 (69) | 639 (162) | 529 (192) |
| Free T, pg/ml | 44 (13) | 116 (35) | 84 (24) |
| LH, IU/liter | 7.1 (8.2) | 3.2 (1.5) | 5.2 (2.3) |
| SHBG, nmol/liter | 37.0 (17.7) | 45.2 (17.2) | 47.0 (16.8) |
| Hemoglobin, g/dl | 13.8 (1.3) | 14.9 (1.0) | 14.7 (0.8) |
| Hematocrit, % | 40.6 (3.6) | 43.4 (3.0) | 45.0 (2.7) |
| PSA ng/ml | 1.15 (0.76) | 0.69 (0.37) | 0.10 (0.05) |
Abbreviations: BMI, body mass index; PSA, prostate-specific antigen.
Pituitary antibodies with a granular staining pattern were only seen in older men with age-related low T
When reported as present or absent, pituitary antibodies were detected in eight of 100 (8%) participants in the TOM trial, 21 of 50 (42%) participants in the 5-AR trial, and three of 32 (9%) in the PCa study. This overall higher prevalence in the 5-AR cohort was largely due to perinuclear positivity, a nonspecific pattern that is also seen in healthy subjects (22). When reported instead based on the pattern of cytosolic staining, the granular positivity (the pattern most specific for pituitary autoimmunity (22)), was only seen in the TOM trial participants. In particular, this granular positivity was seen in four of 100 (4%) men in the TOM trial cohort, zero of 50 (0%) in the 5-AR cohort, and zero of 32 (0%) in the PCa cohort (P = .001, Figure 1). Supplemental Table 1 summarizes the characteristics of TOM Trial participants based on the antibody status.
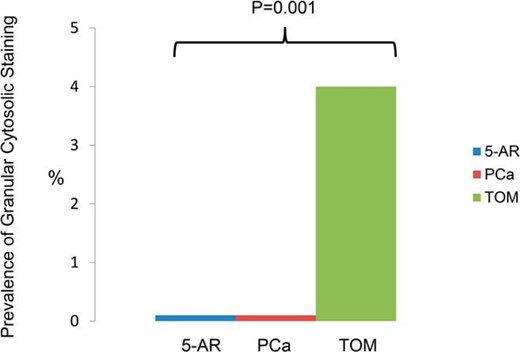
Prevalence of specific antigonadotroph antibody based on the granular cytosolic staining pattern.
Pituitary antibodies with a granular staining pattern recognize gonadotrophs
When assessed for recognition of all five adenohypophyseal cell subtypes by double indirect immunofluorescence, antibodies with a granular staining pattern bound exclusively to gonadotropin-secreting cells. For the FSH double staining, the cells recognized by the participants' serum (Figure 2A, left panel) corresponded to those recognized by the commercial anti-FSH reagent (Figure 2A, middle panel), so that the merging of the green and red signals yielded a yellow colocalization signal (Figure 2A, right panel). Similar findings were obtained with the LH double staining, but here the pituitary cells recognized by the participants' serum (Figure 2B, left panel) were more than those recognized by the commercial anti-LH antibody (Figure 2B, middle panel), so that upon merging there was yellow staining (Figure 2B, right panel, white arrow) but also some residual green staining (Figure 2B, right panel, pink arrow).
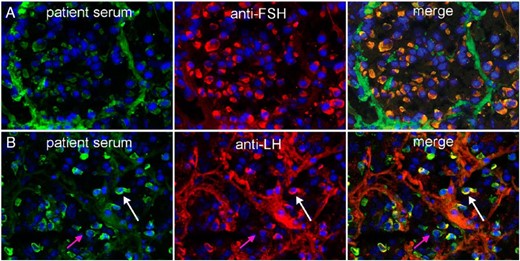
Recognition of gonadotropin-secreting cells by pituitary antibodies.
A, The top three panels represent the staining obtained using participant's serum (left), commercial FSHβ antibody (center), and the merged staining (right). All anterior pituitary cells recognized by the participant's antibodies (green) were also recognized by the anti-FSHβ (red), yielding a yellow color in the merged panel. B, The bottom three panels represent the staining obtained using participant's serum (left), commercial LHβ antibody (center), and the merged staining (right). Most anterior pituitary cells recognized by the participant's antibodies (green) were also recognized by anti-LHβ (red) (white arrow), whereas a few cells were recognized only by the participant's serum (pink arrow).
Although it is traditionally believed that the same gonadotroph secretes both LH and FSH (24), the above findings confirm the existence of monohormonal subsets of gonadotrophs (25). We extended these findings by costaining a normal human pituitary gland with anti-FSH and anti-LH antibodies. In a representative high-power field (Figure 3) containing six gonadotrophs, three cells secreted only FSH (cells 1, 4, and 6), two secreted both FSH and LH (cells 2 and 3), whereas one predominantly secreted LH (cell 5).
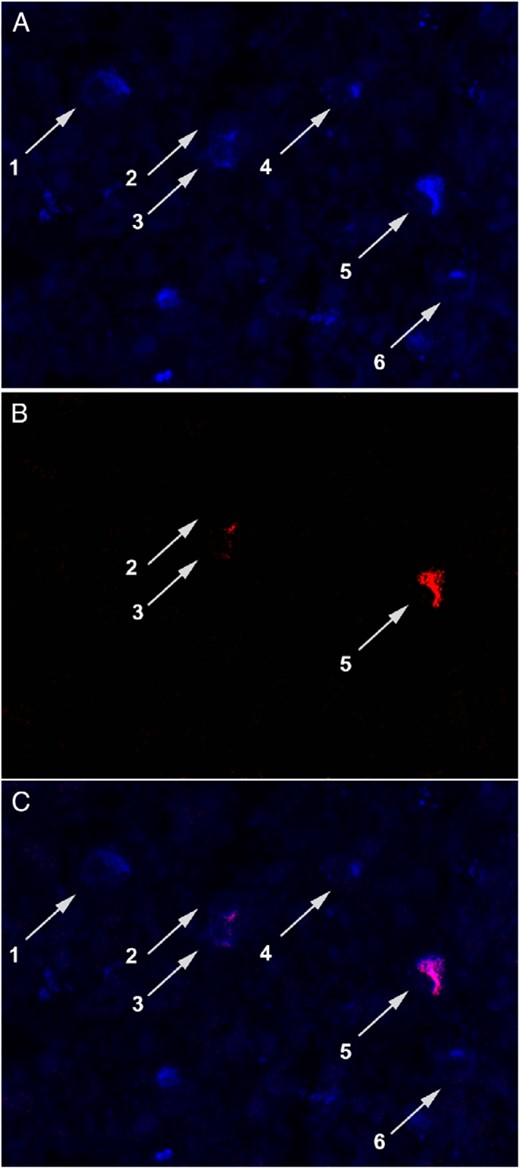
Monohormonal and bihormonal production by the gonadotrophs.
A, Staining with a commercial anti-FSHβ antibody. B, Staining with a commercial anti-LHβ antibody. C, Merged staining. The figure shows that that some gonadotrophs expressed both FSH and LH (cells 2 and 3), others expressed only FSH (cells 1, 4, and 6), and others mainly LH (cell 5).
Serum antibody titers to FSH and LH (measured by ELISA) in those participants of the TOM Trial who had positive antibodies are depicted in Supplemental Figure 1.
Discussion
This proof-of-concept study is the first report suggesting autoimmunity against the gonadotrophs as an additional mechanism that contributes to the pathogenesis of age-related decline in T levels. In contrast to the classic (pathologic) androgen deficiency that results from testicular or hypothalamic-pituitary disease, the age-related decline in T remains unexplained. Older men with low T generally manifest biochemical features consistent with a secondary cause as gonadotropins are low or inappropriately normal (6, 26), implicating central mechanisms. Indeed, aging is associated with blunting of circadian rhythm (10), decreased LH pulse amplitude, and increased frequency of LH pulses (6, 27). In this study, we found that pituitary antibodies with a granular pattern (highly specific for autoimmunity) were not only more common in the TOM cohort (men with age-related low T) compared to their younger and older eugonadal counterparts, but also specifically recognized the gonadotrophs, thus providing a novel mechanism for the age-related decline in T levels. In addition to its novelty, the other strengths of this study, such as the use of the state-of-the-art immunofluorescence methodology and the evaluation of both young and older eugonadal participants (control groups) provide credibility to our observations.
Pituitary autoantibodies were first reported in humans in 1965 by the now abandoned complement consumption assay (28), and then in 1975 by indirect immunofluorescence (12), which remains the most commonly used technique. Over the ensuing four decades, 128 published studies measured pituitary antibodies using immunofluorescence (22). Of these, 25 (20%) studies used double immunofluorescence not only to determine the presence of pituitary antibodies, but also to determine the type(s) of anterior pituitary cells they recognized (29). Fourteen of these 25 studies assessed antibodies to gonadotrophs (summarized in Supplemental Table 2), yielding variable results (17, 18, 29). This variability is in part explained by the wide spectrum of diseases affecting the gonads that were evaluated in those studies, ranging from cryptorchidism (15, 30) to autoimmune polyglandular syndrome 1 (31, 32). Variability could also have resulted from the different pituitary gland species used as substrate for the immunofluorescence. We have recently shown that the human pituitary is the best substrate for pituitary antibody detection by immunofluorescence, and that a granular staining pattern is the one most commonly associated with autoimmunity (22). In the current study, the granular staining pattern was only seen in older men in the TOM cohort who had low serum T levels.
In addition to its diagnostic value, pituitary autoantibodies are a topic of ongoing research because of their pathogenic value. In human autoimmune diseases, direct evidence of antibodies playing a pathogenic role is available in certain conditions where antibodies are transferred from the mother to the fetus such as in neonatal Graves' disease (33), myasthenia gravis (34), and lupus (35). Furthermore, pathogenicity can also be inferred from the findings that in certain conditions antibodies precede the onset of overt clinical symptoms by several years. This predictive role of antibodies was first demonstrated for type 1 diabetes (36) and later confirmed in systemic lupus erythematous (37) and Hashimoto's thyroiditis (38). The predictive role of pituitary antibodies was shown in a study that evaluated participants with normal pituitary function and stratified them based on the presence or absence of pituitary antibodies, and followed them prospectively every 6 months for 5 years (19). Approximately 75% of the participants with positive pituitary antibodies at baseline developed some degree of pituitary dysfunction and the type of hormonal deficiency corresponded to the antibody directed against the particular cell type (19), suggesting that these antibodies are pathogenic. To the contrary, none of the subjects without pituitary antibodies manifested any hormonal deficiency. It is thus conceivable that the gonadotroph antibodies in older men with age-related decline in T might be pathogenic. These results also suggest a window of opportunity for the prevention of T decline as the onset of a clinical syndrome can be averted by immunomodulation. Indeed, treatment with glucocorticoids in patients with subclinical autoimmune adrenalitis, characterized by positive 21-hydroxylase antibodies and impaired cortisol response to ACTH, showed disappearance of antibodies and complete normalization of adrenal function (39). It is estimated that every year, approximately 481 000 new cases of androgen deficiency are diagnosed in men age 40–69 year old in the United States (40). If gonadotroph antibodies are pathogenic, then based on the 4% prevalence found in this study, pituitary autoimmunity might be responsible for age-related low T in approximately 20 000 of such men; this decline could potentially be prevented with immunomodulatory therapies. Future studies aimed at preventing the age-related decline in serum T using immunomodulatory agents in men with positive antigonadotroph antibodies might be of interest.
In summary, we report the presence of specific antigonadotroph antibodies in a subset of older men with low serum T levels associated with aging. The findings of this proof-of-concept study offer a novel mechanism that might contribute to age-related decline in T levels.
Acknowledgments
The authors thank the staff of the General Clinical Research Center of the Boston University Medical Center.
This work was supported in part by patient donations to the Johns Hopkins Hypophysitis Research Center. The 5-AR trial was funded by the National Institute of Child Health and Human Development, National Institutes of Health (NIH). The TOM trial was funded by the National Institute on Aging, NIH. The PCa study was funded by the National Cancer Institute, NIH.
Disclosure Summary: S.Ba. has previously received a research grant from Abbvie Pharmaceuticals on an investigator-initiated clinical trial and previously consulted for Eli Lilly and Takeda. S.Bh. has previously received a research grant from Abbvie Pharmaceuticals. None of the other authors have anything to disclose.
P.C. and S.B. are joint senior authors.
Abbreviations
- 5-AR
5α-reductase
- PCa
prostate cancer
- T
testosterone
- TOM
Testosterone in Older Men with Mobility Limitation.



