-
PDF
- Split View
-
Views
-
Cite
Cite
Dominik Wiese, Katharina Kampe, Jens Waldmann, Anna E. Heverhagen, Detlef K. Bartsch, Volker Fendrich, C-Reactive Protein as a New Prognostic Factor for Survival in Patients With Pancreatic Neuroendocrine Neoplasia, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 3, 1 March 2016, Pages 937–944, https://doi.org/10.1210/jc.2015-3114
Close - Share Icon Share
Abstract
Patients with pancreatic neuroendocrine neoplasia (pNEN) show great variability in prognosis and treatment response. Additional prognostic markers might help in individual therapeutic decision making.
The objective of the study was to investigate the association between preoperative plasma levels of C-reactive protein (CRP) and overall survival (OS) in pNEN.
This was a single-center, retrospective analysis of long-term prospective patient-database.
The study was conducted at a tertiary referral center.
All 149 patients with sporadic pNENs were eligible for retrospective analysis.
Cumulative overall survival, compared between patients with elevated and normal CRP levels, was measured.
Median OS for patients with elevated CRP levels was 1093 days (SE 1261, 95% confidence interval [CI] 0–3565), compared with 6859 days (SE 1252, 95% CI 4405–9313) for patients with normal CRP levels. Log rank test showed a significant correlation between CRP and OS (P < .001). In univariate Cox regression, patients with elevated CRP levels had a significantly higher hazard ratio for death (3.27; 95%-CI 1.74–6.16; P < .001). This finding persisted after multivariable adjustment. Furthermore, OS was associated with the presence of liver metastases (hazard ratio 3.17; 95% CI 1.88–5.35; P < .001), incomplete resection (R1/R2 status; hazard ratio 3.99; 95% CI 2.16–7.35; P < .001) and Ki-67 percentage (hazard ratio 5.05; 95% CI 2.17–11.76; P < .001).
CRP is an independent prognostic marker in patients with pNEN. Pretreatment CRP measurements should be considered for incorporation into prospective studies of outcome in patients with pNENs and clinical trials of systemic therapies for these tumors.
Pancreatic neuroendocrine neoplasias (pNENs) are a rare subset of pancreatic tumors (1), comprised of functioning tumors (gastrinoma, insulinoma, other rare functioning tumors) with their individual associated hormonal syndromes (2), and of nonfunctioning pancreatic neuroendocrine tumors or carcinomas (NF-pNET/-pNEC) (3). Positive immunohistochemistry for synaptophysin acts as defining marker for all neuroendocrine neoplasias, in combination with basic histology and other neuroendocrine markers like chromogranin A (4, 5). Diagnosis of hormonal syndromes like Zollinger-Ellison syndrome and organic hyperinsulinism in gastrinoma and insulinoma patients is based on clinical symptoms and specific functional tests (2). Treatment of most pNENs is founded on surgical resection, with highly proliferative pancreatic neuroendocrine carcinomas being an exception (3, 6–10). In addition to surgery, a multitude of chemotherapeutic, radiotherapeutic, and interventional regimen have been implemented for metastasized pNENs in recent decades (11–16). A characteristic of pNENs is their heterogeneity in individual prognosis (1). A significant influence on prognosis could be shown for macroscopically complete resection, the Ki-67 index as proliferation marker, and the presence or progression of liver metastases (17–22).
For many cancer entities, inflammation has been shown to play an important role in pathogenesis and progression (23). Such findings have for instance been published for ductal pancreatic adenocarcinoma (24), squamous cell and adenocarcinoma of the esophagus (25), melanoma (26), soft tissue sarcoma (27), or gastric cancer (28). Furthermore, in all of the above-mentioned entities, C-reactive protein (CRP) levels were reported to be of significant prognostic value (24–28). Other inflammatory markers and indices like neutrophil to lymphocyte ratio and the Glasgow prognostic score have also been successfully tried for further individual risk stratification in tumor patients (29, 30).
We report here for the first time that CRP is an independent prognostic marker in patients with pNENs.
Materials and Methods
Study design
This study is part of an ongoing prospective investigation designed to identify molecular, genetic, and environmental factors that influence pNEN risk and clinical outcome. All individuals provided informed consent under an institutional review board-approved protocol. Peripheral blood was collected at study entry.
For the present study, our prospective database of surgical patients with pNENs (March 1987 through December 2014) was retrospectively analyzed with focus on Ki-67 percentage (<5% vs ≥5%), existence of liver metastases (M0 vs M1), resection margins (R0 vs R1/R2), and initial preoperative CRP levels (<5 mg/L vs ≥5 mg/L). In patients with pNENs, Zollinger-Ellison-Syndrome was established by clinical symptoms, an elevated fasting serum gastrin level (>125 pg/mL), a positive secretin stimulation test defined as an increase of serum gastrin concentration to greater than 200 pg/mL together with low pH in the stomach, and a positive immunohistochemistry for gastrin in the tumor cells. The diagnosis of insulinoma required a symptomatic hypoglycemia (<40 mg/dL) with concomitant endogenous hyperinsulinism (>20 μU/mL) during a supervised fasting test and a positive immunohistochemistry for insulin in the tumor cells. In cases of insufficiently elevated insulin levels, the amended insulin to glucose ratio was used as an additional diagnostic criterion to still identify insulinoma patients. Lesions were considered as nonfunctioning pNENs (NF-pNENs), if there were no clinical symptoms of hormonal excess present and plasma hormone levels except those of pancreatic polypeptide were within normal limits. Patients with multiple endocrine neoplasia (MEN) were excluded from analysis. Length of follow-up and overall survival (OS) was measured from the date of blood draw to the date of last contact or death, respectively.
Experiments
Plasma was obtained from whole-blood draws prior to tumor-specific treatment. CRP measurements were performed as turbidimetric assay. Cutoff values for normal and elevated CRP levels were set according to the hospital's central laboratory standards (normal CRP: <5 mg/L). The Ki-67 index in the tumors' immunohistochemistry stainings were taken from the hospital's standard pathology reports. For further statistical analysis, the threshold for Ki-67 indices was set at 5%, taking recent findings into consideration, that this might hold a higher prognostic value than the formerly used 2% cutoff between G1- and G2-pNEN (31). Presence of liver metastases was determined in radiological (ultrasound, computed tomography, magnetic resonance imaging, positron emission tomography-computed tomography) and histological (open or minimally invasive biopsy or resection) staging. R status was confirmed integrating radiological workups, if present: macroscopically remaining tumor masses (eg, peritoneal carcinomatosis), and microscopic resection margins in pathology reports. Visible remaining or unresected tumor masses were classified as R2, macroscopically complete resection with tumor infiltrating the microscopic resection margins was classified as R1, macroscopically and microscopically complete resection was classified as R0. T status of the primary tumor was determined according to the European Neuroendocrine Tumor Society classification (32).
Statistical analysis
Cumulative OS in correlation to initial CRP levels and the established risk factors was plotted as Kaplan-Meier curve for each subgroup and compared via log rank test. Hazard ratios for each prognostic factor were calculated in a univariate Cox analysis. A χ2 test was used to determine P values for hazard ratios of each prognostic factor and to investigate the relationship between CRP levels and the other prognostic factors. Multivariate analysis was performed as backward stepwise Cox regression to examine a possible model of independent risk factors. All calculations and plots were performed using IBM SPSS Statistics, version 22 (IBM). Further labeling and tables were created in Microsoft Office 2010 (Microsoft Corp).
Results
Clinical features
After exclusion of 51 MEN1 patients, there remained 149 patients with sporadic pNENs, including insulinoma (n = 56), gastrinoma (n = 31), NF-pNEN (n = 60), and rare functioning pNEN (n = 2). One hundred of these patients (67%) had a pNEN with signs of malignancy (lymphatic and/or hepatic metastasis and/or carcinomatous/invasive histology of primary). In all 200 patients, 237 operations, predominantly enucleations and distal pancreatic resections, were performed. A total of 159 of all 200 patients (79.5%) were alive in their latest follow-up, 37.5% with no evidence of disease and 42% with disease. The 5-year survival rate of patients with sporadic pNEN was 72.1%. Characteristics of these 149 pNEN patients (after exclusion of MEN1 patients) are summarized in Table 1.
| n = 149 . | Patients . | |
|---|---|---|
| n . | % . | |
| Sex | ||
| Male | 73 | 49.0 |
| Female | 76 | 51.0 |
| Age, y | ||
| Median | 54 (range 17–87) | |
| <65 | 119 | 79.9 |
| ≥65 | 30 | 20.1 |
| Resection | ||
| R0 | 71 | 68.9 |
| R1 | 7 | 6.8 |
| R2 | 25 | 24.3 |
| Metastases (hepatic) | ||
| M0 | 110 | 73.8 |
| M1 | 39 | 26.2 |
| Ki-67 | ||
| <5% | 60 | 70.6 |
| ≥5% | 25 | 29.4 |
| CRP, mg/L | ||
| <5 | 67 | 69.1 |
| ≥5 | 30 | 30.9 |
| Entity | ||
| NF-pNEN | 60 | 40.3 |
| Insulinoma | 56 | 37.6 |
| Gastrinoma | 31 | 20.8 |
| Rare functioning | 2 | 1.3 |
| Tumor size | ||
| T1 | 60 | 46.2 |
| T2 | 42 | 32.3 |
| T3/4 | 28 | 21.5 |
| n = 149 . | Patients . | |
|---|---|---|
| n . | % . | |
| Sex | ||
| Male | 73 | 49.0 |
| Female | 76 | 51.0 |
| Age, y | ||
| Median | 54 (range 17–87) | |
| <65 | 119 | 79.9 |
| ≥65 | 30 | 20.1 |
| Resection | ||
| R0 | 71 | 68.9 |
| R1 | 7 | 6.8 |
| R2 | 25 | 24.3 |
| Metastases (hepatic) | ||
| M0 | 110 | 73.8 |
| M1 | 39 | 26.2 |
| Ki-67 | ||
| <5% | 60 | 70.6 |
| ≥5% | 25 | 29.4 |
| CRP, mg/L | ||
| <5 | 67 | 69.1 |
| ≥5 | 30 | 30.9 |
| Entity | ||
| NF-pNEN | 60 | 40.3 |
| Insulinoma | 56 | 37.6 |
| Gastrinoma | 31 | 20.8 |
| Rare functioning | 2 | 1.3 |
| Tumor size | ||
| T1 | 60 | 46.2 |
| T2 | 42 | 32.3 |
| T3/4 | 28 | 21.5 |
| n = 149 . | Patients . | |
|---|---|---|
| n . | % . | |
| Sex | ||
| Male | 73 | 49.0 |
| Female | 76 | 51.0 |
| Age, y | ||
| Median | 54 (range 17–87) | |
| <65 | 119 | 79.9 |
| ≥65 | 30 | 20.1 |
| Resection | ||
| R0 | 71 | 68.9 |
| R1 | 7 | 6.8 |
| R2 | 25 | 24.3 |
| Metastases (hepatic) | ||
| M0 | 110 | 73.8 |
| M1 | 39 | 26.2 |
| Ki-67 | ||
| <5% | 60 | 70.6 |
| ≥5% | 25 | 29.4 |
| CRP, mg/L | ||
| <5 | 67 | 69.1 |
| ≥5 | 30 | 30.9 |
| Entity | ||
| NF-pNEN | 60 | 40.3 |
| Insulinoma | 56 | 37.6 |
| Gastrinoma | 31 | 20.8 |
| Rare functioning | 2 | 1.3 |
| Tumor size | ||
| T1 | 60 | 46.2 |
| T2 | 42 | 32.3 |
| T3/4 | 28 | 21.5 |
| n = 149 . | Patients . | |
|---|---|---|
| n . | % . | |
| Sex | ||
| Male | 73 | 49.0 |
| Female | 76 | 51.0 |
| Age, y | ||
| Median | 54 (range 17–87) | |
| <65 | 119 | 79.9 |
| ≥65 | 30 | 20.1 |
| Resection | ||
| R0 | 71 | 68.9 |
| R1 | 7 | 6.8 |
| R2 | 25 | 24.3 |
| Metastases (hepatic) | ||
| M0 | 110 | 73.8 |
| M1 | 39 | 26.2 |
| Ki-67 | ||
| <5% | 60 | 70.6 |
| ≥5% | 25 | 29.4 |
| CRP, mg/L | ||
| <5 | 67 | 69.1 |
| ≥5 | 30 | 30.9 |
| Entity | ||
| NF-pNEN | 60 | 40.3 |
| Insulinoma | 56 | 37.6 |
| Gastrinoma | 31 | 20.8 |
| Rare functioning | 2 | 1.3 |
| Tumor size | ||
| T1 | 60 | 46.2 |
| T2 | 42 | 32.3 |
| T3/4 | 28 | 21.5 |
CRP is an independent prognostic marker in patients with pancreatic neuroendocrine neoplasias
Preoperative blood levels of CRP were significantly associated with OS in pNENs (P < .001) (Figure 1). Median OS for patients with elevated CRP levels was 1093 days (SE 1261, 95% confidence interval [CI] 0–3565), compared with 6859 days (SE 1252, 95% CI 4405–9313) for patients with normal CRP levels. To test for a possible bias through the different pNEN entities (better prognosis in small NF-pNENs and insulinomas), this analysis was performed again, including only the 100 patients with confirmed lymphatic and/or hepatic metastases and/or carcinomatous primary (malign pNENs). A Kaplan-Meier analysis and log rank test still showed a significant association between CRP levels and OS (P < .001) in this subgroup. This finding still persisted after repeating the analysis for the subgroup of all NF-pNENs (P = .002).
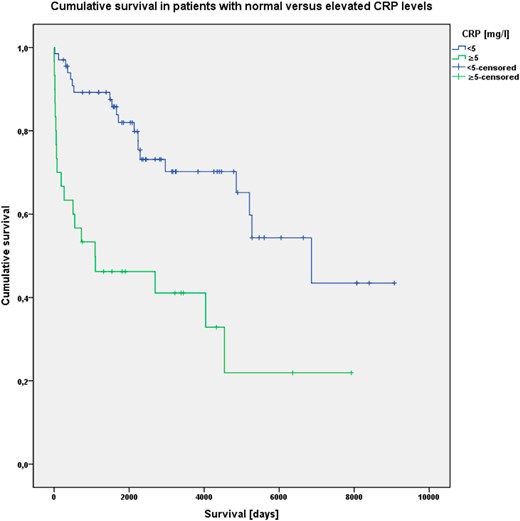
Kaplan-Meier analysis of cumulative OS in patients with normal (<5 mg/L) and elevated (≥5 mg/L) CRP levels; patients still alive at latest follow-up are censored after their respective time of survival.
Correlation of survival and other factors
Furthermore, cumulative OS was significantly associated with the presence of liver metastases (P < .001), R status (P < .001) and Ki-67 index greater than 5%. (P < .001) (Figures 2–4).
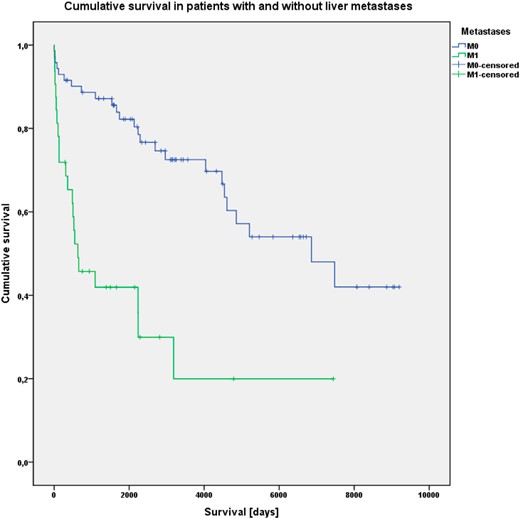
Kaplan-Meier analysis of cumulative OS in patients without (M0) and with (M1) liver metastases.
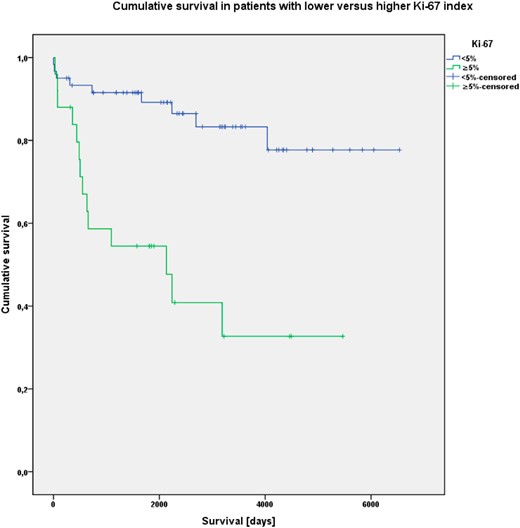
Kaplan-Meier analysis of cumulative OS in patients with Ki-67 index less than 5% and 5% or greater.
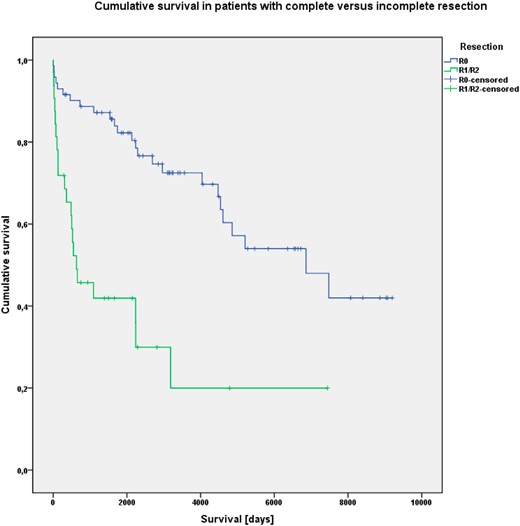
Kaplan-Meier analysis of cumulative OS in patients with (R0) or without (R1/R2) complete tumor resection.
Interaction between CRP and established prognostic factors
After cross-tabulation and Pearson χ2 tests, analyzing the distribution of patients with normal and elevated CRP in the respective subgroups of each other factor, CRP levels were not significantly related to the Ki67 index (P = .116), R status (P = .886), or M status (P = .893).
There was, however, a significant relation between elevated CRP levels and tumor size (T status; P < .001) (Table 2).
Relationship Between CRP Levels and Other Factors (Ki67 Index, R Status, M Status, T Status) (P Values via Pearson-χb Test)
| . | CRP, mg/L . | P Value . | |
|---|---|---|---|
| <5 . | ≥5 . | ||
| Ki-67 | |||
| <5% | 37 | 11 | .116 |
| ≥5% | 11 | 8 | |
| Resection | |||
| R0 | 34 | 18 | .886 |
| R1/R2 | 14 | 8 | |
| Metastases | |||
| M0 | 50 | 22 | .893 |
| M1 | 17 | 8 | |
| Tumor size | |||
| T1/2 | 51 | 16 | <.001 |
| T3/4 | 4 | 12 | |
| . | CRP, mg/L . | P Value . | |
|---|---|---|---|
| <5 . | ≥5 . | ||
| Ki-67 | |||
| <5% | 37 | 11 | .116 |
| ≥5% | 11 | 8 | |
| Resection | |||
| R0 | 34 | 18 | .886 |
| R1/R2 | 14 | 8 | |
| Metastases | |||
| M0 | 50 | 22 | .893 |
| M1 | 17 | 8 | |
| Tumor size | |||
| T1/2 | 51 | 16 | <.001 |
| T3/4 | 4 | 12 | |
Relationship Between CRP Levels and Other Factors (Ki67 Index, R Status, M Status, T Status) (P Values via Pearson-χb Test)
| . | CRP, mg/L . | P Value . | |
|---|---|---|---|
| <5 . | ≥5 . | ||
| Ki-67 | |||
| <5% | 37 | 11 | .116 |
| ≥5% | 11 | 8 | |
| Resection | |||
| R0 | 34 | 18 | .886 |
| R1/R2 | 14 | 8 | |
| Metastases | |||
| M0 | 50 | 22 | .893 |
| M1 | 17 | 8 | |
| Tumor size | |||
| T1/2 | 51 | 16 | <.001 |
| T3/4 | 4 | 12 | |
| . | CRP, mg/L . | P Value . | |
|---|---|---|---|
| <5 . | ≥5 . | ||
| Ki-67 | |||
| <5% | 37 | 11 | .116 |
| ≥5% | 11 | 8 | |
| Resection | |||
| R0 | 34 | 18 | .886 |
| R1/R2 | 14 | 8 | |
| Metastases | |||
| M0 | 50 | 22 | .893 |
| M1 | 17 | 8 | |
| Tumor size | |||
| T1/2 | 51 | 16 | <.001 |
| T3/4 | 4 | 12 | |
Uni- and multivariate analyses confirm CRP as a prognostic marker for OS
Univariate analysis of the whole cohort showed significantly elevated hazard ratios regarding a shortened overall survival for CRP of 5 mg/L or greater (hazard ratio [HR] 3.27; P < .001), incomplete resection (HR 3.99; P < .001), Ki-67 ≥ 5% (HR 5.05; P < .001), and presence of liver metastases (HR 3.17; P < .001). Age at diagnosis of 65 years or older was also associated with a significantly higher HR (HR 2.95; P < .001), whereas female gender was not (HR 0.73; P = .227) (Table 3). Subgroup analysis revealed that HRs were significantly elevated for patients with gastrinoma (HR 2.87; P = .007) and NF-pNEN (HR 3.62; P < .001).
Univariate Analysis of HRs in Patients With Elevated CRP, Incomplete Resection, Higher Ki-67, Presence of Liver Metastases, Older Age, Different pNEN Entities, and Female Sex (Cox Regression)
| . | HR . | 95% CI of HR . | P Value . | |
|---|---|---|---|---|
| Lower . | Upper . | |||
| CRP ≥5 mg/L | 3.27 | 1.74 | 6.16 | <.001 |
| R1/R2 | 3.99 | 2.16 | 7.35 | <.001 |
| Ki-67 ≥5% | 5.05 | 2.17 | 11.76 | <.001 |
| M1 | 3.17 | 1.88 | 5.35 | <.001 |
| Age ≥65 y | 2.95 | 1.72 | 5.06 | <.001 |
| Insulinoma | 1.00 | |||
| NF-PNEN | 3.62 | 1.81 | 7.24 | <.001 |
| Gastrinoma | 2.87 | 1.33 | 6.19 | .007 |
| Female sex | 0.73 | 0.44 | 1.22 | .227 |
| . | HR . | 95% CI of HR . | P Value . | |
|---|---|---|---|---|
| Lower . | Upper . | |||
| CRP ≥5 mg/L | 3.27 | 1.74 | 6.16 | <.001 |
| R1/R2 | 3.99 | 2.16 | 7.35 | <.001 |
| Ki-67 ≥5% | 5.05 | 2.17 | 11.76 | <.001 |
| M1 | 3.17 | 1.88 | 5.35 | <.001 |
| Age ≥65 y | 2.95 | 1.72 | 5.06 | <.001 |
| Insulinoma | 1.00 | |||
| NF-PNEN | 3.62 | 1.81 | 7.24 | <.001 |
| Gastrinoma | 2.87 | 1.33 | 6.19 | .007 |
| Female sex | 0.73 | 0.44 | 1.22 | .227 |
Univariate Analysis of HRs in Patients With Elevated CRP, Incomplete Resection, Higher Ki-67, Presence of Liver Metastases, Older Age, Different pNEN Entities, and Female Sex (Cox Regression)
| . | HR . | 95% CI of HR . | P Value . | |
|---|---|---|---|---|
| Lower . | Upper . | |||
| CRP ≥5 mg/L | 3.27 | 1.74 | 6.16 | <.001 |
| R1/R2 | 3.99 | 2.16 | 7.35 | <.001 |
| Ki-67 ≥5% | 5.05 | 2.17 | 11.76 | <.001 |
| M1 | 3.17 | 1.88 | 5.35 | <.001 |
| Age ≥65 y | 2.95 | 1.72 | 5.06 | <.001 |
| Insulinoma | 1.00 | |||
| NF-PNEN | 3.62 | 1.81 | 7.24 | <.001 |
| Gastrinoma | 2.87 | 1.33 | 6.19 | .007 |
| Female sex | 0.73 | 0.44 | 1.22 | .227 |
| . | HR . | 95% CI of HR . | P Value . | |
|---|---|---|---|---|
| Lower . | Upper . | |||
| CRP ≥5 mg/L | 3.27 | 1.74 | 6.16 | <.001 |
| R1/R2 | 3.99 | 2.16 | 7.35 | <.001 |
| Ki-67 ≥5% | 5.05 | 2.17 | 11.76 | <.001 |
| M1 | 3.17 | 1.88 | 5.35 | <.001 |
| Age ≥65 y | 2.95 | 1.72 | 5.06 | <.001 |
| Insulinoma | 1.00 | |||
| NF-PNEN | 3.62 | 1.81 | 7.24 | <.001 |
| Gastrinoma | 2.87 | 1.33 | 6.19 | .007 |
| Female sex | 0.73 | 0.44 | 1.22 | .227 |
Multivariate analysis of the four prognostic factors (CRP, Ki-67, R status, and M status) revealed elevated CRP levels (P = .007) and the presence of liver metastases (P = .001) as independent risk factors for reduced overall survival with a significantly higher HR in this model (Table 4).
Multivariate Analysis of HRs in a Model With All Four Evaluated Prognostic Factors, Elevated CRP, Higher Ki-67, Presence of Liver Metastases, and Incomplete Resection (Cox Regression)
| . | HR . | 95% CI of HR . | P Value . | |
|---|---|---|---|---|
| Lower . | Upper . | |||
| CRP ≥5 mg/L | 4.02 | 1.47 | 11.03 | .007 |
| Ki-67 ≥5% | 1.37 | 0.43 | 4.29 | .595 |
| M1 | 6.20 | 1.86 | 14.51 | .001 |
| R1/R2 | 1.29 | 0.23 | 7.17 | .772 |
| . | HR . | 95% CI of HR . | P Value . | |
|---|---|---|---|---|
| Lower . | Upper . | |||
| CRP ≥5 mg/L | 4.02 | 1.47 | 11.03 | .007 |
| Ki-67 ≥5% | 1.37 | 0.43 | 4.29 | .595 |
| M1 | 6.20 | 1.86 | 14.51 | .001 |
| R1/R2 | 1.29 | 0.23 | 7.17 | .772 |
Multivariate Analysis of HRs in a Model With All Four Evaluated Prognostic Factors, Elevated CRP, Higher Ki-67, Presence of Liver Metastases, and Incomplete Resection (Cox Regression)
| . | HR . | 95% CI of HR . | P Value . | |
|---|---|---|---|---|
| Lower . | Upper . | |||
| CRP ≥5 mg/L | 4.02 | 1.47 | 11.03 | .007 |
| Ki-67 ≥5% | 1.37 | 0.43 | 4.29 | .595 |
| M1 | 6.20 | 1.86 | 14.51 | .001 |
| R1/R2 | 1.29 | 0.23 | 7.17 | .772 |
| . | HR . | 95% CI of HR . | P Value . | |
|---|---|---|---|---|
| Lower . | Upper . | |||
| CRP ≥5 mg/L | 4.02 | 1.47 | 11.03 | .007 |
| Ki-67 ≥5% | 1.37 | 0.43 | 4.29 | .595 |
| M1 | 6.20 | 1.86 | 14.51 | .001 |
| R1/R2 | 1.29 | 0.23 | 7.17 | .772 |
Because of the observed significant relation between primary tumor size and CRP levels, the same multivariate analysis was performed, including T3/T4 stadium (tumor size >4 cm in diameter and/or invasion of adjacent organ tissue) as an additional (fifth) potentially prognostic factor. However, elevated CRP levels (P = .032) and presence of liver metastases (P = .001) still remained the only independent factors in this model.
Discussion
CRP is an acute-phase protein, produced in the liver (33). Acute-phase response and CRP synthesis is triggered mainly through secretion of IL-6 from macrophages and T cells (33). Any type of chronic or acute inflammatory process stemming from infection, autoimmune disease, trauma, or neoplasia can activate this IL-6 and acute-phase response, making CRP levels a sensitive but very unspecific marker (33).
In this retrospective analysis, Ki-67 index, R status, and M status were confirmed to strongly correlate with OS in pNEN, similar to previous findings (17–22). Also, for the first time, we can show a strong correlation between pretreatment CRP levels and OS in pNEN. Drastically worse OS rates (1093 vs 6895 d) after resection as well as significantly higher HRs in uni- and multivariate analysis for patients with CRP levels of 5 mg/L or greater underlined the significance of this new, possibly independent risk factor. In this study, CRP was chosen as a cheap, sensitive, and widely available laboratory parameter for inflammation. Furthermore, it was simply the most frequently available inflammatory marker in the partly very old patient data sets and had been thoroughly investigated for other malign diseases as mentioned above (24–28). Nevertheless, a possible prognostic value of other inflammatory indices (eg, Glasgow prognostic score, neutrophil to lymphocyte ratio) would be interesting to verify in pNEN as well.
In patients with progressive pNENs, the mentioned heterogeneity of tumor behavior often produces uncertainty when it comes to making therapeutic decisions (1–3). The established markers, integrated in the current World Health Organization classification and European Neuroendocrine Tumor Society guidelines have repeatedly been proven to be of great value for estimation of the general disease progression and prognosis (3, 32, 34). However, especially when it comes to predicting success of different adjuvant treatment options (transarterial chemoembolization, selective internal radiation therapy, peptide receptor radionuclide therapy, different chemotherapy regimen or targeted therapies), new prognostic factors like pretreatment CRP could possibly provide additional information for these decisions. Again, a potential connection between pretreatment CRP levels and different responses to certain therapies could only be investigated in a prospective, controlled setting.
For some cancers, a bad prognosis in cases with peritumoral and systemic inflammation has been attributed to poor susceptibility to chemotherapeutic agents through the inflammatory and mesenchymal shield around the tumor (35). For ductal pancreatic adenocarcinoma in particular, signal transducer and activator of transcription-3 activation via IL-6 transsignaling in an inflammatory environment has been described to play a major role in tumorigenesis and progression (36). With IL-6 being the major activator of the CRP response, this poses a possible link to the findings of worse outcome associated with elevated CRP levels in our patients.
However, there are some limitations to this study. Its retrospective design, the necessity to achieve acceptable patient numbers in a monocentric study for these rare tumors and the heterogeneity in patient data, eg, different pNEN entities, call for a confirmatory prospective study, ideally in a multicenter setting.
Whereas there have been these and other discoveries in understanding the interaction between inflammation and tumorigenesis in ductal adenocarcinoma of the pancreas and some other cancers, there is still much to learn. However, the possible role of inflammation in pNEN remains completely unclear. Virtually no studies exist on this matter, possibly because pNENs are generally viewed as tumors with little to no inflammatory response. Given the apparent prognostic importance of CRP levels in patients with pNENs, the main stimulator of CRP and IL-6 as well as macrophage activation in pNEN should be targets for further research.
As for the role of CRP itself in cancer patients, causality from higher CRP levels to carcinogenesis seems unlikely in the light of past Mendelian randomization studies (37). These showed no difference in cancer incidence for people with genetic polymorphisms that lead to altered CRP levels. Thus, CRP is most likely a byproduct of carcinogenic or cancer progression mechanisms (37).
In conclusion, we show for the first time that CRP is an independent prognostic marker in patients with pNENs. CRP measurement should be considered for incorporation into prospective outcome studies and clinical trials of systemic therapies for patients with pNENs.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- CI
confidence interval
- CRP
C-reactive protein
- HR
hazard ratio
- MEN
multiple endocrine neoplasia
- NF-pNEN
nonfunctioning pNEN
- OS
overall survival
- pNEN
pancreatic neuroendocrine neoplasia.



