-
PDF
- Split View
-
Views
-
Cite
Cite
Berdine R. Martin, George P. McCabe, Linda McCabe, George S. Jackson, Marie Noelle Horcajada, Elizabeth Offord-Cavin, Munro Peacock, Connie M. Weaver, Effect of Hesperidin With and Without a Calcium (Calcilock) Supplement on Bone Health in Postmenopausal Women, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 3, 1 March 2016, Pages 923–927, https://doi.org/10.1210/jc.2015-3767
Close - Share Icon Share
Abstract
Citrus fruits contain unique flavanones. One of the most abundant of the flavanones, hesperidin, has been shown to prevent bone loss in ovariectomized rats.
The objective of the study was to measure the effect of hesperidin with or without calcium supplementation on bone calcium retention in postmenopausal women.
The study was a double-blind, placebo-controlled, randomized-order crossover design of 500 g hesperidin with or without 500 mg calcium supplement in 12 healthy postmenopausal women. Bone calcium retention was determined from urinary excretion of the rare isotope, 41Ca, from bone.
Calcium plus hesperidin, but not hesperidin alone, improved bone calcium retention by 5.5% (P < .04).
Calcium supplementation (Calcilock), in combination with hesperidin, is effective at preserving bone in postmenopausal women.
Both environment and genes influence bone mass and determine the risk of developing osteoporosis (1, 2). Women are at high risk for bone fracture after menopause due to an increased loss of bone in response to decreased estrogen secretion (1). It is well established that nutrition has an important role in bone health. Research has shown that calcium, vitamin D, and several micronutrients are essential for maintaining optimum bone mineral density (BMD) and strength (3). Fruits and vegetables containing various polyphenolic compounds, especially flavonoids, have been associated with improved BMD in part because of an effect on cell signaling pathways that regulate the cells responsible for bone formation and resorption (4–6)
Citrus fruits contain hesperidin (hesperetin-7-O-rutinoside), a flavanone that is especially abundant in oranges (31–43.2 mg/100 g). Preclinical studies in rats and mice have shown positive effects of hesperidin on various bone parameters (7). Dietary supplementation hesperidin (0.5%, wt/wt) of 3-month-old rats resulted in increased bone mass, and the same diet in 6-month-old rats prevented ovariectomy-induced bone loss (8). When the diet of 20-month-old male rats was supplemented with 0.5% hesperidin for 3 months, both femoral bone density and trabecular bone volume increased (9).
Because there is a paucity of studies of the effect of hesperidin on bone in humans, we studied the effect of hesperidin and its interaction with dietary calcium on reducing bone loss in 12 healthy postmenopausal women using the isotopic tracer, 41Ca, method (10).
Subjects and Methods
Subjects
Healthy women, who were at least 4 years postmenopausal as a result of natural or surgical menopause established by questionnaire and validated by serum FSH, were enrolled in the study. Women who were on estrogen replacement therapy, treatment for osteoporosis, or taking dietary calcium supplements were not eligible for the study. All participants gave written informed consent, and the study was approved by the Purdue University and Indiana University Purdue University Institutional Review Boards.
Study design and methods
The study design was randomized-order, double-blind crossover with one baseline and three treatment phases (Figure 1). Interventions consisted of four biscuits daily that contained either: 1) 500 g hesperidin; 2) 500 g hesperidin + 500 mg calcium as Calcilock; or 3) placebo (Nestle Research and Development). Study interventions were assigned to each subject in random order. With three interventions (A, B, and C) there were six possible orders (ABC, ACB, BAC, BCA, CAB, and CBA). A file with the six interventions was randomly sorted to give the orders for the first six subjects. The process was repeated for the six additional subjects. Biscuits were provided at the beginning of each intervention phase. Subjects were instructed to consume one serving (two biscuits) in the morning and one serving in the evening for each day of the 50-day intervention phase. Each intervention phase was followed by a 50-day washout period. An initial 50-day baseline period preceded the first treatment period (Figure 1). During each 50-day period, subjects collected 24-hour urine every 10 days (five collections per phase). Subjects submitted 4-day diet records including 3 weekdays and 1 weekend day at the end of each intervention phase. Records were analyzed using the Nutrition Data System for Research (version 2011).
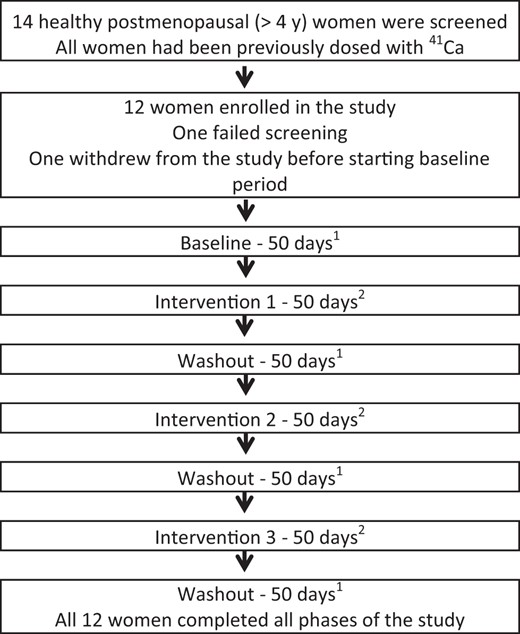
Hesperidin and bone health: study design.
1 During each 50-day period, 24-hour urine was collected every 10 days. Clinical visits occurred at the end of each 50-day period when fasting urine and blood were collected for biochemical analysis. 2 Subjects were assigned product intervention (placebo, 500 g hesperidin, or 500 g hesperidin plus 500 mg calcium supplement) in random order. Subjects consumed two biscuits in the morning and two biscuits in the evening for 50 days.
At the end of each baseline, intervention, and washout phase, there was a clinical visit. Subjects returned any uneaten products. Fasting (>8 hours) serum and urine samples were collected to evaluate mineral and safety biochemistry and biochemical markers of bone turnover including serum C-telopeptide (Crosslaps ELISA; Immunodiagnostic Systems) (coefficient of variation [CV] = 4.2%), PTH (two-site immunoassay; Nichols Institute Diagnostics) (CV = 7.1%), total 25-hydroxyvitamin D (liquid chromatography-tandem mass spectrometry; Novilytic LLC) (CV = 4.54%), and urine deoxypyridinoline (Microvue EIA; Quidel Corp) (CV = 6.9%). Serum and urine creatinine, calcium, and inorganic phosphorus were measured by routine chemistry. Anthropometric measures were taken using standard weight scales, stadiometer, and calipers. Total body, dual hip, and lumbar spine BMDs were measured at the baseline visit (iDXA, software version 4.3e; GE Lunar Corp).
All urine samples were prepared for 41Ca analysis as previously described (11). Briefly, calcium was precipitated by adjusting the pH to at least 10 using ammonium hydroxide and then adding ammonium oxalate to form calcium oxalate (CaCsO4). The supernatant was decanted, and the precipitate was collected on filter paper and dried. The 41Ca:Ca ratios were determined in the filtrate by accelerator mass spectrometry as previously described (12).
Statistical analysis
The power of the protocol was based on the ability to detect changes in 41Ca excretion in urine. With at least 10 subjects, there was 80% power to detect a 10% improvement in net calcium retention. Daily urinary 41Ca during an intervention was compared with the change predicted from baseline and nonintervention periods. Daily urinary 41Ca:Ca ratios were transformed using the natural logarithm. For each subject, a linear regression using nonintervention data was used to predict ratios for intervention periods. For each subject, deviations of the observed intervention value from the corresponding predicted value were averaged to determine the intervention effects. The values were averaged for all 12 subjects, and 95% confidence intervals (CIs) were calculated. The estimates and CIs were expressed as relative change in bone calcium retention (percentage) as described previously (13). Statistical significance (P < .05) indicates significant deviation from the predicted change. The bootstrap method was used to verify the accuracy of the asymptotic approximations used. Biomarkers were secondary outcomes and were analyzed using ANOVA methods based on the crossover design.
Results
Fourteen women were screened for the study. Twelve women (mean age, 66.3 years; postmenopausal, 16.8 years; and body mass index, 25.7 kg/m2) were enrolled in the study (Table 1). All 12 women completed all baseline, three completed intervention, and three completed washout phases. Mean compliance with the consumption of the biscuits was 92.5%.
| Characteristic . | Mean . | SD . | Range . |
|---|---|---|---|
| Age, y | 66.3 | 8.0 | 52.9–78.7 |
| Years post menopause | 16.8 | 8.1 | 6–32 |
| Height, cm | 164.9 | 5.5 | 157–172 |
| Weight, kg | 69.7 | 19.0 | 51–121 |
| Body mass index, kg/m2 | 25.7 | 7.7 | 17.6–47.2 |
| Total body BMD, g/cm2 | 1.02 | 0.12 | 0.82–1.25 |
| Total body BMC, g | 2095 | 301 | 1752–2760 |
| Body fat, % | 39.4 | 8.4 | 26.2–55.5 |
| Characteristic . | Mean . | SD . | Range . |
|---|---|---|---|
| Age, y | 66.3 | 8.0 | 52.9–78.7 |
| Years post menopause | 16.8 | 8.1 | 6–32 |
| Height, cm | 164.9 | 5.5 | 157–172 |
| Weight, kg | 69.7 | 19.0 | 51–121 |
| Body mass index, kg/m2 | 25.7 | 7.7 | 17.6–47.2 |
| Total body BMD, g/cm2 | 1.02 | 0.12 | 0.82–1.25 |
| Total body BMC, g | 2095 | 301 | 1752–2760 |
| Body fat, % | 39.4 | 8.4 | 26.2–55.5 |
| Characteristic . | Mean . | SD . | Range . |
|---|---|---|---|
| Age, y | 66.3 | 8.0 | 52.9–78.7 |
| Years post menopause | 16.8 | 8.1 | 6–32 |
| Height, cm | 164.9 | 5.5 | 157–172 |
| Weight, kg | 69.7 | 19.0 | 51–121 |
| Body mass index, kg/m2 | 25.7 | 7.7 | 17.6–47.2 |
| Total body BMD, g/cm2 | 1.02 | 0.12 | 0.82–1.25 |
| Total body BMC, g | 2095 | 301 | 1752–2760 |
| Body fat, % | 39.4 | 8.4 | 26.2–55.5 |
| Characteristic . | Mean . | SD . | Range . |
|---|---|---|---|
| Age, y | 66.3 | 8.0 | 52.9–78.7 |
| Years post menopause | 16.8 | 8.1 | 6–32 |
| Height, cm | 164.9 | 5.5 | 157–172 |
| Weight, kg | 69.7 | 19.0 | 51–121 |
| Body mass index, kg/m2 | 25.7 | 7.7 | 17.6–47.2 |
| Total body BMD, g/cm2 | 1.02 | 0.12 | 0.82–1.25 |
| Total body BMC, g | 2095 | 301 | 1752–2760 |
| Body fat, % | 39.4 | 8.4 | 26.2–55.5 |
A 5.5% improvement in net calcium bone retention was found for hesperidin with the calcium supplement (P = .04; Figure 2). For hesperidin alone and for placebo, the changes were not statistically significant (P = .34, P = .39). The 95% CI for the combination treatment did not contain zero and thus can be considered an effective treatment for increasing net bone calcium retention. No significant intervention effects were found for the biomarkers, or mineral biochemistry (Table 2) other than PTH was less with the combination treatment than with hesperidin alone or placebo.
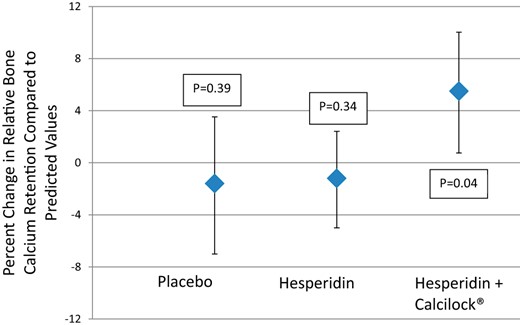
Effect of hesperidin with and without calcium on change in relative bone calcium retention in postmenopausal women (mean ± 95% CI).
Biochemical Markers From Fasting Serum and Urine Samples in Postmenopausal Women Fed Hesperidin With or Without Calcium Supplementation Compared to a Placebo
| Marker . | Placebo . | Hesperidin . | Hesperidin + Calcilock . |
|---|---|---|---|
| C-telopeptide, ng/mL | 0.50 ± 0.21 | 1.3 ± 0.8 | 0.7 ± 0.4 |
| Deoxypyridinoline, nmol/mmol creatinine | 7.1 ± 1.1 | 15.6 ± 2.6 | 8.4 ± 4.7 |
| PTH, pg/mL | 33.95 ± 6.1 | 21.5 ± 1.7b | 24.6 ± 2.4a |
| Alkaline phosphatase, U/L | 70.0 ± 25.8 | 92.3 ± 25.3 | 70.8 ± 25.4 |
| Total 25-hydroxyvitamin D, pg/mL | 25.9 ± 2.8 | 25.5 ± 8.4 | 36.9 ± 7.1 |
| Serum Ca, mg/dL | 9.8 ± 0.3 | 10.1 ± 0.2 | 9.7 ± 0.5 |
| Serum P, mg/dL | 3.8 ± 0.5 | 4.3 ± 0.5 | 4.0 ± 0.4 |
| Serum creatinine, mg/dL | 0.9 ± 0.06 | 0.9 ± 0.2 | 1.0 ± 0.03 |
| Marker . | Placebo . | Hesperidin . | Hesperidin + Calcilock . |
|---|---|---|---|
| C-telopeptide, ng/mL | 0.50 ± 0.21 | 1.3 ± 0.8 | 0.7 ± 0.4 |
| Deoxypyridinoline, nmol/mmol creatinine | 7.1 ± 1.1 | 15.6 ± 2.6 | 8.4 ± 4.7 |
| PTH, pg/mL | 33.95 ± 6.1 | 21.5 ± 1.7b | 24.6 ± 2.4a |
| Alkaline phosphatase, U/L | 70.0 ± 25.8 | 92.3 ± 25.3 | 70.8 ± 25.4 |
| Total 25-hydroxyvitamin D, pg/mL | 25.9 ± 2.8 | 25.5 ± 8.4 | 36.9 ± 7.1 |
| Serum Ca, mg/dL | 9.8 ± 0.3 | 10.1 ± 0.2 | 9.7 ± 0.5 |
| Serum P, mg/dL | 3.8 ± 0.5 | 4.3 ± 0.5 | 4.0 ± 0.4 |
| Serum creatinine, mg/dL | 0.9 ± 0.06 | 0.9 ± 0.2 | 1.0 ± 0.03 |
Data are expressed as mean ± SD.
< Placebo, P < .05.
< Placebo, P < .02.
Biochemical Markers From Fasting Serum and Urine Samples in Postmenopausal Women Fed Hesperidin With or Without Calcium Supplementation Compared to a Placebo
| Marker . | Placebo . | Hesperidin . | Hesperidin + Calcilock . |
|---|---|---|---|
| C-telopeptide, ng/mL | 0.50 ± 0.21 | 1.3 ± 0.8 | 0.7 ± 0.4 |
| Deoxypyridinoline, nmol/mmol creatinine | 7.1 ± 1.1 | 15.6 ± 2.6 | 8.4 ± 4.7 |
| PTH, pg/mL | 33.95 ± 6.1 | 21.5 ± 1.7b | 24.6 ± 2.4a |
| Alkaline phosphatase, U/L | 70.0 ± 25.8 | 92.3 ± 25.3 | 70.8 ± 25.4 |
| Total 25-hydroxyvitamin D, pg/mL | 25.9 ± 2.8 | 25.5 ± 8.4 | 36.9 ± 7.1 |
| Serum Ca, mg/dL | 9.8 ± 0.3 | 10.1 ± 0.2 | 9.7 ± 0.5 |
| Serum P, mg/dL | 3.8 ± 0.5 | 4.3 ± 0.5 | 4.0 ± 0.4 |
| Serum creatinine, mg/dL | 0.9 ± 0.06 | 0.9 ± 0.2 | 1.0 ± 0.03 |
| Marker . | Placebo . | Hesperidin . | Hesperidin + Calcilock . |
|---|---|---|---|
| C-telopeptide, ng/mL | 0.50 ± 0.21 | 1.3 ± 0.8 | 0.7 ± 0.4 |
| Deoxypyridinoline, nmol/mmol creatinine | 7.1 ± 1.1 | 15.6 ± 2.6 | 8.4 ± 4.7 |
| PTH, pg/mL | 33.95 ± 6.1 | 21.5 ± 1.7b | 24.6 ± 2.4a |
| Alkaline phosphatase, U/L | 70.0 ± 25.8 | 92.3 ± 25.3 | 70.8 ± 25.4 |
| Total 25-hydroxyvitamin D, pg/mL | 25.9 ± 2.8 | 25.5 ± 8.4 | 36.9 ± 7.1 |
| Serum Ca, mg/dL | 9.8 ± 0.3 | 10.1 ± 0.2 | 9.7 ± 0.5 |
| Serum P, mg/dL | 3.8 ± 0.5 | 4.3 ± 0.5 | 4.0 ± 0.4 |
| Serum creatinine, mg/dL | 0.9 ± 0.06 | 0.9 ± 0.2 | 1.0 ± 0.03 |
Data are expressed as mean ± SD.
< Placebo, P < .05.
< Placebo, P < .02.
There was no indication that dietary intake varied from treatment to treatment when macro- and micronutrients from the diet records plus supplements were analyzed other than calcium that was greater in the hesperidin plus Calcilock phase (Table 3). Although there was wide variation in the age of the participants as well as menopausal years and body mass index (Table 1), there was no correlation with these variables and net bone calcium retention as measured by the 41Ca method (P < .05).
Dietary and Supplemental Intake in Postmenopausal Women Fed Hesperidin With or Without Calcium Supplementation Compared to a Placebo
| Nutrient . | Placebo . | Hesperidin . | Hesperidin + Calcilockb . |
|---|---|---|---|
| Energy, kcal/d | 1993 ± 565 | 1882 ± 330 | 1870 ± 509 |
| Protein, g/d | 79 ± 23 | 76 ± 16 | 71 ± 15 |
| Fat, g/d | 79 ± 21 | 74 ± 18 | 78 ± 31 |
| Carbohydrate, g/d | 244 ± 83 | 231 ± 39 | 227 ± 67 |
| Dietary fiber, g/d | 22 ± 7 | 21 ± 7 | 20 ± 6 |
| Sodium, mg/d | 3235 ± 1132 | 2972 ± 724 | 2960 ± 756 |
| Calcium, mg/d | 1062 ± 353 | 1048 ± 228 | 1451 ± 359a |
| Iron, mg/d | 15 ± 6 | 14 ± 4 | 13 ± 4 |
| Phosphorus, mg/d | 1348 ± 341 | 1335 ± 310 | 1522 ± 356 |
| Magnesium, mg/d | 312 ± 83 | 313 ± 95 | 393 ± 82 |
| Zinc, mg/d | 12 ± 5 | 10 ± 3 | 11 ± 4 |
| Vitamin C, mg/d | 110 ± 61 | 96 ± 49 | 84 ± 38 |
| Vitamin K-1, μg/d | 161 ± 99 | 168 ± 108 | 135 ± 78 |
| Vitamin D, μg/d | 6 ± 3 | 5 ± 2 | 8 ± 3 |
| Hesperidin, mg/d | 0 | 508 | 508 |
| Citrus, servings/d | 0.4 ± 0.4 | 0.3 ± 0.3 | 0.3 ± 0.4 |
| Nutrient . | Placebo . | Hesperidin . | Hesperidin + Calcilockb . |
|---|---|---|---|
| Energy, kcal/d | 1993 ± 565 | 1882 ± 330 | 1870 ± 509 |
| Protein, g/d | 79 ± 23 | 76 ± 16 | 71 ± 15 |
| Fat, g/d | 79 ± 21 | 74 ± 18 | 78 ± 31 |
| Carbohydrate, g/d | 244 ± 83 | 231 ± 39 | 227 ± 67 |
| Dietary fiber, g/d | 22 ± 7 | 21 ± 7 | 20 ± 6 |
| Sodium, mg/d | 3235 ± 1132 | 2972 ± 724 | 2960 ± 756 |
| Calcium, mg/d | 1062 ± 353 | 1048 ± 228 | 1451 ± 359a |
| Iron, mg/d | 15 ± 6 | 14 ± 4 | 13 ± 4 |
| Phosphorus, mg/d | 1348 ± 341 | 1335 ± 310 | 1522 ± 356 |
| Magnesium, mg/d | 312 ± 83 | 313 ± 95 | 393 ± 82 |
| Zinc, mg/d | 12 ± 5 | 10 ± 3 | 11 ± 4 |
| Vitamin C, mg/d | 110 ± 61 | 96 ± 49 | 84 ± 38 |
| Vitamin K-1, μg/d | 161 ± 99 | 168 ± 108 | 135 ± 78 |
| Vitamin D, μg/d | 6 ± 3 | 5 ± 2 | 8 ± 3 |
| Hesperidin, mg/d | 0 | 508 | 508 |
| Citrus, servings/d | 0.4 ± 0.4 | 0.3 ± 0.3 | 0.3 ± 0.4 |
Data are expressed as mean ± SD.
P = .005, hesperidin + Calcilock > hesperidin or placebo.
Calcilock contributes 250 mg calcium, 125 mg phosphorus, 45 mg magnesium, 1.25 mg zinc, 9 mg vitamin C, 12 μg vitamin K-1, and 0.75 μg vitamin D. Each subject consumed two servings per day.
Dietary and Supplemental Intake in Postmenopausal Women Fed Hesperidin With or Without Calcium Supplementation Compared to a Placebo
| Nutrient . | Placebo . | Hesperidin . | Hesperidin + Calcilockb . |
|---|---|---|---|
| Energy, kcal/d | 1993 ± 565 | 1882 ± 330 | 1870 ± 509 |
| Protein, g/d | 79 ± 23 | 76 ± 16 | 71 ± 15 |
| Fat, g/d | 79 ± 21 | 74 ± 18 | 78 ± 31 |
| Carbohydrate, g/d | 244 ± 83 | 231 ± 39 | 227 ± 67 |
| Dietary fiber, g/d | 22 ± 7 | 21 ± 7 | 20 ± 6 |
| Sodium, mg/d | 3235 ± 1132 | 2972 ± 724 | 2960 ± 756 |
| Calcium, mg/d | 1062 ± 353 | 1048 ± 228 | 1451 ± 359a |
| Iron, mg/d | 15 ± 6 | 14 ± 4 | 13 ± 4 |
| Phosphorus, mg/d | 1348 ± 341 | 1335 ± 310 | 1522 ± 356 |
| Magnesium, mg/d | 312 ± 83 | 313 ± 95 | 393 ± 82 |
| Zinc, mg/d | 12 ± 5 | 10 ± 3 | 11 ± 4 |
| Vitamin C, mg/d | 110 ± 61 | 96 ± 49 | 84 ± 38 |
| Vitamin K-1, μg/d | 161 ± 99 | 168 ± 108 | 135 ± 78 |
| Vitamin D, μg/d | 6 ± 3 | 5 ± 2 | 8 ± 3 |
| Hesperidin, mg/d | 0 | 508 | 508 |
| Citrus, servings/d | 0.4 ± 0.4 | 0.3 ± 0.3 | 0.3 ± 0.4 |
| Nutrient . | Placebo . | Hesperidin . | Hesperidin + Calcilockb . |
|---|---|---|---|
| Energy, kcal/d | 1993 ± 565 | 1882 ± 330 | 1870 ± 509 |
| Protein, g/d | 79 ± 23 | 76 ± 16 | 71 ± 15 |
| Fat, g/d | 79 ± 21 | 74 ± 18 | 78 ± 31 |
| Carbohydrate, g/d | 244 ± 83 | 231 ± 39 | 227 ± 67 |
| Dietary fiber, g/d | 22 ± 7 | 21 ± 7 | 20 ± 6 |
| Sodium, mg/d | 3235 ± 1132 | 2972 ± 724 | 2960 ± 756 |
| Calcium, mg/d | 1062 ± 353 | 1048 ± 228 | 1451 ± 359a |
| Iron, mg/d | 15 ± 6 | 14 ± 4 | 13 ± 4 |
| Phosphorus, mg/d | 1348 ± 341 | 1335 ± 310 | 1522 ± 356 |
| Magnesium, mg/d | 312 ± 83 | 313 ± 95 | 393 ± 82 |
| Zinc, mg/d | 12 ± 5 | 10 ± 3 | 11 ± 4 |
| Vitamin C, mg/d | 110 ± 61 | 96 ± 49 | 84 ± 38 |
| Vitamin K-1, μg/d | 161 ± 99 | 168 ± 108 | 135 ± 78 |
| Vitamin D, μg/d | 6 ± 3 | 5 ± 2 | 8 ± 3 |
| Hesperidin, mg/d | 0 | 508 | 508 |
| Citrus, servings/d | 0.4 ± 0.4 | 0.3 ± 0.3 | 0.3 ± 0.4 |
Data are expressed as mean ± SD.
P = .005, hesperidin + Calcilock > hesperidin or placebo.
Calcilock contributes 250 mg calcium, 125 mg phosphorus, 45 mg magnesium, 1.25 mg zinc, 9 mg vitamin C, 12 μg vitamin K-1, and 0.75 μg vitamin D. Each subject consumed two servings per day.
Discussion
In this randomized-order, placebo-controlled, double-blind clinical trial in postmenopausal women, hesperidin plus a nutritional supplement (Calcilock) formulated into biscuits improved net bone calcium retention by 5.5%, but hesperidin alone had no benefit. This study employed the novel and sensitive approach of measuring urinary appearance of the rare isotope, 41Ca, from bone to determine the effect of interventions on net bone calcium retention (or loss) relative to predicted values from nonintervention periods and compared with a placebo period. All three intervention periods and recovery periods were tested in each of the 12 participants in 350 days. The more traditional approach of a parallel arm study measuring changes in BMD would have involved an intervention period of 2 or more years and 60 or more participants per arm to have similar power to our study. Thus, both methods give an estimate of changes in bone calcium due to intervention, but the power of the crossover design and the sensitivity of 41Ca analysis greatly reduce the time and number of participants required. However, bone densitometry could give site-specific changes in bone, whereas calcium tracers reflect whole body metabolism. The longer intervention periods in bone density trials span several bone remodeling cycles, which may be more reflective of the long-term impact of an intervention. The 50-day intervention periods that we used in this study span a little more than one remodeling cycle and may therefore reflect a remodeling transient, which could mean a one-time benefit that could disappear upon cessation (14). Nevertheless, this is a useful screening approach to identify effective interventions to pursue in the longer, more expensive trials monitoring BMD.
The benefit of hesperidin plus Calcilock intervention on bone calcium retention of 5.5% theoretically is equivalent to a benefit in net calcium balance or total bone mineral content (BMC) because calcium is a constant fraction of BMC. This provides a practical and substantial countermeasure to women stable to menopause who on average lose 0.5 to 2.0% BMC annually, depending on the bone site (15). The lack of benefit of the hesperidin alone intervention suggests that the benefit is due to calcium rather than the hesperidin, although this was not directly tested. However, a similar study in Caucasian women using the same sensitive 41Ca technology to measure the effect of an added 750 mg Ca per day showed a positive effect on bone, which was confirmed by using a linear three-compartment model. An increase of 56% (P < .001) was seen in the Ca transfer rate from the fast exchanging Ca pool to the slow exchanging bone pool. As would be expected, the final model predicted a significant decrease in urinary 41Ca excretion at the end of the treatment period, which corresponds to improved bone calcium retention (16). Consistent with our observed lack of effect of 500 mg hesperidin, an unpublished 2-year randomized, controlled trial described in a conference proceeding (17) found no benefit to BMD at any site including total hip, femoral neck, lumbar spine, or whole body. A benefit of calcium of this magnitude in women with average intake of approximately 1 g/d may be surprising given that the plateau intake for calcium retention in this age group is approximately 1.2 g/d (18). Yet calcium supplementation has benefits to calcium retention and bone parameters in similar populations (19, 20).
The dose and form of hesperidin as well as the inflammatory status of the subjects may influence effects on bone. The same dose of hesperidin as that used in this study was effective in reducing two inflammatory markers, C-reactive protein and serum amyloid A protein, in patients with metabolic syndrome (21). Hesperitin-7-glucoside, a metabolite of hesperidin, may have been more effective than hesperidin because it is more bioavailable (22, 23). The current study provides little mechanistic insight because the purported effects of flavonoids on osteoblast and osteoclast differentiation were not studied. For example, Kim et al (24) showed hesperidin suppressed NF-κB activation. We powered our study for the main outcome, which is a more sensitive measure than for biochemical markers of bone turnover or regulators of calcium metabolism. The bone resorption marker, deoxypyridinoline, was suppressed by hesperidin in a male senescent rat model (9). Serum PTH (P < .05) was much less in the active intervention, a classic response to increased dietary calcium (19).
In summary, in postmenopausal women stable to menopause, Calcilock, a supplement including 500 mg calcium with hesperidin, was effective in improving bone calcium retention, whereas hesperidin alone was not effective. The benefit may be exclusively due to calcium, but a limitation of the study was the lack of a calcium-alone intervention, so an interaction cannot be ruled out. The novel use of a rare calcium isotope, 41Ca, allowed multiple interventions to be directly compared in a crossover design in the same subjects with substantially reduced intervention duration and sample size than a traditional trial of change in BMD.
Acknowledgments
The authors thank D. Maish for phlebotomy and clinical services.
Author Contributions: C.M.W., M.N.H., and E.O.-C. developed hypotheses; B.R.M., C.M.W., M.N.H., and G.P.M. designed the research; B.R.M., C.M.W., and M.P. conducted the research; G.S.J. performed the accelerator mass spectrometer measures; G.P.M., L.M., and B.R.M. analyzed the data; B.R.M., C.M.W., M.P., and G.P.M. wrote the paper; B.R.M. and C.M.W. had primary responsibility for the final content. All authors read and approved the final manuscript.
The study was registered in Clinicaltrials.gov (NCT01881204).
Disclosure Summary: This study was supported by Nestec, Ltd (Vevey, Switzerland). M.N.H and E.O.-C. are research scientists at the Nestle Research Center. C.M.W. is a member of the Scientific Advisory Board for Pharmavite LLC. The other authors have nothing to disclose.



