-
PDF
- Split View
-
Views
-
Cite
Cite
Tsutomu Abe, Mitsuhide Naruse, William F. Young, Nobuya Kobashi, Yoshihiro Doi, Akihiro Izawa, Kei Akama, Yuki Okumura, Miho Ikenaga, Hiroyuki Kimura, Hideo Saji, Kuniaki Mukai, Hiroki Matsumoto, A Novel CYP11B2-Specific Imaging Agent for Detection of Unilateral Subtypes of Primary Aldosteronism, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 3, 1 March 2016, Pages 1008–1015, https://doi.org/10.1210/jc.2015-3431
Close - Share Icon Share
Abstract
Although adrenal vein sampling is the standard method to distinguish unilateral from bilateral forms of primary aldosteronism, it is an invasive and technically difficult procedure. 11C-metomidate (MTO)-positron emission tomography was reported as a potential replacement for adrenal vein sampling. However, MTO has low selectivity for CYP11B2 over CYP11B1.
This study aimed to determine the selectivity of 18F-CDP2230, a new imaging agent, for CYP11B2 over CYP11B1 and determine whether the biodistribution profile of 18F-CDP2230 is favorable for imaging CYP11B2.
The IC50 of CDP2230 for the enzymatic activities of CYP11B2 and CYP11B1 was determined using cells with stable expression of either enzyme. In vitro autoradiography of human adrenal sections with aldosterone-producing adenomas was performed to confirm the specific binding ability of 18F-CDP2230 to CYP11B2-expressing regions. Furthermore, positron emission tomography and magnetic resonance imaging were performed to evaluate the biodistribution of 18F-CDP2230 in rats.
Although CDP2230 showed a significantly lower affinity for CYP11B2 and CYP11B1 than did MTO analogues, its selectivity for CYP11B2 over CYP11B1 was higher than that of MTO analogues. In vitro autoradiography revealed that the binding of 18F-CDP2230 to CYP11B2-expressing regions in the adrenal gland was more specific than that of 123I-IMTO. Moreover, the biodistribution study showed that 18F-CDP2230 accumulated in adrenal glands with low background uptake.
Our study showed a high selectivity of 18F-CDP2230 for CYP11B2 over CYP11B1 with a favorable biodistribution for imaging CYP11B2. 18F-CDP2230 is a promising imaging agent for detecting unilateral subtypes of primary aldosteronism.
Primary aldosteronism (PA) is the most common and surgically curable form of endocrine hypertension, accounting for 5%–10% of all patients with hypertension (1–3). Approximately 60% of patients with PA exhibit bilateral aldosterone hypersecretion, and mineralocorticoid receptor antagonists are the treatment of choice for such patients (3). The remaining 40% of patients with PA have unilateral aldosterone hypersecretion (eg, aldosterone-producing adenoma [APA]). In these patients, hypertension and hyperaldosteronism can be cured by unilateral laparoscopic adrenalectomy (3). Therefore, an accurate method for distinguishing unilateral from bilateral adrenal disease is critical for patients who wish to pursue the surgical option. Currently, adrenal vein sampling is the criterion standard test to make this distinction (3), but it is an invasive and technically demanding procedure. The success rate of bilateral adrenal vein catheterization varies from 8% to 95%, depending on technical expertise and dedication (4, 5). Alternative tests to lateralize the source of aldosterone hypersecretion that are widely available and not operator dependent are needed. 11C-metomidate (MTO) (Figure 1), a positron emission tomography (PET) imaging agent, has been reported to distinguish unilateral APA from bilateral idiopathic hyperplasia with high sensitivity and specificity (6). The iodine-123-labeled analog, 123I-iodometomidate (IMTO) (Figure 1), has also been reported to detect APAs by single photon emission tomography-computed tomography (CT) imaging (7). However, 11C-MTO and 123I-IMTO show low selectivity for CYP11B2 over CYP11B1 expressed in the zona fasciculata. Therefore, dexamethasone pretreatment is required to decrease its accumulation in normal adrenal tissues (6, 8). In addition, PET imaging agents labeled with carbon-11 can only be used in medical centers near a cyclotron because of the very short half-life of carbon-11. Therefore, a fluorine-18-labeled imaging agent with a higher selectivity for CYP11B2 over CYP11B1 would be optimal for detecting unilateral subtypes of PA.
In this study, we synthesized 18F-CDP2230 (Figure 1), a novel imaging agent for PA, and evaluated its selectivity for CYP11B2 by using cells with stable overexpression of either enzyme with nonradiolabeled CDP2230, MTO, and IMTO. Furthermore, we conducted in vitro autoradiography (ARG) using human adrenal sections with APAs to assess the binding of 18F-CDP2230 and 123I-IMTO to APAs. We determined whether 18F-CDP2230 has favorable biodistribution for imaging of CYP11B2 by conducting a biodistribution study and performing PET/magnetic resonance imaging (MRI) studies in rats.
Materials and Methods
Radiosynthesis of 123I-IMTO and 18F-CDP2230
The radiosynthesis of 123I-IMTO and 18F-CDP2230 is described in Supplemental Methods. The chemical reaction formulae used for the radiosynthesis of these compounds from their precursors are shown in Supplemental Figure 1.
Human adrenal tissues
The experiments using human tissues were approved by the ethical committees of Kyoto Medical Center and Nihon Medi-Physics Co. Ltd. Adrenalectomy was performed as the treatment for PA, Cushing syndrome (CS), and pheochromocytoma (Pheo) at Kyoto Medical Center. All patients provided written informed consent for the use of their specimens in this study. The experiments were performed in the Research Center of Nihon Medi-Physics Co. Ltd.
Determination of IC50 values for CYP11B2 and CYP11B1 activities
V79 lung fibroblast cells derived from Chinese hamsters, which reportedly lack endogenous cytochrome P450 activity (9), were purchased from the European Collection of Authenticated Cell Cultures through DS Pharma Biomedical. Expression of human CYP11B1 or CYP11B2 in V79 cells was induced by the Mammalian replication initiation region/Matrix attachment region method, as reported previously (10). Plasmid pcDNA3.31-CYP11B1 or pcDNA3.31-CYP11B2 was used for transfection with geneticin and blasticidin-S used for selection of stably transfected cells.
The V79 cells were cultured in DMEM containing 4.5-g/L D-glucose, L-glutamine, 110-mg/L sodium pyruvate, 10% fetal bovine serum, and penicillin/streptomycin/neomycin. Cells with stable expression of CYP11B1 (V79-B1) and CYP11B2 (V79-B2) were seeded in 96-well microplates and cultured overnight. For evaluation of CYP11B1 inhibitory activity, corticosterone and nonradiolabeled MTO, IMTO, or CDP2230 were added to the V79-B1 culture medium and incubated for 1 hour. For evaluation of CYP11B2 inhibitory activity, 11-deoxycortisol and MTO, IMTO, or CDP2230 were added to the V79-B2 culture medium and incubated for 4 hours. The final concentrations of corticosterone and 11-deoxycortisol were 100nM. DMEM containing 0.1% (vol/vol) dimethylsulfoxide was used for dissolving the substrates and tested compounds. The concentrations of MTO, IMTO, and CDP2230 ranged from 10−3nM to 104nM. After incubation, the concentrations of cortisol, the CYP11B1 metabolite, and aldosterone, the CYP11B2 metabolite, in the culture medium were determined using ELISA kits (Cayman Chemical). The inhibitory effects of MTO, IMTO, and CDP2230 on the production of cortisol and aldosterone were expressed as a percentage of the concentration in vehicle-treated controls. Sigmoid curves and the IC50 values for MTO, IMTO, and CDP2230 were calculated using GraphPad Prism version 5.0 (GraphPad Software). IC50 values are expressed as the mean ± SD. The selectivity for CYP11B2 over CYP11B1 was evaluated using a selectivity factor calculated as the IC50 value for cortisol production divided by the IC50 value for aldosterone production.
Immunohistochemistry and in vitro ARG with human tissue
Immunohistochemical staining of CYP11B1 and CYP11B2 was performed as described previously (11). In brief, human adrenal tissues were embedded with Tissue-Tek O.C.T compound (Sakura Finetechnical Co. Ltd.) and sectioned at 7 μm with a cryostat (Leica Biosystems). Immunohistochemical staining of CYP11B1 (brown) and CYP11B2 (blue) was performed with anti-CYP11B2 and anti-CYP11B1 antibodies (11) using Chem Mate ENVISION kits (Dako).
For in vitro ARG, frozen sections were immersed in PBS containing 1% wt/vol bovine serum albumin and 5–33 kBq/mL of 123I-IMTO or 18F-CDP2230 at room temperature for 10 minutes, followed by washing. For the displacement study with CDP2230, 5μM nonradiolabeled CDP2230 was added to PBS. The molar concentration of CDP2230 was approximately 10 million times that of the radiolabeled tracer. After drying, the sections were exposed to a BAS-SR2040 imaging plate (Fujifilm) for 16–20 hours. ARG images were obtained using the Typhoon FLA 7000 IP System (GE Healthcare). For quantitative comparison of 123I-IMTO and 18F-CDP2230 images, radioactive binding in the region of interest was corrected by integration calculations using the half-life of iodine-123 and fluorine-18, concentration of radioactivity, and duration of contact with the BAS-SR2040 imaging plate.
Biodistribution studies for 123I-IMTO and 18F-CDP2230 in rats
Wistar rats (male, 8 wk) were purchased from Charles River Laboratories Japan, Inc. All animal studies, including PET and PET/MRI with 18F-CDP2230, were approved by the Laboratory for Animal Care and Use Committee at Nihon Medi-Physics research center and were conducted in accordance with institutional guidelines. The rats were injected with approximately 3.7 MBq of 123I-IMTO or 18F-CDP2230 via the tail vein under anesthesia with 1.0%–2.0% isoflurane (Mylan, Inc) in air and were killed by exsanguination under anesthesia 10 minutes, 30 minutes, and 1 hour later. Blood was collected, and the whole brain, heart, lungs, stomach, liver, spleen, small intestine, large intestine, kidneys, bladder including urine, muscle, adrenal glands, and testes were excised and weighed along with the carcass. In addition, the thyroid glands and thigh bones were excised to evaluate the accumulation of iodine-123 or fluorine-18, respectively. Radioactivity was measured using a single-channel γ-counter (Ohyo Koken Kogyo) and represented as a percentage of the injected dose per gram of tissue.
PET and PET/MRI with 18F-CDP2230
Wistar rats (female, 8 wk; Charles River Laboratories Japan, Inc) were injected with 18.5 MBq of 18F-CDP2230 via the tail vein for PET using a small-animal PET scanner (eXplore Vista DR; SEDECAL). The animals were anesthetized with 1.0%–2.0% isoflurane (Mylan, Inc) in 70% nitrous oxide and 30% oxygen and placed with their adrenal glands positioned at the center of the axial field of view. Dynamic PET with frame duration of 10 minutes was performed over 1 hour after injection, with the body temperature of the rats maintained at approximately 37°C using a heater system. All PET images were reconstructed using a first 3-dimensional-ordered subset expectation maximization graphical user interface (GE Healthcare). Two iterations and 50 subsets were used. Standardized uptake values (SUVs) were calculated as tissue radioactivity (kBq/mL) divided by the administered radioactivity per gram of body weight for PET images obtained over 6 10-minute phases. The slice thickness and transverse resolution were 0.775 and 0.3875 mm, respectively.
Immediately after PET, the animals underwent MRI using a 2.0T MRI system (BioSpec 2.0/31; Bruker-BioSpin MRI GmbH) equipped with shielded gradients and a standard volume transmission/reception coil (Bruker-BioSpin MRI GmbH). The conditions for anesthesia, positioning and heating were the same as those used for PET. Sagittal proton density-weighted images (repetition time, 4000 ms; effective echo time, 24.5 ms; rare factor, 4; matrix size, 256 × 96; field of view, 180 × 45 mm; slice thickness, 1 mm) were acquired to obtain morphological information.
In vitro stability of 18F-CDP2230 in human and rat plasma
18F-CDP2230 was added to human or rat plasma (Kohjin Bio Co. Ltd.) to achieve a concentration of 1.6 MBq/mL. After incubation at 37°C for 30 or 60 minutes, 1 mL of plasma was diluted to 2 mL in methanol and centrifuged (1800g, 10 minutes, 4°C). Then, 10 μL of each supernatant were spotted on a thin-layer chromatography plate (silica gel 60 F254; Merck). The mobile phase comprised ethyl acetate, methanol, and diethylamine (10:2:1, vol/vol). Densitometric scanning was performed using Rita Star (raytest).
Statistical analysis
All data are presented as the mean ± SD. Statistical comparisons of IC50 values for CYP11B1 and CYP11B2 among groups and analysis of the data from the biodistribution study were performed using one-way ANOVA followed by post hoc Tukey tests. Differences with a probability of less than 1% were considered statistically significant (P < .01).
Results
IC50 values for CYP11B2 and CYP11B1 activities in V79 cells with stable CYP11B2 or CYP11B1 gene transfection
Inhibition curves for production of aldosterone and cortisol by MTO, IMTO, and CDP2230 are provided in Supplemental Figure 2. MTO and IMTO showed almost the same inhibitory activities for CYP11B1 and CYP11B2, which were significantly higher (P < .002) than those of CDP2230 (Table 1), the selectivity factors for both compounds were approximately 1. In contrast, the selectivity factor for CDP2230 was 15.8, indicating that the selectivity for CYP11B2 over CYP11B1 was higher for CDP2230 than for MTO and IMTO.
| Compound . | IC50 for CYP11B2 (nM) . | IC50 for CYP11B1 (nM) . | Selectivity Factora . |
|---|---|---|---|
| MTO | 0.59 ± 0.64b | 0.24 ± 0.05b | 0.41 |
| IMTO | 0.24 ± 0.14b | 0.28 ± 0.22b | 1.16 |
| CDP2230 | 6.90 ± 2.93 | 109 ± 5.99 | 15.8 |
| Compound . | IC50 for CYP11B2 (nM) . | IC50 for CYP11B1 (nM) . | Selectivity Factora . |
|---|---|---|---|
| MTO | 0.59 ± 0.64b | 0.24 ± 0.05b | 0.41 |
| IMTO | 0.24 ± 0.14b | 0.28 ± 0.22b | 1.16 |
| CDP2230 | 6.90 ± 2.93 | 109 ± 5.99 | 15.8 |
The results are expressed as the mean ± SD of 3–6 independent experiments.
IC50 for CYP11B1/IC50 for CYP11B2.
Significantly different from the value for CDP2230 (P < .01).
| Compound . | IC50 for CYP11B2 (nM) . | IC50 for CYP11B1 (nM) . | Selectivity Factora . |
|---|---|---|---|
| MTO | 0.59 ± 0.64b | 0.24 ± 0.05b | 0.41 |
| IMTO | 0.24 ± 0.14b | 0.28 ± 0.22b | 1.16 |
| CDP2230 | 6.90 ± 2.93 | 109 ± 5.99 | 15.8 |
| Compound . | IC50 for CYP11B2 (nM) . | IC50 for CYP11B1 (nM) . | Selectivity Factora . |
|---|---|---|---|
| MTO | 0.59 ± 0.64b | 0.24 ± 0.05b | 0.41 |
| IMTO | 0.24 ± 0.14b | 0.28 ± 0.22b | 1.16 |
| CDP2230 | 6.90 ± 2.93 | 109 ± 5.99 | 15.8 |
The results are expressed as the mean ± SD of 3–6 independent experiments.
IC50 for CYP11B1/IC50 for CYP11B2.
Significantly different from the value for CDP2230 (P < .01).
In vitro ARG findings
123I-IMTO showed almost uniform binding in the whole adrenal section. However, 18F-CDP2230 specifically accumulated in the region that was pathologically confirmed as APA (Figure 2, A–C). The density of radioactivity in a CYP11B2-positive region in a specimen from a 5-mm APA (Figure 2A) was higher than that in specimens from 9- and 10-mm APAs (Figure 2, B and C). Radioactivity was also observed in aldosterone-producing cell clusters (APCCs) (11) in the subcapsular portion (Figure 2C).
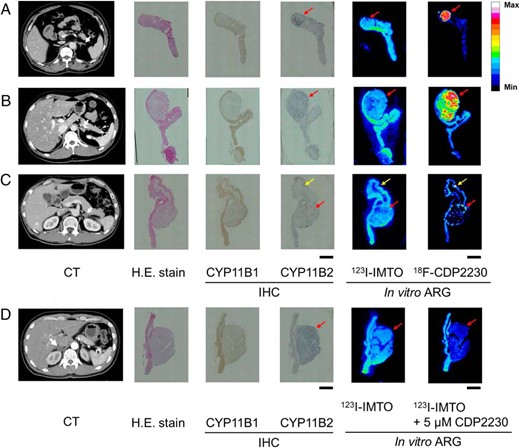
In vitro ARG of 123I-IMTO and 18F-CDP2230 using human APA tissue.
Frozen serial sections of adrenal glands from 4 PA patients (A–D) were used for H.E. stain, immunohistochemical examination of CYP11B1 (brown) and CYP11B2 (blue), and in vitro ARG. CT images of PA patients before adrenalectomy are shown with white arrows indicating adrenal tumors. The maximum diameters of adenomas on CT images in A–D are 5, 9, 10, and 22 mm, respectively. The frozen sections were incubated in buffer containing 123I-IMTO in the presence or absence of CDP2230 (D). The CDP2230 concentration was 5μM. 18F-CDP2230 shows selective binding to CYP11B2-positive regions, APA (A–D, red arrows) and APCC (C, yellow arrows). Scale bars, 5 mm. H.E., hematoxylin and eosin; IHC, immunohistochemistry; Max, maximum; Min, minimum.
To further examine the selectivity of CDP2230 for CYP11B2 over CYP11B1, a displacement study was conducted. 123I-IMTO uniformly bound to both CYP11B1- and CYP11B2-positive regions (Figure 2D). With addition of nonradiolabeled CDP2230, the amount of 123I-IMTO binding to CYP11B2-positive regions decreased, whereas that to CYP11B1-positive regions remained unchanged (Figure 2D).
To confirm the selectivity of 18F-CDP2230 for CYP11B2 over CYP11B1, we conducted in vitro ARG using CS and Pheo surgical specimens. 123I-IMTO homogeneously bound to both CYP11B1- and CYP11B2-positive regions, similar to the binding in APA tissues. In contrast, 18F-CDP2230 did not bind to CYP11B1-positive regions in these specimens (Figure 3).
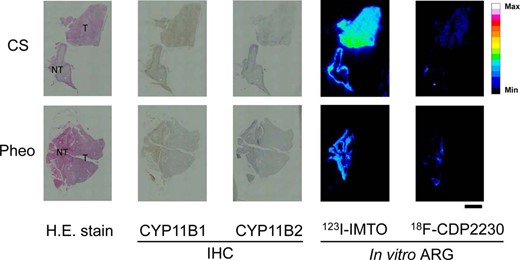
In vitro ARG of 123I-IMTO and 18F-CDP2230 using human adrenal tissue derived from CS and Pheo.
Frozen serial sections of adrenal glands from patients with CS and Pheo were used for H.E. stain, immunohistochemical examination of CYP11B1 (brown) and CYP11B2 (blue), and in vitro ARG. Scale bars, 5 mm. H.E., hematoxylin and eosin; IHC, immunohistochemistry; T, tumor; NT, normal tissue; Max, maximum; Min, minimum.
Biodistribution of 123I-IMTO and 18F-CDP2230 in rats
Accumulation of 18F-CDP2230 in the adrenal glands of rats increased gradually after administration. In contrast, the accumulation of 123I-IMTO remained unchanged (Table 2). The adrenal gland to blood ratio of 18F-CDP2230 accumulation was higher than that of 123I-IMTO, whereas the adrenal gland to surrounding organ (liver and small intestine) ratio of 123I-IMTO accumulation was higher than that of 18F-CDP2230 (Table 2). The accumulation of 18F-CDP2230 in the thigh bones and 123I-IMTO in the thyroid glands as indicators of degradation remained low throughout the evaluation period (Table 2).
Biodistribution of I-IMTO and rF-CDP2230 in Rats at 10, 30, and 60 Minutes After Injection
| . | 10 Minutes . | 30 Minutes . | 60 Minutes . | |||
|---|---|---|---|---|---|---|
| I-IMTO . | rF-CDP2230 . | I-IMTO . | rF-CDP2230 . | I-IMTO . | rF-CDP2230 . | |
| Tissue distribution (percentage of the injected dose per gram of tissue) | ||||||
| Blood | 1.40 ± 0.13 | 0.08 ± 0.01 | 2.55 ± 0.16a | 0.08 ± 0.01 | 2.91 ± 0.09a | 0.09 ± 0.01 |
| Whole brain | 0.30 ± 0.02 | 0.01 ± 0.00 | 0.18 ± 0.02a | 0.01 ± 0.00 | 0.11 ± 0.00a,b | 0.02 ± 0.00 |
| Heart | 0.48 ± 0.07 | 0.29 ± 0.07 | 0.74 ± 0.03a | 0.25 ± 0.06 | 0.77 ± 0.03a | 0.25 ± 0.03a |
| Lungs | 0.72 ± 0.05 | 0.60 ± 0.08 | 0.90 ± 0.09 | 0.39 ± 0.06a | 1.01 ± 0.03a | 0.43 ± 0.05a |
| Stomach | 0.52 ± 0.18 | 0.58 ± 0.16 | 0.46 ± 0.11 | 0.74 ± 0.08 | 0.41 ± 0.05 | 1.06 ± 0.22a,b |
| Liver | 3.29 ± 0.08 | 5.58 ± 1.07 | 1.82 ± 0.04a | 5.90 ± 0.72 | 1.37 ± 0.06a,b | 4.13 ± 0.83b |
| Spleen | 0.30 ± 0.02 | 3.65 ± 0.35 | 0.43 ± 0.04a | 4.05 ± 1.26 | 0.39 ± 0.03 | 3.92 ± 1.07 |
| Sm. intestine | 0.62 ± 0.03 | 1.05 ± 0.11 | 0.68 ± 0.06 | 1.87 ± 0.18a | 0.79 ± 0.11 | 2.98 ± 0.38a,b |
| Lg. intestine | 0.10 ± 0.02 | 0.16 ± 0.02 | 0.12 ± 0.01 | 0.21 ± 0.02 | 0.14 ± 0.02 | 0.46 ± 0.23a |
| Kidneys | 0.70 ± 0.01 | 1.70 ± 0.47 | 1.10 ± 0.12a | 1.09 ± 0.19a | 1.10 ± 0.10a | 1.06 ± 0.13a |
| Bladder, including urine | 0.25 ± 0.27 | 0.24 ± 0.09 | 0.39 ± 0.08 | 1.19 ± 0.97 | 0.68 ± 0.30 | 2.16 ± 1.06a |
| Muscle | 0.18 ± 0.05 | 0.26 ± 0.27 | 0.18 ± 0.02 | 0.10 ± 0.01 | 0.16 ± 0.02 | 0.09 ± 0.01 |
| Adrenal glands | 22.14 ± 6.98 | 9.24 ± 1.45 | 17.66 ± 7.43 | 20.28 ± 4.23a | 19.70 ± 8.14 | 20.40 ± 6.35a |
| Testes | 0.52 ± 0.08 | 0.05 ± 0.01 | 0.32 ± 0.04a | 0.06 ± 0.03 | 0.33 ± 0.02a | 0.08 ± 0.01a,b |
| Carcass | 0.29 ± 0.00 | 0.21 ± 0.03 | 0.31 ± 0.03 | 0.16 ± 0.01 | 0.34 ± 0.01 | 0.18 ± 0.03 |
| Thyroids | 1.30 ± 1.05 | — | 0.81 ± 0.16 | — | 1.29 ± 0.25 | — |
| Thighbones | — | 0.61 ± 0.07 | — | 0.59 ± 0.05 | — | 0.70 ± 0.10 |
| Tissue distribution ratios relative to adrenal gland | ||||||
| Adrenal gland/blood | 15.93 ± 4.94 | 126.00 ± 32.86 | 6.84 ± 2.49 | 258.85 ± 54.92a | 6.73 ± 2.57 | 236.62 ± 77.01 |
| Adrenal gland/liver | 6.72 ± 2.00 | 1.70 ± 0.38 | 9.74 ± 4.16 | 3.48 ± 0.80 | 14.20 ± 5.28 | 4.94 ± 1.41a |
| Adrenal gland/kidney | 31.76 ± 10.16 | 5.66 ± 1.31 | 16.66 ± 8.95 | 18.89 ± 4.50a | 18.20 ± 8.58 | 19.20 ± 6.01a |
| Adrenal gland/Sm. intestine | 35.65 ± 9.43 | 8.81 ± 1.41 | 25.49 ± 8.31 | 10.83 ± 1.77 | 24.59 ± 8.26 | 7.00 ± 2.62 |
| . | 10 Minutes . | 30 Minutes . | 60 Minutes . | |||
|---|---|---|---|---|---|---|
| I-IMTO . | rF-CDP2230 . | I-IMTO . | rF-CDP2230 . | I-IMTO . | rF-CDP2230 . | |
| Tissue distribution (percentage of the injected dose per gram of tissue) | ||||||
| Blood | 1.40 ± 0.13 | 0.08 ± 0.01 | 2.55 ± 0.16a | 0.08 ± 0.01 | 2.91 ± 0.09a | 0.09 ± 0.01 |
| Whole brain | 0.30 ± 0.02 | 0.01 ± 0.00 | 0.18 ± 0.02a | 0.01 ± 0.00 | 0.11 ± 0.00a,b | 0.02 ± 0.00 |
| Heart | 0.48 ± 0.07 | 0.29 ± 0.07 | 0.74 ± 0.03a | 0.25 ± 0.06 | 0.77 ± 0.03a | 0.25 ± 0.03a |
| Lungs | 0.72 ± 0.05 | 0.60 ± 0.08 | 0.90 ± 0.09 | 0.39 ± 0.06a | 1.01 ± 0.03a | 0.43 ± 0.05a |
| Stomach | 0.52 ± 0.18 | 0.58 ± 0.16 | 0.46 ± 0.11 | 0.74 ± 0.08 | 0.41 ± 0.05 | 1.06 ± 0.22a,b |
| Liver | 3.29 ± 0.08 | 5.58 ± 1.07 | 1.82 ± 0.04a | 5.90 ± 0.72 | 1.37 ± 0.06a,b | 4.13 ± 0.83b |
| Spleen | 0.30 ± 0.02 | 3.65 ± 0.35 | 0.43 ± 0.04a | 4.05 ± 1.26 | 0.39 ± 0.03 | 3.92 ± 1.07 |
| Sm. intestine | 0.62 ± 0.03 | 1.05 ± 0.11 | 0.68 ± 0.06 | 1.87 ± 0.18a | 0.79 ± 0.11 | 2.98 ± 0.38a,b |
| Lg. intestine | 0.10 ± 0.02 | 0.16 ± 0.02 | 0.12 ± 0.01 | 0.21 ± 0.02 | 0.14 ± 0.02 | 0.46 ± 0.23a |
| Kidneys | 0.70 ± 0.01 | 1.70 ± 0.47 | 1.10 ± 0.12a | 1.09 ± 0.19a | 1.10 ± 0.10a | 1.06 ± 0.13a |
| Bladder, including urine | 0.25 ± 0.27 | 0.24 ± 0.09 | 0.39 ± 0.08 | 1.19 ± 0.97 | 0.68 ± 0.30 | 2.16 ± 1.06a |
| Muscle | 0.18 ± 0.05 | 0.26 ± 0.27 | 0.18 ± 0.02 | 0.10 ± 0.01 | 0.16 ± 0.02 | 0.09 ± 0.01 |
| Adrenal glands | 22.14 ± 6.98 | 9.24 ± 1.45 | 17.66 ± 7.43 | 20.28 ± 4.23a | 19.70 ± 8.14 | 20.40 ± 6.35a |
| Testes | 0.52 ± 0.08 | 0.05 ± 0.01 | 0.32 ± 0.04a | 0.06 ± 0.03 | 0.33 ± 0.02a | 0.08 ± 0.01a,b |
| Carcass | 0.29 ± 0.00 | 0.21 ± 0.03 | 0.31 ± 0.03 | 0.16 ± 0.01 | 0.34 ± 0.01 | 0.18 ± 0.03 |
| Thyroids | 1.30 ± 1.05 | — | 0.81 ± 0.16 | — | 1.29 ± 0.25 | — |
| Thighbones | — | 0.61 ± 0.07 | — | 0.59 ± 0.05 | — | 0.70 ± 0.10 |
| Tissue distribution ratios relative to adrenal gland | ||||||
| Adrenal gland/blood | 15.93 ± 4.94 | 126.00 ± 32.86 | 6.84 ± 2.49 | 258.85 ± 54.92a | 6.73 ± 2.57 | 236.62 ± 77.01 |
| Adrenal gland/liver | 6.72 ± 2.00 | 1.70 ± 0.38 | 9.74 ± 4.16 | 3.48 ± 0.80 | 14.20 ± 5.28 | 4.94 ± 1.41a |
| Adrenal gland/kidney | 31.76 ± 10.16 | 5.66 ± 1.31 | 16.66 ± 8.95 | 18.89 ± 4.50a | 18.20 ± 8.58 | 19.20 ± 6.01a |
| Adrenal gland/Sm. intestine | 35.65 ± 9.43 | 8.81 ± 1.41 | 25.49 ± 8.31 | 10.83 ± 1.77 | 24.59 ± 8.26 | 7.00 ± 2.62 |
The results are expressed as the mean ± SD of 3–6 independent experiments.
Significantly different from the value at 10 minutes (P < .01).
Significantly different from the value at 30 minutes (P < .01).
Biodistribution of I-IMTO and rF-CDP2230 in Rats at 10, 30, and 60 Minutes After Injection
| . | 10 Minutes . | 30 Minutes . | 60 Minutes . | |||
|---|---|---|---|---|---|---|
| I-IMTO . | rF-CDP2230 . | I-IMTO . | rF-CDP2230 . | I-IMTO . | rF-CDP2230 . | |
| Tissue distribution (percentage of the injected dose per gram of tissue) | ||||||
| Blood | 1.40 ± 0.13 | 0.08 ± 0.01 | 2.55 ± 0.16a | 0.08 ± 0.01 | 2.91 ± 0.09a | 0.09 ± 0.01 |
| Whole brain | 0.30 ± 0.02 | 0.01 ± 0.00 | 0.18 ± 0.02a | 0.01 ± 0.00 | 0.11 ± 0.00a,b | 0.02 ± 0.00 |
| Heart | 0.48 ± 0.07 | 0.29 ± 0.07 | 0.74 ± 0.03a | 0.25 ± 0.06 | 0.77 ± 0.03a | 0.25 ± 0.03a |
| Lungs | 0.72 ± 0.05 | 0.60 ± 0.08 | 0.90 ± 0.09 | 0.39 ± 0.06a | 1.01 ± 0.03a | 0.43 ± 0.05a |
| Stomach | 0.52 ± 0.18 | 0.58 ± 0.16 | 0.46 ± 0.11 | 0.74 ± 0.08 | 0.41 ± 0.05 | 1.06 ± 0.22a,b |
| Liver | 3.29 ± 0.08 | 5.58 ± 1.07 | 1.82 ± 0.04a | 5.90 ± 0.72 | 1.37 ± 0.06a,b | 4.13 ± 0.83b |
| Spleen | 0.30 ± 0.02 | 3.65 ± 0.35 | 0.43 ± 0.04a | 4.05 ± 1.26 | 0.39 ± 0.03 | 3.92 ± 1.07 |
| Sm. intestine | 0.62 ± 0.03 | 1.05 ± 0.11 | 0.68 ± 0.06 | 1.87 ± 0.18a | 0.79 ± 0.11 | 2.98 ± 0.38a,b |
| Lg. intestine | 0.10 ± 0.02 | 0.16 ± 0.02 | 0.12 ± 0.01 | 0.21 ± 0.02 | 0.14 ± 0.02 | 0.46 ± 0.23a |
| Kidneys | 0.70 ± 0.01 | 1.70 ± 0.47 | 1.10 ± 0.12a | 1.09 ± 0.19a | 1.10 ± 0.10a | 1.06 ± 0.13a |
| Bladder, including urine | 0.25 ± 0.27 | 0.24 ± 0.09 | 0.39 ± 0.08 | 1.19 ± 0.97 | 0.68 ± 0.30 | 2.16 ± 1.06a |
| Muscle | 0.18 ± 0.05 | 0.26 ± 0.27 | 0.18 ± 0.02 | 0.10 ± 0.01 | 0.16 ± 0.02 | 0.09 ± 0.01 |
| Adrenal glands | 22.14 ± 6.98 | 9.24 ± 1.45 | 17.66 ± 7.43 | 20.28 ± 4.23a | 19.70 ± 8.14 | 20.40 ± 6.35a |
| Testes | 0.52 ± 0.08 | 0.05 ± 0.01 | 0.32 ± 0.04a | 0.06 ± 0.03 | 0.33 ± 0.02a | 0.08 ± 0.01a,b |
| Carcass | 0.29 ± 0.00 | 0.21 ± 0.03 | 0.31 ± 0.03 | 0.16 ± 0.01 | 0.34 ± 0.01 | 0.18 ± 0.03 |
| Thyroids | 1.30 ± 1.05 | — | 0.81 ± 0.16 | — | 1.29 ± 0.25 | — |
| Thighbones | — | 0.61 ± 0.07 | — | 0.59 ± 0.05 | — | 0.70 ± 0.10 |
| Tissue distribution ratios relative to adrenal gland | ||||||
| Adrenal gland/blood | 15.93 ± 4.94 | 126.00 ± 32.86 | 6.84 ± 2.49 | 258.85 ± 54.92a | 6.73 ± 2.57 | 236.62 ± 77.01 |
| Adrenal gland/liver | 6.72 ± 2.00 | 1.70 ± 0.38 | 9.74 ± 4.16 | 3.48 ± 0.80 | 14.20 ± 5.28 | 4.94 ± 1.41a |
| Adrenal gland/kidney | 31.76 ± 10.16 | 5.66 ± 1.31 | 16.66 ± 8.95 | 18.89 ± 4.50a | 18.20 ± 8.58 | 19.20 ± 6.01a |
| Adrenal gland/Sm. intestine | 35.65 ± 9.43 | 8.81 ± 1.41 | 25.49 ± 8.31 | 10.83 ± 1.77 | 24.59 ± 8.26 | 7.00 ± 2.62 |
| . | 10 Minutes . | 30 Minutes . | 60 Minutes . | |||
|---|---|---|---|---|---|---|
| I-IMTO . | rF-CDP2230 . | I-IMTO . | rF-CDP2230 . | I-IMTO . | rF-CDP2230 . | |
| Tissue distribution (percentage of the injected dose per gram of tissue) | ||||||
| Blood | 1.40 ± 0.13 | 0.08 ± 0.01 | 2.55 ± 0.16a | 0.08 ± 0.01 | 2.91 ± 0.09a | 0.09 ± 0.01 |
| Whole brain | 0.30 ± 0.02 | 0.01 ± 0.00 | 0.18 ± 0.02a | 0.01 ± 0.00 | 0.11 ± 0.00a,b | 0.02 ± 0.00 |
| Heart | 0.48 ± 0.07 | 0.29 ± 0.07 | 0.74 ± 0.03a | 0.25 ± 0.06 | 0.77 ± 0.03a | 0.25 ± 0.03a |
| Lungs | 0.72 ± 0.05 | 0.60 ± 0.08 | 0.90 ± 0.09 | 0.39 ± 0.06a | 1.01 ± 0.03a | 0.43 ± 0.05a |
| Stomach | 0.52 ± 0.18 | 0.58 ± 0.16 | 0.46 ± 0.11 | 0.74 ± 0.08 | 0.41 ± 0.05 | 1.06 ± 0.22a,b |
| Liver | 3.29 ± 0.08 | 5.58 ± 1.07 | 1.82 ± 0.04a | 5.90 ± 0.72 | 1.37 ± 0.06a,b | 4.13 ± 0.83b |
| Spleen | 0.30 ± 0.02 | 3.65 ± 0.35 | 0.43 ± 0.04a | 4.05 ± 1.26 | 0.39 ± 0.03 | 3.92 ± 1.07 |
| Sm. intestine | 0.62 ± 0.03 | 1.05 ± 0.11 | 0.68 ± 0.06 | 1.87 ± 0.18a | 0.79 ± 0.11 | 2.98 ± 0.38a,b |
| Lg. intestine | 0.10 ± 0.02 | 0.16 ± 0.02 | 0.12 ± 0.01 | 0.21 ± 0.02 | 0.14 ± 0.02 | 0.46 ± 0.23a |
| Kidneys | 0.70 ± 0.01 | 1.70 ± 0.47 | 1.10 ± 0.12a | 1.09 ± 0.19a | 1.10 ± 0.10a | 1.06 ± 0.13a |
| Bladder, including urine | 0.25 ± 0.27 | 0.24 ± 0.09 | 0.39 ± 0.08 | 1.19 ± 0.97 | 0.68 ± 0.30 | 2.16 ± 1.06a |
| Muscle | 0.18 ± 0.05 | 0.26 ± 0.27 | 0.18 ± 0.02 | 0.10 ± 0.01 | 0.16 ± 0.02 | 0.09 ± 0.01 |
| Adrenal glands | 22.14 ± 6.98 | 9.24 ± 1.45 | 17.66 ± 7.43 | 20.28 ± 4.23a | 19.70 ± 8.14 | 20.40 ± 6.35a |
| Testes | 0.52 ± 0.08 | 0.05 ± 0.01 | 0.32 ± 0.04a | 0.06 ± 0.03 | 0.33 ± 0.02a | 0.08 ± 0.01a,b |
| Carcass | 0.29 ± 0.00 | 0.21 ± 0.03 | 0.31 ± 0.03 | 0.16 ± 0.01 | 0.34 ± 0.01 | 0.18 ± 0.03 |
| Thyroids | 1.30 ± 1.05 | — | 0.81 ± 0.16 | — | 1.29 ± 0.25 | — |
| Thighbones | — | 0.61 ± 0.07 | — | 0.59 ± 0.05 | — | 0.70 ± 0.10 |
| Tissue distribution ratios relative to adrenal gland | ||||||
| Adrenal gland/blood | 15.93 ± 4.94 | 126.00 ± 32.86 | 6.84 ± 2.49 | 258.85 ± 54.92a | 6.73 ± 2.57 | 236.62 ± 77.01 |
| Adrenal gland/liver | 6.72 ± 2.00 | 1.70 ± 0.38 | 9.74 ± 4.16 | 3.48 ± 0.80 | 14.20 ± 5.28 | 4.94 ± 1.41a |
| Adrenal gland/kidney | 31.76 ± 10.16 | 5.66 ± 1.31 | 16.66 ± 8.95 | 18.89 ± 4.50a | 18.20 ± 8.58 | 19.20 ± 6.01a |
| Adrenal gland/Sm. intestine | 35.65 ± 9.43 | 8.81 ± 1.41 | 25.49 ± 8.31 | 10.83 ± 1.77 | 24.59 ± 8.26 | 7.00 ± 2.62 |
The results are expressed as the mean ± SD of 3–6 independent experiments.
Significantly different from the value at 10 minutes (P < .01).
Significantly different from the value at 30 minutes (P < .01).
PET and PET/MRI findings with 18F-CDP2230 in rats
To confirm that 18F-CDP2230 can image adrenal glands with low background uptake in vivo, we obtained PET and PET/MRI images in rats. Figure 4, A and B, show axial, coronal, and sagittal PET and PET/MRI images, respectively. 18F-CDP2230 accumulated in the adrenal glands 10–20 minutes after administration. Accumulation of 18F-CDP2230 in the adrenal glands increased gradually over time. This finding was consistent with that of the biodistribution study. Accumulation of radioactivity in the adrenal glands was morphologically confirmed by fusion of the PET and MRI images.
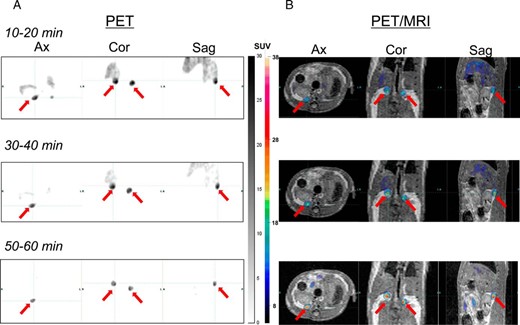
PET (A) and PET/MRI (B) images obtained using 18F-CDP2230 in rats.
Data were acquired for 10–20, 30–40, and 50–60 minutes after administration via the tail vein. The red arrows indicate the adrenal glands. Accumulation of radioactivity in the adrenal glands was morphologically confirmed by fusion of the PET and MRI images. Accumulation of radioactivity is expressed as SUV. Ax, axial section; Cor, coronal section; Sag, sagittal section; min, minutes.
In vitro stability of 18F-CDP2230 in human and rat plasma
No metabolites were detected in human plasma after 30 minutes (Supplemental Figure 3) and 60 minutes of incubation (data not shown). No metabolites were detected in rat plasma after 30 minutes of incubation (Supplemental Figure 3).
Discussion
In this study, we synthesized 18F-CDP2230, a novel imaging agent for PA, and evaluated its selectivity for CYP11B2 over CYP11B1 using cells with stable expression of either enzyme and in vitro ARG of human adrenal sections with APAs, with comparison of the results with those for MTO and IMTO. We also evaluated the biodistribution of 18F-CDP2230 by conducting a biodistribution study and performing PET/MRI in rats. The selectivity for CYP11B2 over CYP11B1 was higher for CDP2230 than for MTO and IMTO. In addition, 18F-CDP2230 showed favorable biodistribution and metabolic stability for CYP11B2 imaging in rats, and it was also stable in human plasma.
Given the similar inhibitory activity for CYP11B1 and CYP11B2 (Table 1) and the limited utility of 11C-MTO, we used 123I-IMTO as a benchmark compound for the present study. Although CDP2230 showed significantly lower affinity for CYP11B2 and CYP11B1 than did MTO and IMTO, its selectivity for CYP11B2 over CYP11B1 was over 15 times higher than those of MTO and IMTO (Table 1). To confirm the selectivity of CDP2230 for CYP11B2 over CYP11B1, in vitro ARG was performed using 3 surgical specimens from patients with PA. 123I-IMTO uniformly bound to CYP11B1-positive regions in normal adrenal tissues and CYP11B2-positive regions in APA tissues, whereas 18F-CDP2230 bound more selectively to APA tissues than 123I-IMTO (Figure 2, A–C). Furthermore, in the displacement study, 123I-IMTO binding decreased only in CYP11B2-positive regions in the presence of nonradiolabeled CDP2230. Thus, we demonstrated superior selectivity of CDP2230 for CYP11B2 over CYP11B1. Although the affinity of CDP2230 for CYP11B2 was significantly lower than that of IMTO (Table 1), the intensity of bound 18F-CDP2230 was much higher than that of 123I-IMTO in human APA tissues (Figure 2, A and B). With regard to the clinical application of 18F-CDP2230 in patients with PA, evaluation by in vitro ARG using human tissue is more appropriate than studies of gene-transfected cells. In in vitro ARG studies, the possibility that 18F-CDP2230 may bind to other proteins overexpressed in APA, but not in normal adrenal tissues, cannot be completely excluded. Although additional studies are required to assess this possibility, the data from gene-transfected cells showed that 18F-CDP2230 is most likely to bind to CYP11B2.
It has been reported that in smaller APAs, the expression density of CYP11B1 decreases and that of CYP11B2 increases (12, 13). In addition, CYP11B2 expression has been reported to be homogeneous in smaller adenomas and heterogeneous in larger adenomas (13). We demonstrated similar findings in our study. These trends were also observed in in vitro ARG with 18F-CDP2230 in APAs; the density of radioactivity was higher and distribution pattern of radioactivity was more homogeneous in smaller APAs (Figure 2A) than in larger ones (Figure 2, B and C). These findings indicate an advantage of nuclear medicine imaging in PA.
Whether 18F-CDP2230 distinguishes APA from hyperplasia remains unknown, because in vitro ARG has not been conducted in the adrenal tissues with hyperplasia. However, 18F-CDP2230 was shown to bind to the APCC in the subcapsular portion (Figure 2C), it is therefore suggested that 18F-CDP2230 would bind to any source of aldosterone production whatever the type of the disease, adenoma or hyperplasia. As is the case with 11C-MTO PET, the SUV ratios of adrenal glands could be used to distinguish unilateral from bilateral disease (6). From this perspective, it may not be problematic that 18F-CDP2230 will not be “too sensitive.” Instead, it is likely that 18F-CDP2230 will be more suitable for the diagnosis of laterality of hyperaldosteronism due to its higher selectivity for CYP11B2 over CYP11B1 than 11C-MTO.
APAs undetectable by CT account for 13%–30% of all APAs (14–16). In addition, CT is based on morphological information and can provide misleading results for 38%–47% of patients with regard to the laterality of the lesion responsible for PA, which can lead to serious and irreversible treatment errors (4, 17). On the basis of the high selectivity of 18F-CDP2230 for CYP11B2 over CYP11B1 and the favorable findings in the biodistribution study, 18F-CDP2230 holds promise as a novel imaging agent that will be superior to 11C-MTO and aid in the detection of CT-negative unilateral disease.
Moreover, 11C-MTO-PET requires dexamethasone pretreatment. Supraphysiological doses of dexamethasone can be problematic, particularly in patients with diabetes mellitus, hypokalemia and infection. The selectivity of CDP2230 for CYP11B2 over CYP11B1 may preclude the requirement for dexamethasone pretreatment.
Conclusions
The selectivity of 18F-CDP2230 for CYP11B2 over CYP11B1 was higher than those of MTO derivatives. The biodistribution of this agent was favorable for CYP11B2 imaging. Considering these findings and the limitations of adrenal vein sampling, we conclude that 18F-CDP2230 is a promising imaging agent that has the potential to be used for noninvasive imaging for detecting the unilateral subtypes of PA with high sensitivity and specificity.
Acknowledgments
We thank Mr Masato Kiriu for radiosynthesis and Mr Norihito Nakata, Dr Shunsuke Meike, and Ms Chihiro Usui for the biodistribution studies at the Research Center, Nihon Medi-Physics Co. Ltd.
Disclosure Summary: T.A., N.K., Y.D., A.I., K.A., Y.O., M.I., and H.M. are employed by Nihon Medi-Physics Co. Ltd.; W.F.Y. consults for Nihon Medi-Physics Co. Ltd.; M.N., H.K., and H.S. have received research grants from Nihon Medi-Physics Co. Ltd.; and K.M. has nothing to declare.
Abbreviations
- APA
aldosterone-producing adenoma
- APCC
aldosterone-producing cell cluster
- ARG
autoradiography
- CS
Cushing syndrome
- CT
computed tomography
- CYP11B1
cytochrome P450 11 beta-hydroxylase
- CYP11B2
cytochrome P450 aldosterone synthase
- IMTO
iodometomidate
- MRI
magnetic resonance imaging
- MTO
metomidate
- PA
primary aldosteronism
- PET
positron emission tomography
- Pheo
pheochromocytoma
- SUV
standardized uptake value.



