-
PDF
- Split View
-
Views
-
Cite
Cite
Thalita G. Alves, Teresa S. Kasamatsu, Ji H. Yang, Maria Cecília Z. Meneghetti, Aline Mendes, Ilda S. Kunii, Susan C. Lindsey, Cléber P. Camacho, Magnus R. Dias da Silva, Rui M. B. Maciel, José Gilberto H. Vieira, João Roberto M. Martins, Macrocalcitonin Is a Novel Pitfall in the Routine of Serum Calcitonin Immunoassay, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 2, 1 February 2016, Pages 653–658, https://doi.org/10.1210/jc.2015-3137
Close - Share Icon Share
Calcitonin (CT) is a sensitive marker of medullary thyroid carcinoma (MTC) and is used for primary diagnosis and follow-up after thyroidectomy. However, persistently elevated CT is observed even after complete surgical removal without evidence of a recurrent or persistent tumor.
To investigate the presence of assay interference in the serum CT of MTC patients who are apparently without a structural disease.
We studied three index MTC cases for CT assay interference and 14 patients with metastatic MTC. The CT level was measured using an immunofluorometric assay. Screening for assay interference was performed by determination of CT levels before and after serum treatment with polyethylene glycol. Additionally, samples were analyzed by chromatography on ultra-performance liquid chromatography and protein A-Sepharose.
Patients with biochemical and structural disease showed CT mean recovery of 84.1% after polyethylene glycol treatment, whereas patients suspected of interference showed recovery from 2–7%. The elution profile on UPLC showed that the immunometric CT from these three patients behaved like a high molecular mass aggregate (>300 kDa). Additionally, when these samples were applied to the protein A-Sepharose, CT immunoreactivity was retained on the column and was only released after lowering the pH.
For the first time, our results show the presence of a novel pitfall in the CT immunoassay: “macrocalcitonin.” Its etiology, frequency, and meaning remain to be defined, but its recognition is of interest and can help clinicians avoid unnecessary diagnostic investigations and treatment during the follow-up of MTC.
Measurement of serum calcitonin (CT) is a major marker of medullary thyroid carcinoma (MTC) used for the primary diagnosis and to predict the outcome (1), and it can also be used as an aid in planning long-term follow-up after thyroidectomy (2, 3). Additionally, in RET mutation carriers, who are at elevated risk for multiple endocrine neoplasia 2, both basal and stimulated serum CT could also help to guide the timing of thyroidectomy and indicate the need for a more extensive initial surgical approach (3). However, such strategies remain controversial (4), especially regarding the limitations of assay methods and the interpretation of results.
Indeed, persistently elevated CT can be observed even after complete surgery with no evidence of a persistent or recurrent tumor in the follow-up, leading to potential misdiagnosis. Despite recent improvements in the immunochemiluminometric CT assays that minimize cross-reactivity with procalcitonin and CT-related peptides, some challenges remain, especially concerning the presence of heterophilic antibodies in patient serum, which can produce elevated CT (5–8).
Thus, some challenges arise in clinical practice. When the postoperative serum CT is undetectable, the risk of persistent or recurrent residual disease is low, and further CT-stimulating tests or imaging techniques are not immediately required. In contrast, if CT levels exceed 500 pg/mL, radiographically identifiable distant metastases are almost always present (9). However, upon modestly increased CT levels (<150 pg/mL), the presence of local or distant metastasis by radiographic imaging is difficult to detect and requires additional and often expensive tests during follow-up (10). An additional challenge occurs when CT levels are persistently increased but unchanged over time.
Here, we describe three patients with MTC whose serum CT in the late postoperative period was modestly increased but showed no evidence of residual tumor. They were diagnosed as having a new interference in the CT immunoassay, which was characterized by falsely increased values and herein termed “macrocalcitonin” (macro-CT).
Patients and Methods
We studied three index MTC cases suspected of having CT assay interference (A, B, and C) and, for comparison, 14 patients with MTC who were followed at the Division of Endocrinology, Department of Medicine, Escola Paulista de Medicina, Federal University of São Paulo, São Paulo, Brazil. The study was approved by the Ethical Committee of Universidade Federal de São Paulo/Escola Paulista de Medicina (CAAE: 12820313.4.0000.5505), and written informed consent was obtained from each subject.
Patient A
In 2003, a 45-year-old white woman attended with a history of multinodular goiter. Cytology was benign, but the patient was submitted to total thyroidectomy due to recent increased volume of the thyroid gland. Histopathology showed an isolated MTC (2.0 cm) with no evidence of capsular, vascular, or extrathyroidal invasion. During the postoperative period, serum CT and cervical ultrasound were unremarkable. In April 2010, the patient was referred to us for further evaluation. Revision of histopathology confirmed the diagnosis of MTC. The presence of a genomic mutation in the gene RET was negative. There were no suspect lymph nodes on cervical ultrasound, and her first biochemical analysis showed a slightly increased serum CT (118 pg/mL). Further biochemical analyzes showed a serum CT ranging from 138–262 pg/mL. Carcinoembryonic antigen measurements were all normal. Neck and thorax tomography, abdomen and pelvis magnetic resonance imaging, and a bone scan showed no abnormalities. During 3 years of follow-up, the patient remained very well with no clinical or imaging evidence of a residual or recurrent tumor, but she maintains persistently increased serum CT levels.
Patient B
In 2011, a 16-year-old boy carrying the V804M RET mutation was referred for evaluation. Physical examination and cervical ultrasound were normal. Basal CT was mildly increased (35 pg/mL). A pentagastrin stimulation test was performed and showed a blunted response (28.4, 35.0, 37.0, 30.0, and 25.7 pg/mL at 0, 2, 5, 10, and 15 min, respectively). Screening for hyperparathyroidism and pheochromocytoma was negative. Despite the low risk of American Thyroid Association Guideline Task Force (ATA) (10) stratification at the time (ATA risk level A), the patient and his family were selected for prophylactic thyroidectomy. He underwent surgery in May 2011, and histopathology was normal, including the absence of C-cell hyperplasia. During 3 years of follow-up, the patient maintained normal cervical ultrasound but had persistently increased serum CT (30–40 pg/mL).
Patient C
A 40-year-old woman carrier of the E768D RET mutation was referred to us in 2010 for evaluation. She had already been submitted to prophylactic thyroidectomy in January 2010, and histopathology revealed a 0.6-cm diameter MTC and bilateral C-cell hyperplasia. Postoperative cervical ultrasound was completely normal with no evidence of mass or suspect lymph nodes. Screening for hyperparathyroidism and pheochromocytoma was negative. The first serum CT showed a value of 40.3 pg/mL. Serum carcinoembryonic antigen was within the normal range. After 3 years of follow-up, there has been no evidence of clinical or imaging recurrence of MTC, but the patient's CT serum remains moderately increased (35–50 pg/mL).
CT measurement and polyethylene glycol (PEG) treatment
Serum CT levels were measured by an in-house two-site immunofluorometric assay using two mouse monoclonal antibodies against two different epitopes of CT. This assay has an analytical sensitivity of 1.0 pg/mL, and the 95% reference values are 5.5 and 11.1 pg/mL for females and males, respectively (11). In thyroidectomized patients who have been treated for differentiated thyroid carcinoma, more than 97.5% had a CT < 6.9 pg/mL (11). Screening for assay interference was carried out by the determination of CT levels before and after treating the serum with PEG (PEG-6000; Labsynth Products Labs Ltd). The PEG protocol was performed as previously described (12, 13). Briefly, 250 μL of 250 g/L PEG-6000 solution was added to 250 μL of each serum sample, vortex mixed for 1 minute, and centrifuged at 9500 × g for 5 minutes at 25°C; the CT level was measured in the supernatant in triplicate. Recovery (percentage) was calculated by the original CT value. We also analyzed the linearity of serum CT measurements after serial dilution with an equal amount of mouse serum to check the possible interference of heterophilic antibodies. Suspect sample CT was also analyzed by a commercially available electrochemiluminescence immunoassay (Elecsys Calcitonin; Roche Diagnostics).
Gel filtration on ultra-performance liquid chromatography
The three serum samples suspected of interference—a sample obtained from the washout from a fine-needle aspiration of a metastatic MTC lymph node and two serum samples from patients with metastatic disease—were analyzed by gel filtration chromatography. For this analysis, samples were chromatographed on a Biosep S-3000 column 30 × 6.7 mm (Phenomenex) coupled to an ultra-performance liquid chromatography system (AKTA; GE Healthcare). After calibrating the column with standard molecular weights (GE Healthcare), serum samples (200 μL) were applied and eluted with a 0.05 m PBS at pH 7.4 and a flow rate of 0.5 mL/min. Aliquots of 0.5 mL were collected separately for CT measurement.
Affinity chromatography on protein A-Sepharose
Possible interferences were also characterized on protein A-Sepharose CL-4B (GE Healthcare), which specifically binds Ig. Initially, 2 mL of protein A-Sepharose was packed into a plastic column and equilibrated in PBS. Serum sample (0.5 mL) and the same volume of standard CT (WHO International Reference Standard for Human Calcitonin 89/620, National Institute for Biological Standards and Control) were applied separately to the column and eluted with PBS; then, 0.5-mL aliquots (n = 10) were collected for subsequent CT measurement. After this procedure, the column was washed 10 times with PBS, followed by the addition of 5 mL of 0.1 m glycine at pH 2.8. Finally, 0.5-mL aliquots (n = 10) were collected, and each was assayed for CT.
Thyroglobulin autoantibodies, thyroperoxidase autoantibodies, and procalcitonin measurement
In the serum samples suspected for interference, we performed thyroglobulin autoantibody and thyroperoxidase autoantibody assays using electrochemiluminescence immunoassay (Roche Diagnostics) and procalcitonin measurement by an ELISA (USCN Life Science Inc).
Results
Patients with biochemical and structural disease presented a CT mean recovery of 84.1% (ranging from 51–135%) after PEG treatment (Table 1). Interestingly, the three suspect patients for interference showed very low CT recovery, ranging from 2–7%. In comparison with the in-house assay, serum levels of CT determined by commercial method showed lower values of 67, 2, and 4 pg/mL for cases A, B, and C, respectively.
Clinical and Laboratory Data of Patients With MTC Enrolled in the CT Immunoassay Interference Study
| Patient ID . | Gender . | Age, y . | RET Mutation . | Years Since Thyroidectomy . | Disease Status . | CT, pg/mL . | CT-PEG, pg/mL . | Recovery, % . |
|---|---|---|---|---|---|---|---|---|
| A | F | 66 | Negative | 12 | B | 261 | 9 | 3.4 |
| B | F | 40 | E761D | 4 | B | 36 | 0.6 | 1.7 |
| C | M | 16 | V804M | 4 | B | 38 | 2.8 | 7.4 |
| 1 | F | 22 | C634Y | 2 | B/S | 21 754 | 18 567 | 85.3 |
| 2 | F | 28 | Negative | 5 | B/S | 19 800 | 18 729 | 94.6 |
| 3 | M | 48 | Negative | 9 | B/S | 14 070 | 12 985 | 92.3 |
| 4 | M | 35 | Negative | 9 | B/S | 11 795 | 7928 | 67.2 |
| 5 | M | 62 | Negative | 9 | B/S | 11 271 | 7642 | 67.8 |
| 6 | F | 44 | S891A | 4 | B/S | 10 087 | 7644 | 75.8 |
| 7 | F | 46 | C634Y | 1 | B/S | 9193 | 5414 | 58.9 |
| 8 | F | 44 | Negative | 7 | B/S | 941 | 848 | 90.1 |
| 9 | F | 67 | Negative | 14 | B/S | 744 | 593 | 79.7 |
| 10 | F | 57 | S891A | 4 | B/S | 429 | 438 | 102.1 |
| 11 | F | 22 | C634Y | 6 | B/S | 249 | 338 | 135.7 |
| 12 | M | 57 | S891A | NP | B/S | 114 | 59 | 51.7 |
| 13 | M | 41 | C634Y | 3 | B/S | 87 | 78 | 89.7 |
| 14 | F | 39 | Negative | 6 | B/S | 57 | 49 | 86.0 |
| Patient ID . | Gender . | Age, y . | RET Mutation . | Years Since Thyroidectomy . | Disease Status . | CT, pg/mL . | CT-PEG, pg/mL . | Recovery, % . |
|---|---|---|---|---|---|---|---|---|
| A | F | 66 | Negative | 12 | B | 261 | 9 | 3.4 |
| B | F | 40 | E761D | 4 | B | 36 | 0.6 | 1.7 |
| C | M | 16 | V804M | 4 | B | 38 | 2.8 | 7.4 |
| 1 | F | 22 | C634Y | 2 | B/S | 21 754 | 18 567 | 85.3 |
| 2 | F | 28 | Negative | 5 | B/S | 19 800 | 18 729 | 94.6 |
| 3 | M | 48 | Negative | 9 | B/S | 14 070 | 12 985 | 92.3 |
| 4 | M | 35 | Negative | 9 | B/S | 11 795 | 7928 | 67.2 |
| 5 | M | 62 | Negative | 9 | B/S | 11 271 | 7642 | 67.8 |
| 6 | F | 44 | S891A | 4 | B/S | 10 087 | 7644 | 75.8 |
| 7 | F | 46 | C634Y | 1 | B/S | 9193 | 5414 | 58.9 |
| 8 | F | 44 | Negative | 7 | B/S | 941 | 848 | 90.1 |
| 9 | F | 67 | Negative | 14 | B/S | 744 | 593 | 79.7 |
| 10 | F | 57 | S891A | 4 | B/S | 429 | 438 | 102.1 |
| 11 | F | 22 | C634Y | 6 | B/S | 249 | 338 | 135.7 |
| 12 | M | 57 | S891A | NP | B/S | 114 | 59 | 51.7 |
| 13 | M | 41 | C634Y | 3 | B/S | 87 | 78 | 89.7 |
| 14 | F | 39 | Negative | 6 | B/S | 57 | 49 | 86.0 |
Abbreviations: F, female; M, male; NP, not performed; B, presenting only biochemical disease; B/S, presenting biochemical and structural disease. Blood serum from patients A, B, and C was suspected for CT immunofluorometric assay interference. RET mutation indicates identified germ line RET mutation. CT was measured by the in-house assay. CT-PEG and CT were measured after PEG treatment.
Clinical and Laboratory Data of Patients With MTC Enrolled in the CT Immunoassay Interference Study
| Patient ID . | Gender . | Age, y . | RET Mutation . | Years Since Thyroidectomy . | Disease Status . | CT, pg/mL . | CT-PEG, pg/mL . | Recovery, % . |
|---|---|---|---|---|---|---|---|---|
| A | F | 66 | Negative | 12 | B | 261 | 9 | 3.4 |
| B | F | 40 | E761D | 4 | B | 36 | 0.6 | 1.7 |
| C | M | 16 | V804M | 4 | B | 38 | 2.8 | 7.4 |
| 1 | F | 22 | C634Y | 2 | B/S | 21 754 | 18 567 | 85.3 |
| 2 | F | 28 | Negative | 5 | B/S | 19 800 | 18 729 | 94.6 |
| 3 | M | 48 | Negative | 9 | B/S | 14 070 | 12 985 | 92.3 |
| 4 | M | 35 | Negative | 9 | B/S | 11 795 | 7928 | 67.2 |
| 5 | M | 62 | Negative | 9 | B/S | 11 271 | 7642 | 67.8 |
| 6 | F | 44 | S891A | 4 | B/S | 10 087 | 7644 | 75.8 |
| 7 | F | 46 | C634Y | 1 | B/S | 9193 | 5414 | 58.9 |
| 8 | F | 44 | Negative | 7 | B/S | 941 | 848 | 90.1 |
| 9 | F | 67 | Negative | 14 | B/S | 744 | 593 | 79.7 |
| 10 | F | 57 | S891A | 4 | B/S | 429 | 438 | 102.1 |
| 11 | F | 22 | C634Y | 6 | B/S | 249 | 338 | 135.7 |
| 12 | M | 57 | S891A | NP | B/S | 114 | 59 | 51.7 |
| 13 | M | 41 | C634Y | 3 | B/S | 87 | 78 | 89.7 |
| 14 | F | 39 | Negative | 6 | B/S | 57 | 49 | 86.0 |
| Patient ID . | Gender . | Age, y . | RET Mutation . | Years Since Thyroidectomy . | Disease Status . | CT, pg/mL . | CT-PEG, pg/mL . | Recovery, % . |
|---|---|---|---|---|---|---|---|---|
| A | F | 66 | Negative | 12 | B | 261 | 9 | 3.4 |
| B | F | 40 | E761D | 4 | B | 36 | 0.6 | 1.7 |
| C | M | 16 | V804M | 4 | B | 38 | 2.8 | 7.4 |
| 1 | F | 22 | C634Y | 2 | B/S | 21 754 | 18 567 | 85.3 |
| 2 | F | 28 | Negative | 5 | B/S | 19 800 | 18 729 | 94.6 |
| 3 | M | 48 | Negative | 9 | B/S | 14 070 | 12 985 | 92.3 |
| 4 | M | 35 | Negative | 9 | B/S | 11 795 | 7928 | 67.2 |
| 5 | M | 62 | Negative | 9 | B/S | 11 271 | 7642 | 67.8 |
| 6 | F | 44 | S891A | 4 | B/S | 10 087 | 7644 | 75.8 |
| 7 | F | 46 | C634Y | 1 | B/S | 9193 | 5414 | 58.9 |
| 8 | F | 44 | Negative | 7 | B/S | 941 | 848 | 90.1 |
| 9 | F | 67 | Negative | 14 | B/S | 744 | 593 | 79.7 |
| 10 | F | 57 | S891A | 4 | B/S | 429 | 438 | 102.1 |
| 11 | F | 22 | C634Y | 6 | B/S | 249 | 338 | 135.7 |
| 12 | M | 57 | S891A | NP | B/S | 114 | 59 | 51.7 |
| 13 | M | 41 | C634Y | 3 | B/S | 87 | 78 | 89.7 |
| 14 | F | 39 | Negative | 6 | B/S | 57 | 49 | 86.0 |
Abbreviations: F, female; M, male; NP, not performed; B, presenting only biochemical disease; B/S, presenting biochemical and structural disease. Blood serum from patients A, B, and C was suspected for CT immunofluorometric assay interference. RET mutation indicates identified germ line RET mutation. CT was measured by the in-house assay. CT-PEG and CT were measured after PEG treatment.
Analyzes by gel filtration on a Biosep S-3000 column showed that CT obtained from washout of fine-needle aspiration of a metastatic MTC lymph node (Figure 1A) had an immunometric CT elution profile between 20 and 23 minutes, which corresponds to peptides of low molecular weight. As expected, the two serum samples from patients with metastatic MTC (patients 10 and 11) showed a similar retention time for immunometric CT when compared with the CT washout sample (Figure 1, B and C). Surprisingly, the patient with low recovery (Figure 1D) had almost all immunometric CT eluted as a high molecular mass aggregate (>300 kDa).
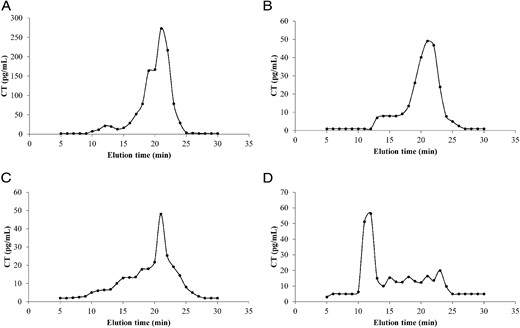
Representative elution profiles of immunometric serum calcitonin (CT) observed through ultra-performance liquid chromatography. Elution distribution from a sample obtained from the washout of fine-needle aspiration of a metastatic MTC lymph node (A) and serum from patients with high (B and C) and low (D) CT recovery after PEG treatment. Sera samples were chromatographed on a Biosep S-3000 column 30 × 6.7 mm (Phenomenex) coupled to an ultra-performance liquid chromatographer.
Figure 2 shows the elution profiles of CT that did and did not bind protein A-Sepharose. As expected, standard CT (Figure 2A) and serum from patients with metastatic MTC (Figure 2, B and C) showed immunoreactivity for CT in early elution steps, confirming that CT was not retained in the column. However, when samples suspected of assay interference were applied to the protein A-Sepharose, CT immunoreactivity was almost completely retained on the column and was only released after the addition of 0.1 m glycine-HCl (Figure 2, D–F), clearly indicating an Ig-CT complex.
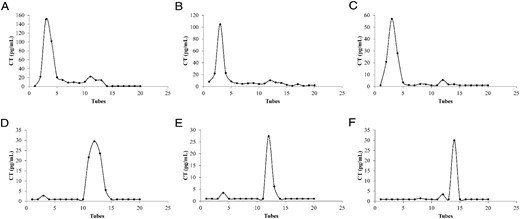
Calcitonin (CT) immunoreactivity after elution via protein A-Sepharose affinity chromatography. WHO International Reference Standard for Human Calcitonin 89/620, National Institute for Biological Standards and Control (A), serum from patients with metastatic MTC (B and C), and patients with MTC carrying interference in CT assay (D–F) obtained through the column and the elution on protein A-Sepharose.
Sera from the three reported cases were negative for thyroglobulin autoantibody, thyroperoxidase autoantibody, and procalcitonin.
Discussion
To our knowledge, the present study demonstrates for the first time the presence of a new interference in the serum CT immunoassay, herein termed macro-CT. This new interference results in a falsely increased CT measurement and represents a new challenge for MTC management.
CT is recognized as the cornerstone for the management of MTC. Screening for MTC in nodular thyroid disease using serum CT measurements has identified this tumor in 0.3–1.5% of cases (4, 10). In this setting, some authors argue for CT serum testing in the context of thyroid nodule evaluation to allow for the early diagnosis of a potentially more aggressive tumor (1, 14, 15) and to improve the sensitivity of fine-needle aspiration cytology, which will actually confirm the diagnosis of MTC in less than 50% of patients (16). However, this issue is controversial, and many questions remain unanswered, especially regarding cost effectiveness, the availability of pentagastrin for stimulation testing, and the difficult interpretation of false-positive serum CT levels (3, 4). In this regard, when evaluating preoperative thyroid nodules with serum CT determination, clinicians should be aware that variable elevations in CT levels may also underlie several clinical conditions, such as autoimmune thyroiditis, chronic renal failure, hypergastrinemia, hyperparathyroidism, and inflammatory diseases and numerous tumors, especially those derived from neuroendocrine tissue from the intestine and lungs (3, 17). Depending on the immunoassay performed, approximately 10% of normal subjects might have CT levels greater than 10 pg/mL (3). Additionally, the serum CT level varies according to age, sex, physical activity, and the use of common drugs such as proton pump blockers, beta blockers, and calcium (17). During the follow-up of thyroidectomized patients for MTC, certain concerns remain because interference in the CT immunoassay cannot be avoided entirely (3).
Thus, despite the high reliability and sensitivity of the new immunochemiluminometric CT measurement (18, 19), some concerns persist, especially regarding the presence of falsely elevated serum CT levels. With these new immunoassays, cross-reactivity with procalcitonin and other related peptides has been almost completely abolished, but the presence of heterophilic antibodies resulting in falsely increased levels is not negligible and occurs in up to 3.7% of cases (20).
In the present study, sera from the three reported cases were negative for antithyroid antibodies and procalcitonin. Furthermore, the presence of heterophilic antibodies was also excluded once the CT results maintained linearity after dilution; this interference was also prevented by adding an excess of mouse serum during the routine CT immunometric assay.
Another possibility for the unexpectedly high level of CT in these three reported cases could be the presence of antibodies against CT in the sera. In fact, the interference of endogenous antibodies during routine immunoassays has already been described for some proteins and peptides, especially for prolactin, insulin, GH, LH, and TSH (21–23), but thus far not for CT. The nature of this phenomenon involves the formation of macroaggregates between the peptide and Ig, mainly IgG, but other molecular modifications, including glycosylation and covalent/noncovalent binding, are also possible (24). Our findings showed a similar mechanism as demonstrated by three strategies: demonstration of low recovery after PEG treatment, the presence of immunoreactivity for CT as a macroaggregate in the gel filtration chromatography, and the presence of CT-Ig complexes by protein A-Sepharose analysis. However, some care may be needed when finding unexpectedly high levels of CT. The macro-CT phenomenon may be patient sample- and assay system-dependent. An example of this was the discrepancy observed between serum CT values obtained by the in-house method compared with the commercial test. These disagreements have also been observed in the description of other autoimmune macroaggregate phenomena, such as for macroprolactin (25–27), macro-TSH (21, 28), and macro-FSH (23). Therefore, it is possible that the same phenomenon is occurring with macro-CT. This could occur due to differences in the affinity of CT for endogenous anti-CT antibody or the antibodies used by each different CT immunoassay. Another possibility is that, for different serum samples, there were anti-CT antibodies directed against different epitopes, which could also influence CT recognition by a specific method (27). Therefore, if the endogenous anti-CT antibody binds to a specific CT peptide site required for recognition of an immunoassay, this method will not recognize CT adequately.
Although the exact etiology and frequency of the macro-CT phenomenon remain to be explored, its discovery opens new perspectives for refining the management of patients with MTC. Additional importance for this work arises if a CT measurement was used in the initial management of thyroid nodules, as proposed by some authors (1, 14, 15). In these cases, additional caution must be taken, especially if the CT measurement was only moderately increased (<150 pg/mL). Finally, rather than a simple immunological artifact, macro-CT recognition is noteworthy because it can help clinicians avoid unnecessary diagnostic investigations and treatments during MTC follow-up.
Acknowledgments
The authors are very grateful to Dr. M. Conceição Mamone for her expertise in thyroid cytopathology, to Alberto L. Machado for his expertise in neck ultrasound, to Gilberto K. Furuzawa for laboratory help, to Dr. Flávia F. Valente for information about patients, and to Angela M. Faria and Yeda Queiroga for efficient laboratory administration. We are also very grateful to the Head and Neck Surgical team, particularly Drs. Flavio C. Hojaij, Marcel Palumbo, and Fabio Brodskyn.
This work was supported by research grants from the São Paulo State Research Foundation-FAPESP (Grants 2006/60402-1 and 2010/51547-1) (to R.M.B.M., J.G.H.V., J.R.M.M., and M.R.D.d.S.), by FAPESP Fellowship Grant 2010/19478 (to T.G.A.), by the Federal Agency of Support and Evaluation of Postgraduate Education (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), and by the National Council for Scientific and Technological Development. J.G.H.V. and R.M.B.M. are investigators of the Fleury Group.
Disclosure Summary: The authors have nothing to disclosure.



