-
PDF
- Split View
-
Views
-
Cite
Cite
Paulo Homem de Mello Bianchi, Gabriela Romanenghi Fanti Carvalho Araujo Gouveia, Elaine M. Frade Costa, Sorahia Domenice, Regina M. Martin, Luciane Carneiro de Carvalho, Tatiana Pelaes, Marlene Inacio, Rodrigo Rocha Codarin, Maria Beatriz Sator de Faria, Rossana Pulcineli Vieira Francisco, Edmund Chada Baracat, Paulo César Serafini, Berenice B. Mendonca, Successful Live Birth in a Woman With 17α-Hydroxylase Deficiency Through IVF Frozen-Thawed Embryo Transfer, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 2, 1 February 2016, Pages 345–348, https://doi.org/10.1210/jc.2015-3201
Close - Share Icon Share
Abstract
Congenital adrenal hyperplasia (CAH) due to 17α-hydroxylase deficiency in 46,XX patients is characterized by primary amenorrhea, absent or incomplete sexual maturation, infertility, low serum levels of estradiol, and elevated progesterone (P). There were no previous reports of singleton live births from such women.
To describe the first successful singleton live birth in a female with CAH due to 17α-hydroxylase deficiency.
A 26-year-old Brazilian woman with CAH associated with 17α-hydroxylase deficiency due to the compound heterozygote mutation (p.W406R/P428L) in the CYP17A1 gene expressed the desire to conceive. In vitro fertilization (IVF) was recommended due to the complexity of the disorder. The first attempt of treatment failed despite the production of viable embryos. At the second IVF attempt, all viable embryos were frozen due to inadequate endometrial development associated with prematurely elevated serum P during ovarian stimulation. Subsequently, a long-acting GnRH agonist and oral dexamethasone were used to lower ovarian and adrenal P overproduction. Once serum levels of P were < 1 ng/mL, endometrial preparation with estradiol valerate and frozen-thawed embryo transfer were performed, resulting in a singleton pregnancy. Estradiol supplementation was completely suspended by 14 weeks of gestation. She delivered at 30 weeks and 4 days due to acute fetal distress. The puerperium was uneventful; the newborn was discharged in good conditions 5 weeks after birth.
A successful live birth was achieved in a woman with 17-hydroxylase deficiency through IVF, cryopreservation of all embryos, and frozen-thawed embryo transfer after adequate endometrial preparation.
Mutations in the CYP17A1 gene account for < 1% of congenital adrenal hyperplasia (CAH) cases approximately (1). Complete enzymatic deficiency in 46,XX patients is associated with hypertension, hypergonadotropic hypogonadism (2), follicular arrest at the early antral stages (3), and hypoplastic uterus, rendering the bearer of the mutation infertile. On the other hand, spontaneous menarche and regular menstrual cycles have been described in women with incomplete 17α-hydroxylase deficiency (17OHD) (4).
Reports on fertility treatments of females with 17OHD are even rarer. A recent review (1) identified five articles reporting on the outcome of assisted reproductive treatments in eight women with incomplete 17OHD. Although there was follicular development and oocytes were harvested in all of those women, treatments did not result in pregnancies because of either in vitro embryo developmental arrest or unsuccessful embryo implantation due to poor endometrial quality. There is one report in the literature of a viable pregnancy in a woman with 17OHD in which embryos produced with donated oocytes were transferred to the uterus (5). The patient had to use a subcutaneous 17 β-estradiol implant associated with cyclical im progesterone administration to achieve adequate endometrial maturation. At the fifth embryo transfer attempt, the treatment resulted in a twin pregnancy that was further complicated with severe pre-eclampsia, hemolysis, elevated liver enzymes low platelet (HELLP) syndrome, and premature delivery; one newborn died minutes after delivery, whereas the other was kept for several weeks at the neonatal intensive care unit and discharged without apparent disabilities.
We report the first live singleton birth from a woman with partial 17OHD, using her own oocytes.
Case Report
In 2009, a 46,XX patient (age, 26 y) with CAH, associated with 17OHD due to compound heterozygous mutation (p.W406R/P428L) in the CYP17A1 gene, expressed the desire to have a child. She had been followed at the Endocrinology Division of Hospital das Clínicas da Faculdade de Medicina da Universidade de Sao Paulo (HCFMUSP) where she presented at age 24 years for inadequate breast development, primary amenorrhea, and blood hypertension, resistant to conventional antihypertensives drugs. Since 2007, she was using oral dexamethasone 0.5 mg/d and hormonal replacement therapy (HRT) with conjugated equine estrogen (CEE) 0.625 mg/d. The biochemical diagnosis of 17OHD was based on basal hypokalemia (K = 3.3 mEq/L), elevated ACTH levels (120 pg/mL), cortisol levels with blunted response after ACTH stimulation (4 to 14 μg/dL), elevated basal progesterone levels (5.2 ng/mL), low testosterone (<11 ng/dL) and androstenedione (<0.2 ng/mL) levels, and low plasma renin activity (0.5 ng/mL/h).
The patient was first referred to a private infertility clinic and underwent in vitro fertilization (IVF). Ovulation induction (OI) succeeded in producing mature oocytes and embryos but did not result in a pregnancy. She temporarily declined another IVF attempt and resumed treatment with dexamethasone and HRT.
Three years later, she was referred to Centro de Reprodução Humana do HCFMUSP, willing to resume the fertility treatment. She was 29 years old, and her body mass index was 37.7 kg/m2. No other infertility factor was identified. Considering the reproductive consequences of 17OHD, IVF was again recommended.
Figure 1 summarizes the treatment strategy. Oral dexamethasone acetate (0.5 mg/d) was maintained during all treatment phases. After signing an informed consent, the patient began HRT with oral estradiol valerate (4 mg/d). Twelve days later, daily natural micronized progesterone (200 mg/d) was added vaginally. Beginning on the 19th day of HRT, leuprolide acetate (0.5 mg sc) was administered daily to achieve down-regulation of pituitary FSH and LH secretion (long GnRH agonist protocol). HRT was suspended 3 days later, and she experienced menstrual bleeding. A transvaginal ultrasound (TVUS) was performed on the second day of the menstrual cycle. Endometrial thickness was 6 mm, and 32 follicles < 8 mm were identified. Serum estradiol was < 13 pg/mL, and progesterone was 1.7 ng/mL. The daily dose of leuprolide was reduced to 0.25 mg, and OI started with recombinant FSH (rFSH) 112.5 U/d associated with oral CEE (0.625 mg/d). Follicular development was assessed by serial TVUS on days 5, 7, 9, and 11 of OI. The daily dose of rFSH was not altered throughout OI (total, 1237.5 U).
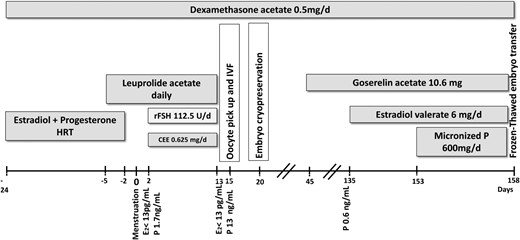
At the end of OI, there were four follicles ≥ 15 mm; serum estradiol was < 13 pg/mL, but progesterone was 13.1 ng/mL. Recombinant human chorionic gonadotropin (rhCG) was administered to induce final follicular maturation, and 36 hours later, four mature oocytes were retrieved through transvaginal follicular aspiration guided by ultrasound under anesthesia. The oocytes were fertilized by a fresh processed semen sample (64 million motile spermatozoa after processing) 3 hours after oocyte retrieval. The resulting embryos were cultured for 5 days; two achieved the blastocyst stage. Because elevated serum progesterone levels at the end of OI are associated with lower implantation rates (6), all embryos were cryopreserved (vitrification) (7).
One month after oocyte retrieval, the patient received 10.6 mg of the depot goserelin acetate (GnRH agonist) subcutaneously to reduce ovarian progesterone production. Also, oral dexamethasone (0.5 mg/d) was maintained to diminish adrenal progesterone production. Three months later, serum progesterone was 0.6 ng/mL, and endometrial preparation was started with oral estradiol valerate (6 mg/d). When endometrial thickness reached 7.9 mm on TVUS (18th day of endometrial stimulation), natural micronized progesterone (600 mg/d) was administered vaginally, and 5 days later, the two frozen embryos were thawed and transferred to the uterus. β-hCG tested positive 14 days later, and a singleton pregnancy was confirmed on TVUS performed at the sixth week of gestation.
According to gestational age, the patient received different regimens of estradiol and micronized progesterone supplementation. From the 11th week of gestation on, the estradiol daily dose was reduced gradually; serum estradiol levels remained stable, indicating normal placental steroidogenesis. Estradiol supplementation was completely suspended by 14 weeks of gestation. Vaginal progesterone supplementation and oral dexamethasone acetate (0.5 mg/d) were maintained through the entire duration of pregnancy. Table 1 summarizes hormonal and treatment data of the patient before, during, and after pregnancy.
Hormonal Data of the Patient With 17-Hydroxylase Deficiency Before, During, and After Pregnancy
| LH, U/L . | FSH, U/L . | E2, pg/mL . | P, ng/mL . | hCG, mU/L . | Condition . | Treatment . |
|---|---|---|---|---|---|---|
| 13.7 | 8.2 | 22 | 5.2 | Basal | Pretreatment | |
| 18.3 | 6.7 | 4.0 | Dex suppression | 0.5 mg, 4 × day for 4 d | ||
| 19.1 | 7.4 | 28 | 6.0 | Before pregnancy | Dex 0.5 mg + CEE 0.625 mg (oral) | |
| <0.6 | <0.1 | 208 | 37.3 | 185 274 | 7 wk pregnant | Dex 0.5 mg + EV 2 mg (oral) + EV 6 mg (vaginal) + MP 300 mg (vaginal) |
| <0.6 | <0.1 | 421 | 46 | 158 046 | 8 wk pregnant | Dex 0.5 mg + EV 2 mg (oral) + E2 6 mg (transdermal) + MP 600 mg (vaginal) |
| <0.6 | <0.1 | 546 | >60 | 189 108 | 11 wk pregnant | Dex 0.5 mg + EV 2 mg (oral) + E2 4 mg (transdermal) + MP 600 mg (vaginal) |
| <0.6 | <0.1 | 381 | 55.8 | 153 755 | 12 wk pregnant | Dex 0.5 mg + E2 2 mg (transdermal) + MP 600 mg (vaginal) |
| <0.6 | <0.1 | 482 | >60 | 112 461 | 14 wk pregnant | Dex 0.5 mg + MP 600 mg (vaginal) |
| <0.6 | <0.1 | 930 | >60 | 23 wk pregnant | Dex 0.5 mg + MP 200 mg (vaginal) + β-blocker 10 mg | |
| 11.8 | 10 | 17.3 | 8.6 | 24 d after birth | Dex 0.375 mg + captopril 50 mg |
| LH, U/L . | FSH, U/L . | E2, pg/mL . | P, ng/mL . | hCG, mU/L . | Condition . | Treatment . |
|---|---|---|---|---|---|---|
| 13.7 | 8.2 | 22 | 5.2 | Basal | Pretreatment | |
| 18.3 | 6.7 | 4.0 | Dex suppression | 0.5 mg, 4 × day for 4 d | ||
| 19.1 | 7.4 | 28 | 6.0 | Before pregnancy | Dex 0.5 mg + CEE 0.625 mg (oral) | |
| <0.6 | <0.1 | 208 | 37.3 | 185 274 | 7 wk pregnant | Dex 0.5 mg + EV 2 mg (oral) + EV 6 mg (vaginal) + MP 300 mg (vaginal) |
| <0.6 | <0.1 | 421 | 46 | 158 046 | 8 wk pregnant | Dex 0.5 mg + EV 2 mg (oral) + E2 6 mg (transdermal) + MP 600 mg (vaginal) |
| <0.6 | <0.1 | 546 | >60 | 189 108 | 11 wk pregnant | Dex 0.5 mg + EV 2 mg (oral) + E2 4 mg (transdermal) + MP 600 mg (vaginal) |
| <0.6 | <0.1 | 381 | 55.8 | 153 755 | 12 wk pregnant | Dex 0.5 mg + E2 2 mg (transdermal) + MP 600 mg (vaginal) |
| <0.6 | <0.1 | 482 | >60 | 112 461 | 14 wk pregnant | Dex 0.5 mg + MP 600 mg (vaginal) |
| <0.6 | <0.1 | 930 | >60 | 23 wk pregnant | Dex 0.5 mg + MP 200 mg (vaginal) + β-blocker 10 mg | |
| 11.8 | 10 | 17.3 | 8.6 | 24 d after birth | Dex 0.375 mg + captopril 50 mg |
Abbreviations: E2, estradiol; P, progesterone; Dex, dexamethasone; EV, estradiol valerate; MP, micronized progesterone.
Hormonal Data of the Patient With 17-Hydroxylase Deficiency Before, During, and After Pregnancy
| LH, U/L . | FSH, U/L . | E2, pg/mL . | P, ng/mL . | hCG, mU/L . | Condition . | Treatment . |
|---|---|---|---|---|---|---|
| 13.7 | 8.2 | 22 | 5.2 | Basal | Pretreatment | |
| 18.3 | 6.7 | 4.0 | Dex suppression | 0.5 mg, 4 × day for 4 d | ||
| 19.1 | 7.4 | 28 | 6.0 | Before pregnancy | Dex 0.5 mg + CEE 0.625 mg (oral) | |
| <0.6 | <0.1 | 208 | 37.3 | 185 274 | 7 wk pregnant | Dex 0.5 mg + EV 2 mg (oral) + EV 6 mg (vaginal) + MP 300 mg (vaginal) |
| <0.6 | <0.1 | 421 | 46 | 158 046 | 8 wk pregnant | Dex 0.5 mg + EV 2 mg (oral) + E2 6 mg (transdermal) + MP 600 mg (vaginal) |
| <0.6 | <0.1 | 546 | >60 | 189 108 | 11 wk pregnant | Dex 0.5 mg + EV 2 mg (oral) + E2 4 mg (transdermal) + MP 600 mg (vaginal) |
| <0.6 | <0.1 | 381 | 55.8 | 153 755 | 12 wk pregnant | Dex 0.5 mg + E2 2 mg (transdermal) + MP 600 mg (vaginal) |
| <0.6 | <0.1 | 482 | >60 | 112 461 | 14 wk pregnant | Dex 0.5 mg + MP 600 mg (vaginal) |
| <0.6 | <0.1 | 930 | >60 | 23 wk pregnant | Dex 0.5 mg + MP 200 mg (vaginal) + β-blocker 10 mg | |
| 11.8 | 10 | 17.3 | 8.6 | 24 d after birth | Dex 0.375 mg + captopril 50 mg |
| LH, U/L . | FSH, U/L . | E2, pg/mL . | P, ng/mL . | hCG, mU/L . | Condition . | Treatment . |
|---|---|---|---|---|---|---|
| 13.7 | 8.2 | 22 | 5.2 | Basal | Pretreatment | |
| 18.3 | 6.7 | 4.0 | Dex suppression | 0.5 mg, 4 × day for 4 d | ||
| 19.1 | 7.4 | 28 | 6.0 | Before pregnancy | Dex 0.5 mg + CEE 0.625 mg (oral) | |
| <0.6 | <0.1 | 208 | 37.3 | 185 274 | 7 wk pregnant | Dex 0.5 mg + EV 2 mg (oral) + EV 6 mg (vaginal) + MP 300 mg (vaginal) |
| <0.6 | <0.1 | 421 | 46 | 158 046 | 8 wk pregnant | Dex 0.5 mg + EV 2 mg (oral) + E2 6 mg (transdermal) + MP 600 mg (vaginal) |
| <0.6 | <0.1 | 546 | >60 | 189 108 | 11 wk pregnant | Dex 0.5 mg + EV 2 mg (oral) + E2 4 mg (transdermal) + MP 600 mg (vaginal) |
| <0.6 | <0.1 | 381 | 55.8 | 153 755 | 12 wk pregnant | Dex 0.5 mg + E2 2 mg (transdermal) + MP 600 mg (vaginal) |
| <0.6 | <0.1 | 482 | >60 | 112 461 | 14 wk pregnant | Dex 0.5 mg + MP 600 mg (vaginal) |
| <0.6 | <0.1 | 930 | >60 | 23 wk pregnant | Dex 0.5 mg + MP 200 mg (vaginal) + β-blocker 10 mg | |
| 11.8 | 10 | 17.3 | 8.6 | 24 d after birth | Dex 0.375 mg + captopril 50 mg |
Abbreviations: E2, estradiol; P, progesterone; Dex, dexamethasone; EV, estradiol valerate; MP, micronized progesterone.
Pregnancy was complicated by pre-eclampsia, gestational diabetes (requiring insulin administration), cholestasis gravidarum (requiring ursacol administration), and cellulitis of the lower right extremity. At 30 weeks and 4 days, an emergency cesarean section was performed due to acute fetal distress. A true umbilical knot was identified, and a live normal male newborn was delivered: weight, 1945 g; length, 43.5 cm; Apgar score, 7/10/10. The puerperium was uneventful. Five weeks after delivery, the newborn was discharged in good condition and taken home.
Discussion
The difficulties in achieving a viable pregnancy in women with 17OHD are at least partially explained by the effects of estradiol deficiency and progesterone excess, accentuated by OI, on follicular development and endometrial maturation.
Complete estradiol deficiency is associated with follicular developmental arrest (3), yet there is evidence of adequate oocyte maturation and viable embryo formation in a low estradiol follicular milieu (8). In the case reported above, the patient had incomplete 17OHD and, although serum estradiol was < 13 pg/mL at the end of OI, some intrafollicular estrogen activity probably had occurred secondary to the concomitant administration of CEE and/or via limited endogenous production. Previous functional studies in transiently transfected COS-7 cells expressing the cDNA for CYP17A1 wild type or for p.W406R and p.P428L mutants showed absent 17-hydroxylase activity for the W406R mutant and residual hydroxylase activity for the p.P428L mutant. Activities of these CYP17A1 mutants in Saccharomyces cerevisiae confirm these results (9).
Evidence suggests that premature elevation of serum progesterone negatively influences live birth rates in IVF (6). This effect is only observed in fresh embryo transfer cycles and is independent of other prognostic factors. It was not observed, however, when embryos originating in IVF cycles with elevated progesterone were used in oocyte donation programs or were frozen for subsequent frozen-thawed embryo transfer (FET) (6). Also, several studies reporting on the outcomes of OI during the luteal phase of the menstrual cycle demonstrated that progesterone does not preclude follicular growth and oocyte viability, and it is possible to achieve live births after FET (10). Therefore, the predominant effect of the premature elevation of progesterone in fresh IVF cycles is not on the oocyte, but rather is on the endometrium, and it can be overcome with cryopreservation and FET.
In conclusion, the strategy described above resulted, for the first time, in a live birth from a female patient with 17OHD through IVF and FET, after adequate hormonal control and endometrial preparation maneuvers.
Acknowledgments
This work was partially supported by grants from Fundação de Amparo a Pesquisa do estado de Sao Paulo (2013/02162–8) and from Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (301339/2008–9; to B.B.M).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- CAH
congenital adrenal hyperplasia
- CEE
conjugated equine estrogen
- FET
frozen-thawed embryo transfer
- HRT
hormonal replacement therapy
- IVF
in vitro fertilization
- 17OHD
17α-hydroxylase deficiency
- OI
ovulation induction
- rFSH
recombinant FSH
- rhCG
recombinant humanchorionic gonadotropin
- TVUS
transvaginal ultrasound.



