-
PDF
- Split View
-
Views
-
Cite
Cite
Niklas Zethraeus, Anna Dreber, Eva Ranehill, Liselott Blomberg, Fernand Labrie, Bo von Schoultz, Magnus Johannesson, Angelica Lindén Hirschberg, Combined Oral Contraceptives and Sexual Function in Women—a Double-Blind, Randomized, Placebo-Controlled Trial, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 11, 1 November 2016, Pages 4046–4053, https://doi.org/10.1210/jc.2016-2032
Close - Share Icon Share
There is a lack of knowledge about how oral contraceptives may affect sexual function.
To determine whether there is a causal effect of oral contraceptives on sexuality. We hypothesized that a widely used pill impairs sexuality.
A double-blind, randomized, placebo-controlled trial. Enrollment began in February 2012 and was completed in August 2015.
Karolinska University Hospital, Stockholm, Sweden.
A total of 340 healthy women, aged 18–35 years, were randomized to treatment, and 332 completed the study.
A combined oral contraceptive (150 μg levonorgestrel and 30 μg ethinylestradiol) or placebo for 3 months of treatment.
The primary outcome was the aggregate score on the Profile of Female Sexual Function (PFSF). Secondary outcomes were the seven domains of the PFSF, the Sexual Activity Log, and the Personal Distress Scale.
Overall sexual function was similar in women in the oral contraceptive and placebo groups. The PFSF domains desire (−4.4; 95% confidence interval [CI], −8.49 to −0.38; P = .032), arousal (−5.1; 95% CI, −9.63 to −0.48; P = .030), and pleasure (−5.1; 95% CI, −9.97 to −0.32; P = .036) were significantly reduced in comparison to placebo, whereas orgasm, concern, responsiveness, and self-image were similar between groups. The mean frequency of satisfying sexual episodes and personal distress were also similar between groups.
This study shows no negative impact of a levonorgestrel-containing oral contraceptive on overall sexual function, although three of seven sexual function domains were adversely affected.
The oral contraceptive pill (OC) has been on the market for more than 50 years and has had a tremendous impact on women’s reproductive health and role in modern society. OC treatment is recognized as a highly effective contraceptive method and a generally safe medication. Still, there is a remarkable lack of basic knowledge about how OC use may affect women’s quality of life, sexuality, and behavior. In clinical practice, many women report negative side effects like mood changes and decreased libido leading to the discontinuation of medication (1–4) and a subsequent risk of unwanted pregnancies and induced abortions. Sexual dissatisfaction has been reported to be among the best predictors of OC discontinuation, although other symptoms such as irregular bleeding, breast tenderness, and mood changes also are common causes (1, 4).
According to a recent systematic review on the influence of combined OCs on sexual function (5), most OC users showed no significant change or increase in libido (85%), whereas 15% reported a decreased libido. However, most studies were retrospective, prospective, uncontrolled, or randomized comparisons between different types of OCs without a placebo group (5). Only two small placebo-controlled randomized studies were published between the years 1978 to 2011, both with inconclusive results (6, 7). The current lack of knowledge might reflect low interest from the pharmaceutical industry to perform placebo-controlled studies and the considerable financial costs to perform large-scale trials.
The aim of the present study was to address a lack of knowledge regarding a possible causal effect of OC treatment on sexual function in women. We thus performed a double-blind, randomized, and placebo-controlled trial on a levonorgestrel-containing combined OC in 340 young, healthy women. The effect of OC use on sexual function was explored, and our hypothesis was that OCs decrease sexual function (6, 7). The primary outcome measure was the aggregate score on the validated Profile of Female Sexual Function (PFSF) instrument (8). As secondary outcome measures, we investigated each of the seven domains of the PFSF, the Sexual Activity Log (SAL), and the Personal Distress Scale (PDS) (8). In exploratory analyses, changes in sexual function were related to changes in serum levels of total and free T.
Subjects and Methods
This was a single-center, randomized, double-blind, placebo-controlled, parallel study conducted at the Karolinska University Hospital, Stockholm, Sweden. Women fulfilling all the inclusion criteria (age, 18–35 years; body mass index [BMI], 19–30 kg/m2; regular menstrual cycle, 25–33 days; using nonhormonal contraceptive at study start; and being fluent in Swedish) and none of the exclusion criteria (smoking, hypertension, risk factors for thromboembolism and cardiovascular disease, severe migraine, diabetes mellitus, liver disease, pancreatitis, history of hormone-dependent cancer, undiagnosed bleeding, and pregnancy) were included in the study. The women were instructed to use nonhormonal contraceptive methods during the study.
The study (EudraCT no. 2010-020824-23), which was approved by the local ethics committee in Stockholm (2010/1075-31/1) and the Swedish Medical Products Agency (151:2010/44213), was carried out in accordance with “good clinical practice and the World Medical Association Declaration of Helsinki – Ethical principles for medical research involving human subjects.” All participants gave written informed consent.
Participants were randomly assigned to treatment with placebo or a combined OC (Neovletta) containing 150 μg levonorgestrel and 30 μg ethinylestradiol during a period of 3 months. Randomization was provided by a contract organization (APL, Pharma Specials). A balanced block randomization [1:1] was carried out with 10 participants in each block of fixed size. The study investigators, research coordinators, and participating women were blinded to treatment allocation.
Participants took one capsule every day for 21 days, followed by a 7-day break. Baseline data collection was performed in the early follicular phase of the menstrual cycle (cycle days 1–7), before the start of treatment, and final data collection was performed at the end of the third treatment cycle. At both occasions, a blood sample (20 mL) was collected for analyses of hormones and binding proteins, and participants filled out three surveys on sexual function: the weekly SAL (8), the PFSF (8–11), and the PDS (8, 12).
Our primary outcome measure of sexual function consisted of the average of the normalized score on the seven PFSF domains and was denoted “PFSF total” with a range from 0 to 100. The normalized score on each PFSF domain was defined as a secondary outcome measure. A score < 40 in the sexual-desire domain indicated low sexual desire (8). The SAL measure (the number of satisfying sexual episodes over a 1-week period) and the PDS measure (normalized as 0 [no distress] to 100 [maximum distress]) were also defined as secondary outcome measures. A score > 40 indicated distress (8).
Measurement of T and estradiol in serum was performed by the highly sensitive and specific liquid chromatography tandem mass spectrometry method (13). Serum concentrations of FSH and LH were determined by fluorescence immunoassays, and SHBG concentration was determined by chemiluminescent enzyme immunometric assay. Concentrations of free T were calculated from values of total T, SHBG, and a fixed albumin concentration based on an equation system derived from the law of mass action (14).
The data analysis was conducted according to a predefined protocol posted at the Open Science Framework website (freely available at https://osf.io/he8nb/). The main analysis was carried out as an intention-to-treat analysis including all women that completed the data collection.
To test whether there was a statistically significant difference in the average value of an outcome measure between the OC and placebo groups, we used an independent samples t test (not assuming equal variances). The tests were based on the difference in the value of the outcome measure between the final data collection and the baseline data collection for each participant, and all statistical tests were two-sided (α level, 0.05). We also estimated the correlation between the pre- and post-treatment change in serum levels of total and free T and the change in sexual function using the Pearson and Spearman correlation coefficients. The sample size of 340 gave a statistical power of 80% to find a significant difference at the 5% level in overall sexual function (PFSF total) of 4.3 U, measured on the PFSF total normalized scale between 0 and 100.
In the Supplemental Data, we provide a complete methods section with details about the study population, study design, treatment, sexual function measurements, safety assessments, hormone measurements, and statistical analysis.
Results
Baseline characteristics
Enrollment began in February 2012 and was completed in August 2015. Of about 1000 women initially screened by phone, 347 were assessed for eligibility at a screening visit, and 340 of these were included and scheduled for their first visit at baseline. All women who showed up at the baseline visit (n = 340) were randomly assigned to either the OC or placebo group. Seven participants were lost to follow-up, and one participant did not complete the data collection due to an administrative error (Figure 1). All women who completed the data collection at follow-up (n = 332) were included in our main analysis. Three of these women discontinued treatment but completed the data collection.

Enrollment and outcomes. *, One participant initially randomized to the treatment group discontinued treatment (after 7 weeks) due to an egg donation but was later randomized into the study again. This explains the uneven number of participants in the two groups. †, All participants who completed the data collection at follow-up were included in our main analysis (n = 164 in the OC group, and n = 168 in the placebo group).
Baseline characteristics were similar among the two treatment groups (Table 1). The mean score for overall sexual function (the average of the seven PFSF domains) did not differ significantly between the two groups, but there was a significant difference in two of the domains (orgasm, P = .02; and concern, P = .02). The 1-week frequency of satisfying sexual episodes and distress (PDS) did not differ significantly between the two treatment groups.
| Characteristic . | Placebo Group . | OC Group . |
|---|---|---|
| n | 171 | 169 |
| Age, y | 24.1 ± 4.0 | 23.3 ± 3.6 |
| Education, n (%)a | ||
| Primary school | 5 (2.9) | 6 (3.6) |
| Secondary school | 53 (31.2) | 58 (34.7) |
| University studies (ongoing) | 64 (37.6) | 56 (33.5) |
| University degree | 48 (28.2) | 47 (28.1) |
| Subjects in stable relationship, n (%)a | 100 (58.8) | 97 (57.7) |
| BMI, kg/m2 | 22.6 ± 2.8 | 22.5 ± 3.1 |
| Low sexual desire, n (%) | ||
| <40 in the sexual desire domain of PFSF | 33 (19.3) | 30 (17.8) |
| Sexual distress, n (%) | ||
| >40 in the PDS | 24 (14.0) | 26 (15.4) |
| Score on PFSF totalb | 69.4 ± 15.9 | 66.9 ± 15.0 |
| Score on PFSF domainsb | ||
| Desire | 56.9 ± 19.5 | 56.7 ± 18.1 |
| Arousal | 78.3 ± 21.0 | 77.9 ± 21.0 |
| Orgasm | 66.4 ± 25.3 | 59.9 ± 26.5 |
| Pleasure | 68.5 ± 26.1 | 68.7 ± 20.8 |
| Concern | 73.9 ± 20.2 | 68.2 ± 23.4 |
| Responsiveness | 78.2 ± 16.6 | 76.2 ± 17.3 |
| Self-image | 64.0 ± 20.3 | 60.7 ± 20.5 |
| Score on PDSc | 16.5 ± 22.4 | 19.6 ± 24.4 |
| No. of satisfying sexual episodes over 1-wk period (SAL) | 2.6 ± 2.5 | 2.5 ± 2.5 |
| Serum levels of blood sample testsd | ||
| FSH, IU/L | 5.4 ± 1.7 | 5.4 ± 1.7 |
| LH, IU/L | 4.9 ± 2.4 | 4.8 ± 2.2 |
| Total T, pg/mL | 246.4 ± 93.7 | 244.3 ± 88.7 |
| SHBG, nmol/L | 78.1 ± 46.7 | 72.6 ± 27.3 |
| Free T, pg/mL | 3.2 ± 1.3 | 3.2 ± 1.3 |
| Estradiol, pg/mL | 33.5 ± 19.1 | 34.4 ± 25.1 |
| Characteristic . | Placebo Group . | OC Group . |
|---|---|---|
| n | 171 | 169 |
| Age, y | 24.1 ± 4.0 | 23.3 ± 3.6 |
| Education, n (%)a | ||
| Primary school | 5 (2.9) | 6 (3.6) |
| Secondary school | 53 (31.2) | 58 (34.7) |
| University studies (ongoing) | 64 (37.6) | 56 (33.5) |
| University degree | 48 (28.2) | 47 (28.1) |
| Subjects in stable relationship, n (%)a | 100 (58.8) | 97 (57.7) |
| BMI, kg/m2 | 22.6 ± 2.8 | 22.5 ± 3.1 |
| Low sexual desire, n (%) | ||
| <40 in the sexual desire domain of PFSF | 33 (19.3) | 30 (17.8) |
| Sexual distress, n (%) | ||
| >40 in the PDS | 24 (14.0) | 26 (15.4) |
| Score on PFSF totalb | 69.4 ± 15.9 | 66.9 ± 15.0 |
| Score on PFSF domainsb | ||
| Desire | 56.9 ± 19.5 | 56.7 ± 18.1 |
| Arousal | 78.3 ± 21.0 | 77.9 ± 21.0 |
| Orgasm | 66.4 ± 25.3 | 59.9 ± 26.5 |
| Pleasure | 68.5 ± 26.1 | 68.7 ± 20.8 |
| Concern | 73.9 ± 20.2 | 68.2 ± 23.4 |
| Responsiveness | 78.2 ± 16.6 | 76.2 ± 17.3 |
| Self-image | 64.0 ± 20.3 | 60.7 ± 20.5 |
| Score on PDSc | 16.5 ± 22.4 | 19.6 ± 24.4 |
| No. of satisfying sexual episodes over 1-wk period (SAL) | 2.6 ± 2.5 | 2.5 ± 2.5 |
| Serum levels of blood sample testsd | ||
| FSH, IU/L | 5.4 ± 1.7 | 5.4 ± 1.7 |
| LH, IU/L | 4.9 ± 2.4 | 4.8 ± 2.2 |
| Total T, pg/mL | 246.4 ± 93.7 | 244.3 ± 88.7 |
| SHBG, nmol/L | 78.1 ± 46.7 | 72.6 ± 27.3 |
| Free T, pg/mL | 3.2 ± 1.3 | 3.2 ± 1.3 |
| Estradiol, pg/mL | 33.5 ± 19.1 | 34.4 ± 25.1 |
Values are expressed as means ± SD unless stated otherwise.
There were three missing values on the education variable (two in the OC group and one in the placebo group), and two missing values on the “in a stable relationship” variable (one in the OC group and one in the placebo group).
PFSF total (and each PFSF domain) range from 0 to 100, with higher values indicating greater sexual function. PFSF total is defined as the average of the normalized score on each PFSF domain where each domain is given the same weight.
PDS scores range from 0 to 100, with higher scores indicating more distress.
In the OC group, there were two missing values for FSH, LH, SHBG, and free T and one missing value for both total T and estradiol.
| Characteristic . | Placebo Group . | OC Group . |
|---|---|---|
| n | 171 | 169 |
| Age, y | 24.1 ± 4.0 | 23.3 ± 3.6 |
| Education, n (%)a | ||
| Primary school | 5 (2.9) | 6 (3.6) |
| Secondary school | 53 (31.2) | 58 (34.7) |
| University studies (ongoing) | 64 (37.6) | 56 (33.5) |
| University degree | 48 (28.2) | 47 (28.1) |
| Subjects in stable relationship, n (%)a | 100 (58.8) | 97 (57.7) |
| BMI, kg/m2 | 22.6 ± 2.8 | 22.5 ± 3.1 |
| Low sexual desire, n (%) | ||
| <40 in the sexual desire domain of PFSF | 33 (19.3) | 30 (17.8) |
| Sexual distress, n (%) | ||
| >40 in the PDS | 24 (14.0) | 26 (15.4) |
| Score on PFSF totalb | 69.4 ± 15.9 | 66.9 ± 15.0 |
| Score on PFSF domainsb | ||
| Desire | 56.9 ± 19.5 | 56.7 ± 18.1 |
| Arousal | 78.3 ± 21.0 | 77.9 ± 21.0 |
| Orgasm | 66.4 ± 25.3 | 59.9 ± 26.5 |
| Pleasure | 68.5 ± 26.1 | 68.7 ± 20.8 |
| Concern | 73.9 ± 20.2 | 68.2 ± 23.4 |
| Responsiveness | 78.2 ± 16.6 | 76.2 ± 17.3 |
| Self-image | 64.0 ± 20.3 | 60.7 ± 20.5 |
| Score on PDSc | 16.5 ± 22.4 | 19.6 ± 24.4 |
| No. of satisfying sexual episodes over 1-wk period (SAL) | 2.6 ± 2.5 | 2.5 ± 2.5 |
| Serum levels of blood sample testsd | ||
| FSH, IU/L | 5.4 ± 1.7 | 5.4 ± 1.7 |
| LH, IU/L | 4.9 ± 2.4 | 4.8 ± 2.2 |
| Total T, pg/mL | 246.4 ± 93.7 | 244.3 ± 88.7 |
| SHBG, nmol/L | 78.1 ± 46.7 | 72.6 ± 27.3 |
| Free T, pg/mL | 3.2 ± 1.3 | 3.2 ± 1.3 |
| Estradiol, pg/mL | 33.5 ± 19.1 | 34.4 ± 25.1 |
| Characteristic . | Placebo Group . | OC Group . |
|---|---|---|
| n | 171 | 169 |
| Age, y | 24.1 ± 4.0 | 23.3 ± 3.6 |
| Education, n (%)a | ||
| Primary school | 5 (2.9) | 6 (3.6) |
| Secondary school | 53 (31.2) | 58 (34.7) |
| University studies (ongoing) | 64 (37.6) | 56 (33.5) |
| University degree | 48 (28.2) | 47 (28.1) |
| Subjects in stable relationship, n (%)a | 100 (58.8) | 97 (57.7) |
| BMI, kg/m2 | 22.6 ± 2.8 | 22.5 ± 3.1 |
| Low sexual desire, n (%) | ||
| <40 in the sexual desire domain of PFSF | 33 (19.3) | 30 (17.8) |
| Sexual distress, n (%) | ||
| >40 in the PDS | 24 (14.0) | 26 (15.4) |
| Score on PFSF totalb | 69.4 ± 15.9 | 66.9 ± 15.0 |
| Score on PFSF domainsb | ||
| Desire | 56.9 ± 19.5 | 56.7 ± 18.1 |
| Arousal | 78.3 ± 21.0 | 77.9 ± 21.0 |
| Orgasm | 66.4 ± 25.3 | 59.9 ± 26.5 |
| Pleasure | 68.5 ± 26.1 | 68.7 ± 20.8 |
| Concern | 73.9 ± 20.2 | 68.2 ± 23.4 |
| Responsiveness | 78.2 ± 16.6 | 76.2 ± 17.3 |
| Self-image | 64.0 ± 20.3 | 60.7 ± 20.5 |
| Score on PDSc | 16.5 ± 22.4 | 19.6 ± 24.4 |
| No. of satisfying sexual episodes over 1-wk period (SAL) | 2.6 ± 2.5 | 2.5 ± 2.5 |
| Serum levels of blood sample testsd | ||
| FSH, IU/L | 5.4 ± 1.7 | 5.4 ± 1.7 |
| LH, IU/L | 4.9 ± 2.4 | 4.8 ± 2.2 |
| Total T, pg/mL | 246.4 ± 93.7 | 244.3 ± 88.7 |
| SHBG, nmol/L | 78.1 ± 46.7 | 72.6 ± 27.3 |
| Free T, pg/mL | 3.2 ± 1.3 | 3.2 ± 1.3 |
| Estradiol, pg/mL | 33.5 ± 19.1 | 34.4 ± 25.1 |
Values are expressed as means ± SD unless stated otherwise.
There were three missing values on the education variable (two in the OC group and one in the placebo group), and two missing values on the “in a stable relationship” variable (one in the OC group and one in the placebo group).
PFSF total (and each PFSF domain) range from 0 to 100, with higher values indicating greater sexual function. PFSF total is defined as the average of the normalized score on each PFSF domain where each domain is given the same weight.
PDS scores range from 0 to 100, with higher scores indicating more distress.
In the OC group, there were two missing values for FSH, LH, SHBG, and free T and one missing value for both total T and estradiol.
Outcomes
We hypothesized that OCs would reduce sexual function. The effect for our primary outcome measure, the overall sexual function (PFSF total), was not significantly different between the OC and placebo groups (mean difference = −2.5; 95% confidence interval [CI], −5.54 to 0.45; P = .096) (Figure 2A).
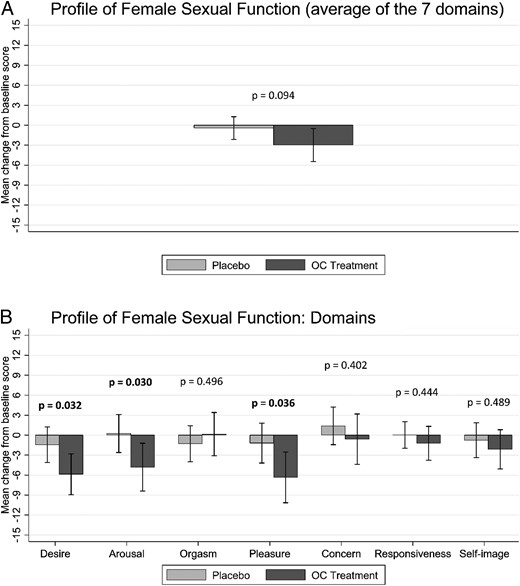
PFSF. Mean changes from baseline to end of follow-up in scores for the PFSF total in the placebo group and the OC treatment group. A, Results for PFSF total (the average of the score on each domain). B, Results for each of the seven PFSF domains. Scores on each domain range from 0 to 100, with higher scores indicating increased sexual functioning. Error bars denote 95% CI.
However, we did find a statistically significant negative impact of OC on sexual function in the hypothesized direction for the following PFSF domains: desire (mean difference, −4.4; 95% CI, −8.49 to −0.38; P = .032), arousal (mean difference, −5.1; 95% CI, −9.63 to −0.48; P = .031), and pleasure (mean difference, −5.1; 95% CI, −9.97 to −0.32; P = .037) (Figure 2B). For the other four PFSF domains of orgasm (mean difference, 1.45; 95% CI, −2.75 to 5.66; P = .497), concern (mean difference, −2.00; 95% CI, −6.72 to 2.71; P = .403), responsiveness (mean difference, −1.25; 95% CI, −4.49 to 1.98; P = .446), and self-image (mean difference, −1.38; 95% CI, −5.30 to 2.54; P = .409), we did not find any significant difference between the OC and placebo groups (Figure 2B).
There was no significant difference between the OC and placebo groups for the PDS (mean difference, 3.63; 95% CI, −0.47 to 7.74; P = .083) and SAL (mean difference, −0.57; 95% CI, −1.15–0.01; P = .054).
The results of two predefined robustness tests described in the Supplemental Data implied only marginal changes to effect size and statistical significance when we also included individuals who did not complete the data collection (n = 340) or only included participants who did not discontinue treatment (n = 329). The primary outcome measure PFSF total was not significantly different in any of the two robustness tests (P values ranged from 0.097 to 0.112), and the three significant PFSF domains were significant also in both robustness tests (P values ranged from 0.031 to 0.043).
Before treatment, the proportion of women having low sexual desire (<40 on the sexual desire domain of PFSF) was 18% in the OC group and 19% in the placebo group, and distress (>40 on the PDS) was 15% in the OC group and 14% in the placebo group (Table 1). After treatment, 32% in the OC group and 22% in the placebo group had low sexual desire, whereas 21% in the OC group and 15% in the placebo group had distress. The primary outcome measure and all of the secondary outcome measures were not statistically significant in these subgroups, but it should be noted that the study was not powered for comparisons within subgroups.
Hormone levels
Serum levels of FSH, LH, and total and free T decreased as expected after 3 months of active treatment, and the change in hormone levels was significantly different compared to placebo, supporting the theory that participants were compliant to study treatment (P < .001) (Figure 3 and Supplemental Table 1).
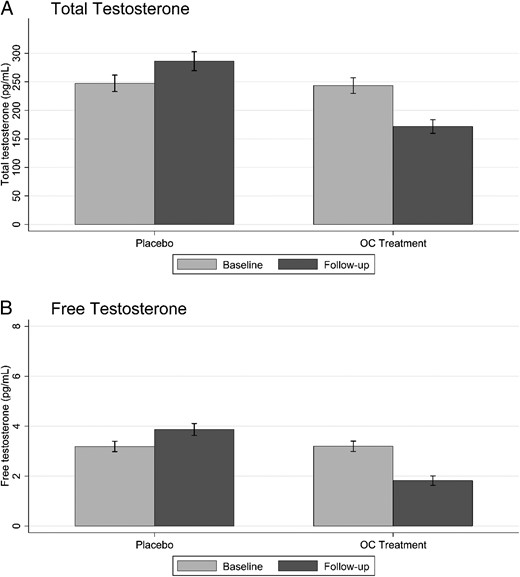
Serum levels of total and free T. Mean values of total T (A) and free T (B) at baseline and follow-up in the placebo and OC treatment groups. Error bars denote 95% CI.
We also tested whether the size of pre-/post-treatment changes in serum levels of total and free T were correlated with the change in sexual function. None of these correlations were significant in the OC group (P > .05), and only one was significant in the placebo group (between the change in the SAL measure and the change in free T; P < .05) (Supplemental Tables 2 and 3).
Safety and adverse events
We detected adverse events in 21.0% of the women in the OC group and 12.0% in the placebo group (Supplemental Table 4). In total, there were four serious adverse events, including two pregnancies and one ovarian cyst in the placebo group and one panic disorder in the OC group. The most common reported adverse events in the OC group were bleeding disturbances (n = 14), anxiety and mood changes (n = 12), acne (n = 5), and appetite changes (n = 3). In the placebo group, anxiety and mood changes (n = 4) were the most commonly reported adverse events.
Discussion
This is the first adequately sized randomized, placebo-controlled trial on effects of a combined OC on sexual function in women. We found no significant reduction in overall sexual function by a levonorgestrel-containing combined OC in comparison to placebo, but we did find impairment in the domains of sexual desire, arousal and pleasure, whereas orgasm, concern, responsiveness, and self-image were not significantly affected. In addition, the mean frequency of satisfying sexual episodes and personal distress were not significantly different between groups. Thus, our results indicate that OC use may decrease some aspects of sexual function.
The size of the change in the PFSF domains sexual desire, arousal, and pleasure corresponds to changes of −7.8%, −6.5%, and −7.4%, respectively. The effect size expressed as Cohen’s D was 0.24 for desire, 0.24 for arousal, and 0.22 for pleasure, corresponding to small (0.2) effect sizes in terms of Cohen’s D terminology (15). Our results could be compared with a large European questionnaire study of medical students showing that OC use was associated with lower female sexual function index score than for other contraceptive methods but higher than for those using no contraceptives (16). Because this was not a randomized study, the findings could be attributable to potential bias factors such as sexual activity and being in a stable relationship or not.
Female sexuality is complex, reflecting the interaction of many factors including physical, emotional, psychosocial, and cultural determinants (17). A potential mechanism for a decrease in libido after OC use has been suggested to be the estrogen-induced increase in the production of SHBG (5). Raised levels of this protein will increase the binding of circulating T and thereby reduce the levels of free, biologically active T (18). Furthermore, OCs inhibit the production of androgens in the ovaries and adrenal glands (19). Because T treatment has been shown to increase libido in women with hypoactive sexual desire disorder (8, 20), reduced levels of bioavailable T may impair sexual function.
In the present study, we found an increase in SHBG and consequently a decrease in free T by OC treatment. Furthermore, levels of total T were reduced. However, there were no significant correlations between the change in sexual function and the change in total and free T in the OC group. This is in agreement with a previous prospective study showing no clear association between T levels and sexuality in women before and during OC treatment (21). However, some studies have shown correlations between T levels and sexual interest for women taking OCs (22, 23). Sexual hormones have been suggested to modulate subtle aspects of behavior and arousal involved in sexual expectation (24). Such subtle modulation may not have been detected by the instruments used in the present investigation. The role of endogenous T in women’s sexuality needs to be further explored (25).
A possible alternative mechanism whereby OCs might affect sexual function is a direct negative effect of the progestin component on the brain. Sex hormones have been classified as neurosteroids (26). They act via genomic effects, binding to their specific nuclear receptors, but could also influence brain functions via more rapid, nongenomic mechanisms and indirectly modulate brain functions via neurotransmitter systems, such as the serotonin and γ-aminobutyric acid systems (27). Premenstrual symptoms, including irritability, tension, depression, and fatigue have been related to an increase of progesterone metabolites during the menstrual cycle (28). Several studies have shown that different progestins and combined OCs can provoke similar symptoms (29). Therefore, a direct negative effect on libido and sexual behavior in women cannot be excluded. This possibility has so far attracted little attention, and basic research in this field is warranted.
Different combined OCs are equally effective but may vary in risk profile, side effects, and compliance. Most combined OCs contain ethinylestradiol, a synthetic estrogen, and a few recently developed OCs contain estradiol. Meanwhile, many different progestins are combined with the estrogen component. The progestins used mainly belong to two chemical families: derivatives of 19-nortestosterone and derivatives of 17-α-hydroxyprogesterone. Derivatives of 19-nortestosterone have varying degrees of androgenic activity, whereas derivatives of 17-α-hydroxyprogesterone lack androgenic effects and may even exert antiandrogenic activity. Progestins with androgenic activity in combination with ethinylestradiol (second-generation OC) have been suggested to imply a lower risk of vascular thromboembolism and are therefore recommended as the first choice for contraception in some countries (30). In the present study, we chose the combination of 150 μg levonorgestrel and 30 μg ethinylestradiol. This is an androgenic second-generation pill in a 21/7 (hormone-containing/hormone-free) regimen and the most commonly prescribed OC in Sweden.
Negative side effects like mood changes, acne, increased appetite, and weight gain may vary between OCs according to the progestin component and also by the type of regimen. Adverse effects seem to be most common during the last week of active treatment and the tablet-free interval (31). Symptoms may be more frequent when androgenic OCs in a 21/7 regimen are used (32, 33). In contrast, placebo-controlled studies have reported a positive mood effect in women with premenstrual syndrome by a 24/4 combination with ethinylestradiol and the antiandrogenic progestin drospirenone (34). Consequently, our findings for a specific estrogen/progestin combination cannot be generalized to be valid for all OCs. Large-scale and placebo-controlled studies on nonandrogenic OCs are needed. If data from alternative OCs show less or no adverse effects on sexual function, this could influence the risk-benefit analysis as made by health care providers and counselors. Sexual dissatisfaction is an important reason for OC discontinuation and risk of unwanted pregnancies (1, 4). An OC without adverse effects on sexual function might well be preferred as a first choice of treatment.
The large number of participants and the placebo-controlled group are strengths of the present study. Data collection by using well established and validated instruments for assessment of sexual function (8–12) and the highly specific method for T analysis (13) represent further strengths of the study. However, the duration of treatment was quite short, only 3 months, and one could argue that some of the adverse effects recorded might vanish over time. Still, a further deterioration could also be possible. Also, the use of OCs will influence the amount and cyclicity of menstrual bleeding, and therefore patient blinding in reality may not have been complete. Although specific questions on these issues were avoided, women may have suspected that they were given active treatment. Thus, we cannot rule out that subjective opinions and expectations might have had some influence on the results. Such bias, however, could work in both directions. Moreover, some positive effects of OC use in real life, such as not having barrier contraception and being less concerned about getting pregnant, could not be assessed in the present study. Furthermore, the occurrence of two pregnancies in the placebo group, despite the strong requirement for nonhormonal contraception, illustrates the importance of using highly effective contraception, such as OCs.
The present data support the theory that a levonorgestrel-containing OC can have an adverse effect on some aspects of sexual function in young women. Although the effect was quite small, it may represent a clinically important side effect for individual women. This possible side effect should be recognized and considered during contraceptive counseling and may be relevant for the choice of hormonal method. Future placebo-controlled studies are needed to evaluate effects on sexual function by alternative OC combinations.
Acknowledgments
We thank Elisabeth Berg and Mesfin Tessma for valuable comments on the analysis protocol. We are grateful to Berit Legerstam, Siv Rödin Andersson, and Agneta Berge for research assistance and to Johan Almenberg for proofreading the manuscript.
There was no support for this work from the pharmaceutical industry. The study was supported by research grants from the Jan Wallander and Tom Hedelius Foundation (Grants P2010-0133:1, P2012-0002:1, and P2013-0156:1), the Knut and Alice Wallenberg Foundation (Wallenberg Academy Fellows grant, to A.D.), the Swedish Council for Working Life and Social Research (Grant 2006–1623), the Swiss National Science Foundation (Grant 100010-149451), the Swedish Research Council (Grant 20324), the Karolinska Institutet, and the regional agreement on medical training and clinical research (ALF) between the Stockholm County Council and Karolinska Institutet (Grant 20130313).
Clinical Trial Registration No.: EudraCT number: 2010-020824-23.
Disclosure Summary: F.L. reports personal fees from EndoCeutics Inc. No other disclosures were reported.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- OC
oral contraceptive pill
- PDS
Personal Distress Scale
- PFSF
Profile of Female Sexual Function
- SAL
Sexual Activity Log.
References
Author notes
N.Z., A.D., and E.R. contributed equally to this work.



