-
PDF
- Split View
-
Views
-
Cite
Cite
Livia Lenzini, Giacomo Rossitto, Giuseppe Maiolino, Claudio Letizia, John W. Funder, Gian Paolo Rossi, A Meta-Analysis of Somatic KCNJ5 K+ Channel Mutations In 1636 Patients With an Aldosterone-Producing Adenoma, The Journal of Clinical Endocrinology & Metabolism, Volume 100, Issue 8, 1 August 2015, Pages E1089–E1095, https://doi.org/10.1210/jc.2015-2149
Close - Share Icon Share
Due to selection biases and inadequate statistical power, individual studies may fail to identify the clinical features of patients with an aldosterone-producing adenoma (APA) harboring KCNJ5 mutations. When this failure occurs, meta-analysis can provide significant outcome data.
The objective was to determine the clinical features of these APA patients.
We systematically searched the PubMed, Scopus, Web of Science, and Cochrane databases library in January 2015 applying the Population, Intervention, Comparison, and Outcome (PICO) strategy. The standardized differences in mean and corresponding 95% confidence interval of continuous variables were computed by random-effects modeling.
We performed a meta-analysis of all available studies on somatic KCNJ5 mutations in APA.
We could identify 13 studies that recruited 1636 patients (age 49 ± 4 years; 55% females).
Differences between APA with and without KCNJ5 mutations in gender, plasma renin activity, plasma aldosterone, tumor size, serum potassium, and blood pressure were investigated.
The overall prevalence of KCNJ5 mutations was 43% (range = 12–80%). Their rate was lower (P < .003) in the studies done in Europe, the United States, and Australia (35%) than in Japan and China (63%); it correlated (r = 0.60, P = .029) with the mean daily urinary sodium excretion. Compared with the wild-type, the mutated APA patients were younger (45 ± 3 vs 52 ± 5 yrs), had higher plasma aldosterone (42 ± 8 vs 33 ± 8 ng/dl), larger tumors (16.1 ± 6.4 versus 14.9 ± 7.4 mm), and were more often females (67% vs 44%) (all P < .05).
Meta-analysis showed that more pronounced hyperaldosteronism, young age, female gender, and larger tumors are the phenotypic features of APA patients with KCNJ5 mutations. No significant differences in blood pressure and serum K+ was found, which suggests that these clinical features do not help in identifying mutated APA patients.
Primary aldosteronism (PA) is the most common cause of endocrine arterial hypertension. In addition to high blood pressure (BP), patients with PA retain sodium and water, and have low-to-undetectable plasma renin and angiotensin II (1); in addition, they may be hypokalemic. These factors would be expected to lower aldosterone production to normal levels. Hyperaldosteronism, however, persists, which was a puzzling phenomenon until Choi et al discovered mutations in the KCNJ5 gene coding for the Kir 3.4 K+ channel (2). Subsequently, we and others found elevated activating autoantibodies for angiotensin II type 1 receptors in the plasma of some PA patients (3, 4).
In aldosterone-producing cells of the zona glomerulosa the Kir 3.4 K+ channel is highly expressed and plays a key role in maintaining cell membrane hyperpolarization. After the first report of mutations in the selectivity filter of the channel, several other mutations were identified both in the same “hot spot” region of the gene and outside this region (5, 6) (for a review, see (7)). These mutations were found to impair the selectivity of the filter for K+ and to allow Na+ influx, leading to zona glomerulosa cell membrane depolarization, opening of T type Ca2+ channels, and activation of the Na+/Ca2+ exchanger (5); the ensuing Ca2+ influx activates aldosterone synthesis and secretion. Why these mutations are found in this region of the gene is unclear; similarly unclear is why these KCNJ5 mutations are often reported to be more common in patients from Asia (Japan and China) than elsewhere.
Individual studies may not be adequate to identify the clinical and phenotypic features of aldosterone-producing adenoma (APA) patients with KCNJ5 mutations or to explore geographical differences, for multiple reasons. The latter includes selection biases, race- and ethnicity-related factors, and inadequate statistical power of small samples. Most of these limitations can be overcome by meta-analysis, which therefore can provide novel insights in this area. We thus sought to test this hypothesis by undertaking a meta-analysis of all available studies about KCNJ5 mutations in APA with the following two aims: 1) to determine if there is geographical heterogeneity in the rate of mutations and 2) to ascertain the clinical and pathological correlates of KCNJ5 somatic mutations.
Materials and Methods
All the details about search and selection processes, the specific type of study, and analyses are described in Supplemental Table 1. We searched the PubMed, Scopus, Web of Science, and Cochrane databases library in January 2015 using the Population, Intervention, Comparison, and Outcome (PICO) strategy (8) and the following predefined terms: population, primary aldosteronism patients with sporadic aldosterone-producing adenoma; intervention, adrenalectomy, sequencing for KCNJ5 mutation; control, APA without KCNJ5 mutations; and outcome, clinical and pathological correlates of KCNJ5 mutation.
Exclusion criteria included the lack of adequate information on patient selection, insufficient data on the genotyping technique, or detection of germinal and/or single nucleotide polymorphism. For each study, data on demographic and clinical characteristics of the patients, including BP values and tumor size, were gathered. The mean/median values and measures of spread were obtained from the individual studies. Variables for which measures (mean, standard deviation, SEM) were not reported in the original publication (5) were calculated from original data with SPSS software (version 22, Italy). To document the predictors of KCNJ5 mutation rate, we used stepwise backward (Wald) linear regression analysis to include any potentially relevant variables.
For meta-analysis, we elected to use a commercially available software package (Comprehensive Meta-analysis, Statistical Solution, Biostat, Englewood, NJ) to allow for independent replication of results. For continuous variables, the standardized difference in mean between mutated APA and wild-type APA and corresponding 95% confidence intervals were computed by random-effects modeling. For all studies, heterogeneity was assessed (9), and statistical significance (P value) set at .05. The funnel plot and related statistics (eg, fail-Safe N, Orwin fail-safe N, rank correlation and regression procedures) were used to investigate if publication bias had occurred.
Results
KCNJ5 mutation prevalence
Thirteen studies comprising 1636 APA patients were identified (2, 5, 6, 10–19). One study was excluded because KCNJ5 mutations reported in PA patients were germ-line mutations or single nucleotide polymorphisms (20). Another study reported data on the variables of interest for only two KCNJ5 mutations of the 16 APAs that were genotyped. Hence, it could not be meta-analyzed, but used only for calculation of the summary data (19). Two studies collected data in more than one continent (10, 11): the first includes samples from nine European centers and one Australian center; therefore, because only 9% of the patients were from Australia this study was considered as done in Europe. The second study included patients from the United Kingdom and Australia (11), which were considered separately. In another study (13), the genetic screening of mutations in KCNJ5 was previously described for all patients from the Torino, Ancona, Padova B, Pisa, Würzburg, and Berlin cohorts (12, 16, 21). Thus, only clinical data from the new samples (n = 199) were considered to avoid duplication of cases. Figure 1 shows the flow chart of the study and rates of the KCNJ5 mutation in each of the reports under analysis.
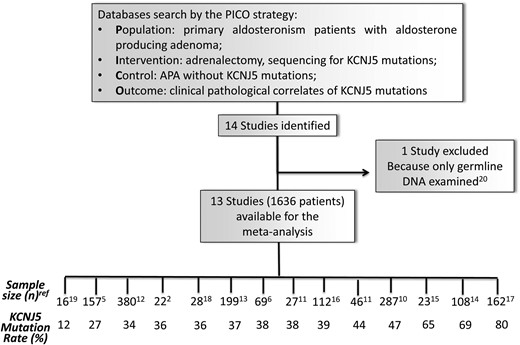
Flow chart of the study with the size and prevalence of KCNJ5 mutations in aldosterone-producing adenoma (APA) in each study.
The clinical features of patients are summarized in Table 1 and Supplemental Table 2. Overall, the prevalence of KCNJ5 was 43% (range, 12–80%). The studies done in Europe, the United States, and Australia, which showed similar rates of prevalence, were grouped together. To determine if the prevalence of these mutations varied according to the geographical area and dietary habits, the average prevalence rate seen in these continents was compared with that reported in Japan and China. Although in Asia there were some difference among studies, the prevalence of KCNJ5 mutations in Asia was higher there (63%, P < .003) than in Europe + the United States + Australia (35%) (Figure 2A).
![The box-and-whisker plot shows the prevalence of KCNJ5 mutations in aldosterone-producing adenoma (APA) according to three geographical macro-areas (America with Europe [Eu], Australia [Aus], and Asia [As]) (A), and the relation between such prevalence and 24-hour sodium (Na) intake (B) of the studies.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jcem/100/8/10.1210_jc.2015-2149/7/m_zeg9991518970002.jpeg?Expires=1750269728&Signature=N3d~dZ8InyapeCBVARpbFa4wAuRZGTBCONSKD9eWxPCe4PqpLwkuYeqpdPjb0eTtvWzba39TG79HJJ5JLOE3ubgyLzqDn0NfwK3eqA7bw9x4zEAdOCvP7XodqWbVyYWPINVyktqh7ffGD4ek7lyCrqoZ4F3Q1IaRUgWhVwSBDmrR68csMRztI-i3kzZmfVLE4Z9gE~mmPgci4i4pfzD6QfDXSHBJSHYYzj08zVw~dp6zfNs5Q8cmtTiaho7~Az4RE69TBWdjj9y9uphjH8daSwHN7p333k8iBL-OJ3Z4O5oFi8-dYsuKlL1bqLOEooGyZm2EIh5b1SdJRVs1J4Zv7A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
The box-and-whisker plot shows the prevalence of KCNJ5 mutations in aldosterone-producing adenoma (APA) according to three geographical macro-areas (America with Europe [Eu], Australia [Aus], and Asia [As]) (A), and the relation between such prevalence and 24-hour sodium (Na) intake (B) of the studies.
In panel B, round circles report data from European studies, squares from American studies, rhombus from Australian studies, and triangles from Asian studies.
Clinical and demographics characteristics of the APA patients recruited in the 13 meta-analyzed studies
| . | KCNJ5 Mutated (n = 725) . | KCNJ5 Wild Type (n = 911) . |
|---|---|---|
| Age (years) | 45 ± 3 | 52 ± 5 |
| Females/males (%) | 69/31 | 44/56 |
| PAC (ng/dl) | 41.8 ±8.4 | 32.8 ± 7.4 |
| PRA (ng/ml/hr) ARR (ng/dl)/ (ng/ml/hr) | 0.29 ± 0.09 | 0.42 ± 0.20 |
| ARR (ng/dl)/ (ng/ml/hr) | 157 ± 55 | 85 ± 55 |
| Serum K+ (mEq/L) | 3.04 ± 0.30 | 3.17 ± 0.20 |
| Systolic BP (mm Hg) | 151 ± 10 | 156 ± 9 |
| Diastolic BP (mm Hg) | 93 ± 5 | 92 ± 9 |
| Tumor size (mm) | 16.3 ± 6.1 | 14.9 ±7.4 |
| . | KCNJ5 Mutated (n = 725) . | KCNJ5 Wild Type (n = 911) . |
|---|---|---|
| Age (years) | 45 ± 3 | 52 ± 5 |
| Females/males (%) | 69/31 | 44/56 |
| PAC (ng/dl) | 41.8 ±8.4 | 32.8 ± 7.4 |
| PRA (ng/ml/hr) ARR (ng/dl)/ (ng/ml/hr) | 0.29 ± 0.09 | 0.42 ± 0.20 |
| ARR (ng/dl)/ (ng/ml/hr) | 157 ± 55 | 85 ± 55 |
| Serum K+ (mEq/L) | 3.04 ± 0.30 | 3.17 ± 0.20 |
| Systolic BP (mm Hg) | 151 ± 10 | 156 ± 9 |
| Diastolic BP (mm Hg) | 93 ± 5 | 92 ± 9 |
| Tumor size (mm) | 16.3 ± 6.1 | 14.9 ±7.4 |
For significant differences please refer to the forest plots. All items are mean ± standard deviation.
Abbreviations: ARR, aldo renin ratio; BP, blood pressure. PAC, plasma aldosterone; PRA, plasma renin activity; Serum K+, serum potassium.
Clinical and demographics characteristics of the APA patients recruited in the 13 meta-analyzed studies
| . | KCNJ5 Mutated (n = 725) . | KCNJ5 Wild Type (n = 911) . |
|---|---|---|
| Age (years) | 45 ± 3 | 52 ± 5 |
| Females/males (%) | 69/31 | 44/56 |
| PAC (ng/dl) | 41.8 ±8.4 | 32.8 ± 7.4 |
| PRA (ng/ml/hr) ARR (ng/dl)/ (ng/ml/hr) | 0.29 ± 0.09 | 0.42 ± 0.20 |
| ARR (ng/dl)/ (ng/ml/hr) | 157 ± 55 | 85 ± 55 |
| Serum K+ (mEq/L) | 3.04 ± 0.30 | 3.17 ± 0.20 |
| Systolic BP (mm Hg) | 151 ± 10 | 156 ± 9 |
| Diastolic BP (mm Hg) | 93 ± 5 | 92 ± 9 |
| Tumor size (mm) | 16.3 ± 6.1 | 14.9 ±7.4 |
| . | KCNJ5 Mutated (n = 725) . | KCNJ5 Wild Type (n = 911) . |
|---|---|---|
| Age (years) | 45 ± 3 | 52 ± 5 |
| Females/males (%) | 69/31 | 44/56 |
| PAC (ng/dl) | 41.8 ±8.4 | 32.8 ± 7.4 |
| PRA (ng/ml/hr) ARR (ng/dl)/ (ng/ml/hr) | 0.29 ± 0.09 | 0.42 ± 0.20 |
| ARR (ng/dl)/ (ng/ml/hr) | 157 ± 55 | 85 ± 55 |
| Serum K+ (mEq/L) | 3.04 ± 0.30 | 3.17 ± 0.20 |
| Systolic BP (mm Hg) | 151 ± 10 | 156 ± 9 |
| Diastolic BP (mm Hg) | 93 ± 5 | 92 ± 9 |
| Tumor size (mm) | 16.3 ± 6.1 | 14.9 ±7.4 |
For significant differences please refer to the forest plots. All items are mean ± standard deviation.
Abbreviations: ARR, aldo renin ratio; BP, blood pressure. PAC, plasma aldosterone; PRA, plasma renin activity; Serum K+, serum potassium.
To determine if this difference could be due to environmental factors, we looked at differences in sodium intake across the studied populations. Given the lack of data on sodium intake in the studies, we used the mean sodium urinary excretion values of the centers from literature (22–24). This analysis showed that the average sodium intake in Asia was higher than in the rest of the world (4.9 ± 0.39 vs 3.4 ± 0.23 g/24 h, P = .002). Interestingly, bivariate correlation analysis showed that the mean daily urinary sodium excretion was correlated with the rate of KCNJ5 mutations (r = 0.60, P = .03) (Figure 2B). Moreover, stepwise backward (Wald) linear regression analysis (with gender, plasma aldosterone concentration, tumor size, serum K+, and BP as variables initially in the model) showed that daily urinary sodium excretion was the only independent variable that remained in the model and was strongly associated with the reported rate of KCNJ5 mutation (F = 60.64, P = .008, adjusted r2 = 0.445), indicating that a higher average sodium intake may cause a more severe phenotype and/or an earlier detection of the disease in patients with APA.
Results of the meta-analysis
We tested the heterogeneity among the studies by comparing the Q values with the degree of freedom Q for all the analyzed variables. This showed that most variables did not show heterogeneity among studies with the exception of plasma aldosterone concentrations and diastolic BP. Compared with wild-type patients, those with KCNJ5 mutations had higher plasma levels of aldosterone (42 ng/dl ± 8 vs 33 ng/dl ± 8) (Figure 3A), were younger (45 ± 3 yrs 52 ± 5 yrs) (Figure 3B), more often female (67% vs 44%) (Figure 3C), and had bigger tumors (16.1 ± 6.4 mm vs 14.9 ± 7.4 mm)(Figure 3D). In contrast, no association of mutation status with systolic or diastolic BP and serum potassium levels was found.
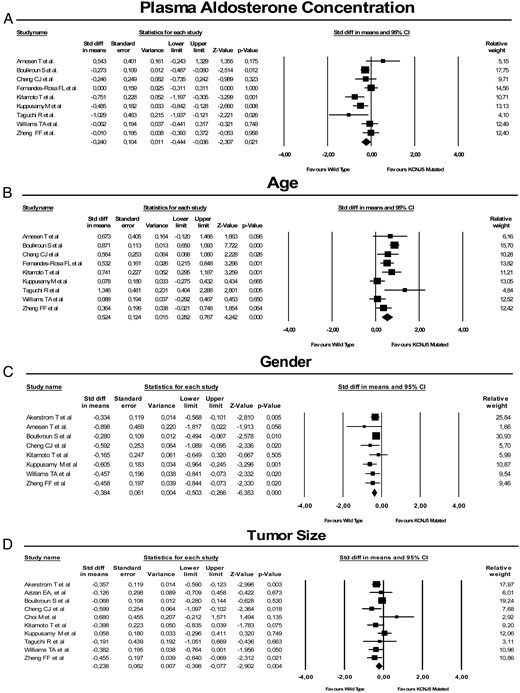
The forest plot shows the standardized differences (Std diff) of the mean of plasma aldosterone concentration (A), age (B), gender (C), and tumor size (D) between wild-type and KCNJ5-mutated aldosterone-producing adenoma (APA).
For each study, the symbols are proportional to the study size and the 95% confidence interval (CI) is also displayed. The relative weight of each study for the meta-analysis is also shown.
Discussion
This meta-analysis included 13 studies that recruited a total of 1636 APA patients who underwent adrenalectomy and sequencing of tumor DNA for KCNJ5 mutations at centers scattered across four continents, albeit predominantly in Europe and Asia. This study represents the largest collection of such patients reported. Accordingly, the summary data reported in Table 1 furnish an accurate picture of the features of the APA patients adrenalectomized and genotyped for KCNJ5 mutations at major referral centers worldwide to date.
It is therefore worth noting that the average age (49 years), the almost even gender distribution, and the average BP, plasma aldosterone, and size of the tumors were similar to those currently encountered in clinical practice in patients with APA. Therefore on the whole, these findings indicate that this cohort is not unrepresentative of the population of APA patients. In fact, by using funnel plot and related statistics, we could find no evidence for a publication bias, thus making the occurrence of such type of bias unlikely. Therefore, with the strength provided by a large dataset, this meta-analysis disclosed some novel information that was unable to be gathered by the individual studies.
KCNJ5 mutations by continent
When the rate of KCNJ5 mutations was examined according to study rate, these mutations appear more common in Japan and China than in Europe, America, and Australia (Figure 2A).
These data thus support the reported prevalence of KCNJ5 mutations being markedly skewed, on average almost 2-fold higher in studies carried out in Asia than elsewhere. These differences may thus provide a clue to the mechanism(s) responsible for mutation occurrence at hot spots in the Kir3.4 gene. Whether this skewed distribution is due to “nature” (eg, ethnic or racial factors) or to “nurture” (eg, environmental factors) cannot be answered by a meta-analysis. Moreover, the lack of information on dietary habits, and particularly sodium intake, in all of the studies that we included in the meta-analysis does not help in answering this question. However, when the average sodium intake of the population in the regions where the studies were performed (22) was entered in a regression model, it was significantly correlated with the rate of KCNJ5 mutations, both significantly higher in Japan and China than elsewhere (Figure 2B).
The observation that the rate of prevalence differed only slightly between Europe, North America, and Australia (eg, three continents geographically far apart, but sharing a good deal of ethnic/racial factors) would suggest that these factors can be as important as the environmental factors as determinants of the KCNJ5 mutations. These continents can also share similar sodium intakes, thus suggesting a role of this potentially important environmental factor. However, considering the lack of information on patients' ethnicity and related dietary differences in the individual studies, this conclusion should only be seen as hypothesis-generating because attribution of an “average” sodium intake to the patients in a given country is a rough oversimplification.
In addition, it is our opinion that differences in the strategy being exploited in different centers/countries for selecting and/or referring the PA patients for adrenalectomy may well also play a key role. Many, but not all, Japanese centers, for example, report very high (∼70%) rates of PA patients having APA in keeping with findings in the referred patients submitted to adrenal vein sampling of the Primary Aldosteronism Prevalence in Italy Study (25): because APA usually exhibit a more severe phenotype, particularly if they are large in size, a higher rate of mutation-bearing APA are likely to be detected under such circumstances. The difference of mean tumor size between mutated and wild-type APA (Figure 4) also supports the contention that PA patients with more marked hyperaldosteronism (from larger tumors) could have been selected beforehand at the Asian centers. The disparity within these centers—with one reporting levels similar to those in the rest of the world—is also consistent with this possibility.
Clinical features of APA patients with KCNJ5 mutations
A forest plot of the standardized differences of the mean of plasma aldosterone concentrations between wild-type and mutated APA (Figure 3A) provided unequivocal evidence that somatic KCNJ5 mutations are associated with higher plasma aldosterone concentrations. This finding, which supports results of some (12, 14), although not all, previous studies (6, 13, 16, 17) suggests that these mutations in APA translate into a higher rate of constitutive aldosterone production. Likewise, a forest plot of the standardized differences of the mean of age at adrenalectomy between wild-type APA and mutated APA (Figure 3B) highlighted a significantly younger age in mutated APA than in wild-type APA patients. Thus, the present results support the conclusion that these mutations occur more commonly at a younger than at an older age and/or are detected sooner because of a more severe phenotype.
In keeping with findings in the largest previous studies (5, 6, 10, 12, 13, 16), but not in all studies (18), our meta-analysis also detected a significant association of KCNJ5 mutations with female gender. Even though these findings may not be totally unexpected given the effects of estradiol on aldosterone secretion (26), the precise mechanisms underlying this association deserve further specific research.
We identified a direct association of KCNJ5 mutations with a larger tumor size (Figure 3D), indicating that either the growth of the APA implies a higher chance of KCNJ5 mutation development or that mutations were investigated in larger than smaller tumors. Our meta-analysis showed no significant differences in systolic and diastolic BPs and serum K+ levels between wild-type APA and mutated APA patients. The lack of association of KCNJ5 mutations with BP and serum K+ levels in spite of the more prominent hyperaldosteronism in mutated tumors is intriguing, but can have several explanations. For example, robust methods for measuring BP (eg, 24-hour BP monitoring) were not exploited in the meta-analysis studies, thus raising the possibility of an inaccurate assessment of this phenotype. As regards serum K+ levels, it is probable that most patients were on mineralocorticoid receptor antagonists and/or other K+-sparing agents at the time of diagnosis, which might explain the lack of association with KCNJ5 mutation status as well as the lack of BP differences between wild type and mutated APA. Whatever the explanations might be, these clinical features do not help to identify patients with mutation-benign APA.
Limitations and strengths
Practically all meta-analyses in this study rely on summary data and therefore could not catch the individual variability present in each study. Second, because the average sodium intake was not reported in the individual studies, the data on urinary sodium intake were obtained from epidemiological surveys, which might not accurately mirror the sodium intake in the recruited patients. Moreover, even though we could find no evidence for a publication bias, whether the features of the PA patients genotyped so far truly portray those of the general population of APA patients remains to be determined because the KCNJ5 genotyping has so far confined to major referral centers actively involved in primary aldosteronism research. Finally, although we took due care to exclude studies with duplicated data, whether and to what extent this occurred could not be determined with certainty. These potential limitations are accompanied by some major strengths, such as using a systematic strategy to search the relevant literature and painstaking care to gather all the relevant summary values and related measures of spread.
Conclusions
In summary, based on the largest collection of KCNJ5-genotyped APA examined to date, elsewhere this study provided clear-cut evidence that APA patients with mutations in the Kir3.4 channel are young and present with higher plasma aldosterone levels and larger tumors than wild-type APA patients. At variance, although KCNJ5 mutations have been associated with drug-resistant hypertension (27), we could find no significant differences of systolic and diastolic BP values between APA patients with and without such mutations. The determinants of the seemingly higher rate of KCNJ5 mutations in Japan and China, which could be related to different protocols for patient selection or to sodium intake, is worth further research to eventually solve the question of “nature or nurture.”
Acknowledgments
We thank the Foundation for Advanced Research In Hypertension and Cardiovascular diseases (www.forica.it).
This work was supported by FORICA (The Foundation for Advanced Research in Hypertension and Cardiovascular Diseases), the University of Padua (to GPR), and research grant Project GR-20091524351 by the Young Research Program of the Italy's Health Ministry (to L.L.).
Disclosure Summary: All authors have nothing to disclose.



