-
PDF
- Split View
-
Views
-
Cite
Cite
Roberta Lupoli, Matteo Nicola Dario Di Minno, Anna Tortora, Alessandra Scaravilli, Marianna Cacciapuoti, Livia Barba, Alessandro Di Minno, Pasquale Ambrosino, Gelsy Arianna Lupoli, Giovanni Lupoli, Primary and Secondary Hemostasis in Patients With Subclinical Hypothyroidism: Effect of Levothyroxine Treatment, The Journal of Clinical Endocrinology & Metabolism, Volume 100, Issue 7, 1 July 2015, Pages 2659–2665, https://doi.org/10.1210/jc.2015-1726
Close - Share Icon Share
Subclinical hypothyroidism (SH) is associated with some abnormalities in primary and secondary hemostasis.
The objective of the study was to evaluate changes in primary and secondary hemostasis induced by levothyroxine (L-T4) treatment in SH patients.
This was a prospective cohort study with a 6-month follow-up.
Outpatients were referred to “Federico II” University of Naples.
Subjects with a SH without previous/ongoing L-T4 therapy participated in the study.
Changes in major hemostatic/fibrinolytic variables and platelet reactivity [mean platelet volume (MPV), arachidonic acid (AA), or ADP concentrations inducing a ≥ 50% irreversible aggregation (AC-50%)] in SH patients before and after a 6-month L-T4 treatment.
At baseline, 41 SH patients showed higher levels of factor VII activity (123.9 ± 20.4 vs 107.7 ± 12.2, P < .001), plasminogen activator inhibitor-1 (33.6 ± 13.9 vs 22.5 ± 5.74, P < .001) and tissue plasminogen activator (5.56 ± 2.22 vs 4.75 ± 1.61, P = .010), with lower levels of D-dimer (220.3 ± 67.1 vs 252.1 ± 72.4, P = .017) compared with healthy controls. SH patients also showed a higher MPV (9.92 ± 1.15 vs 8.9 ± 0.9, P < .001) and AC-50% to AA (0.18 ± 0.12 vs 0.36 ± 0.10, P < .001) and to ADP (1.5 ± 0.6 vs 1.9 ± 1.3, P = .024). After a 6-month L-T4 therapy, a reduction of factor VII activity (from 123.9 ± 20.4 to 102.6 ± 14.3, P < .001), plasminogen activator inhibitor-1 (33.6 ± 13.9 to 19.4 ± 7.6, P < .001), and tissue plasminogen activator (5.56 ± 2.22 to 1.91 ± 4:43, P = .002) was found in SH subjects, with a marginal increase in D-dimer (from 220.3 ± 67.1 to 245.2 ± 103.1, P = .053). AC-50% to AA (from 0.18 ± 0.12 to 0.54 ± 0.3, P < .001) and to ADP (from 1.5 ± 0.6 to 1.86 ± 0.3, P = .042) were reduced, paralleled by a significant reduction of MPV (from 9.92 ± 1.15 to 9.10 ± 1.23, P = .016).
SH patients exhibit a prothrombotic status, which is reverted by a 6-month L-T4 treatment.
Thyroid function disorders are associated with different hemostatic abnormalities, involving primary hemostasis (largely represented by platelet adhesion, activation, and aggregation), secondary hemostasis (ie, the coagulative phase), and fibrinolysis. These modifications may range from subclinical laboratory abnormalities to clinically relevant hemorrhagic or thrombotic events (1). Whereas consistent results are available about the thrombotic risk in hyperthyroid subjects, data about hypothyroidism are inconclusive. In detail, most of the evidence suggests that overt hyperthyroidism is associated with hypofibrinolysis and, in turn, with an increased risk of thrombosis (2). Conversely, perhaps due to the different severity of the disease, discordant data about hypothyroidism are reported. Whereas an increased fibrinolytic activity is reported in some patients with severe hypothyroidism, a hypercoagulable state in patient with moderate/mild hypothyroidism has been reported (2). Subclinical hypothyroidism (SH) is associated with an abnormally high risk of cardiovascular and cerebrovascular disease (3). However, data about this condition are even fewer and contrasting. Although no significant changes in hemostasis parameters were observed in patients with SH (4, 5) compared with healthy controls, the large majority of the data argues for a state of hypercoagulability and hypofibrinolysis in this clinical setting (6–12). A recent study showed an increased mean platelet volume (MPV) in patients with SH as compared with controls (13). With MPV being a surrogate marker of platelet reactivity, this suggests that SH can be associated with an increased platelet reactivity, which in turn contributes to an increased cardiovascular risk in these patients. Nevertheless, no study specifically assessing platelet reactivity in SH patients is currently available. The aim of our study was to prospectively evaluate changes in primary and secondary hemostasis in SH patients after a 6-month replacement treatment with appropriate doses of levothyroxine (L-T4).
Materials and Methods
In a 24-months period (January 2011 through January 2013), consecutive patients referred to the Endocrinology outpatient clinic of Federico II University Hospital (Naples, Italy) for a thyroid function evaluation were screened for the enrollment in this study.
Patients aged 18–60 years with SH were enrolled for the present study. Biochemical diagnosis of SH (confirmed on the basis of two hormonal assays at a 8–10 wk distance) was based on TSH levels greater than 4.5 μU/mL and less than 10 μU/mL and free T3 (FT3) and free T4 (FT4) within the normal laboratory range in the absence of previous or ongoing L-T4 therapy. Major exclusion criteria were known inherited alterations in primary and/or secondary hemostasis; treatment with anticoagulant or antiplatelet drugs; personal and/or family history of arterial or venous thrombosis; other conditions known to impact hemostatic variables levels (liver disease, active inflammatory processes, pregnancy, malignancy, hematological diseases, puerperium, oral contraceptive intake, and hormone replacement therapy); history of chronic infectious disease (including hepatitis B and C); and unstable medical conditions.
For each enrolled subject, a family and personal clinical summary (including information about a history of bleeding and smoking habits), a clinical examination (including thyroid examination, height and weight measurements, and blood pressure evaluation after 10 min rest), and a thyroid ultrasound examination were performed. For each enrolled patient, the Bleeding Score Questionnaire was also administered to assess the presence of clinical signs due to clotting abnormalities (presence of epistaxis, gastrointestinal bleeding, prolonged bleeding after dental extractions, etc) (14). In addition, in each patient a venous blood sample was drawn from the antecubital vein without venous stasis via a 19-gauge scalp-vein needle at 8:30–9:00 am after 12–15 hours of overnight fasting. Serum FT3, FT4, TSH, thyroglobulin antibodies, and thyroperoxidase antibodies were assayed in all subjects and were measured with an electrochemiluminescence immunoassay (Elecsys E170; Roche Diagnostics). The TSH assay has an analytical sensitivity of 0.005 mIU/L and a functional sensitivity of 0.014 mIU/L (reference range 0.27–4.2 mIU/L). The FT4 assay has an analytical sensitivity of 0.30 pmol/L (reference range 0.93–1.7 ng/dL). The FT3 assay has an analytical sensitivity of 0.60 pmol/L (reference range 2.4–4.4 pg/mL).
In each case, fibrinolytic [plasminogen activator inhibitor-1 (PAI-1) and tissue plasminogen activator (t-PA)] and hemostatic variables [prothrombin time, activated partial thromboplastin time (seconds), fibrinogen, D-dimer, coagulation factors VII and VIII, and von Willebrand factor (vWF)] were determined.
According to validated protocols (15), PAI-1 and t-PA antigens (Imulyze) were measured by ELISA methods using kits from Biopool-Menarini (16). Prothrombin time and activated partial thromboplastin time were measured by coagulation analyzers using kits from Dade Behring Marburg GmbH. Fibrinogen activity was evaluated by the Clauss clotting method using the kit from Mascia Brunelli. Factor VII (FVII) and factor VIII (FVIII) activity were evaluated by a clotting assay (Dade-Behring). The vWF antigen was evaluated with a commercially available kit (INNOVANCE VWF Ac kit; Siemens Healthcare Diagnostics). Antithrombin activity was measured using a commercial kit (Berichrom ATIII; Behringwerke) (17, 18). Levels of D-dimer were assessed with the INNOVANCE D-dimer kit (Siemens Healthcare Diagnostics).
MPV was measured in a nine-volume blood sample collected in one volume of 3.8% trisodium citrate [1:4 (vol/vol)]. A Cell-Dyn 3500 (Abbott) was used for complete blood counts (13).
All subjects underwent ex vivo platelet aggregation assessment by means of light transmittance aggregometry (Chrono-Log; Mascia Brunelli) with increasing doses of arachidonic acid (AA) and ADP to measure the minimum dose of each proaggregating agent needed to achieve an irreversible aggregation greater than 50% within 5 minutes (AC-50%) (19, 20). All laboratory assessments were performed by technicians unaware of the patients' treatment and disease.
After baseline evaluations, SH patients started L-T4 treatment titrated to normalize TSH levels. After 6 months from TSH normalization, all the laboratory assessments were repeated in each SH patient.
The data obtained at baseline were compared with those achieved in a control group of healthy subjects matched (1:1) for age, sex, and body weight with SH patients. In line with previous studies (15, 21), the variability of laboratory methods was assessed in random samples collected twice within a 1-month period from 260 subjects without any known endocrine, cardiovascular, or inflammatory disease, recruited in the same time period from the hospital staff. All changes in laboratory measures in patients with SH have been adjusted for the percentage of variability of the method found in the test group. The present study protocol has been approved by the Federico II University local ethic committee.
Statistical analysis
Statistical analysis was performed with the SPSS 17 system (SPSS Inc). Continuous data were expressed as means ± SD; categorical variables were expressed as percentages. To compare continuous variables, an independent-sample t test was performed. In case of values with a skewed distribution, Mann-Whitney U test was used to compare means. The χ2 test was used to analyze categorical data. All the results are presented as two-tailed values with statistical significance for values of P < .05.
Results
Among the 78 subjects with SH screened for the inclusion in this study, 37 were excluded because of the presence of at least one exclusion criteria [alteration in primary and/or secondary hemostasis (n = 1); treatment with anticoagulant or antiplatelet drugs (n = 3), personal and/or family history of arterial or venous thrombosis (n = 14); active inflammatory processes (n = 2), pregnancy (n = 9), or oral contraceptive intake (n = 5)]. In addition, three patients were lost during the follow-up and were excluded from the analysis.
Baseline clinical and demographic data of the 41 SH subjects who completed the study and of the 41 matched controls are reported in Table 1.
| . | SH Patients (n = 41) . | Controls (n = 41) . | P Value . |
|---|---|---|---|
| Female gender, n (%) | 33 (80.5) | 33 (80.5) | 1.000 |
| Age, y | 41.4 ± 13.0 | 42.2 ± 11.9 | .772 |
| BMI, kg/m2 | 25.9 ± 4.8 | 24.8 ± 4.2 | .273 |
| Smoking habit, n (%) | 8 (19.5) | 11 (26.8) | .434 |
| Systolic bp, mm Hg | 128 ± 15 | 123 ± 14 | .123 |
| Diastolic bp, mm Hg | 74 ± 10 | 75 ± 8 | .618 |
| FT3, pg/mLa | 3.1 ± 0.7 | 3.3 ± 0.5 | .140 |
| FT4, ng/dLa | 1.1 ± 0.3 | 1.2 ± 0.6 | .343 |
| TSH, μUI/mLa | 7.3 ± 4.8 | 2.1 ± 0.9 | <.001 |
| FVII, % | 123.9 ± 20.4 | 107.7 ± 12.2 | <.001 |
| FVIII, % | 120.4 ± 19.3 | 119.3 ± 17.4 | .716 |
| vWF, % | 119.5 ± 16.6 | 128.9 ± 19.9 | .002 |
| D-dimer, ng/mL | 220.3 ± 67.1 | 252.1 ± 72.4 | .017 |
| PAI, ng/mLa | 33.6 ± 13.9 | 22.5 ± 5.74 | <.001 |
| t-PA, ng/mLa | 5.56 ± 2.22 | 4.75 ± 1.61 | .010 |
| AC-50%-AA, mmola | 0.18 ± 0.12 | 0.36 ± 0.10 | <.001 |
| AC-50%-ADP, μmola | 1.5 ± 0.6 | 1.9 ± 1.3 | .024 |
| MPV, fl | 9.92 ± 1.15 | 8.9 ± 0.9 | <.001 |
| . | SH Patients (n = 41) . | Controls (n = 41) . | P Value . |
|---|---|---|---|
| Female gender, n (%) | 33 (80.5) | 33 (80.5) | 1.000 |
| Age, y | 41.4 ± 13.0 | 42.2 ± 11.9 | .772 |
| BMI, kg/m2 | 25.9 ± 4.8 | 24.8 ± 4.2 | .273 |
| Smoking habit, n (%) | 8 (19.5) | 11 (26.8) | .434 |
| Systolic bp, mm Hg | 128 ± 15 | 123 ± 14 | .123 |
| Diastolic bp, mm Hg | 74 ± 10 | 75 ± 8 | .618 |
| FT3, pg/mLa | 3.1 ± 0.7 | 3.3 ± 0.5 | .140 |
| FT4, ng/dLa | 1.1 ± 0.3 | 1.2 ± 0.6 | .343 |
| TSH, μUI/mLa | 7.3 ± 4.8 | 2.1 ± 0.9 | <.001 |
| FVII, % | 123.9 ± 20.4 | 107.7 ± 12.2 | <.001 |
| FVIII, % | 120.4 ± 19.3 | 119.3 ± 17.4 | .716 |
| vWF, % | 119.5 ± 16.6 | 128.9 ± 19.9 | .002 |
| D-dimer, ng/mL | 220.3 ± 67.1 | 252.1 ± 72.4 | .017 |
| PAI, ng/mLa | 33.6 ± 13.9 | 22.5 ± 5.74 | <.001 |
| t-PA, ng/mLa | 5.56 ± 2.22 | 4.75 ± 1.61 | .010 |
| AC-50%-AA, mmola | 0.18 ± 0.12 | 0.36 ± 0.10 | <.001 |
| AC-50%-ADP, μmola | 1.5 ± 0.6 | 1.9 ± 1.3 | .024 |
| MPV, fl | 9.92 ± 1.15 | 8.9 ± 0.9 | <.001 |
bp, blood pressure; fl, femtoliter.
Mann-Whitney U test.
| . | SH Patients (n = 41) . | Controls (n = 41) . | P Value . |
|---|---|---|---|
| Female gender, n (%) | 33 (80.5) | 33 (80.5) | 1.000 |
| Age, y | 41.4 ± 13.0 | 42.2 ± 11.9 | .772 |
| BMI, kg/m2 | 25.9 ± 4.8 | 24.8 ± 4.2 | .273 |
| Smoking habit, n (%) | 8 (19.5) | 11 (26.8) | .434 |
| Systolic bp, mm Hg | 128 ± 15 | 123 ± 14 | .123 |
| Diastolic bp, mm Hg | 74 ± 10 | 75 ± 8 | .618 |
| FT3, pg/mLa | 3.1 ± 0.7 | 3.3 ± 0.5 | .140 |
| FT4, ng/dLa | 1.1 ± 0.3 | 1.2 ± 0.6 | .343 |
| TSH, μUI/mLa | 7.3 ± 4.8 | 2.1 ± 0.9 | <.001 |
| FVII, % | 123.9 ± 20.4 | 107.7 ± 12.2 | <.001 |
| FVIII, % | 120.4 ± 19.3 | 119.3 ± 17.4 | .716 |
| vWF, % | 119.5 ± 16.6 | 128.9 ± 19.9 | .002 |
| D-dimer, ng/mL | 220.3 ± 67.1 | 252.1 ± 72.4 | .017 |
| PAI, ng/mLa | 33.6 ± 13.9 | 22.5 ± 5.74 | <.001 |
| t-PA, ng/mLa | 5.56 ± 2.22 | 4.75 ± 1.61 | .010 |
| AC-50%-AA, mmola | 0.18 ± 0.12 | 0.36 ± 0.10 | <.001 |
| AC-50%-ADP, μmola | 1.5 ± 0.6 | 1.9 ± 1.3 | .024 |
| MPV, fl | 9.92 ± 1.15 | 8.9 ± 0.9 | <.001 |
| . | SH Patients (n = 41) . | Controls (n = 41) . | P Value . |
|---|---|---|---|
| Female gender, n (%) | 33 (80.5) | 33 (80.5) | 1.000 |
| Age, y | 41.4 ± 13.0 | 42.2 ± 11.9 | .772 |
| BMI, kg/m2 | 25.9 ± 4.8 | 24.8 ± 4.2 | .273 |
| Smoking habit, n (%) | 8 (19.5) | 11 (26.8) | .434 |
| Systolic bp, mm Hg | 128 ± 15 | 123 ± 14 | .123 |
| Diastolic bp, mm Hg | 74 ± 10 | 75 ± 8 | .618 |
| FT3, pg/mLa | 3.1 ± 0.7 | 3.3 ± 0.5 | .140 |
| FT4, ng/dLa | 1.1 ± 0.3 | 1.2 ± 0.6 | .343 |
| TSH, μUI/mLa | 7.3 ± 4.8 | 2.1 ± 0.9 | <.001 |
| FVII, % | 123.9 ± 20.4 | 107.7 ± 12.2 | <.001 |
| FVIII, % | 120.4 ± 19.3 | 119.3 ± 17.4 | .716 |
| vWF, % | 119.5 ± 16.6 | 128.9 ± 19.9 | .002 |
| D-dimer, ng/mL | 220.3 ± 67.1 | 252.1 ± 72.4 | .017 |
| PAI, ng/mLa | 33.6 ± 13.9 | 22.5 ± 5.74 | <.001 |
| t-PA, ng/mLa | 5.56 ± 2.22 | 4.75 ± 1.61 | .010 |
| AC-50%-AA, mmola | 0.18 ± 0.12 | 0.36 ± 0.10 | <.001 |
| AC-50%-ADP, μmola | 1.5 ± 0.6 | 1.9 ± 1.3 | .024 |
| MPV, fl | 9.92 ± 1.15 | 8.9 ± 0.9 | <.001 |
bp, blood pressure; fl, femtoliter.
Mann-Whitney U test.
Among the 41 SH patients, 31 (70.4%) were affected by Hashimoto thyroiditis, documented by positivity of antithyroid antibodies (thyroglobulin antibodies and/or thyroperoxidase antibodies).
There were no significant differences between the two study groups for gender, mean age, body mass index (BMI), smoking habits, and systolic/diastolic blood pressure.
At baseline evaluation, compared with control subjects, SH patients showed higher levels of FVII activity, PAI-1, and t-PA, paralleled by lower levels of D-dimer (Table 1).
Although the mean plasma vWF levels were lower in SH patients than in controls, the Bleeding Score Questionnaire was negative in both cases and controls, excluding any clinical relevance of the mild reduction in vWF.
The assessment of ex vivo platelet aggregation with ADP and AA stimulation showed a AC-50% significantly lower in cases than in controls, clearly suggesting a platelet hyperreactivity in SH patients (Table 1). In line with these results, the MPV value was significantly higher in the SH group than in the control group (Table 1).
Six-month follow-up
All 41 patients were compliant with the suggested L-T4 treatment. Six months after restoring normal TSH values with an L-T4 mean dose of 30.75 μg/d (range 25–75 μg/d), TSH mean value decreased from 6.7 ± 1.8 to 2.1 ± 0.4 μUI/mL (P < .001), and FT3 and FT4 resulted unchanged (from 3.1 ± 0.7 and 3.0 ± 0.6 pg/mL, P = 1.00, and from 1.1 ± 0.3 to 1.2 ± 0.2 ng/dL, P = .08, respectively), being already normal at the baseline assessment.
As to the hemostatic parameters evaluated, whereas no variations of FVIII were found (from 120.4% ± 9.3% and 116.5% ± 16.1%, P = .18), we reported a significant reduction of FVII activity (from 123.9 ± 20.4 to 102.6 ± 14.3, P < .001) and an increase of vWF (from 119.5% ± 16.6% to 122.1% ± 10.2%, P = .04) (Table 2). No changes were found in the Bleeding Score Questionnaire after the 6-month L-T4 treatment.
| . | Before L-T4 Treatment . | After 6-Month L-T4 Treatment . | P Value . |
|---|---|---|---|
| FVII, % | 123.9 ± 20.4 | 102.6 ± 14.3 | <.001 |
| FVIII, % | 120.41 ± 9.3 | 116.5 ± 16.1 | .182 |
| vWF, % | 119.5 ± 16.6 | 122.1 ± 10.2 | .044 |
| D-dimer, ng/mL | 220.3 ± 67.1 | 245.2 ± 103.1 | .053 |
| PAI, ng/mL | 33.6 ± 13.9 | 19.4 ± 7.6 | <.001 |
| t-PA, ng/mL | 5.56 ± 2.22 | 4.43 ± 1.91 | .002 |
| AC-50%-AA, mmol | 0.18 ± 0.12 | 0.54 ± 0.3 | <.001 |
| AC-50%-ADP, μmol | 1.5 ± 0.6 | 1.86 ± 0.3 | .042 |
| MPV, fl | 9.92 ± 1.15 | 9.10 ± 1.23 | .016 |
| . | Before L-T4 Treatment . | After 6-Month L-T4 Treatment . | P Value . |
|---|---|---|---|
| FVII, % | 123.9 ± 20.4 | 102.6 ± 14.3 | <.001 |
| FVIII, % | 120.41 ± 9.3 | 116.5 ± 16.1 | .182 |
| vWF, % | 119.5 ± 16.6 | 122.1 ± 10.2 | .044 |
| D-dimer, ng/mL | 220.3 ± 67.1 | 245.2 ± 103.1 | .053 |
| PAI, ng/mL | 33.6 ± 13.9 | 19.4 ± 7.6 | <.001 |
| t-PA, ng/mL | 5.56 ± 2.22 | 4.43 ± 1.91 | .002 |
| AC-50%-AA, mmol | 0.18 ± 0.12 | 0.54 ± 0.3 | <.001 |
| AC-50%-ADP, μmol | 1.5 ± 0.6 | 1.86 ± 0.3 | .042 |
| MPV, fl | 9.92 ± 1.15 | 9.10 ± 1.23 | .016 |
fl, femtoliter.
| . | Before L-T4 Treatment . | After 6-Month L-T4 Treatment . | P Value . |
|---|---|---|---|
| FVII, % | 123.9 ± 20.4 | 102.6 ± 14.3 | <.001 |
| FVIII, % | 120.41 ± 9.3 | 116.5 ± 16.1 | .182 |
| vWF, % | 119.5 ± 16.6 | 122.1 ± 10.2 | .044 |
| D-dimer, ng/mL | 220.3 ± 67.1 | 245.2 ± 103.1 | .053 |
| PAI, ng/mL | 33.6 ± 13.9 | 19.4 ± 7.6 | <.001 |
| t-PA, ng/mL | 5.56 ± 2.22 | 4.43 ± 1.91 | .002 |
| AC-50%-AA, mmol | 0.18 ± 0.12 | 0.54 ± 0.3 | <.001 |
| AC-50%-ADP, μmol | 1.5 ± 0.6 | 1.86 ± 0.3 | .042 |
| MPV, fl | 9.92 ± 1.15 | 9.10 ± 1.23 | .016 |
| . | Before L-T4 Treatment . | After 6-Month L-T4 Treatment . | P Value . |
|---|---|---|---|
| FVII, % | 123.9 ± 20.4 | 102.6 ± 14.3 | <.001 |
| FVIII, % | 120.41 ± 9.3 | 116.5 ± 16.1 | .182 |
| vWF, % | 119.5 ± 16.6 | 122.1 ± 10.2 | .044 |
| D-dimer, ng/mL | 220.3 ± 67.1 | 245.2 ± 103.1 | .053 |
| PAI, ng/mL | 33.6 ± 13.9 | 19.4 ± 7.6 | <.001 |
| t-PA, ng/mL | 5.56 ± 2.22 | 4.43 ± 1.91 | .002 |
| AC-50%-AA, mmol | 0.18 ± 0.12 | 0.54 ± 0.3 | <.001 |
| AC-50%-ADP, μmol | 1.5 ± 0.6 | 1.86 ± 0.3 | .042 |
| MPV, fl | 9.92 ± 1.15 | 9.10 ± 1.23 | .016 |
fl, femtoliter.
In addition, we found a significant decrease of PAI (33.6 ± 13.9 to 19.4 ± 7.6, P < .001) with a parallel reduction of t-PA (5.56 ± 2.22 to 1.91 ± 4:43, P = .002). As a further expression of improvement of fibrinolysis, a trend toward an increase in D-dimer (from 220.3 ± 67.1 to 245.2 ± 103.1, P = .053) was reported (Table 2).
By evaluating the percentage changes in each hemostatic and fibrinolytic variable (Figure 1), the most significant change has been found in fibrinolytic parameters (PAI-1 and t-PA), which was paralleled by an increase in D-dimer levels.
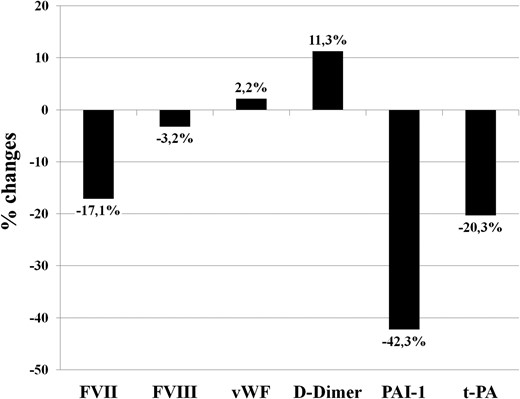
Percentage changes in hemostatic and fibrinolytic variables in patients with subclinical hypothyroidism after a 6-month treatment with L-T4.
As to platelet aggregation, a significant increase was found in AC-50% induced by AA (0.18 ± 0.12 to 0.54 ± 0.3, P < .001) and by ADP (1.5 ± 0.6 to 1.86 ± 0.3, P = .042). This was paralleled by a significant reduction in MPV (from 9.92 ± 1.15 femtoliter to 9.10 ± 1.23 femtoliter, P = .016).
Discussion
This is the first study, to our best knowledge, extensively assessing primary and secondary hemostasis parameters in patients with SH. Interestingly, we found that patients with SH report alterations in primary hemostasis (increased MPV and platelet hyperreactivity), secondary hemostasis (hypercoagulability evidenced by the increase in FVII), and fibrinolysis (hypofibrinolysis documented by increased PAI-1 levels), consistently suggesting a prothrombotic state in this clinical subset. It is also noteworthy that all prothrombotic changes are reverted after replacement therapy with L-T4, further confirming the interrelationship between hemostatic balance and thyroid function.
The main finding of the present study is that patients with SH display a prothrombotic tendency. Indeed, compared with controls, SH patients showed higher levels of FVII, PAI-1, and t-PA together with lower D-dimer levels. Overall, this pattern of alterations is suggestive of a hypercoagulable state with a reduction of fibrinolysis (22). In particular, the total fibrinolytic potential of human blood, determined by the balance between t-PA and PAI-1, has been widely recognized as a predictor of both venous and arterial thrombosis (23). Moreover, increased levels of t-PA might represent a compensatory reaction to a hypofibrinolysis due to an increased inhibitory effect of PAI-1 (23). The clinical impact of increased PAI-1 levels lies in the association between high PAI-1 concentrations and the risk of myocardial infarction (MI) (24). In a nested case-control design on 234 patients, the increase in PAI-1 was associated with a higher risk of MI (odds ratio 1.04, 95% confidence interval 1.01–1.08) (24). This association has also been confirmed in a recent meta-analysis (25). Overall, the relevance of our results can be better understood when we consider that the risk of MI increases approximately 1% of each 1 ng/mL increase in PAI-1 levels (26). In our study population, we have found an approximately 11 ng/mL difference in PAI-1 levels among SH patients and controls, which, according to above-reported data, should confer an approximately 11% increase in the thrombotic risk in this clinical setting.
In addition to the evaluation of fibrinolytic balance, in this study we reported changes in a series of other hemostatic parameters. In particular, we found an approximately 13% increase in levels of FVII, which is the clotting factor with the shortest half-life, and it is recognized to be associated with a prothrombotic state and with an increased risk of venous and arterial thrombosis (15). In detail, some data showed that a 14.7% increase in FVII levels, is associated with a 4% increase in the risk of MI and a 6% increase in the risk of stroke (27).
These observations are in line with some data available in literature that show an impaired fibrinolysis in SH patients. However, most of these studies assessed only secondary hemostasis without providing data on the primary phase of the coagulation (2, 6, 8, 9, 11, 12).
Interestingly, we found that SH patients had a higher MPV value that is a marker of increased platelet turnover and, then, a predictor of cardiovascular risk.(28) More in detail, MPV, being associated with an increased secretion of vasoactive substances and prothrombotic factors (thromboxane A2, serotonin, and ATP) that induce platelet aggregation, might be considered an indirect marker of platelet hyperreactivity (2, 29–31). Although this evidence might suggest the hypothesis of an increased platelet reactivity in SH patients (13), studies specifically assessing platelet function are currently lacking. Accordingly, with the aim to confirm and extend this hypothesis, in the present study, we specifically assessed ex vivo platelet aggregation in SH patients using different proaggregating agonists, and we have been able to directly demonstrate that patients with SH had a platelet hyperreactivity compared with controls. This result is an acquisition not yet present in the literature that helps clarify the thrombotic risk in this clinical setting. Indeed, platelet hyperreactivity correlates with an elevated incidence of arterial and venous thrombosis and represents a well-established marker of cardiovascular risk (32–34).
Some recent data clearly suggest that patients with over hypothyroidism exhibit an acquired von Willebrand disease with a clinically relevant bleeding tendency (35, 36). In our sample, although vWF produces lower results in patients with SH than in controls, its levels are not low enough to cause hemorrhagic manifestations (37) as confirmed by the Bleeding Score Questionnaire, which is specific for von Willebrand disease and which was negative both in cases and in controls. Indeed, the decrease in vWF found in overt hypothyroidism was of a greater magnitude than that we found in SH and was associated with prolonged bleeding time and bleeding manifestations. Moreover, effects of L-T4 on vWF levels were more relevant in overt hypothyroidism than in SH patients (38).
Thus, the bleeding tendency, which is well known in patients with overt hypothyroidism, is not present in the present setting of patients with SH.
Therefore, it is evident that in patients with SH, although there is a nonsignificant prohemorrhagic tendency, a more relevant prothrombotic state, mainly due to an impairment of fibrinolysis, can be detected.
Whereas the pathophysiological mechanisms underlying the relationship between SH and a hypercoagulable state are unknown, a strong confirmation of this association emerges from the evidence that replacement therapy with L-T4 is able to revert the alterations observed.
In fact, after 6 months of euthyroid status induced by L-T4 therapy, we reported a significant reduction in FVII, PAI-1, and t-PA. In particular, the most relevant variation occurred in PAI-1, and, in line with previous data, changes in t-PA seem to mirror those found in PAI-1 (16).
In parallel, we observed a normalization of MPV and platelet reactivity, confirming the improvement of the primary hemostasis pattern.
Some potential limitations of the present study need to be addressed. In detail, a series of other clinical conditions are known to impact primary and secondary hemostasis. Most of the confounding factors have been avoided according to exclusion criteria. Moreover, any potential impact of obesity on the hemostatic and fibrinolytic balance (39) is likely to have been excluded, considering that the BMI of our study sample was within normal ranges of reference.
It is known that hemostatic parameters change over time. The results achieved after the 6-month treatment may be, at least in part, accounted for such variability. However, as detailed in Materials and Methods, all results have been adjusted for the variability of each measurement observed in a large validation sample. Thus, we are confident that the impact of such variability on our results is marginal. The lack of the assessment of a clinical outcome (incidence of MI, stroke, or other thrombotic events) is a limitation of the present study. However, the aim of our research was to evaluate mechanisms leading to a hypercoagulable state in SH patients. Our results, by highlighting an impaired fibrinolysis and a hypercoagulable state, are in line with the increased risk of cardiovascular events in SH documented in some literature studies (40–43).
In conclusion, in our study we report that subclinical hypothyroidism is associated with a prothrombotic state, as revealed by the presence of alterations in primary hemostasis (high MPV and platelet reactivity), secondary hemostasis (increased FVII levels), and fibrinolysis (high levels of PAI-1 and t-PA). These abnormalities of the hemocoagulative profile appear to be present in patients already at the moment of diagnosis. For this reason, it could be important for an early detection of subclinical thyroid dysfunction with an adequate screening of additional cardiovascular risk factors. Furthermore, based on our results, the prompt treatment of SH with appropriate replacement therapy is needed to revert the hemostatic abnormalities reducing, in turn, the hypercoagulability.
Acknowledgments
The authors contributed the following: R.L. and M.N.D.D.M. conceived and designed the study, performed the statistical analysis, interpreted the results, and drafted the manuscript; A.T., A.S., M.C., L.B., A.D.M., and P.A. acquired the clinical data and drafted the manuscript; G.A.L. and G.L. interpreted the results and performed the critical revision of the manuscript. All authors read and approved the final version of the manuscript.
Disclosure Summary: The authors have nothing to disclose.
R.L. and M.N.D.D.M. contributed equally to this work.
G.A.L. and G.L. share coseniorship of this work.
Abbreviations
- AA
arachidonic acid
- AC-50%
minimum dose of each proaggregating agent needed to achieve an irreversible aggregation greater than 50% within 5 minutes
- BMI
body mass index
- FT3
free T3
- FT4
free T4
- FVII
factor VII
- FVIII
factor VIII
- L-T4
levothyroxine
- MI
myocardial infarction
- MPV
mean platelet volume
- PAI-1
plasminogen activator inhibitor-1
- SH
subclinical hypothyroidism
- t-PA
tissue plasminogen activator
- vWF
von Willebrand factor.



