-
PDF
- Split View
-
Views
-
Cite
Cite
Giuseppe Pasqualetti, Gennaro Pagano, Giuseppe Rengo, Nicola Ferrara, Fabio Monzani, Subclinical Hypothyroidism and Cognitive Impairment: Systematic Review and Meta-Analysis, The Journal of Clinical Endocrinology & Metabolism, Volume 100, Issue 11, 1 November 2015, Pages 4240–4248, https://doi.org/10.1210/jc.2015-2046
Close - Share Icon Share
The association between subclinical hypothyroidism (sHT) and cognitive impairment or risk of dementia is not well-defined, especially in the elderly, where the assessment of central nervous system function is challenging. The aim of this systematic review and meta-analysis was to evaluate the possible effect of sHT on cognitive decline and the risk of dementia.
Cognitive function was the primary outcome, evaluated as composite endpoint of incidence or prevalence of dementia or difference of Mini Mental State Examination, Wechsler Adult Intelligence Scale, and Wechsler Memory Scale-Revised scores.
Thirteen studies were included in the meta-analysis. A significant risk of cognitive alteration was observed only in sHT individuals younger than age 75 years: composite endpoint odds ratio (OR) 1.56 (95% confidence interval [CI] 1.07–2.27, P = .02, I2 = 82.5%), risk of dementia OR 1.81 (95% CI 1.43–2.28, P < .01, I2 = 35%). Mean serum thyroid-stimulating hormone (TSH) levels and the OR of composite endpoint were positively correlated. No significant effect of sHT was found when considering all the studies as a whole: composite endpoint OR 1.26 (95% CI 0.96–1.66, P = .09, I2 = 87.2%), risk of dementia OR 1.42 (95% CI, 0.97–2.07, P = .07, I2 = 66.8%), Mini Mental State Examination mean difference −0.059 (95% CI −0.464 to 0.346 P = .78, I2 = 51.8%).
This meta-analysis demonstrates a relationship between sHT and cognitive impairment only in individuals younger than 75 years of age and those with higher TSH concentrations. No correlation was found while considering all the studies as a whole. The lack of utilization of age-related serum TSH reference ranges and consequent potential misdiagnosis of sHT in older people may account for this.
Subclinical thyroid dysfunction is common a condition in the general population, especially in older people. In fact, mild thyroid failure or subclinical hypothyroidism (sHT), defined as serum thyroid-stimulating hormone (TSH) concentration above the upper limit of the reference range in the face of normal free T4 (FT4) and free T3 levels, represents one of the most frequently observed diseases in the elderly (1–3). Several pathological entities, mainly represented by chronic autoimmune thyroiditis, are known to be associated with sHT pathogenesis (1). It has been estimated that sHT affects 5–10% of adult population with an increased prevalence in women and older people (4–6). However, it has been shown that serum TSH rises in normal healthy elderly individuals and, by excluding people whose serum TSH is below the 97.5th percentile for age (around 7 mIU/L in octogenarians) the actual prevalence of sHT is likely lower (6–8). Most subjects with sHT do not show specific symptoms, whereas others experience symptoms that resemble those observed in overt hypothyroidism, although to a lesser extent (1, 8). Considering the subtle clinical presentation of sHT, the evaluation of the impact of mild thyroid failure on cognition could be a challenge, especially in specific populations such as older people or patients with chronic diseases. The effect of sHT on cognitive function has been investigated in several preclinical studies, and a growing body of evidence has suggested a relevant link between thyroid hormones and central nervous system (CNS) (9). However, the actual association between sHT and cognitive impairment or risk of dementia has not been completely elucidated, especially in the elderly, in whom the assessment of CNS function is more challenging (9, 10). In this setting, conflicting data have been reported in large, population-based studies, leading to uncertain conclusions regarding the association between mild thyroid failure and memory/cognition impairment (11–17). Moreover, no meta-analyses on this topic have been published. For these reasons, we carried out a systematic review and meta-analysis to evaluate the effect of sHT on cognition and on the risk of dementia.
Materials and Methods
The study was designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (18) and recommendations from the Cochrane Collaboration and Meta-analysis of Observational Studies in Epidemiology (19) using a methodology extensively described in a previous publication of our group written following previous studies (20, 21).
Search strategy
The MEDLINE, Web of Science, Cochrane CENTRAL, and Scopus databases were searched for articles in all languages published until November 2014. Gray literature (ie, everything that is not peer-reviewed and controlled by commercial publishers) was not considered as a priority asset of our systematic review, and only formally published English articles were selected. Studies were identified and evaluated by three of the authors (G.Pas, G.Pag., F.M.) using the major medical subject heading “hypothyroidism” and “dementia” combined with the following text and key words (the following is an example for MEDLINE): (subclinical AND (“hypothyroidism” [MeSH Terms] OR (“hypothyroidism” [MeSH Terms] OR “hypothyroidism”))) AND (((((((((“dementia” [MeSH Terms] OR “alzheimer disease” [MeSH Terms]) OR (“dementia” [MeSH Terms] OR “dementia” OR “dementias”)) OR (“dementia” [MeSH Terms] OR “dementia” OR “amentia”)) OR (“dementia” [MeSH Terms] OR “dementia” OR “amentia”)) OR (“dementia” [MeSH Terms] OR “dementia” OR (“senile” AND “paranoid” AND “dementia”))) OR (“dementia” [MeSH Terms] OR “dementia” OR (“senile” AND “paranoid” AND “dementias”))) OR (“dementia” [MeSH Terms] OR “dementia” OR (“familial” AND “dementia”) OR “familial dementia”)) OR (“dementia” [MeSH Terms] OR “dementia” OR (“familial” AND “dementia”) OR “familial dementia”)) OR ((((“Mild Cognitive Impairment” [Mesh] OR (cognitive AND impairment)) OR (cognitive AND impairments)) OR (“mild cognitive impairment” [MeSH Terms] OR (“mild” AND “cognitive” AND “impairment”) OR “mild cognitive impairment” OR (“mild” AND “cognitive” AND “impairments”) OR “mild cognitive impairments”)) OR cognitive)). Additional eligible studies were identified screening the reference lists of studies included in our analysis.
Study selection
All selected titles and abstracts were independently reviewed by two authors (G.Pas., G.Pag.). Studies were excluded if the title and/or abstract were not appropriate for the aim of the review. Full texts were subsequently obtained for eligible studies or when the relevance of an article could not definitively be excluded. Disagreement was resolved by consensus and by opinion of a third reviewer (F.M.), when necessary. Selected studies were eligible if they met the following criteria: published data, cross-sectional, case control, or longitudinal analysis enrolling at least 15 patients with subclinical hypothyroidism, well-defined normal upper limit of TSH value, and defined and unique commercial product to run TSH assay. Reviews, case reports, nonhuman studies, and abstracts or conference proceedings were excluded.
Risk of bias in included studies
The quality of the included studies was assessed by Newcastle-Ottawa Scale (NOS) (22). NOS is characterized by eight items including selection, comparability, and exposure (case-control studies), or outcome (cohort studies). The scale ranged from zero to nine stars, with the highest degree representing the greatest methodological quality. Disagreement was resolved by consensus and by opinion of a third reviewer (F.M.). The presence of publication bias was explored by performing the test for asymmetry of the funnel plot by Egger, which consists of the linear regression of normalized estimate effect (divided by its standard error) against precision (reciprocal of the standard error of the estimate) (23, 24).
Data extraction
Two reviewers (G.Pas, G.Pag.) independently completed the data extraction by using a standardized form. Disagreement was resolved by consensus and the opinion of a third reviewer (F.M.), when required. Detailed information was recorded on study year, author first name, type of study, sHT definition, commercial product used to run the TSH assay, cognitive function evaluation (prevalence or incidence of dementia, Mini Mental State Examination [MMSE], Wechsler Memory Scale-Revised, total memory quotient, and Wechsler Adult Intelligence Scale scores, sample size, and characteristics of patients (including possible thyroid medication).
Outcomes
Primary outcome (cognitive function) was assessed as composite endpoint, derived from one of the following parameters as available in each study: incidence or prevalence of dementia, reduced MMSE, Wechsler Memory Scale-Revised, total memory quotient, and Wechsler Adult Intelligence Scale scores. Secondary outcome was the mean difference of MMSE score and the prevalence of dementia.
Statistical analysis
The odds ratio (OR) was calculated for each outcome of the composite endpoint except MMSE, in which the mean difference of scores was assessed. The results were pooled using the inverse variance method. When standard deviation (SD) was not available, missing SD was imputed and changes in endpoint measures from baseline to follow-up were obtained according to the Cochrane Handbook (25).
For binary outcomes, OR and 95% confidence intervals (CI) were calculated for each outcome by using the intention-to-treat principle. The choice to use OR was driven by the retrospective design of the meta-analysis based on published studies that varied in design, subjects' population, primary outcome measure, and research quality (26). Heterogeneity was assessed using I2 statistic that accounts of between-study (or interstudy) variability as opposed to within-study (or intrastudy) variability. Because of latent clinical heterogeneity, random effect model was used to synthesize data instead of a fixed-effect model, independently from statistical evidence of heterogeneity (27). Heterogeneity was considered substantial if I2 value was greater than 25% (28).
All reported test results were two-tailed and P value < .05 was considered significant. Data analysis was performed with STATA (Statacorp, version 12.0).
Sensitivity analysis
To investigate the influence of individual studies on the summary risk estimate, we undertook one-study-removed analysis by omitting one study in each turn and recalculating the pooled estimates on remaining studies using the metaninf command (29). To explore the influence of potential effect modifiers on composite endpoint, a meta-regression analysis was performed with the metareg command to test age, sex (male %), and baseline TSH (STATA) (30).
For all meta-regression analyses, a random effect model was used to take into account the mean of a distribution of effects across studies. In fact, the random effect model more appropriately provides wider confidence intervals for the regression coefficients than a fixed effect analysis, if residual heterogeneity exists (31).
The weight used for each study was the inverse of the sum of the within trial variance and the residual between trial variance to correspond to a random effect analysis. To estimate the additive (between-study) component of variance τ-2, the restricted maximum likelihood method was used to take into account the occurrence of residual heterogeneity, not explained by the potential effect modifiers (31).
Results
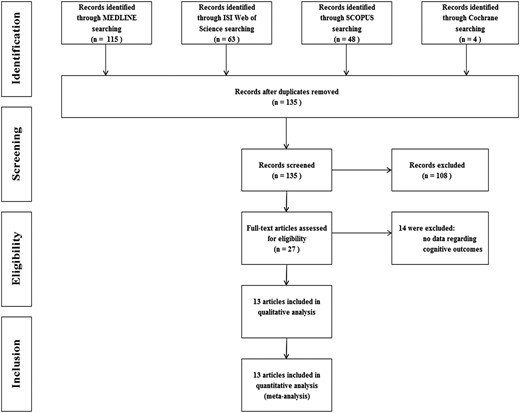
A total of 135 articles were identified by the initial search up to November 4, 2014, 27 of which were retrieved for more detailed evaluation and 13 were finally included in the meta-analysis (Figure 1). In detail, we found four cross-sectional, one case-control, and seven cohort studies, with an age range from 50.3 to 81.2 years (12, 14, 15, 17, 32–40) (Table 1). Heterogeneity among studies was observed for the method of TSH measurement and for the upper limit of normal TSH level, with values ranging from 2.1 to 5.5 mIU/L. The mean values of serum TSH ranged from 5.5 to 12.0 mIU/L in the subclinical hypothyroid groups and from 1.2 to 2.3 mIU/L in the control groups (Table 1). The composite endpoint was obtained from all the studies, the MMSE score in eight (14, 17, 34–37, 39, 40), and the risk of dementia in six studies (12, 17, 37–40) (Figure 2). The quality of included studies was moderate or good, varying from five to seven NOS stars (Table 1).
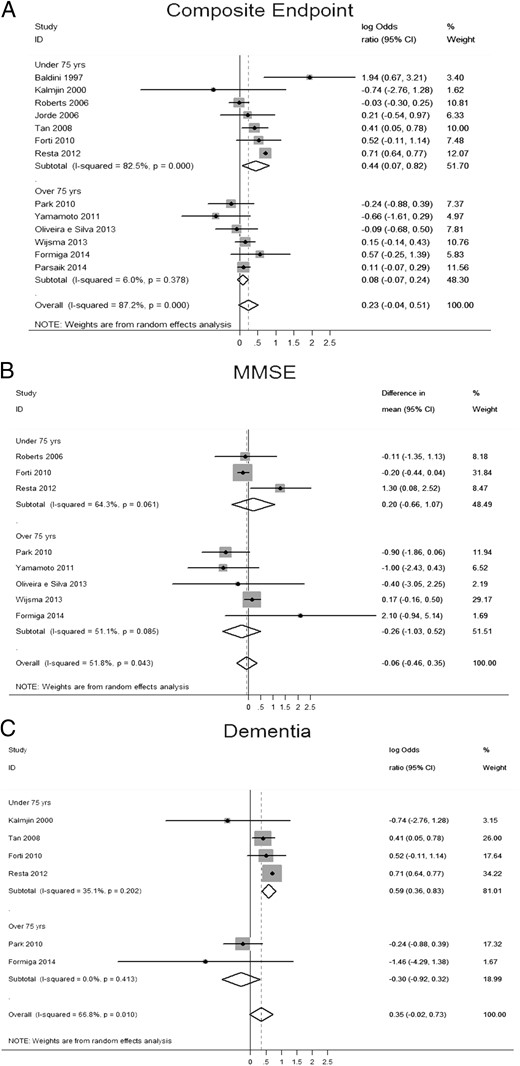
Pooled analyses (log of OR) for: (A) primary composite endpoint (incidence or prevalence of dementia, MMSE score, miscellaneous cognitive function scales, Wechsler Memory Scale-Revised score, and total memory quotient); (B) MMSE score (standardized mean difference); (C) incidence or prevalence of dementia (log of OR).
Reported data are stratified by age (older and younger than 75 years).
| Study . | Type . | sHT Definition . | TSH Assay . | Thyroid Medication Allowed (Yes, No) . | TSH Upper Limit >97.5th Percentile Adjusted for Agea . | Main Cognitive Endpoint . | Number sHT . | Number Controls . | sHT Female (%) . | Controls Female (%) . | sHT Mean Age (Years) . | Controls Mean Age (Years) . | Newcastle-Ottawa Scale . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baldini et al (1997) (33) | Case control | TSH exceeding the upper normal limits by 20% (4.6 mU/liter) or more (in at least two consecutive occasions) associated with normal FT4 and FT3 | IRMA Allegro HS-TSH, Nichols Institute Diagnostics, San Juan Capistrano, CA | No | Yes | Total memory quotient | 19 | 17 | 100 | 100 | 52.2 | 50.3 | 5 |
| Formiga et al (2014) (40) | Cohort study | TSH >5 mU/liter, FT4 10–26 pmol/liter | MABs Roche Diagnostics | No | No | Dementia | 20 | 282 | 55 | 60.3 | 85 | 85 | 7 |
| Jorde et al (2006) (15) | Cohort study | TSH 5–10 mU/liter, FT4 9–22 pmol/liter in at least two consecutive occasions | Hoffman-La Roche | No | Yes | Composite cognitive function | 28 | 102 | 30 | 78.68 | 61.7 | 61.3 | 7 |
| Park et al (2010) (17) | Cohort study | TSH >4.1 mU/liter, FT4 0.7–1.8 ng/liter | TSH: CIS Bio International, Gif-sur-Yvette | No | No | Dementia | 164 | 754 | 55.5 | 56.6 | 76.8 | 76.5 | 6 |
| Parsaik et al (2014) (32) | Cross-sectional | TSH 5–10 mU/liter, FT4 1.01–1.79 ng/dl | TSH Mayo Clinic Protocol | No | No | Wechsler Memory Scale-Revised | 141 | 1450 | 49.6 | 43.9 | 81.7 | 80.0 | 6 |
| Roberts et al (2006) (14) | Cohort study | TSH >5.5 mU/liter, FT4 9–20 pmol/liter | Adiva Centaur Bayer Diagnostic | No | No | MMSE | 168 | 5554 | 64 | 50 | 74 | 73 | 6 |
| Wijsma et al (2013) (34) | Cohort study | TSH >4.5 mU/liter, FT4 12–18 pmol/liter in at least two consecutive occasions | Roche Elecsys 2010 | No | No | MMSE | 161 | 4.928 | 64 | 48.6 | 75.6 | 75.3 | 6 |
| Yamamoto et al (2011) (35) | Cohort study | NA | NA | No | NA | MMSE | 15 | 214 | 53,3 | 65,9 | 80,1 | 80,9 | 5 |
| Silva et al (2013) (36) | Cross-sectional | TSH 4–19.9 U/liter, FT4 0.8–1.19 ng/dl | Immunolite 2000 | No | No | MMSE | 43 | 235 | 81.4 | 76.6 | 81.2 | 80.3 | 6 |
| Tan et al (2008) (12) | Cohort study | TSH >1.8–2.1 mU/literb | London Diagnostics, Eden Prairie, Minnesota | Yes, maximum 6% of the whole cohort | No | Dementia | 559 | 1133 | 59.4 | 57.9 | 72 | 71 | 6 |
| Forti et al (2012) (37) | Cross-sectional | TSH >4.50 mU/liter, FT4 10.3–25.7 pmol/liter | Roche Elecsys 2010 | Yes | No | Dementia | 217 | 423 | 93 | 113 | 74 | 71.9 | 6 |
| Resta et al (2012) (39) | Cross-sectional | TSH 3.6 mU/liter, FT4 8–17 pmol/liter | Vedere ILSA Study Ref 24 | No | No | Dementia | 42 | 283 | 64.3 | 44.2 | 74.3 | 74.3 | 6 |
| Kalmijn et al (2000) (38) | Cohort study | TSH >4 mU/liter, FT4 11–25 pmol/liter | TSH Lumitest (Hennin, Berlin, Germany) | Yes, maximum 0.4% of the whole cohort | No | Dementia | 170 | 1552 | NA | 6 |
| Study . | Type . | sHT Definition . | TSH Assay . | Thyroid Medication Allowed (Yes, No) . | TSH Upper Limit >97.5th Percentile Adjusted for Agea . | Main Cognitive Endpoint . | Number sHT . | Number Controls . | sHT Female (%) . | Controls Female (%) . | sHT Mean Age (Years) . | Controls Mean Age (Years) . | Newcastle-Ottawa Scale . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baldini et al (1997) (33) | Case control | TSH exceeding the upper normal limits by 20% (4.6 mU/liter) or more (in at least two consecutive occasions) associated with normal FT4 and FT3 | IRMA Allegro HS-TSH, Nichols Institute Diagnostics, San Juan Capistrano, CA | No | Yes | Total memory quotient | 19 | 17 | 100 | 100 | 52.2 | 50.3 | 5 |
| Formiga et al (2014) (40) | Cohort study | TSH >5 mU/liter, FT4 10–26 pmol/liter | MABs Roche Diagnostics | No | No | Dementia | 20 | 282 | 55 | 60.3 | 85 | 85 | 7 |
| Jorde et al (2006) (15) | Cohort study | TSH 5–10 mU/liter, FT4 9–22 pmol/liter in at least two consecutive occasions | Hoffman-La Roche | No | Yes | Composite cognitive function | 28 | 102 | 30 | 78.68 | 61.7 | 61.3 | 7 |
| Park et al (2010) (17) | Cohort study | TSH >4.1 mU/liter, FT4 0.7–1.8 ng/liter | TSH: CIS Bio International, Gif-sur-Yvette | No | No | Dementia | 164 | 754 | 55.5 | 56.6 | 76.8 | 76.5 | 6 |
| Parsaik et al (2014) (32) | Cross-sectional | TSH 5–10 mU/liter, FT4 1.01–1.79 ng/dl | TSH Mayo Clinic Protocol | No | No | Wechsler Memory Scale-Revised | 141 | 1450 | 49.6 | 43.9 | 81.7 | 80.0 | 6 |
| Roberts et al (2006) (14) | Cohort study | TSH >5.5 mU/liter, FT4 9–20 pmol/liter | Adiva Centaur Bayer Diagnostic | No | No | MMSE | 168 | 5554 | 64 | 50 | 74 | 73 | 6 |
| Wijsma et al (2013) (34) | Cohort study | TSH >4.5 mU/liter, FT4 12–18 pmol/liter in at least two consecutive occasions | Roche Elecsys 2010 | No | No | MMSE | 161 | 4.928 | 64 | 48.6 | 75.6 | 75.3 | 6 |
| Yamamoto et al (2011) (35) | Cohort study | NA | NA | No | NA | MMSE | 15 | 214 | 53,3 | 65,9 | 80,1 | 80,9 | 5 |
| Silva et al (2013) (36) | Cross-sectional | TSH 4–19.9 U/liter, FT4 0.8–1.19 ng/dl | Immunolite 2000 | No | No | MMSE | 43 | 235 | 81.4 | 76.6 | 81.2 | 80.3 | 6 |
| Tan et al (2008) (12) | Cohort study | TSH >1.8–2.1 mU/literb | London Diagnostics, Eden Prairie, Minnesota | Yes, maximum 6% of the whole cohort | No | Dementia | 559 | 1133 | 59.4 | 57.9 | 72 | 71 | 6 |
| Forti et al (2012) (37) | Cross-sectional | TSH >4.50 mU/liter, FT4 10.3–25.7 pmol/liter | Roche Elecsys 2010 | Yes | No | Dementia | 217 | 423 | 93 | 113 | 74 | 71.9 | 6 |
| Resta et al (2012) (39) | Cross-sectional | TSH 3.6 mU/liter, FT4 8–17 pmol/liter | Vedere ILSA Study Ref 24 | No | No | Dementia | 42 | 283 | 64.3 | 44.2 | 74.3 | 74.3 | 6 |
| Kalmijn et al (2000) (38) | Cohort study | TSH >4 mU/liter, FT4 11–25 pmol/liter | TSH Lumitest (Hennin, Berlin, Germany) | Yes, maximum 0.4% of the whole cohort | No | Dementia | 170 | 1552 | NA | 6 |
The 97.5th percentile of TSH adjusted for age was obtained by the general National Health and Nutrition Examination Survey cohort values as by Boucai et al (8).
The limits indicated the third percentile for men and women, respectively.
FT3, free T3; NA, not available.
| Study . | Type . | sHT Definition . | TSH Assay . | Thyroid Medication Allowed (Yes, No) . | TSH Upper Limit >97.5th Percentile Adjusted for Agea . | Main Cognitive Endpoint . | Number sHT . | Number Controls . | sHT Female (%) . | Controls Female (%) . | sHT Mean Age (Years) . | Controls Mean Age (Years) . | Newcastle-Ottawa Scale . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baldini et al (1997) (33) | Case control | TSH exceeding the upper normal limits by 20% (4.6 mU/liter) or more (in at least two consecutive occasions) associated with normal FT4 and FT3 | IRMA Allegro HS-TSH, Nichols Institute Diagnostics, San Juan Capistrano, CA | No | Yes | Total memory quotient | 19 | 17 | 100 | 100 | 52.2 | 50.3 | 5 |
| Formiga et al (2014) (40) | Cohort study | TSH >5 mU/liter, FT4 10–26 pmol/liter | MABs Roche Diagnostics | No | No | Dementia | 20 | 282 | 55 | 60.3 | 85 | 85 | 7 |
| Jorde et al (2006) (15) | Cohort study | TSH 5–10 mU/liter, FT4 9–22 pmol/liter in at least two consecutive occasions | Hoffman-La Roche | No | Yes | Composite cognitive function | 28 | 102 | 30 | 78.68 | 61.7 | 61.3 | 7 |
| Park et al (2010) (17) | Cohort study | TSH >4.1 mU/liter, FT4 0.7–1.8 ng/liter | TSH: CIS Bio International, Gif-sur-Yvette | No | No | Dementia | 164 | 754 | 55.5 | 56.6 | 76.8 | 76.5 | 6 |
| Parsaik et al (2014) (32) | Cross-sectional | TSH 5–10 mU/liter, FT4 1.01–1.79 ng/dl | TSH Mayo Clinic Protocol | No | No | Wechsler Memory Scale-Revised | 141 | 1450 | 49.6 | 43.9 | 81.7 | 80.0 | 6 |
| Roberts et al (2006) (14) | Cohort study | TSH >5.5 mU/liter, FT4 9–20 pmol/liter | Adiva Centaur Bayer Diagnostic | No | No | MMSE | 168 | 5554 | 64 | 50 | 74 | 73 | 6 |
| Wijsma et al (2013) (34) | Cohort study | TSH >4.5 mU/liter, FT4 12–18 pmol/liter in at least two consecutive occasions | Roche Elecsys 2010 | No | No | MMSE | 161 | 4.928 | 64 | 48.6 | 75.6 | 75.3 | 6 |
| Yamamoto et al (2011) (35) | Cohort study | NA | NA | No | NA | MMSE | 15 | 214 | 53,3 | 65,9 | 80,1 | 80,9 | 5 |
| Silva et al (2013) (36) | Cross-sectional | TSH 4–19.9 U/liter, FT4 0.8–1.19 ng/dl | Immunolite 2000 | No | No | MMSE | 43 | 235 | 81.4 | 76.6 | 81.2 | 80.3 | 6 |
| Tan et al (2008) (12) | Cohort study | TSH >1.8–2.1 mU/literb | London Diagnostics, Eden Prairie, Minnesota | Yes, maximum 6% of the whole cohort | No | Dementia | 559 | 1133 | 59.4 | 57.9 | 72 | 71 | 6 |
| Forti et al (2012) (37) | Cross-sectional | TSH >4.50 mU/liter, FT4 10.3–25.7 pmol/liter | Roche Elecsys 2010 | Yes | No | Dementia | 217 | 423 | 93 | 113 | 74 | 71.9 | 6 |
| Resta et al (2012) (39) | Cross-sectional | TSH 3.6 mU/liter, FT4 8–17 pmol/liter | Vedere ILSA Study Ref 24 | No | No | Dementia | 42 | 283 | 64.3 | 44.2 | 74.3 | 74.3 | 6 |
| Kalmijn et al (2000) (38) | Cohort study | TSH >4 mU/liter, FT4 11–25 pmol/liter | TSH Lumitest (Hennin, Berlin, Germany) | Yes, maximum 0.4% of the whole cohort | No | Dementia | 170 | 1552 | NA | 6 |
| Study . | Type . | sHT Definition . | TSH Assay . | Thyroid Medication Allowed (Yes, No) . | TSH Upper Limit >97.5th Percentile Adjusted for Agea . | Main Cognitive Endpoint . | Number sHT . | Number Controls . | sHT Female (%) . | Controls Female (%) . | sHT Mean Age (Years) . | Controls Mean Age (Years) . | Newcastle-Ottawa Scale . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baldini et al (1997) (33) | Case control | TSH exceeding the upper normal limits by 20% (4.6 mU/liter) or more (in at least two consecutive occasions) associated with normal FT4 and FT3 | IRMA Allegro HS-TSH, Nichols Institute Diagnostics, San Juan Capistrano, CA | No | Yes | Total memory quotient | 19 | 17 | 100 | 100 | 52.2 | 50.3 | 5 |
| Formiga et al (2014) (40) | Cohort study | TSH >5 mU/liter, FT4 10–26 pmol/liter | MABs Roche Diagnostics | No | No | Dementia | 20 | 282 | 55 | 60.3 | 85 | 85 | 7 |
| Jorde et al (2006) (15) | Cohort study | TSH 5–10 mU/liter, FT4 9–22 pmol/liter in at least two consecutive occasions | Hoffman-La Roche | No | Yes | Composite cognitive function | 28 | 102 | 30 | 78.68 | 61.7 | 61.3 | 7 |
| Park et al (2010) (17) | Cohort study | TSH >4.1 mU/liter, FT4 0.7–1.8 ng/liter | TSH: CIS Bio International, Gif-sur-Yvette | No | No | Dementia | 164 | 754 | 55.5 | 56.6 | 76.8 | 76.5 | 6 |
| Parsaik et al (2014) (32) | Cross-sectional | TSH 5–10 mU/liter, FT4 1.01–1.79 ng/dl | TSH Mayo Clinic Protocol | No | No | Wechsler Memory Scale-Revised | 141 | 1450 | 49.6 | 43.9 | 81.7 | 80.0 | 6 |
| Roberts et al (2006) (14) | Cohort study | TSH >5.5 mU/liter, FT4 9–20 pmol/liter | Adiva Centaur Bayer Diagnostic | No | No | MMSE | 168 | 5554 | 64 | 50 | 74 | 73 | 6 |
| Wijsma et al (2013) (34) | Cohort study | TSH >4.5 mU/liter, FT4 12–18 pmol/liter in at least two consecutive occasions | Roche Elecsys 2010 | No | No | MMSE | 161 | 4.928 | 64 | 48.6 | 75.6 | 75.3 | 6 |
| Yamamoto et al (2011) (35) | Cohort study | NA | NA | No | NA | MMSE | 15 | 214 | 53,3 | 65,9 | 80,1 | 80,9 | 5 |
| Silva et al (2013) (36) | Cross-sectional | TSH 4–19.9 U/liter, FT4 0.8–1.19 ng/dl | Immunolite 2000 | No | No | MMSE | 43 | 235 | 81.4 | 76.6 | 81.2 | 80.3 | 6 |
| Tan et al (2008) (12) | Cohort study | TSH >1.8–2.1 mU/literb | London Diagnostics, Eden Prairie, Minnesota | Yes, maximum 6% of the whole cohort | No | Dementia | 559 | 1133 | 59.4 | 57.9 | 72 | 71 | 6 |
| Forti et al (2012) (37) | Cross-sectional | TSH >4.50 mU/liter, FT4 10.3–25.7 pmol/liter | Roche Elecsys 2010 | Yes | No | Dementia | 217 | 423 | 93 | 113 | 74 | 71.9 | 6 |
| Resta et al (2012) (39) | Cross-sectional | TSH 3.6 mU/liter, FT4 8–17 pmol/liter | Vedere ILSA Study Ref 24 | No | No | Dementia | 42 | 283 | 64.3 | 44.2 | 74.3 | 74.3 | 6 |
| Kalmijn et al (2000) (38) | Cohort study | TSH >4 mU/liter, FT4 11–25 pmol/liter | TSH Lumitest (Hennin, Berlin, Germany) | Yes, maximum 0.4% of the whole cohort | No | Dementia | 170 | 1552 | NA | 6 |
The 97.5th percentile of TSH adjusted for age was obtained by the general National Health and Nutrition Examination Survey cohort values as by Boucai et al (8).
The limits indicated the third percentile for men and women, respectively.
FT3, free T3; NA, not available.
Primary and secondary outcomes
Overall, no significant difference was found for the composite endpoint: OR 1.26 (95% CI 0.96–1.66, P = .09, I2 = 87.2%). The mean difference for MMSE score was −0.059 (95% CI −0.464 to 0.346, P = .78, I2 = 51.8%) and the OR for dementia 1.42 (95% CI 0.97–2.07, P = .07, I2 = 66.8%). However, the analysis of the composite endpoint and the risk of dementia showed significant results when age was taken into account. A significant association between sHT and cognitive alterations or dementia was obtained in studies with mean age of the enrolled population younger than 75 years, whereas no effect was observed in the six studies enrolling older people (Figure 2). In detail, in the former studies, the OR of the composite endpoint and risk of dementia was 1.56 (95% CI 1.07–2.27, P = .02, I2 = 82.5%) and 1.81 (95% CI 1.43–2.28, P < .01, I2 = 35%), respectively. Accordingly, the meta-regression analysis of OR of the composite endpoint and mean age confirmed a significant association between age and the composite endpoint (Figure 3). It is worth noting that the risk of composite endpoint was positively related with the degree of TSH elevation (ß = 0.28, P = .005; data available for five studies), whereas no effect was obtained by stratifying for gender. Finally, the secondary analysis performed using the mean difference of MMSE scores as endpoint failed to show any association between sHT and cognitive function, regardless of age and gender stratification.
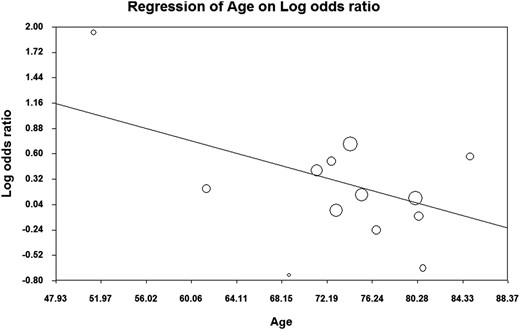
Sensitivity analysis and publication bias
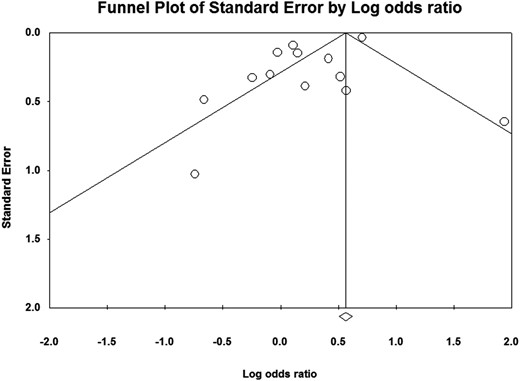
One-study-removed analysis showed a significant effect of sHT on the composite endpoint (OR 1.324, 95% C.I. 1.004–1.746, P = .047) only when the study by Yamamoto et al was removed (2011). However, by using the mean difference of MMSE scores and risk of dementia as endpoints, a significant effect was never obtained. Publication bias was detected by Egger's linear regression method for both primary composite outcome and risk of dementia (P = .02 and P = .01, respectively), whereas the analysis on MMSE scores did not show any publication bias (P = .38) (Figure 4).
Discussion
Overall, this systematic review and meta-analysis indicates that mild thyroid failure is not associated with cognitive alterations, defined by the diagnosis of dementia or the decline of cognitive function at MMSE. During the past 3 decades, a large number of studies investigated the relationship between sHT and cognitive decline; however, these studies presented a huge heterogeneity in the enrolled population, especially for age, gender, and clinical setting. These differences need to be taken into account when analyzing the clinical endpoints of the single study and, as suggested by our analysis on composite endpoint and risk of dementia, mild thyroid failure may lead to cognitive alterations and increased risk of dementia in individuals younger than age 75 years (9, 41). This finding should be interpreted with caution considering that it was not confirmed by analyzing the age stratified mean difference of MMSE scores. However, it is worth mentioning that the low sensitivity of MMSE, especially for detecting low/mild cognition impairment and the ceiling effect of this scale (42, 43), made the MMSE score inconclusive for very slight deficit of cognitive function (12, 33, 39, 44, 45). Several basic and clinical studies have suggested a strong relationship between thyroid hormones and CNS development and function (9). However, conflicting data exist on the relationship between sHT and cognitive impairment (46). Naturalistic studies have provided evidences of the negative effect of full-blown hypothyroidism on cognitive function, especially during the childbearing period and in infants (47, 48). Moreover, some studies documented a specific alteration of the memory domain in sHT patients (12, 33, 39, 44), whereas others reported possible or no effect on other cognitive domains (14, 15, 17, 44, 45, 49, 50). Based on the heterogeneity of these results, we created a composite outcome, comprehensive of different endpoints (dementia, cognitive function, and intelligence scales) to obtain a sufficient statistical power and to guarantee reliability of the results. By using this composite endpoint, an association between sHT and the risk of dementia was found in patients younger than age 75 years but not in older people. A possible explanation of this discrepancy could be the overdiagnosis of sHT in the elderly resulting from a lack of utilization of age-related serum TSH reference ranges in all the studies included in the meta-analysis (51). Indeed, the physiological shift of the serum TSH distribution curve toward upper values during normal aging may lead to misclassification of older subjects as having sHT (6, 8). According to the National Health and Nutrition Examination Survey study, the 97.5th percentile for normal serum TSH is 3.9 mIU/L in individuals younger than 49 years and 6.3 in those older than 80 (8). In other words, it is likely that by the same serum TSH value young individuals had “true” mild thyroid failure, whereas older ones (>80 years) did not. This could have led to an underestimation of the risk of cognitive alteration associated with sHT in the elderly. Another interesting result of the present meta-analysis is represented by the significant positive relationship between the OR for the composite endpoint and serum TSH value in sHT groups, although this result has been observed in a secondary evaluation of five studies. This finding would suggest that cognitive decline is increasingly related to the extent of thyroid failure, in line with the recommendation of the international guidelines for the diagnosis of dementia (10, 52). Finally, we did not confirm a gender susceptibility to sHT, as reported by Tan et al in a longitudinal study (12).
The inclusion of individuals taking thyroid hormone replacement therapy was allowed only in three studies (enrolling subjects younger than age 75 years) (Table 1). An association of sHT with cognitive alterations and increased risk of dementia was nonetheless shown in two of the three studies. Thus, the inclusion of patients taking LT4 therapy seems not to interfere with the main result of the meta-analysis.
In this setting, it could be interesting to evaluate the potential effect of LT4 replacement therapy on cognition. But scanty data exist on this topic, generally obtained in young individuals, without randomized study design (45).
Taken together, we can conclude from our results that a possible interaction between mild thyroid deficiency and CNS function could be present in subjects younger than age 75 years, whereas no effect was documented in the oldest old (>75 years). In this setting, it is worth noting that a similar figure has been described also for cardiovascular and stroke risk of sHT patients (53–55).
Strengths and limitations of this meta-analysis
Some limitations are present in this meta-analysis. First, we focused only on English-language literature. The included studies were not restricted to specific range of age and sex and were designed as naturalistic analysis (cross-sectional, case control, and cohort studies) with different data collection (prospective and retrospective). We should also mention as a possible bias the heterogeneity of included studies in terms of sHT definition (based on the upper limit of TSH) and in cognitive evaluation (diagnosis of dementia and cognitive scales). Moreover, none of the meta-analyzed studies used age adjusted expected ranges, leading to the possible misclassification of older subjects as having sHT. Moreover, the diagnosis of sHT was generally based on a single measurement of TSH, without at least a second confirmatory assessment, with potential misclassification of euthyroid subjects only suffering from transient elevation of serum TSH. Finally, the meta-analysis presented significant regression bias regarding the primary composite endpoint and risk of dementia. Despite these limitations, the present meta-analysis has increased the statistical power by pooling results of the single studies. Therefore, the total number of the cases and controls was sufficiently large to support the study conclusion of an age-related increased risk for dementia in sHT subjects.
Conclusions
This systematic review and meta-analysis demonstrates a relationship between sHT and cognitive function only documented in individuals younger than 75 years and those with higher TSH concentrations. No correlation was found while considering all the studies as a whole. The lack of utilization of age-related serum TSH reference ranges and consequent potential overdiagnosis of sHT in older people may account for this. Future studies using multiple age-related TSH reference ranges will provide a better understanding of the risk of cognitive deficits in sHT patients.
Acknowledgments
The study was partially supported by grants from the University of Pisa (to F.M.).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- CI
confidence interval
- CNS
central nervous system
- FT4
free T4
- MMSE
Mini Mental State Examination
- NOS
Newcastle-Ottawa Scale
- OR
odds ratio
- sHT
subclinical hypothyroidism
- SD
standard deviation
- TSH
thyroid-stimulating hormone.



