-
PDF
- Split View
-
Views
-
Cite
Cite
Mariana Carvalho Sturaro, Rafael Araújo, Larissa Sobrinho Aniceto, Gabrielli Rodrigues de Medeiros, Gleyce Hellen de Almeida de Souza, Simone Simionatto, Cephalosporin-based combination therapies for combating ESKAPE pathogens: a patent review, Journal of Applied Microbiology, Volume 136, Issue 5, May 2025, lxaf107, https://doi.org/10.1093/jambio/lxaf107
Close - Share Icon Share
Abstract
ESKAPE (namely Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter) pathogens pose a major threat to global health. The World Health Organization highlights the need for new antimicrobial strategies, including combination therapies, to address their resistance. Cephalosporins, due to their broad-spectrum activity and safety profile, are widely used in hospitals and serve as strong candidates for such regimens. While many studies explore cephalosporin combinations, there is a lack of systematic reviews focused on patent literature. This study analyses patent filings related to cephalosporin-based combination therapies targeting ESKAPE pathogens. The Espacenet database was thoroughly searched using the keywords “combination,” “antibiotics,” and “cephalosporin” yielding 666 patent applications filed up to June 2024. Based on the inclusion criteria, 30 patents were selected for further analysis. Notably, most patents were filed in China and the USA, accounting for up to 30% and 17%, respectively. Most patents were filed by private companies and classified under the International Patent Classification code A61K, indicating their pharmaceutical applications. Additionally, in 2022, the highest number of patents were filed in this area. However, clinical data were included in only two patents, reflecting a broader challenge: the high cost of development limits real-world validation of these combinations. Despite this, the patent landscape offers valuable insights into emerging strategies for combating ESKAPE pathogens with cephalosporin-based therapies.
Introduction
Multidrug-resistant (MDR) ESKAPE pathogens—Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species—pose a serious global health threat (Kulshrestha et al. 2024). Their infections are hard to treat, costly, and often deadly due to limited antibiotic options (de Souza et al. 2024). ESKAPE bacteria rapidly develop resistance through various mechanisms (Denissen et al. 2022), prompting the World Health Organization to prioritize new treatments (De Oliveira et al. 2020). However, antibiotic development typically takes 10–15 years and involves high costs, making progress slow (Freire-Moran et al. 2011). As a result, alternative strategies such as combination therapies are urgently needed (Miller and Arias 2024).
In contrast to antibiotic monotherapy, using commercially available antibiotic combinations is a more cost-effective approach. It leverages existing knowledge of drug composition and safety, helping to expedite the development of anti-infection therapies (Coates et al. 2020). Discovering synergistic interactions among antibiotics can facilitate the overcoming of microbial resistance (Sharafi et al. 2024), broadening the antibacterial spectrum (Hanci and Igan 2023), and reduce necessary treatment dosages, thereby minimizing toxicity risks to patients and also the rise of antimicrobial resistance (Li et al. 2024). Cephalosporins, a class of beta-lactam antibiotics originally derived from the fungus Acremonium, exhibit potent antibacterial activity against various pathogens and relatively low toxicity, making them promising candidates for combination therapies (Marshall and Blair 1999, Stewart et al. 2020). Their extensive use has led to the emergence of resistant bacterial strains, complicating treatment regimens (Piras et al. 2021, Morán-Díaz et al. 2023, Tan et al. 2023). However, cephalosporins remain essential antibacterial agents, especially given the limited development of new therapeutic options.
Despite several studies on cephalosporin combination therapies targeting ESKAPE pathogens, only a small number have led to patent filings, with even fewer advancing to clinical trials. Given the limited advancements in this field, reviewing relevant patents is crucial for addressing the scarcity of available sources. Unlike research articles, patents offer a unique perspective on the translation of scientific knowledge into market-ready innovations, making them accessible to society. Furthermore, patents serve as a valuable resource for uncovering novel drug formulations, mechanisms of action, and potential therapeutic applications, providing insights into the practical application of research into healthcare solutions (Carneiro et al. 2022). In this context, patent reviews are crucial for monitoring new developments, understanding current technologies, and predicting future trends (Braga et al. 2023).
In this study, we conducted a review of patent literature to identify and analyze innovations in cephalosporin combination therapies targeting ESKAPE pathogens. This review aims to gain valuable insights by highlighting progress, identifying research gaps, and guiding the development of novel antimicrobial treatments.
Materials and methods
Patents search
This review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Fig. 1). Patents filed up to June 2024 were retrieved from the Espacenet online database provided by the European Patent Office. No meta-analyses or detailed risk-of-bias assessments were performed (de Souza et al. 2023). Key search terms were carefully selected after an in-depth analysis of various three-word combinations. The combinations that yielded the highest number of relevant patents were chosen to maximize the study’s scope. The final search strategy was conducted using the following key terms: “combination” and “antibiotics” and “cephalosporin.” The resulting patent applications were examined by reviewing their titles, abstracts, and full texts.
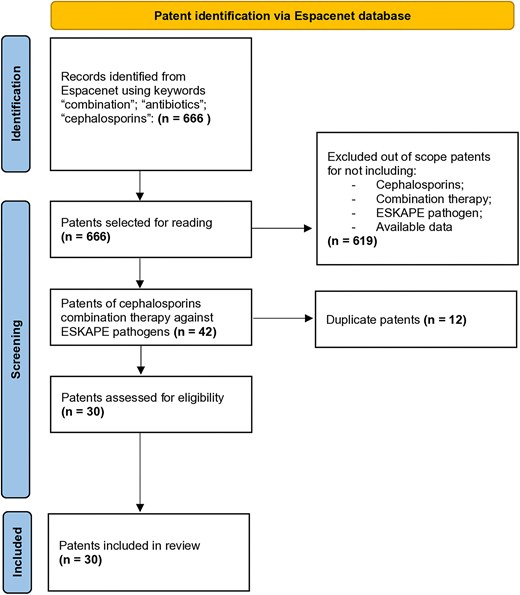
PRISMA flow diagram detailing the selection and screening process of the patent review. The diagram outlines each step from initial identification of patents to screening for relevance and final inclusion in the analysis. This systematic review ensures transparency and reproducibility.
Study selection
Three independent reviewers (RA, LSA, and GRM) carefully screened the patent applications identified through the database search to determine their eligibility for inclusion. In cases of uncertainty, a fourth reviewer’s (MCS) opinion was requested. The inclusion criteria were as follows: (1) patents featuring at least one antimicrobial combination containing a cephalosporin antibiotic; (2) patents involving cephalosporin combinations tested against ESKAPE pathogens; and (3) patents with potential applications in healthcare settings. Conversely, the exclusion criteria included (1) patents that did not involve cephalosporin combinations or ESKAPE pathogens and (2) duplicate entries and patents with unavailable content. During the study selection process, we encountered challenges, including language barriers and missing full-text data. Consequently, the Google Patents database was used to retrieve missing content. Patents that remained inaccessible were excluded. A total of 30 patents meeting the inclusion criteria were selected for further analysis (Table S1). The countries of origin, year of filing, applicant type, and International Patent Classification (IPC) code were examined. Data were compiled and analyzed via GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA), and RStudio (Posit PBC, Boston, MA, USA).
Patent features
Geographical distribution of patents
Priority countries refer to nations where a patent application is first submitted, establishing the earliest filing date. The number of priority filings reflects the level of research and development activity in a specific technological field within a given region (Parihar et al. 2020). An analysis of patent filings revealed that the priority countries for cephalosporin combination therapy patents were China (30%, 9 patents) and the USA (17%, 5 patents) (Fig. 2a). This finding aligns with data from the Worldwide Intellectual Property Office (WIPO) (https://www.wipo.int/ipstats/en/statistics/country_profile), which shows that China and the USA are the leading patent filers, with 1 586 339 and 515 281 applications, respectively, in 2022. These high numbers of patent filings are attributable to several factors, including robust innovation ecosystems, significant investments in research and development, strong intellectual property frameworks, highly skilled workforces, and advanced technological infrastructure, which collectively contribute to their dominance.
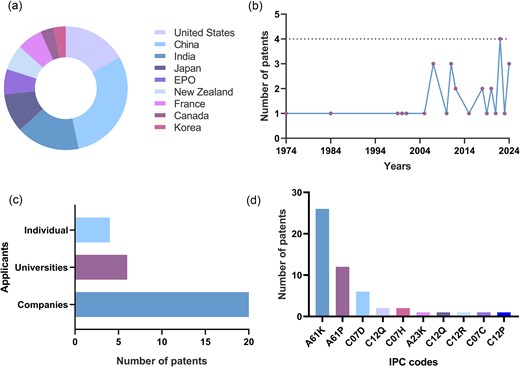
Selected patent features. (a) Geographical distribution of selected patents illustrating global innovation trends. The USA and China dominate patent filings, highlighting their leading roles in this field. EPO: European Patent Office. (b) Publication trends of selected patents. The data reveal a sharp increase in patent filings, which peaked in 2022, reflecting increased research and development efforts. (c) Applicant categories for selected patents. Private companies dominate patent filings, underscoring their pivotal role in innovation in this domain. (d) Interrelationships among the IPC codes for selected patents. The International Patent Classification (IPC) codes provide insights into the nature of patented inventions. Most patents fall under the A61K code, covering medical, dental, and toiletry preparations, highlighting the therapeutic focus of these innovations.
Trends of cephalosporin combination therapy patents
A total of 30 cephalosporin combination therapy patents were filed between 1974 and 2024 (Fig. 2b). The emergence of antimicrobial resistance in 1974 sparked initial efforts to develop therapeutic approaches using cephalosporin combinations. Over the last five decades, various combination antimicrobial therapies have been reported worldwide as time- and cost-efficient strategies. Patent application filings rose significantly in the 21st century, peaking in 2022. This surge may be linked to increased research funding and technological advances in the healthcare sector following the COVID-19 pandemic, which boosted scientific exploration and data generation.
Patent applicants and classifications
Numerous studies have investigated combination therapies to combat ESKAPE pathogens. An analysis of patent applicants revealed that private companies were the primary filers (20 patents), reflecting the economic importance of this strategy (Fig. 2c). Conversely, universities filed significantly fewer patents (6 patents), underscoring the need for increased investment in academic research to advance knowledge and foster innovation in this field. Sustained academic funding is essential for driving innovation, advancing scientific knowledge, and addressing pressing global challenges.
The IPC code, established by the WIPO, serves as a global system for classifying patents. Cephalosporin combination therapy patents are classified under IPC sections A (Human Necessities) and C (Chemistry) (Fig. 2d). Most of these patents were categorized under the IPC codes A61K (preparations for medical, dental, or toiletry purposes), A61P (specific therapeutic activity of chemical compounds or medicinal preparations), and C07D (heterocyclic compounds). These classifications underscore the significant role of drug combinations in medical and chemical innovations, highlighting their importance in pharmaceutical applications (Zona Rubio et al. 2025).
Patents detailed analyses
This patent analysis thoroughly examined various innovations related to cephalosporin combinations aimed at combating ESKAPE pathogens. Considering the nature of the associated compounds and their intended applications, the patents are categorized and detailed in the following sections.
Cephalosporin combinations with newly developed antimicrobial compounds
Several patents included in this review reported the development of novel antimicrobial compounds or cephalosporin derivatives (Table 1). For example, patent EP0911030A2 describes the use of vinyl-pyrrolidinone cephalosporin derivatives in combination with carbapenems (namely, imipenem and meropenem) and beta-lactamase inhibitors (BLIs) (such as clavulanic acid, tazobactam, and sulbactam) to combat a broad spectrum of bacteria, including methicillin-resistant S. aureus (MRSA), K. pneumoniae, Escherichia coli, Serratia marcescens, and P. aeruginosa (Angehrn et al. 1999). Patent IN3216MU2013A describes the development of nitrogen-containing compounds associated with BLIs and other antibiotics, including cephalosporins, to combat bacterial infections (Deshpande et al. 2015).
Included patents that described the development of new compounds, or cephalosporins derivates, and their respective combination therapy model.
| New developed drug . | Antibiotic in combination . | ESKAPE pathogen . | Mechanism of action . | In vivo assay . | Reference . |
|---|---|---|---|---|---|
| Vinyl-pyrrolidinone cephalosporin derivatives | Carbapenems and beta-lactamase inhibitors | MRSA, K. pneumoniae, E. coli, S. marcescens, and P. aeruginosa | ̶ | ̶ | Angehrn et al. (1999) |
| Nitrogen-containing compounds | Cephalosporins and beta-lactamase inhibitors | Acinetobacter, E. coli, Pseudomonas aeruginosa, S. aureus, Klebsiella | ̶ | ̶ | Deshpande et al. (2015) |
| Cephalosporin derivatives with a siderophore group | Not specified | Klebsiella pneumoniae, A. baumannii, and P. aeruginosa | Outer membrane increased permeability | Rat infection model | Cho et al. (2012) |
| Homodimeric tobramycin adjuvant | Cefotaxime or ceftazidime | Pseudomonas aeruginosa, K. pneumoniae, A. Baumannii, and E. coli | Outer membrane increased permeability | Galleria mellonella toxicity model | Schweizer; Idowu (2020) |
| Synthetic derivatives of rifamycin | Cephalosporins | Gram-negative and Gram-positive bacteria | ̶ | ̶ | Konopka and Gelzer (1974) |
| Novel aminoglycosides | Cephalosporins | Klebsiella, Acinetobacter, S. aureus, Pseudomonas | A-RNA site binding | ̶ | Haddad et al. (2005) |
| Novel carbapenems (C-19393 S2 and H2) | Cephalosporins | Escherichia coli | Beta-lactamase inhibition | ̶ | Imada et al. (1984) |
| New developed drug . | Antibiotic in combination . | ESKAPE pathogen . | Mechanism of action . | In vivo assay . | Reference . |
|---|---|---|---|---|---|
| Vinyl-pyrrolidinone cephalosporin derivatives | Carbapenems and beta-lactamase inhibitors | MRSA, K. pneumoniae, E. coli, S. marcescens, and P. aeruginosa | ̶ | ̶ | Angehrn et al. (1999) |
| Nitrogen-containing compounds | Cephalosporins and beta-lactamase inhibitors | Acinetobacter, E. coli, Pseudomonas aeruginosa, S. aureus, Klebsiella | ̶ | ̶ | Deshpande et al. (2015) |
| Cephalosporin derivatives with a siderophore group | Not specified | Klebsiella pneumoniae, A. baumannii, and P. aeruginosa | Outer membrane increased permeability | Rat infection model | Cho et al. (2012) |
| Homodimeric tobramycin adjuvant | Cefotaxime or ceftazidime | Pseudomonas aeruginosa, K. pneumoniae, A. Baumannii, and E. coli | Outer membrane increased permeability | Galleria mellonella toxicity model | Schweizer; Idowu (2020) |
| Synthetic derivatives of rifamycin | Cephalosporins | Gram-negative and Gram-positive bacteria | ̶ | ̶ | Konopka and Gelzer (1974) |
| Novel aminoglycosides | Cephalosporins | Klebsiella, Acinetobacter, S. aureus, Pseudomonas | A-RNA site binding | ̶ | Haddad et al. (2005) |
| Novel carbapenems (C-19393 S2 and H2) | Cephalosporins | Escherichia coli | Beta-lactamase inhibition | ̶ | Imada et al. (1984) |
– Inaccessible or not found data. MRSA: Methicillin-resistant S. aureus.
Included patents that described the development of new compounds, or cephalosporins derivates, and their respective combination therapy model.
| New developed drug . | Antibiotic in combination . | ESKAPE pathogen . | Mechanism of action . | In vivo assay . | Reference . |
|---|---|---|---|---|---|
| Vinyl-pyrrolidinone cephalosporin derivatives | Carbapenems and beta-lactamase inhibitors | MRSA, K. pneumoniae, E. coli, S. marcescens, and P. aeruginosa | ̶ | ̶ | Angehrn et al. (1999) |
| Nitrogen-containing compounds | Cephalosporins and beta-lactamase inhibitors | Acinetobacter, E. coli, Pseudomonas aeruginosa, S. aureus, Klebsiella | ̶ | ̶ | Deshpande et al. (2015) |
| Cephalosporin derivatives with a siderophore group | Not specified | Klebsiella pneumoniae, A. baumannii, and P. aeruginosa | Outer membrane increased permeability | Rat infection model | Cho et al. (2012) |
| Homodimeric tobramycin adjuvant | Cefotaxime or ceftazidime | Pseudomonas aeruginosa, K. pneumoniae, A. Baumannii, and E. coli | Outer membrane increased permeability | Galleria mellonella toxicity model | Schweizer; Idowu (2020) |
| Synthetic derivatives of rifamycin | Cephalosporins | Gram-negative and Gram-positive bacteria | ̶ | ̶ | Konopka and Gelzer (1974) |
| Novel aminoglycosides | Cephalosporins | Klebsiella, Acinetobacter, S. aureus, Pseudomonas | A-RNA site binding | ̶ | Haddad et al. (2005) |
| Novel carbapenems (C-19393 S2 and H2) | Cephalosporins | Escherichia coli | Beta-lactamase inhibition | ̶ | Imada et al. (1984) |
| New developed drug . | Antibiotic in combination . | ESKAPE pathogen . | Mechanism of action . | In vivo assay . | Reference . |
|---|---|---|---|---|---|
| Vinyl-pyrrolidinone cephalosporin derivatives | Carbapenems and beta-lactamase inhibitors | MRSA, K. pneumoniae, E. coli, S. marcescens, and P. aeruginosa | ̶ | ̶ | Angehrn et al. (1999) |
| Nitrogen-containing compounds | Cephalosporins and beta-lactamase inhibitors | Acinetobacter, E. coli, Pseudomonas aeruginosa, S. aureus, Klebsiella | ̶ | ̶ | Deshpande et al. (2015) |
| Cephalosporin derivatives with a siderophore group | Not specified | Klebsiella pneumoniae, A. baumannii, and P. aeruginosa | Outer membrane increased permeability | Rat infection model | Cho et al. (2012) |
| Homodimeric tobramycin adjuvant | Cefotaxime or ceftazidime | Pseudomonas aeruginosa, K. pneumoniae, A. Baumannii, and E. coli | Outer membrane increased permeability | Galleria mellonella toxicity model | Schweizer; Idowu (2020) |
| Synthetic derivatives of rifamycin | Cephalosporins | Gram-negative and Gram-positive bacteria | ̶ | ̶ | Konopka and Gelzer (1974) |
| Novel aminoglycosides | Cephalosporins | Klebsiella, Acinetobacter, S. aureus, Pseudomonas | A-RNA site binding | ̶ | Haddad et al. (2005) |
| Novel carbapenems (C-19393 S2 and H2) | Cephalosporins | Escherichia coli | Beta-lactamase inhibition | ̶ | Imada et al. (1984) |
– Inaccessible or not found data. MRSA: Methicillin-resistant S. aureus.
Patent KR101719556B1 describes the development of 68 novel cephalosporin derivatives containing a siderophore group—a molecule produced by microorganisms to scavenge iron from the environment. These derivatives were designed to combat MDR Gram-negative bacteria, including K. pneumoniae, A. baumannii, and notably P. aeruginosa. The siderophore group enhances the antibiotic’s activity by facilitating its uptake through the bacterial outer membrane. Most of these derivatives have low minimum inhibitory concentrations against the tested bacterial strains. Among them, compounds 4 and 8 exhibited significantly enhanced antimicrobial efficacy in vivo, with high bioavailability and favorable therapeutic outcomes in rats infected with P. aeruginosa. Additionally, these cephalosporin derivatives have been explored for use in combination regimens or as antibiotic delivery platforms, thereby expanding their therapeutic potential (Cho et al. 2012).
Similarly, patent WO2020232534A1 describes the development of a non-toxic broad-spectrum homodimeric tobramycin adjuvant designed to permeabilize the bacterial outer membrane, thereby enhancing the efficacy of associated antibiotics (Schweizer and Idowu 2020). Notably, the homodimeric tobramycin adjuvant exhibited no signs of hemotoxicity, cytotoxicity, or toxicity in an in vivo Galleria mellonella model at a dose of 200 mg/kg. Furthermore, it demonstrated a synergistic interaction with cephalosporins, such as cefotaxime and ceftazidime, yielding fractional inhibitory concentration indices (FICIs) ≤0.5 (Idowu et al. 2019). Patent CA946284A describes the development of synthetic derivatives of rifamycin and their combinations with various antibiotics, including cephalosporins. These combinations were designed to target both Gram-negative and Gram-positive bacteria among the ESKAPE pathogens, thereby providing a broad-spectrum approach (Konopka and Gelzer 1974).
Patent US2005171035A1 describes the development and use of aminoglycosides as part of a novel antibiotic strategy. These aminoglycosides were used in combination with various antibiotic classes, including cephalosporins, to effectively combat a broad spectrum of bacterial pathogens (Haddad et al. 2005). Novel aminoglycosides use the neamine mechanism, binding to bacterial A-site RNA to boost the effectiveness of antimicrobial agents (Haddad et al. 2002). Patent SU1075984A3 describes the development of the novel carbapenem antibiotics C-19393 S2 and H2, derived from Streptomyces griseus subsp. cryophilus, along with their antimicrobial applications. They act as BLIs, thereby sensitizing resistant microorganisms and improving antibiotic efficacy in combination therapy (Imada et al. 1984). Cephalosporins and penicillin are referred to as particularly promising adjuncts for C-19393 S2 and H2 (Imada et al. 1980).
This review highlights progress in developing novel antimicrobial compounds and cephalosporin derivatives aimed at fighting MDR bacteria and expanding treatment options. While many patents focus on development methods, few fully explore antimicrobial potential through in vitro, in vivo, or clinical testing—only two of seven patents, for example, included in vivo assays, and most lacked detailed mechanistic data or defined drug combinations. Cephalosporins show promising synergy against ESKAPE pathogens, but more research is needed to confirm their safety and efficacy through clinical trials. Regulatory hurdles and limited funding remain major barriers. Addressing these through increased investment and interdisciplinary collaboration is essential for translating combination therapy innovations into real-world health settings.
Combination of cephalosporins with commercial antibiotics
Among the 30 reviewed patents, 11 focused on commercial antibiotic combinations as promising antimicrobial strategies. These combinations use well-established drugs with proven efficacy, safety profiles, and known pharmacological properties. Notably, third-generation cephalosporins were the most frequently used class (Fig. 3), likely because of their broad-spectrum activity and favorable safety profile (Sturaro et al. 2024). Patent CN1305375A describes the combination of penicillin, amoxicillin, or amikacin with cephalosporins, particularly cefixime or cefdinir, for treating mixed respiratory infections caused by Streptococcus, Moraxella, Haemophilus, and/or Klebsiella species (Yoshimi et al. 2001). Interestingly, cefixime-amikacin exhibited a synergistic effect with a mean FICI of 0.6, demonstrating potent antibacterial activity in a murine respiratory tract infection model (Matsumoto 1998).
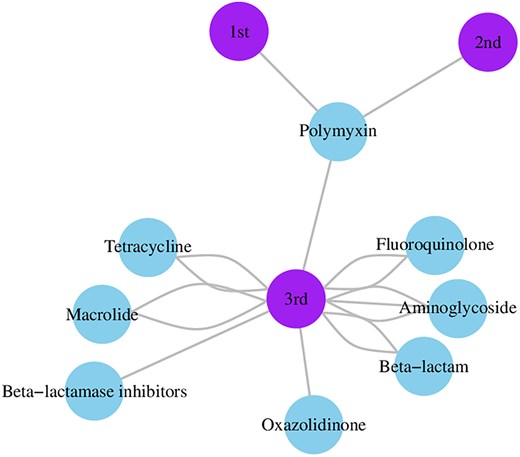
Commercial antibiotic combination network graphic illustrating the relationships between antibiotic classes and cephalosporin generations in patented combinations. Nodes represent either antibiotic classes (including β-lactamase inhibitors) or cephalosporin generations (first, second, or third). Edges between nodes indicate co-occurrence in patented combinations, and the number of connections reflects their frequency in the dataset. Third-generation cephalosporins appear most frequently in combination patents, particularly alongside aminoglycosides.
Patent WO0057882A1 describes various effective combinations of antibiotics targeting MRSA, notably cefdinir with oxytetracycline hydrochloride, ofloxacin, gentamicin sulfate, clarithromycin, or erythromycin. These combinations showed potent synergy in checkerboard assays, as indicated by a low FICI (Yokota 2000). Patent WO2007086013A1 describes a pharmacological formulation combining ceftazidime with tazobactam (a BLI) and further enhanced with linezolid. The developed formulation exhibited broad-spectrum activity against resistant Gram-negative and Gram-positive bacteria. The patent also recommends multiple administration routes—oral, topical, and parenteral—to maximize clinical utility and effectiveness (Srinivas 2007a). Similarly, WO2007086012A1 describes the combination of cefpodoxime (a cephalosporin component) and clavulanic acid (a BLI) to expand antimicrobial applications (Srinivas 2007b). Furthermore, CN113194943A describes a pharmaceutical composition incorporating BLIs and cephalosporins to sensitize resistant bacteria (Sun et al. 2021).
CN102292079A describes the use of ceftaroline, a fifth-generation cephalosporin, combined with various antibiotics—including beta-lactams, aminoglycosides, tetracycline, sulfonamide, trimethoprim, fluoroquinolone, vancomycin, macrolide, polymyxin, chloramphenicol, and lincosamide—aiming to treat skin infections and community-acquired pneumonia with a broad-spectrum and effective approach (Donald 2011). Patent US2020289610A1 describes the use of cephalosporins, such as cefazolin, cefuroxime, ceftazidime, cephalexin, cephaloridine, cefamandole, cefsulodin, cefonicid, cefoperazone, cefprozil, and ceftriaxone, in combination with polymyxins. The combination of cefuroxime, polymyxin B, and amikacin has demonstrated activity against MRSA; further, polymyxin B combined with cefuroxime, ceftazidime, and levofloxacin exhibited bactericidal properties with low toxicity (Gardner 2020).
Patent NZ535648A describes the use of an oxazolidinone antibiotic, commonly prescribed for diabetic foot infections in mammals, in combination with cephalosporins via multiple administration routes. This approach increases the antimicrobial activity against resistant Gram-positive bacteria, including S. aureus, S. epidermidis, and S. hemolyticus (Norden 2007). Patent EP3560489A1 describes the use of various antibiotic combinations as targeted therapies and pharmaceutical formulations against bacterial infections, emphasizing the use of cephalosporins. The synergistic effects of cephalosporins combined with aminoglycosides, macrolides, and fluoroquinolones enhance the treatment efficacy against Gram-positive and Gram-negative bacteria, including MDR strains (Gontao et al. 2019).
This review highlights recurring challenges in the development of patents for antibiotic combinations, particularly barriers to real-world applications. A major issue is the limited investment in in vivo assays, as most patents focus solely on the in vitro antimicrobial activity of combinations. Furthermore, many patents lack detailed insights into the mechanisms of antibacterial action, which limits their practical applicability. Overall, the patents underscore the potential of combining cephalosporins with commercial antibiotics to strengthen antibacterial therapy. While many of these combinations are already used in clinical practice, their efficacy and safety remain insufficiently evaluated. Closing this gap through rigorous scientific studies could support future patent developments and facilitate clinical translation.
Cephalosporin combinations with repurposed compounds
Several patents have described the combination of cephalosporins with non-antibiotic compounds (Table 2) as part of a drug repurposing strategy to expand their therapeutic applications. For example, patent CN108125954A describes the use of amlodipine, an antihypertensive agent, along with cephalosporins to combat A. baumannii and MRSA infections (Ziyue 2018). Amlodipine exhibited strong BLI activity, providing a broad beta-lactamase coverage compared with clavulanic acid and sulbactam. This property helps counteract antimicrobial resistance mediated by lytic enzymes. Additionally, amlodipine exhibited a strong synergistic effect with cefuroxime against MRSA, achieving a remarkably low FICI of 0.125 within 22 h of exposure (Yi et al. 2019).
Reviewed patents of cephalosporins in combination with repurposed compounds.
| Combination . | . | . | . | |
|---|---|---|---|---|
| Cephalosporin . | Repurposed compound . | Nature of the repurposed compound . | Microorganism . | Reference . |
| Ceftazidime, ceftriaxone, or cefuroxime | Amlodipine | Antihypertensive medicine | Acinetobacter baumannii and MRSA | Ziyue (2018) |
| Cefoperazone/sulbactam or ceftazidime | A-pinene | Essential oils component | Acinetobacter baumannii | Zeng et al. (2024) |
| Ceftriaxone | Plant-based MDRi | Plants extracts (from a variety of specimens) | Gram-negative and Gram-positive bacteria | Lakshmisubramanian (2023) |
| Cefotaxime | Dimetridazole | Antiprotozoal | Escherichia coli | Wei et al. (2022a) |
| Cefotaxime | Dimenidazole | Antiprotozoal | Klebsiella pneumoniae | Wei et al. (2022b) |
| Cefazolin | TRN1029, TRN1030, TRN1031, TRN1032 or TRN1033 | Fully human antibody or antigen-binding fragment | MSSA | Liao et al. (2024) |
| Cefazolin, cephalexin, cefoxitin, cefotaxime, Cefuroxime, or ceftazidime | PGLa | Polypeptide | Gram-negative and Gram-positive bacteria | Huping et al. (2022) |
| Cefotaxime | Peptide | Snake venom | Acinetobacter baumannii or E. coli | Zhiliang et al. (2024) |
| ̶ | Bicarbonate | Weak base | Gram-negative and Gram-positive bacteria | Brown et al. (2018) |
| Combination . | . | . | . | |
|---|---|---|---|---|
| Cephalosporin . | Repurposed compound . | Nature of the repurposed compound . | Microorganism . | Reference . |
| Ceftazidime, ceftriaxone, or cefuroxime | Amlodipine | Antihypertensive medicine | Acinetobacter baumannii and MRSA | Ziyue (2018) |
| Cefoperazone/sulbactam or ceftazidime | A-pinene | Essential oils component | Acinetobacter baumannii | Zeng et al. (2024) |
| Ceftriaxone | Plant-based MDRi | Plants extracts (from a variety of specimens) | Gram-negative and Gram-positive bacteria | Lakshmisubramanian (2023) |
| Cefotaxime | Dimetridazole | Antiprotozoal | Escherichia coli | Wei et al. (2022a) |
| Cefotaxime | Dimenidazole | Antiprotozoal | Klebsiella pneumoniae | Wei et al. (2022b) |
| Cefazolin | TRN1029, TRN1030, TRN1031, TRN1032 or TRN1033 | Fully human antibody or antigen-binding fragment | MSSA | Liao et al. (2024) |
| Cefazolin, cephalexin, cefoxitin, cefotaxime, Cefuroxime, or ceftazidime | PGLa | Polypeptide | Gram-negative and Gram-positive bacteria | Huping et al. (2022) |
| Cefotaxime | Peptide | Snake venom | Acinetobacter baumannii or E. coli | Zhiliang et al. (2024) |
| ̶ | Bicarbonate | Weak base | Gram-negative and Gram-positive bacteria | Brown et al. (2018) |
– Not specified, authors have cited a wide range of cephalosporins. MRSA: Methicillin-resistant S. aureus. MSSA: Methicillin-sensitive S. aureus.
Reviewed patents of cephalosporins in combination with repurposed compounds.
| Combination . | . | . | . | |
|---|---|---|---|---|
| Cephalosporin . | Repurposed compound . | Nature of the repurposed compound . | Microorganism . | Reference . |
| Ceftazidime, ceftriaxone, or cefuroxime | Amlodipine | Antihypertensive medicine | Acinetobacter baumannii and MRSA | Ziyue (2018) |
| Cefoperazone/sulbactam or ceftazidime | A-pinene | Essential oils component | Acinetobacter baumannii | Zeng et al. (2024) |
| Ceftriaxone | Plant-based MDRi | Plants extracts (from a variety of specimens) | Gram-negative and Gram-positive bacteria | Lakshmisubramanian (2023) |
| Cefotaxime | Dimetridazole | Antiprotozoal | Escherichia coli | Wei et al. (2022a) |
| Cefotaxime | Dimenidazole | Antiprotozoal | Klebsiella pneumoniae | Wei et al. (2022b) |
| Cefazolin | TRN1029, TRN1030, TRN1031, TRN1032 or TRN1033 | Fully human antibody or antigen-binding fragment | MSSA | Liao et al. (2024) |
| Cefazolin, cephalexin, cefoxitin, cefotaxime, Cefuroxime, or ceftazidime | PGLa | Polypeptide | Gram-negative and Gram-positive bacteria | Huping et al. (2022) |
| Cefotaxime | Peptide | Snake venom | Acinetobacter baumannii or E. coli | Zhiliang et al. (2024) |
| ̶ | Bicarbonate | Weak base | Gram-negative and Gram-positive bacteria | Brown et al. (2018) |
| Combination . | . | . | . | |
|---|---|---|---|---|
| Cephalosporin . | Repurposed compound . | Nature of the repurposed compound . | Microorganism . | Reference . |
| Ceftazidime, ceftriaxone, or cefuroxime | Amlodipine | Antihypertensive medicine | Acinetobacter baumannii and MRSA | Ziyue (2018) |
| Cefoperazone/sulbactam or ceftazidime | A-pinene | Essential oils component | Acinetobacter baumannii | Zeng et al. (2024) |
| Ceftriaxone | Plant-based MDRi | Plants extracts (from a variety of specimens) | Gram-negative and Gram-positive bacteria | Lakshmisubramanian (2023) |
| Cefotaxime | Dimetridazole | Antiprotozoal | Escherichia coli | Wei et al. (2022a) |
| Cefotaxime | Dimenidazole | Antiprotozoal | Klebsiella pneumoniae | Wei et al. (2022b) |
| Cefazolin | TRN1029, TRN1030, TRN1031, TRN1032 or TRN1033 | Fully human antibody or antigen-binding fragment | MSSA | Liao et al. (2024) |
| Cefazolin, cephalexin, cefoxitin, cefotaxime, Cefuroxime, or ceftazidime | PGLa | Polypeptide | Gram-negative and Gram-positive bacteria | Huping et al. (2022) |
| Cefotaxime | Peptide | Snake venom | Acinetobacter baumannii or E. coli | Zhiliang et al. (2024) |
| ̶ | Bicarbonate | Weak base | Gram-negative and Gram-positive bacteria | Brown et al. (2018) |
– Not specified, authors have cited a wide range of cephalosporins. MRSA: Methicillin-resistant S. aureus. MSSA: Methicillin-sensitive S. aureus.
Patent CN117442734A describes a pharmaceutical composition combining α-pinene, a monoterpene derived from essential oils, with beta-lactam antibiotics to combat MDR A. baumannii. α-Pinene demonstrated antimicrobial activity against Gram-positive bacteria, and its combination with meropenem showed synergy in checkerboard assays, inhibited biofilm formation, and was effective in a murine infection model. The inventors also proposed combinations of α-pinene with cephalosporins, such as cefoperazone/sulbactam or ceftazidime (Zeng et al. 2024). Similarly, patent WO2023047421A1 describes plant-based compositions combined with cephalosporins, particularly ceftriaxone, to target resistant Gram-negative and positive pathogens (Lakshmisubramanian 2023).
Patent CN114652716A describes the use of dimetridazole, an antiprotozoal agent, with cefotaxime, a third-generation cephalosporin, to combat drug-resistant E. coli in veterinary settings. Although E. coli is not officially classified as an ESKAPE pathogen, its increasing pathogenicity and ability to acquire antimicrobial resistance warrant its inclusion as an active member of the ESKAPE group (Ayobami et al. 2022, Craven et al. 2024). The combination had a FICI of 0.3125–0.375, indicating synergy, with potential benefits for bacterial sensitization and dosage reduction (Wei et al. 2022a). Similarly, patent CN114831994A describes the efficacy of a cefotaxime-dimenidazole combination against drug-resistant K. pneumoniae, with a FICI of 0.25–0.375, further demonstrating synergistic antimicrobial activity (Wei et al. 2022b).
Some patents propose more innovative strategies. Patent CN117771379A describes the combination of a fully human antibody or antigen-binding fragment with cephalosporins to target S. aureus (Liao et al. 2024). Patent CN114432428A describes the use of polypeptide PGLa combined with cephalosporins to enhance bacterial sensitivity (Huping et al. 2022). Patent CN117343131A describes an innovative combination of snake venom peptides (SVPs) and cephalosporins for treating A. baumannii and E. coli infections. In particular, cefotaxime combined with SVPs yielded a FICI of 0.31 against an ESBL-positive E. coli strain, demonstrating synergistic antimicrobial activity (Zhiliang et al. 2024). Patent WO2018141063A1 describes an innovative use of bicarbonate as an enhancer for various antimicrobial agents, including cephalosporins, fluoroquinolones, macrolides, and tetracyclines (Brown et al. 2018), by disrupting the pH gradient of the proton motive force in bacterial cytoplasmic membranes (Farha et al. 2018).
These patents underscore the potential of cephalosporin combinations with repurposed compounds to address antibiotic resistance. Such strategies improve cephalosporin efficacy and offer promising avenues for more effective antimicrobial treatments. However, translating repurposed compounds into clinical practice remains challenging due to stringent regulatory requirements, limited funding, scalability issues, skepticism from healthcare providers and patients, and the need for optimized dosing regimens. In addition, the reviewed patents lack toxicity data and information on clinical progression. Considering the need for new antimicrobial therapies, addressing these gaps is essential to fully realize the potential of drug repurposing in combination treatments and advancing the fight against resistant infections.
Identification methodologies using cephalosporin combinations
Some studies included in this review aimed to develop detection methods for resistant ESKAPE bacteria using cephalosporin combinations. Although these combinations indirectly target pathogens, they aid in the selection of the most effective treatment, making this process more robust (Fig. 4). For instance, patent US2011165604A1 describes a reaction medium that uses antibiotic combinations at subinhibitory concentrations to identify MRSA. Notably, all generations of cephalosporins and carbapenems were tested, with cefoxitin combinations—particularly with ceftriaxone, cefotaxime, ertapenem, cefoperazone, or cefpodoxime—enhancing both the specificity and sensitivity of MRSA detection and isolation (Orenga et al. 2011).
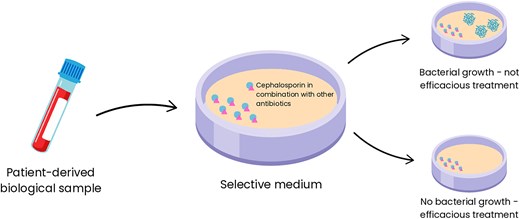
Representation of the mechanism by which selective media containing cephalosporin combinations enable precise selection of antibiotic regimens for treating infections caused by ESKAPE pathogens. The diagram illustrates how the selective media functions to isolate and identify resistant bacterial strains. By leveraging the activity of specific cephalosporin combinations, the media inhibits nontarget organisms while promoting the growth of resistant strains, allowing clinicians to determine the most effective antibiotic therapies for combating these high-priority pathogens.
Patent CN102586390A describes a specialized culture medium incorporating cephalosporins and other antibiotics capable of distinguishing three different types of microorganisms, particularly Gram-negative bacteria, from a single biological sample, representing a significant advancement in diagnostic microbiology (Orenga et al. 2012). Patent CA3175879A1 outlines a system that uses molecular and phenotypic methods to identify ESKAPE pathogens in polymicrobial infections and guide anti-infective therapy selection (Baunoch et al. 2022). These patents highlight significant advancements in the use of cephalosporin combinations to identify resistant ESKAPE pathogens, improving diagnostic accuracy and supporting effective treatment strategies. However, extensive studies are required to validate these methodologies in healthcare settings.
Cephalosporin combination therapies with clinical trials
Only two patent-related studies have progressed to clinical trials (Fig. 5). One example, NZ555076A, investigated an amikacin-cefepime combination for treating infections caused by E. coli, K. pneumoniae, Streptococcus pneumoniae, Enterococcus faecalis, P. aeruginosa, and S. aureus (Chaudhary 2010). In a clinical trial with 200 patients diagnosed with nosocomial pneumonia, participants were divided into two equal groups: one received amikacin-cefepime, the other cefepime alone, both administered intravenously for 7–10 days. The combination group showed significantly better outcomes, with 89% achieving clinical success versus 71% in the monotherapy group. Bacteriological eradication was also higher (90% vs. 66%), particularly in P. aeruginosa infections, where success reached 92% compared to 46% in the cefepime-only group (P < 0.05). Both treatments were well tolerated, with no major adverse effects or significant changes in laboratory parameters reported (Chaudhary et al. 2008b).
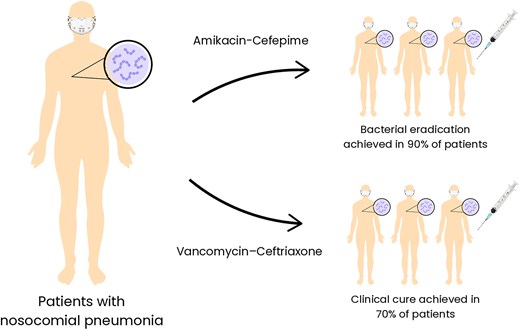
Representation of the two patented antibiotic combinations undergoing clinical trials and their therapeutic outcomes. The amikacin-cefepime combination demonstrated remarkable efficacy, eradicating bacterial pathogens in 90% of the patients included in the study. Similarly, the vancomycin-ceftriaxone combination achieved a clinical cure in 70% of the patients. The figure highlights the potential of these patented combinations in addressing infections caused by bacterial pathogens and their promising results in clinical applications.
Patent US2011257079A1 describes a pharmacological composition combining glycopeptides and cephalosporins for treating drug-resistant Staphylococci non-ocular infections, via parenteral administration, particularly highlighting the vancomycin-ceftriaxone combination (Chaudhary 2011). Phase II clinical trial demonstrated that vancomycin-ceftriaxone was effective, with 70% of the patients achieving clinical cure within 7 days. Significant reductions in the total leukocyte count and erythrocyte sedimentation rate further indicated infection recovery. This combination is particularly effective due to its broad antibacterial spectrum, targeting both Gram-positive and Gram-negative pathogens. The treatment was well tolerated, with no major adverse effects reported. Key safety biochemical parameters, including liver and kidney function, showed no significant changes post-treatment, indicating low toxicity. Mild side effects, such as pain at the injection site, nausea, and dizziness, were observed in <5% of patients (Chaudhary et al. 2008a).
The amikacin-cefepime and vancomycin-ceftriaxone combinations illustrate the promise of cephalosporin-based therapies in treating severe and drug-resistant infections. Sustaining the effectiveness of cephalosporin combinations requires ongoing monitoring of bacterial resistance and side effects in larger and more diverse patient populations. Improvements in drug delivery systems and formulation strategies could further enhance both safety and therapeutic outcomes. Despite their promise, the complexity and cost of clinical research remain significant obstacles—evident in the fact that only two related patents include clinical data—slowing progress through the later stages of development and limiting real-world application. Since clinical trials are essential for validating safety, efficacy, and practical use, overcoming these barriers is crucial for wider adoption.
Resistance profiles of ESKAPE pathogens
Several patents included in this review focus on combating antimicrobial-resistant bacteria by leveraging the broad-spectrum activity of antibiotic combinations. MRSA is a primary target of cephalosporin combination therapies. According to US2011165604A1, MRSA exhibits resistance to methicillin and oxacillin mediated by mecA, which encodes the modified protein PBP2a. MRSA is a major cause of nosocomial infections and is often associated with severe and potentially fatal health issues. These infections are highly contagious and are often transmitted between patients through healthcare personnel. MRSA is responsible for difficult-to-control endemic outbreaks (Orenga et al. 2011). For this matter, EP0911030A2 targets beta-lactamase–producing MRSA strains (Angehrn et al. 1999). NZ555076A focuses on treating hospital-acquired pneumonia caused by beta-lactam–resistant MRSA (Chaudhary 2010). WO0057882A1 reported combination therapies effective against MRSA and methicillin-resistant S. epidermidis, both of which causing severe infections in immunocompromised or older patients (Yokota 2000). CN108125954A, CN102292079A, and US2020289610A1, also propose various antibiotic combinations to combat MRSA infections (Donald 2011, Ziyue 2018, Gardner 2020).
Resistant Gram-negative bacteria are also a significant focus of cephalosporin combination therapies due to their severe impact on public health. Patent KR101719556B1 targets resistant P. aeruginosa strains with mutations in the outer membrane and porin channels, which reduce antibiotic efficacy (Cho et al. 2012). WO2020232534A1 describes a combination with potent bactericidal activity against carbapenem- and colistin-resistant Gram-negative strains harboring plasmid-borne mcr-1 genes (Schweizer and IDOWU 2020). WO2007086013A1 and WO2007086012A1 patents address penicillin-resistant bacteria, particularly P. aeruginosa and E. coli, which exhibit PBP-mediated resistance (Srinivas 2007a, 2007b).
Further, US2016257684A1 introduces novel strategies to combat ESBL-producing strains of K. pneumoniae, E. coli, and P. aeruginosa (Deshpande et al. 2015). CN117442734A presents a new combination therapy for treating beta-lactamase-producing A. baumannii, particularly targeting carbapenemase oxacillinase (Zeng et al. 2024). CN114831994A focuses on MDR K. pneumoniae, resistant to gentamicin, tetracycline, chloramphenicol, ceftriaxone, cefazolin, cefuroxime, cefepime, and lomefloxacin (Wei et al. 2022b). CN114652716A targets beta-lactam-resistant E. coli (Wei et al. 2022a), whereas CN113194943A addresses multiple beta-lactamase-producing Gram-negative bacteria (Sun et al. 2021). WO2023047421A1 aims to combat MDR A. baumannii, E. coli, K. pneumoniae, and P. aeruginosa, particularly focusing on efflux pump mechanisms and lytic enzyme production (Lakshmisubramanian 2023).
The cephalosporin combination therapies described in these patents exhibit broad-spectrum antimicrobial activity against ESKAPE pathogens, effectively targeting various bacterial resistance mechanisms. This therapeutic approach has significant potential for the development of new antimicrobial therapies, offering promising strategies for managing hard-to-treat, resistant bacterial infections.
Conclusion
In conclusion, patent analyses play a critical role in tracking innovation and guiding research and development efforts toward new and effective solutions for antimicrobial resistance. This analysis of cephalosporin combination therapy patents reveals strong potential against ESKAPE pathogens, showing synergistic effects with novel agents, repurposed compounds, and established antibiotics. These combinations can broaden antimicrobial coverage, reduce toxicity, and improve treatment outcomes. However, the path to clinical use faces major hurdles, including limited in vivo data and a lack of specificity in antibiotic pairings. Most patents remain at the in vitro stage due to the high costs and complexity of clinical trials. To realize the full potential of these therapies, future work must focus on rigorous preclinical and clinical validation, along with strategies for real-world implementation.
Acknowledgments
Canva Pro (Canva Pty Ltd, Sydney, NSW, Australia) was used to design Figs 4 and 5, in accordance with the Content Licensing Terms.
Author contributions
Mariana Carvalho Sturaro (Conceptualization, Data curation, Formal analysis, Investigation, Writing - original draft), Rafael Araújo (Formal analysis, Investigation), Larissa Sobrinho Aniceto (Formal analysis, Investigation), Gabrielli Rodrigues de Medeiros (Formal analysis, Investigation), Gleyce Hellen de Almeida de Souza (Project administration, Supervision, Validation, Visualization, Writing - review & editing), and Simone Simionatto (Funding acquisition, Project administration, Resources, Supervision, Writing - review & editing)
Conflict of interest
None declared.
Funding
The authors are grateful for financial support from National Council for Scientific and Technological Development (CNPq) (408778/2022-9, 307946/2022-3, 444735/2023-2, and 405785/2024-0), Foundation to Support the Development of Education, Science, and Technology of the State of Mato Grosso do Sul (76/2023 and 113/2023), Coordination for the Improvement of Higher Education Personnel (CAPES), and Federal University of Grande Dourados. Sponsors did not take part in data collection, analysis, interpretation, or in manuscript writing.
Data availability
All data are available from the corresponding authors upon reasonable request.



