-
PDF
- Split View
-
Views
-
Cite
Cite
Vishnu Das, Vivek Vinod, Lalitha Biswas, Anil Kumar, Raja Biswas, An update on possible alternative therapeutics for future periodontal disease management, Journal of Applied Microbiology, Volume 134, Issue 1, January 2023, lxac039, https://doi.org/10.1093/jambio/lxac039
Close - Share Icon Share
Abstract
Periodontitis is an inflammatory disease caused by microbial infections of the gum. At an advanced stage, periodontitis can even destroy the alveolar bone. Fusobacterium nucleatum, Prevotella intermedia, Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Capnocytophaga gingivalis, and Pr. nigrescens are the major pathogens in periodontitis. Scaling and root planning are used together with local or systemic antibiotics to treat periodontitis. The difficulty in complete eradication of periodontal pathogens frequently leads to the relapse of the disease. As not many new antibiotics are available in the market, many researchers are now focusing on developing alternative strategies against periodontal microbes. This review provides an overview of the possible use of bacteriophages, lysins, honey, plant extracts, metallic salts, nanoparticles, and vaccines as alternative therapeutic agents against periodontal infections. The information provided here could help in designing alternative therapeutics for the treatment of periodontal infections.
Introduction
Periodontitis is a universal, progressive inflammatory disease, predominantly caused by microbial colonization and biofilm formation along with other systemic factors (Nibali 2015, Frédéric et al. 2018, Cecoro et al. 2020). Periodontitis affects around 30%–35% of the total human population (Khan et al. 2015). Smoking, malnutrition, stress, imperfect alignment of teeth, defective tooth restorations, and prosthetics are some of the factors that contribute to the onset of periodontitis (Chen et al. 2006, Tkacz et al. 2021).
Microbial colonization and biofilm formation provoke prolonged inflammation of soft tissues and degradation of collagen fibres that support the tooth in the alveolar bone (Di Benedetto et al. 2013). In gingivitis, the gums can bleed on probing, lose its texture, and get inflamed (Müller and Barrieshi-Nusair 2005). As gingivitis progresses to periodontitis, the gingiva can recede from the crown and teeth may lose their attachment to the alveolar bone due to its resorption (Fig. 1). Poor oral hygiene or a reduction in microbe clearance by saliva can lead to the formation of biofilm on the surface of enamel and cementum (Jati et al. 2016, Könönen et al. 2019). Individuals with AIDS, defective neutrophil syndromes, diabetes, Papillon–Lefèvre syndrome, and Down syndrome as well as transplantation patients on immune suppressants are at increased risk of developing periodontal diseases (Tkacz et al. 2021). Female hormone changes during pregnancy and oral contraceptive usage have been found to be associated with gingivitis and periodontitis (Zachariasen 1993, Prachi et al. 2019). Smoking, use of tobacco products, and bruxism are also associated with the development of periodontal disease (Silva 2021). Additionally, individuals with periodontal disease are at higher risk for different respiratory infections, cardiovascular diseases, diabetes mellitus, rheumatoid arthritis, Alzheimer’s diseases, and pregnancy-associated complications (Kaur et al. 2012, Komine-Aizawa et al. 2019, Liccardo et al. 2019, Kamer et al. 2020). Gingipains released from Po. gingivalis has been associated with Alzheimer’s disease (Dominy et al. 2019). Prostaglandins released during periodontitis can trigger preterm contractions in pregnancy and in turn cause preterm birth and low birth weight (Tellapragada et al. 2014). Loss of teeth by periodontitis is associated with increased risk of respiratory infections and Chronic obstructive pulmonary disease (COPD) (Nazir 2017).
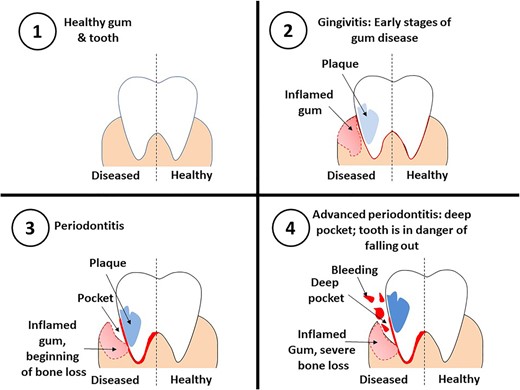
Periodontal disease affects about 20%–50% of the population around the globe. Developing countries have a higher prevalence of periodontal diseases (35%–70%) among adolescents when compared to developed countries (4%–34%). Higher prevalence of periodontal diseases was observed among adolescents from Belarus, Norway, Germany, Iran, and Taiwan. Countries like China, India, and Belarus had no adult without periodontal disease, while Germany and Taiwan had only 1% of adults with no disease. Other countries where periodontal infections are most prevalent includes Nepal, Croatia, Poland, Malaysia, Libya, Iran, and Taiwan (Nazir et al. 2020).
Campylobacter rectus, Eikenella corrodens, Prevotella intermedia, Po. gingivalis, Capnocytophaga gingivalis, Aggregatibacter actinomycetemcomitans, Treponema denticola, Tannerella forsynthia, Parvimonas micra, Pr. melanogeninica, Fusobacterium nucleatum, and Pr. nigrescens are some of the putative periodontal pathogens (Saygun et al. 2011, Frédéric et al. 2018). Some newly identified pathogens that can also cause periodontitis include Pr. denticola, Filifactor alocis, Cryptobacterium curtum, Eubacterium saphenum, Tr. lecithinolyticum, Slackia exigua, Tr. putidum, Pr. disiens, Peptostreptococcus magnus, Enterococcus faecalis, and Po. endodontalis (Teles et al. 2013).
The gold standard for periodontal therapy is scaling and root planning, which is based on the mechanical debridement of root surfaces for the removal of subgingival calculus (calcified dental plaque) and plaque (Drisko 2014). Mechanical removal of biofilm and calculus is performed routinely using hand held ultrasonic instruments (Bastendorf et al. 2021). The benefits of topical [e.g. periochip (chlorhexidine gluconate), Periostat (orally deliverable formulation of doxycycline), and minocycline microspheres] and systemic antimicrobial agents have been reported in a number of reviews (Oringer et al. 2002, Prakasam et al. 2012, Jhinger et al. 2015). However, excess use of antimicrobial agents can lead to the development of drug resistance and other side effects (Jhinger et al. 2015, Teles et al. 2021). The effectiveness of periodontal therapy is dependent on the expertise of the clinician and the successful removal of bacteria residing in the deep pockets, furcations, and other locations (Shaddox and Walker 2010). Therefore, scaling and root planning are frequently used in combination with local or systemic antimicrobial agents. Failure to completely eradicate periodontal pathogens often leads to the relapse of the disease. Because of the inadequacy of current therapy and increased microbial resistance against available antimicrobial agents, there is a need to develop more effective drugs and treatment methods against periodontal microbes.
Nonantibiotic antimicrobial agents
The occurrence of periodontal infections caused by drug resistant strains is on a rise, while the discovery of new classes of antibiotics has stalled, which makes the search for alternative treatment strategies imperative. Several nonconventional ways of treating periodontal infections have shown promising results in vitro and preclinical studies, and a few of them have shown significant clinical outcomes in small clinical trials. The nonconventional nonantibiotic agents (e.g. bacteriophages, lysins, phytochemicals, and herbal extracts) that showed antibacterial efficacy against different periodontal pathogens are discussed here (Fig. 2).
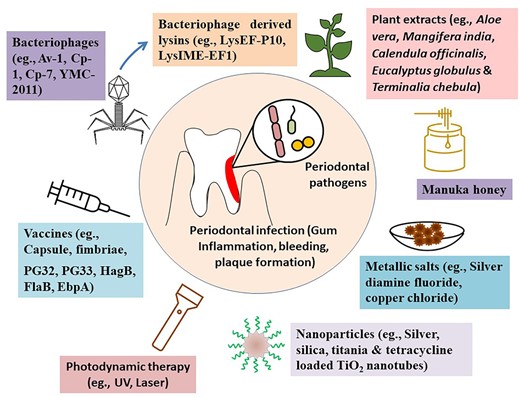
Recent developments in alternative therapeutic strategies against periodontal infections.
Bacteriophages
Phage therapy has gained incredible attention in the past few years, particularly at a time when drug resistance and biofilm infections are on the rise. There are many cases where bacteriophages have been used successfully to treat human diseases (Morozova et al. 2018, Principi et al. 2019). In dentistry, a number of bacteriophages that infect various periodontal pathogens have been isolated from saliva samples and from dental plaque. The advantages of therapeutic phages are that they are highly specific for a particular bacterial species, cause minimal damage to the commensal flora, have self-reproducing capability, and are active against antibiotic-resistant bacteria, although cross reactions have been reported (Torres-Barceló 2018, Principi et al. 2019). Some bacteriophages have the ability to stick to the mucus layers (Van Belleghem et al. 2019). Phages can also improve granulocyte functions by restricting bacterial growth to levels at which our immune system is able to combat the infection (Roach et al. 2017).
The efficacy of phage therapy has been studied for the treatment of periodontal infections caused by Ag. actinomycetemcomitans and En. faecalis. The efficacies of some of these phages have been proven against bacterial biofilms. Lytic phages were also identified against several other dental pathogens, including Actinomyces naeslundii, Ag. actinomycetemcomitans, F. nucleatum, Veillonella sp., Tr. denticola, Streptococcus mutans, St. pneumoniae, St. mitis, St. oralis, St. gordoni, and St. salivarius (Szafrański et al. 2017).
Examples of phages include vB_EfaS_PHB08 against En. faecalis (Yang et al. 2020), phage Av-1 against Ag. naeslundii (Delisle et al. 2006), phage Cp-1 and Cp-7 against St. oralis (Ronda et al. 1989), FnpΦ02 against F. Nucleatum (Machuca et al. 2010), YMC-2011 against St. salivarius (Chou et al. 2017), and M102, M102AD, and ΦAPCM01 against St. mutans (Dalmasso et al. 2015). Virulent phages as antimicrobial agents present several advantages compared to antibiotics, but they also exhibit some limiting properties. The disadvantages of phage therapy include eventual development of phage-resistant bacteria; phages if recognized by our immune surveillance system as unfamiliar antigens can activate adaptive immune responses (Loc-Carrillo and Abedon 2011, Navarro and Muniesa 2017). However, bacteriophages when delivered through the oral route may not elicit significant immune response of any clinical concern, possibly because during oral delivery of bacteriophages chances of their entering into the systemic circulation will be very limited.
Probiotics
Probiotics are live nonpathogenic microorganisms that exert beneficial effects to the host by inhibiting invasion and colonization of pathogenic organisms and modifying the host immune response. Probiotics have been used since many years for the treatment of various human diseases, including rotavirus diarrhoea, inflammatory bowel disease, allergies, and vaginal and respiratory tract infections. Recent studies have also reported antimicrobial activity of several probiotic bacteria against a number of oral pathogens suggesting a possible use of probiotics for the management of periodontal infections. The probiotic bacteria that demonstrated antimicrobial activity against periodontal pathogens include Lactobacillus rhamnosus, L. reuteri, L. helveticus, L. salivaris, L. casei, and Bifidobacteria sp. (Chatterjee et al. 2011, Jayaram et al. 2016). The beneficial effects of these probiotic bacteria were validated in several clinical studies (Table 1), which includes reduced plaque development and gingival inflammation, decrease in growth, and colonization of several periodontal pathogens.
List of human trials using probiotics for the treatment of periodontal diseases.
| Microorganisms . | Therapeutic effects . | References . |
|---|---|---|
| Probiotic drop containing L. rhamnosus, L. reuteri, and Bifidobacterium infantis | Decreased salivary counts of St. mutans | (Tehrani et al. 2016) |
| Fermented milk containing probiotic L. rhamnosus SD11 with maltitol | Reduced salivary counts of St. mutans | (Pahumunto et al. 2020) |
| Chewing gum containing L. reuteri | Reductions in plaque development and gingival inflammation; decrease in growth and colonization of several periodontal pathogens | (Twetman et al. 2009) |
| Lozenge containing L. reuteri | Helpful in controlling pregnancy gingivitis | (Schlagenhauf et al. 2016) |
| Tablets containing L. salivarius | Improvement in pocket probing depth and plaque index; reduced salivary counts of St. mutans | (Nishihara et al. 2014) |
| Mouth rinse containing Weissella cibaria CMS1 | Reduction in dental plaque formation | (Kang et al. 2006) |
| Ice cream containing Bi. lactis Bb-12 | Reduced salivary counts of St. mutans | (Çaglar et al. 2008) |
| Microorganisms . | Therapeutic effects . | References . |
|---|---|---|
| Probiotic drop containing L. rhamnosus, L. reuteri, and Bifidobacterium infantis | Decreased salivary counts of St. mutans | (Tehrani et al. 2016) |
| Fermented milk containing probiotic L. rhamnosus SD11 with maltitol | Reduced salivary counts of St. mutans | (Pahumunto et al. 2020) |
| Chewing gum containing L. reuteri | Reductions in plaque development and gingival inflammation; decrease in growth and colonization of several periodontal pathogens | (Twetman et al. 2009) |
| Lozenge containing L. reuteri | Helpful in controlling pregnancy gingivitis | (Schlagenhauf et al. 2016) |
| Tablets containing L. salivarius | Improvement in pocket probing depth and plaque index; reduced salivary counts of St. mutans | (Nishihara et al. 2014) |
| Mouth rinse containing Weissella cibaria CMS1 | Reduction in dental plaque formation | (Kang et al. 2006) |
| Ice cream containing Bi. lactis Bb-12 | Reduced salivary counts of St. mutans | (Çaglar et al. 2008) |
List of human trials using probiotics for the treatment of periodontal diseases.
| Microorganisms . | Therapeutic effects . | References . |
|---|---|---|
| Probiotic drop containing L. rhamnosus, L. reuteri, and Bifidobacterium infantis | Decreased salivary counts of St. mutans | (Tehrani et al. 2016) |
| Fermented milk containing probiotic L. rhamnosus SD11 with maltitol | Reduced salivary counts of St. mutans | (Pahumunto et al. 2020) |
| Chewing gum containing L. reuteri | Reductions in plaque development and gingival inflammation; decrease in growth and colonization of several periodontal pathogens | (Twetman et al. 2009) |
| Lozenge containing L. reuteri | Helpful in controlling pregnancy gingivitis | (Schlagenhauf et al. 2016) |
| Tablets containing L. salivarius | Improvement in pocket probing depth and plaque index; reduced salivary counts of St. mutans | (Nishihara et al. 2014) |
| Mouth rinse containing Weissella cibaria CMS1 | Reduction in dental plaque formation | (Kang et al. 2006) |
| Ice cream containing Bi. lactis Bb-12 | Reduced salivary counts of St. mutans | (Çaglar et al. 2008) |
| Microorganisms . | Therapeutic effects . | References . |
|---|---|---|
| Probiotic drop containing L. rhamnosus, L. reuteri, and Bifidobacterium infantis | Decreased salivary counts of St. mutans | (Tehrani et al. 2016) |
| Fermented milk containing probiotic L. rhamnosus SD11 with maltitol | Reduced salivary counts of St. mutans | (Pahumunto et al. 2020) |
| Chewing gum containing L. reuteri | Reductions in plaque development and gingival inflammation; decrease in growth and colonization of several periodontal pathogens | (Twetman et al. 2009) |
| Lozenge containing L. reuteri | Helpful in controlling pregnancy gingivitis | (Schlagenhauf et al. 2016) |
| Tablets containing L. salivarius | Improvement in pocket probing depth and plaque index; reduced salivary counts of St. mutans | (Nishihara et al. 2014) |
| Mouth rinse containing Weissella cibaria CMS1 | Reduction in dental plaque formation | (Kang et al. 2006) |
| Ice cream containing Bi. lactis Bb-12 | Reduced salivary counts of St. mutans | (Çaglar et al. 2008) |
Lysins
Although phages are specific and active against antibiotic resistant microbes, the major obstacles of phage therapy include unwanted immunological responses against phages, and the difficulties in their production and quality control processes. The spread of antibiotic resistance and the prevalence of periodontal biofilm infections inspired the scientific community to explore the therapeutic potential of phage-derived lysins. Lysins or endolysins are peptidoglycan (PG) hydrolases derived from bacteriophages that show antimicrobial activity against Multiple drug resistant (MDR) pathogens (Fenton et al. 2010, Murray et al. 2021). Lysins are produced by bacteriophages during the natural life cycle of the bacteriophage. They break down the bacterial PG layer in order to release progeny phages. Structurally, lysins are composed of a PG hydrolase domain and a PG binding domain. Lysins are in general very specific to their host from which they are derived. Therefore, purified lysins can be applied for the targeted killing of selected periodontal pathogens with minimal effects on other commensal bacteria.
Based on their target area, lysins are generally classified into glycosidases, those that attack the bonds of the glycan chain; N-acetylmuramoyl-L-alanine amidases, which attack the amide bonds between the glycan and peptide chain; and endopeptidases that hydrolyze bonds within peptidechains (Vázquez et al. 2018). When bacteria are treated with purified lysins, they cause rapid osmotic lysis and bacterial death. Lysins are also effective against bacterial biofilms (Amankwah et al. 2021). Several lysins were isolated in recent days, which included LysEF-P10, ΦEf11 ORF28, LysIME-EF1, and LysEFm5; and PlyV12, Lys168, and Lys170 (Yoong et al. 2004, Proença et al. 2012, Cheng et al. 2017, Zhang et al. 2019, Zhou et al. 2020). These lysins demonstrated bactericidal activity against En. faecalis. The chimeolysin ClyR comprising of the catalytic domain of PlyC lysin and the cell wall binding domain of PlySs2 lysin demonstrated antimicrobial activity against a number of Gram-positive pathogens, including multiple strains of Enterococci, Staphylococci, Streptococci, St. mutans, and St. sobrinus. The antibacterial activity of ClyR was further validated against St. mutans using a mouse model, and against mixed infections of St. sobrinus and St. mutans in rat models (Xu et al. 2018).
However, only a few studies using lysins were carried out under in vivo conditions. Further studies and clinical trials need to be conducted for successful therapeutic applications of lysins.
Herbal extracts and phytochemicals
A number of herbal extracts have been demonstrated to possess antimicrobial activities. The herbal extracts frequently used for treatment of several dental ailments are Aloe vera, Acacia arabica, Azadirachta indica, Mangifera indica, Berberis vulgaris, Boswellia serrata, Calendula officinalis, Camellia sinensis, Cinnamomum zeylanicum, Copaifera sp., Curcuma longa, Enteromorpha linza, Eucalyptus globulus, Eugenia uniflora, Garcinia mangostana, Glycyrrhiza glabra, Ilex rotunda, Ixora coccinea, Lactuca sativa, Lippia sidoides, Magnolia officinalis, Matricaria chamomilla, Melaleuca sp., Ocimum sanctum, Polygonum aviculare, Punica granatum, Pu. granatum, Rabdosia rubescens, Sa. persica, Schinus terebinthifolius, Scutellaria baicalensis, Streblus asper, Terminalia chebula, and Vaccinium myrtillus (Chandra Shekar et al. 2015, Eid Abdelmagyd et al. 2019).
Aqueous, ethanolic, and methanolic extracts of several plants demonstrated antibacterial activity against periodontal pathogens. These include aqueous and methanolic extracts of M. communis (Dib et al. 2021), and ethanolic and methanolic extracts of gall nut and cloves (Mirpour et al. 2015). Methanolic extract of Nigella sativa seeds (Kiari et al. 2018) and ethanolic extract of Tulsi demonstrated antibacterial activity (Mallikarjun et al. 2016). Chewing sticks derived from Sa. persica have demonstrated favourable effects against periodontal disease conditions (Nagata et al. 2008).
Recent studies have also demonstrated the antimicrobial efficacy of plant-derived essential oils and volatile oils against dental pathogens. Essential oils such as clove oil, eucalyptus oil, lemon essential oil, orange oil, basil oil, tea-tree oil, and myrrh oil showed activity against St. mutans, Ag. actinomycetemcomitans, and F. nucleatum (Carson et al. 2006). Volatile oils such as turmerone, atlantone, and zingiberene were effective when used for subgingival irrigation, improving gingival, and periodontal health (Kapadia et al. 2015). Chewing gums containing eucalyptus extracts demonstrated beneficial effects against periodontal infections (Nagata et al. 2008).
A herbal mouth wash containing ethanolic extracts of Matricaria recutita, Salvia officinalis, Acorus calamus, Mentha piperita, Arnica sp., Quercus sp., and Thymus sp. exhibited antimicrobial and anti-inflammatory properties, and prevented gingivitis and periodontitis in human clinical trials (Schönknecht et al. 2021)
Another herbal mouthwash containing Sa. persica, Te. bellirica, Piper betle, essential oils from Elettaria cardamomum, Gaultheria fragrantissima, and flavouring agents from Trachyspermum ammi and Mentha sp. showed antimicrobial activity against St. mutans, St. sanguinis, Ag. actinomycetemcomitans, Po. gingivalis, and F. nucleatum (Pathan et al. 2017). In a separate study, Gupta et al. demonstrated mouth washes containing O. sanctum extracts that were shown to reduce gingival inflammation (Gupta et al. 2014).
Additionally, both green tea and black tea extracts demonstrated oral health promoting benefits. The active principal antimicrobial compound in green tea is Epigallocatechin-3-gallate and in black tea is theaflavin and theaflavin-3,3′-digallate. Both extracts inhibited the growth of Po. gingivalis and Pr. intermedia. Black tea extracts also demonstrated synergistic antimicrobial action against Po. gingivalis when used in combination with metronidazole and tetracycline (Bedran et al. 2015).
Honey
Honey is one of the oldest traditional medicine. It has anti-inflammatory, antibacterial, antioxidant, and wound-healing properties. Honey comprises numerous different substances, including vitamins, minerals, proteins, polyphenolic compounds, sugar, and other plant derivatives. A number of polyphenolic compounds (methylglyoxal, hydrogen peroxide, β-defensin, chrysin, chlorogenic acid, ellagic acid, ferulic acid, and quercetin) are present in honey, which act synergistically to enhance its potency as an antimicrobial agent (Ahmed et al. 2018). The antimicrobial activity of honey was first reported in 1892 (Ahmed et al. 2018, Albaridi 2019). Honey in general, irrespective of its sources, was found to be active against Campylobacter spp., Ag. actinomycetemcomitans, Ca. rectus, Eu. nodatum, Po. gingivalis, and St. gordonii (Aparna et al. 2012). Manuka honey is the product of the New Zealand scrub plant and is widely used for the treatment of burns, infected wounds, and ulcers (Carter et al. 2016). A random clinical trial (RCT) in patients with periodontitis showed significant reduction in the mean plaque scores, and bleeding sites after 21 days of treatment with Manuka honey (English et al. 2004). The high concentrations of methylglyoxal present in Manuka honey contribute to its superior antimicrobial activity (Johnston et al. 2018). In another RCT, honey produced by Imtenan Co. Ltd., Egypt, showed significant antimicrobial activity against St. mutans and Po. gingivalis (Atwa et al. 2014). The major advantage of using honey as an antimicrobial agent is that no microorganism has yet been reported that has gained resistance to honey. Although the antimicrobial efficacy of honey has been demonstrated against periodontal pathogens, the use of medical honey against periodontal pathogens requires further validation through human clinical trials.
Metallic salts
Metal compounds having low molecular mass exhibit bactericidal and bacteriostatic activities. In vitro antimicrobial assays were performed on metal compounds such as copper chloride, silver diamine fluoride (SDF), silver nitrate, and zinc chloride. Antimicrobial activity of SDF was established against Ca. rectus, Po. gingivalis, Pr. intermedia, Pa. micra, T. forsythia, F. nucleatum, and St. constellatus, whereas silver nitrate demonstrated broader activity against Pr. denticola, Pr. intermedia, Po. gingivalis, Bacteroides forsythus, Ca. gracilis, Ei. corrodens, and Ag. actinomycetemcomitans (Spacciapoli et al. 2001, Mei et al. 2013).
Endodontal medicaments such as Cupral contain copper ions and calcium hydroxide. Detachment of the biofilms was observed when the biofilms made by oral pathogens such as St. gordonni, St. oralis, and Ag. actinomycetemcomitans were treated using Cupral (von Maltzahn et al. 2020). Partially oxidized glutathione capped silver and silver-zeolite demonstrated antibacterial activity against Po. gingivalis (Holden et al. 2016). Silver-zeolite also exhibited antibacterial activity against Pr. intermedia, and Ag. actinomycetemcomitans (Kawahara et al. 2000, Saengmee-anupharb et al. 2013). Other than silver, copper, and zinc metals, cerium compounds containing zeolitic imidazolate framework-8 have antibacterial activity against Po. gingivalis and F. nucleatum (Li et al. 2019).
Nanoparticle formulations
Several Nanoparticles (NPs) (e.g. ZnO NPs, Ag NPs, etc.) have demonstrated antimicrobial properties against both Gram-positive and Gram-negative bacteria (Sirelkhatim et al. 2015, Bruna et al. 2021). NPs are small in size but have a high surface area and show higher chemical reactivity. They have increased optical, magnetic, electrical, mechanical, or biological properties or compared to their macroscopic or microscopic counterparts. NPs can kill microbes by damaging its membrane structure, inducing the production reactive oxygen species (ROS) and interacting with microbial DNA and proteins (Nadar et al. 2022).
Gold, silver, silica, titanium dioxide, glutathione capped silver, silver/gold, cerium-doped zeolitic imidazolate framework NPs and tetracycline-loaded TiO2 nanotubes have been investigated for their antimicrobial efficacy against periodontal pathogens. Gold, silver, silica, and titania NPs showed antimicrobial activity against St. mutans. Tetracycline-loaded TiO2 nanotubes exhibited antimicrobial properties against Po. gingivalis (Besinis et al. 2014, Holden et al. 2016, Sun et al. 2018, Li et al. 2019, Zorraquín-Peña et al. 2020, Lavaee et al. 2021).
Glutathione capped silver, silver/gold (Au:Ag = 0.2), and cerium-doped zeolitic imidazolate framework NPs inhibited the growth of Po. gingivalis (Holden et al. 2016, Li et al. 2019, Zorraquín-Peña et al. 2020). The antimicrobial activity of silver/gold NPs against Po. gingivalis was further enhanced in the presence of hydrogen peroxide (Holden et al. 2016). Cerium-doped zeolitic imidazolate framework NPs also demonstrated antibacterial activity against F. nucleatum (Li et al. 2019).
NPs as antibacterial agents have several advantages: NPs are active against antibiotic resistance bacteria, they can act on multiple microbial targets, and NPs can act as good carriers of antibiotics. There is a huge scope for NPs to be used against periodontal infections (Wang et al. 2017). Several antimicrobial studies have also demonstrated that NPs can disperse bacterial biofilms. It is very likely that NPs will be effective in killing biofilm embedded periodontal pathogens. However, the biofilm dispersion activities of NPs against periodontal pathogens are yet to be proven in clinical trials.
Photodynamic therapy (PDT) and photosensitizers
PDT was introduced in 1904. The principle behind PDT is that a photosensitizer can bind to the target bacteria and can be activated by light of a particular wavelength. Following the activation of the photosensitizer through light, singlet oxygen and other very reactive agents would be produced that are extremely toxic to certain bacteria (Liu et al. 2015).
Antimicrobial PDT has been suggested as an adjunct to conventional treatment. Lasers (diode, argon, helium neon, and gallium arsenide), LED, light bulb, and UV radiation in combination with photosensitizers (e.g. toluidine blue O and methylene blue (MB), eosin, erythrosine and rose bengal, porphyrins, chlorophyll derivatives, including Ce6 in combination with sodium chlorine, pyridinium zinc phthalocyanine, curcumin and hypericin, curcuma, or hydrogen peroxide) generate ROS (e.g. singlet oxygen and free radicals) and have the ability to kill periodontal bacteria in a biofilm (Raghavendra et al. 2009). Porphyromonas gingivalis, Pr. intermedia, and Ag. actinomycetemcomitans were found to be susceptible to UV radiation (Aung et al. 2019).
In the presence of a light source, NPs with photosensitizer formulations showed deeper tissue penetration and enhanced antimicrobial activity. Rose bengal, erythrosine, and indocyanine green-loaded chitosan NPs in the presence of appropriate light sources demonstrated antimicrobial activity against En. faecalis, St. mutans, and Po. gingivalis, respectively (Chen et al. 2012, Shrestha et al. 2014, Higuchi et al. 2021).
Freitas et al. have demonstrated that MB loaded poly (lactic-co-glycolic) (PLGA) NPs exhibit a photodynamic effect on human dental plaque bacteria in the presence of red light (660 nm) (Freitas et al. 2016). The main advantages of PDT over conventional antimicrobial therapies include the immediate action of PDT and the rapid elimination of drug resistant microorganisms.
Vaccines
Currently, no periodontal vaccine is available in the clinics. Recently, several successful animal trials have evaluated the roles of different antigens from Po. gingivalis, F. nucleatum, and Ag. actinomycetemcomitans as vaccine candidates. Heat-killed whole bacteria, capsular polysaccharide and fimbriae, outer membrane proteins (PG32 and PG33), recombinant hemagglutinin B (HagB), hemagglutinin/adhesion domain derived from gingipain, and cysteine protease porphypain-2 have been identified as potential vaccine candidates against Po. gingivalis (Choi et al. 1988, Moritz et al. 1998, Katz et al. 1999, Ross et al. 2004, Zhang et al. 2005). Similarly, fusion protein of Vibrio vulnificus flagellin (FlaB) and a truncated form of the outer membrane protein of F. nucleatum (tFomA), Hgp44 flagellin fusion protein were shown to provide protection against F. nucleatum and Po. gingivalis (Puth et al. 2017, 2019). Likewise, the conjugate vaccine of the capsular-like serotype b-specific polysaccharide antigen (SPA) of Ag. actinomycetemcomitans and bovine serum albumin was capable of generating opsonophagocytic antibodies against the pathogen (Takamatsu-Matsushita et al. 1996), though its vaccine efficacy is yet to be validated in animal systems. A list of the potential vaccine candidates is provided in Table 2. A number of vaccine candidates have been identified from En. faecalis (e.g. EbpA, PpiC, GelE, and VS87_01 105) for the prevention of its systemic and catheter associated bladder infection (Flores-Mireles et al. 2014, Kazemian et al. 2019), but their role in preventing En. faecalis mediated periodontitis has not yet been studied.
List of different vaccine antigens used for the prevention of periodontitis under in vivo conditions.
| Organism . | Vaccine formulation . | Animal model . | Experimental outcome . | References . |
|---|---|---|---|---|
| Po. gingivalis | Conjugate vaccine consisting of capsular polysaccharide and fimbriae | Humanized Severe combined immunodeficient mice (SCID) mice | Decreased alveolar bone loss after Po. gingivalis infection | (Choi et al., 1998) |
| Po. gingivalis | Outer membrane proteins PG32 and PG33 | BALB/c mice | Reduced lesion size in infected mice | (Ross et al. 2004) |
| Po. gingivalis | Purified recombinant hemagglutinin B (rHag B) | Fischer rats | Provided protection against experimental periodontal bone after infection | (Katz et al. 1999) |
| Po. gingivalis | Recombinant hemagglutinin/adhesin HArep domain from the gingipain Kgp (Gingipains are trypsin-like cysteine proteinases) | BALB/c mice | Po. gingivalis invasion of epithelial cells was prevented by Anti-Kgp-rHArep antibodies | (Zhang et al. 2005) |
| Po. gingivalis | Porphypain-2 cysteine protease | Macaca fascicularis | Protection against ligature-induced periodontitis | (Moritz et al. 1998) |
| Po. gingivalis | Hgp44 domain polypeptide of Arginine-specific cysteine proteinase (Arg-gingipain) | BALB/c mice | Decreased alveolar bone loss induced by Po. gingivalis infection | (Puth et al. 2017) |
| F. nucleatum | Flagellin from V. vulnificus FlaB conjugated with truncated FomA major outer membrane protein of F. nucleatum | BALB/c mice | Inhibit alveolar bone loss | (Puth et al. 2019) |
| Ag. actinomycetemcomitans | Capsule-like serotype SPA antigen coupled with bovine serum albumin | BALB/c mice | High serum IgM and IgG responses to SPA subunit. Murine antisera efficiently opsonized Ag. actinomycetemcomitans serotype b | (Takamatsu-Matsushita et al. 1996) |
| F. nucleatum | UV-inactivated bacteria | Female ICR mice | Immunized mice have reduced gum abscess | (Liu et al. 2009) |
| Organism . | Vaccine formulation . | Animal model . | Experimental outcome . | References . |
|---|---|---|---|---|
| Po. gingivalis | Conjugate vaccine consisting of capsular polysaccharide and fimbriae | Humanized Severe combined immunodeficient mice (SCID) mice | Decreased alveolar bone loss after Po. gingivalis infection | (Choi et al., 1998) |
| Po. gingivalis | Outer membrane proteins PG32 and PG33 | BALB/c mice | Reduced lesion size in infected mice | (Ross et al. 2004) |
| Po. gingivalis | Purified recombinant hemagglutinin B (rHag B) | Fischer rats | Provided protection against experimental periodontal bone after infection | (Katz et al. 1999) |
| Po. gingivalis | Recombinant hemagglutinin/adhesin HArep domain from the gingipain Kgp (Gingipains are trypsin-like cysteine proteinases) | BALB/c mice | Po. gingivalis invasion of epithelial cells was prevented by Anti-Kgp-rHArep antibodies | (Zhang et al. 2005) |
| Po. gingivalis | Porphypain-2 cysteine protease | Macaca fascicularis | Protection against ligature-induced periodontitis | (Moritz et al. 1998) |
| Po. gingivalis | Hgp44 domain polypeptide of Arginine-specific cysteine proteinase (Arg-gingipain) | BALB/c mice | Decreased alveolar bone loss induced by Po. gingivalis infection | (Puth et al. 2017) |
| F. nucleatum | Flagellin from V. vulnificus FlaB conjugated with truncated FomA major outer membrane protein of F. nucleatum | BALB/c mice | Inhibit alveolar bone loss | (Puth et al. 2019) |
| Ag. actinomycetemcomitans | Capsule-like serotype SPA antigen coupled with bovine serum albumin | BALB/c mice | High serum IgM and IgG responses to SPA subunit. Murine antisera efficiently opsonized Ag. actinomycetemcomitans serotype b | (Takamatsu-Matsushita et al. 1996) |
| F. nucleatum | UV-inactivated bacteria | Female ICR mice | Immunized mice have reduced gum abscess | (Liu et al. 2009) |
List of different vaccine antigens used for the prevention of periodontitis under in vivo conditions.
| Organism . | Vaccine formulation . | Animal model . | Experimental outcome . | References . |
|---|---|---|---|---|
| Po. gingivalis | Conjugate vaccine consisting of capsular polysaccharide and fimbriae | Humanized Severe combined immunodeficient mice (SCID) mice | Decreased alveolar bone loss after Po. gingivalis infection | (Choi et al., 1998) |
| Po. gingivalis | Outer membrane proteins PG32 and PG33 | BALB/c mice | Reduced lesion size in infected mice | (Ross et al. 2004) |
| Po. gingivalis | Purified recombinant hemagglutinin B (rHag B) | Fischer rats | Provided protection against experimental periodontal bone after infection | (Katz et al. 1999) |
| Po. gingivalis | Recombinant hemagglutinin/adhesin HArep domain from the gingipain Kgp (Gingipains are trypsin-like cysteine proteinases) | BALB/c mice | Po. gingivalis invasion of epithelial cells was prevented by Anti-Kgp-rHArep antibodies | (Zhang et al. 2005) |
| Po. gingivalis | Porphypain-2 cysteine protease | Macaca fascicularis | Protection against ligature-induced periodontitis | (Moritz et al. 1998) |
| Po. gingivalis | Hgp44 domain polypeptide of Arginine-specific cysteine proteinase (Arg-gingipain) | BALB/c mice | Decreased alveolar bone loss induced by Po. gingivalis infection | (Puth et al. 2017) |
| F. nucleatum | Flagellin from V. vulnificus FlaB conjugated with truncated FomA major outer membrane protein of F. nucleatum | BALB/c mice | Inhibit alveolar bone loss | (Puth et al. 2019) |
| Ag. actinomycetemcomitans | Capsule-like serotype SPA antigen coupled with bovine serum albumin | BALB/c mice | High serum IgM and IgG responses to SPA subunit. Murine antisera efficiently opsonized Ag. actinomycetemcomitans serotype b | (Takamatsu-Matsushita et al. 1996) |
| F. nucleatum | UV-inactivated bacteria | Female ICR mice | Immunized mice have reduced gum abscess | (Liu et al. 2009) |
| Organism . | Vaccine formulation . | Animal model . | Experimental outcome . | References . |
|---|---|---|---|---|
| Po. gingivalis | Conjugate vaccine consisting of capsular polysaccharide and fimbriae | Humanized Severe combined immunodeficient mice (SCID) mice | Decreased alveolar bone loss after Po. gingivalis infection | (Choi et al., 1998) |
| Po. gingivalis | Outer membrane proteins PG32 and PG33 | BALB/c mice | Reduced lesion size in infected mice | (Ross et al. 2004) |
| Po. gingivalis | Purified recombinant hemagglutinin B (rHag B) | Fischer rats | Provided protection against experimental periodontal bone after infection | (Katz et al. 1999) |
| Po. gingivalis | Recombinant hemagglutinin/adhesin HArep domain from the gingipain Kgp (Gingipains are trypsin-like cysteine proteinases) | BALB/c mice | Po. gingivalis invasion of epithelial cells was prevented by Anti-Kgp-rHArep antibodies | (Zhang et al. 2005) |
| Po. gingivalis | Porphypain-2 cysteine protease | Macaca fascicularis | Protection against ligature-induced periodontitis | (Moritz et al. 1998) |
| Po. gingivalis | Hgp44 domain polypeptide of Arginine-specific cysteine proteinase (Arg-gingipain) | BALB/c mice | Decreased alveolar bone loss induced by Po. gingivalis infection | (Puth et al. 2017) |
| F. nucleatum | Flagellin from V. vulnificus FlaB conjugated with truncated FomA major outer membrane protein of F. nucleatum | BALB/c mice | Inhibit alveolar bone loss | (Puth et al. 2019) |
| Ag. actinomycetemcomitans | Capsule-like serotype SPA antigen coupled with bovine serum albumin | BALB/c mice | High serum IgM and IgG responses to SPA subunit. Murine antisera efficiently opsonized Ag. actinomycetemcomitans serotype b | (Takamatsu-Matsushita et al. 1996) |
| F. nucleatum | UV-inactivated bacteria | Female ICR mice | Immunized mice have reduced gum abscess | (Liu et al. 2009) |
Conclusion
Periodontitis is the most common infectious disease of the oral cavity and is a worldwide health problem. Also, there has been a rapid increase in the burden of periodontal disease during the last decades. The extensive use of antibiotics may have triggered the rise of drug resistance in oral bacteria. As there are not many new antibiotics in the pipeline for development, there is a significant and growing interest in alternative medicine for periodontal infection control and therapies across the world. Here, we described the roles of bacteriophages, phytochemicals, phototherapy, probiotics, prebiotics, NPs, and vaccines as alternatives to current therapy. Although, there are several advantages associated with alternative therapies, some disadvantages are also associated with these approaches. For example, the major drawback to phage therapy is the possible emergence of phage-resistant bacteria. Experimental data have shown the emergence of phage-resistant bacteria in different animal and human studies. Phage-derived lysins are highly active against periodontal pathogens, but because they are proteins in nature, they can mount humoral immune responses. Phytochemicals and phyto-extracts may act as better alternatives than conventional antibiotics and bacteriophages against periodontal pathogens as most of them act on multiple targets, so it is very unlikely that microbes will develop resistance against them. However, herbal therapies may take longer to cure. Additionally, phytochemicals and plant extracts can cause allergic reactions and other side effects in some cases. Clinical trial results indicated that probiotic strains can provide oral health benefits. Although there are some rare incidences where probiotic strains turned pathogenic. Very few bacteraemia and bacterial endocarditis cases were reported after consumption of probiotic preparations in immunocompromised individuals. Recent studies also indicate various prebiotic substrates can be used to modify oral microbiome composition. In vitro studies indicated prebiotic substrates like N-acetyl-d-glucosamine can induce a favourable compositional shift in oral microbiome; and N-acetyl-d-mannosamine, α-d-lactose, d-(+)-raffinose, and d-(+)-trehalose can downregulate virulence gene expression in periodontal pathogens (Verspecht et al. 2021). Therefore, probiotics and prebiotics provide great scope for clinical translation. Metallic salts and NPs although effective, but at higher concentrations can cause toxicity to host cells. Vaccine development against pathogens is always ideal for the prevention of infectious disease development. However, as a large number of pathogens cause periodontal infections, it will not be so easy to target and develop vaccines against individual pathogens. Despite these few disadvantages, alternative therapeutics possess great opportunities to be considered for the treatment of periodontal infections, as they can act on existing antibiotic resistant microbes. It is of vital importance to validate the efficacy of these alternative medicines in large clinical trials. It is also important to know the efficacy of different alternative medicines against drug resistant infections. Some of these nonantibiotic compounds may also be used to prevent or treat periodontal biofilm formation. Clinical trial data will be able to answer which treatment approach will be best for the management of periodontitis.
Conflicts of interest
None declared.
Funding
We thank the Centre for Nanoscience and Molecular Medicine, Amrita Vishwa Vidyapeetham, Kochi, India, for infrastructural and financial support.
Authors contribution
Conceptualization: V.D. and R.B. Writing original draft: V.D. and V.V. Final editing: L.B., A.K., and R.B.



