-
PDF
- Split View
-
Views
-
Cite
Cite
Qiu-Yun Zhao, Pin-Xian Chen, Ling Yang, Run-Mao Cai, Jia-Hang Zhu, Liang-Xing Fang, Mark A Webber, Hong-Xia Jiang, Transmission of plasmid-borne and chromosomal blaCTX-M-64 among Escherichia coli and Salmonella isolates from food-producing animals via ISEcp1-mediated transposition, Journal of Antimicrobial Chemotherapy, Volume 75, Issue 6, June 2020, Pages 1424–1427, https://doi.org/10.1093/jac/dkaa044
Close - Share Icon Share
Abstract
To clarify the transmission mechanism of the blaCTX-M-64 gene between Escherichia coli and Salmonella isolates from food animals.
A total of 329 E. coli and 60 Salmonella isolates collected from food animals in 2016 were screened for the presence of blaCTX-M-64 genes. The blaCTX-M-64-positive isolates were typed and plasmid and chromosome DNA was sequenced to determine the genetic context of blaCTX-M-64 and the plasmid types present.
The blaCTX-M-64 gene was identified in only three E. coli isolates but was the predominant gene in the Salmonella isolates (n = 9). These 12 CTX-M-64-positive isolates were all resistant to ampicillin, cefotaxime, ceftiofur, ceftriaxone, ceftazidime and florfenicol and 9 were resistant to ciprofloxacin. The blaCTX-M-64 gene was located on transferable IncI2 plasmids and an IncHI2 plasmid in three E. coli and one Salmonella isolate, respectively. The remaining eight Salmonella isolates contained blaCTX-M-64 integrated into the chromosome. Different genetic contexts of blaCTX-M-64 genes were found among the 12 isolates: ISEcp1-blaCTX-M-64-orf477-A/C on IncI2 plasmids of 3 E. coli isolates; ΔISEcp1-blaCTX-M-64-orf477-A/C in the chromosome of 1 Salmonella isolate; and ISEcp1-blaCTX-M-64-orf477 on the IncHI2 plasmid and chromosome of 8 Salmonella isolates.
To the best of our knowledge, this is the first report of chromosomally encoded CTX-M-64 in Salmonella isolates. ISEcp1-mediated transposition is likely to be responsible for the spread of blaCTX-M-64 between different plasmids and chromosomes in Enterobacteriaceae especially E. coli and Salmonella.
Introduction
Multiple CTX-M variants have been found in the same bacterial host and this co-occurrence within the same cell could favour the formation of CTX-M hybrid enzymes.1 So far, hybrid CTX-M ESBL types have been discovered, including CTX-M-64,1 CTX-M-123,2 CTX-M-132 and CTX-M-137,3,4 and the resulting hybrids have demonstrated higher catalytic activities than their parent enzymes.5 The CTX-M-1 and CTX-M-9 group members were most often found together in Escherichia coli from food animals in China, suggesting that E. coli is the likely host for the generation of novel chimeric alleles. Recently we detected hybrid CTX-M-64 among Salmonella from food-producing animals, so we questioned whether the occurrence of blaCTX-M-64 in Salmonella isolated from food-producing animals origin was linked to CTX-M-64-producing E. coli. The detection of blaCTX-M-64 in bacterial isolates from both humans and animals in China raises the possibility that a shared environment may be a significant source of co-transmission between animals and humans.6,7 A first step in determining the transmission mechanisms of hybrid CTX-M enzymes is to identify the genetic contexts of the blaCTX-M-64 genes.
Materials and methods
Bacterial epidemiological background
In this study, a total of 435 rectal swab samples were collected from food animals in Guangdong (one chicken farm and two duck farms) and Shandong (one chicken farm) provinces in China in 2016. All isolates were screened for the blaCTX-M-64 gene using PCR and sequencing and typed using PFGE and MLST as previously described.8 All Salmonella isolates were serotyped using hyperimmune sera by the slide agglutination test (S&A Reagents Lab, Bangkok, Thailand). The MICs of 18 antimicrobial agents for the E. coli and Salmonella isolates were determined using the agar dilution method and the results were interpreted following CLSI (2015, M100-S25)9 and veterinary CLSI (VET01-A4/VET01-S2) guidelines.10
Transfer and location of blaCTX-M-64
To test the transferability of the blaCTX-M-64 gene, broth mating was performed with a plasmid-free E. coli (C600) as recipient. Transconjugants were selected on MacConkey agar (Land Bridge, Beijing, China) supplemented with 1 mg/L cefotaxime and 2000 mg/L streptomycin. For the nine blaCTX-M-64-producing Salmonella isolates, no transconjugants were obtained; an alternative E. coli (DH5α) was used as a recipient for transformation experiments and transformants were selected on MacConkey agar plates supplemented with 1 mg/L cefotaxime. The replicon types for all transconjugants and transformants with hybrid blaCTX-M-64-carrying plasmids were determined by PCR-based replicon typing (PBRT) as described previously.11 Plasmid content (number and size) was estimated by S1-PFGE followed by Southern blot hybridization with a blaCTX-M-64 gene probe as described previously.8 For isolates in which a plasmid was not involved in the mobilization of blaCTX-M-64, the entire DNA was digested with I-CeuI (NEB, Ipswich, MA, USA), followed by PFGE and Southern blotting from I-CeuI-PFGE gels using digoxigenin-labelled probes that were specific for the blaCTX-M-64 gene and the 23S rDNA gene.12
Plasmid and genome sequencing and analysis
Two plasmids, pWG20 and pYC33, from the transconjugant C-WG20 and the Salmonella isolate YC33, respectively, and two genomes encoding blaCTX-M-64 from Salmonella isolates (WY36 and SG2) were fully sequenced using the Illumina HiSeq system. DNA reads were assembled using SOAPdenovo software. The nucleotide and amino acid sequences were annotated by RAST followed by manual review, using BLASTn and BLASTp as described previously.13 The ORF finder program (http://www.ncbi.nlm.nih.gov/orffinder) was also used to identify features.
Nucleotide sequence accession numbers
The partial nucleotide sequences of pWG20, pYC33, WY36 and SG2 and complete sequences of the ColE-like plasmids pYC33-1 and pYC33-2 have been submitted to the GenBank database and have been assigned accession numbers MK704494, MN447699, MN447698, MK649379, MN097153 and MN097152, respectively.
Results and discussion
Prevalence and phenotypes of blaCTX-M-64-carrying isolates
Of 435 samples, 276 were obtained from chickens and 159 from ducks. Three hundred and twenty-nine E. coli were obtained, 137 from chickens (41.64%) and 192 from ducks (58.36%), and 60 Salmonella were obtained, 39 from chickens (65%) and 21 from ducks (35%). A total of 12 CTX-M-64-positive isolates (3 E. coli and 9 Salmonella) were detected. All 12 (100%) of the CTX-M-64-positive isolates were resistant to ampicillin, cefotaxime, ceftiofur, ceftriaxone, ceftazidime and florfenicol and 83.33% were resistant to ciprofloxacin (Table 1).
Characteristics of blaCTX-M-64-positive E. coli and Salmonella isolates from chickens and ducks throughout Shandong and Guangdong provinces in 2016
| Strain . | Species . | Serotype . | Source . | blaCTX-M-64 location . | Plasmid size (kb) . | Replicon type . | MLST . | Resistance phenotypea . |
|---|---|---|---|---|---|---|---|---|
| SF1b | E. coli | ND | chicken | plasmid | ∼360 | IncI2 | NT | AMP/CTX/CTF/CRO/CAZ/CHL/FLF/GEN/APR/TET/CIP/DAN/CST/FOS/STR |
| SF4b | E. coli | ND | chicken | plasmid | ∼360 | IncI2 | NT | AMP/CTX/CTF/CRO/CAZ/CHL/FLF/GEN/APR/TET/CIP/DAN/CST/FOS/STR |
| WG20 | E. coli | ND | duck | plasmid | ∼360 | IncI2 | NT | AMP/CTX/CTF/CRO/CAZ/CHL/FLF/GEN/AMK/APR/TET/DOX/CIP/DAN/CST/STR |
| YC33 | S. enterica | Typhimurium | duck | plasmid | ∼190 | IncHI2 | ST19 | AMP/CTX/CTF/CRO/CAZ/GEN/TET/DOX |
| SG2 | S. enterica | Typhimurium | chicken | chromosome | NA | NA | ST19 | AMP/CTX/CTF/CRO/CAZ/CHL/FLF/TET |
| M77 | S. enterica | Indiana | chicken | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY6 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY18 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY23 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY29 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY35 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY36 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| Strain . | Species . | Serotype . | Source . | blaCTX-M-64 location . | Plasmid size (kb) . | Replicon type . | MLST . | Resistance phenotypea . |
|---|---|---|---|---|---|---|---|---|
| SF1b | E. coli | ND | chicken | plasmid | ∼360 | IncI2 | NT | AMP/CTX/CTF/CRO/CAZ/CHL/FLF/GEN/APR/TET/CIP/DAN/CST/FOS/STR |
| SF4b | E. coli | ND | chicken | plasmid | ∼360 | IncI2 | NT | AMP/CTX/CTF/CRO/CAZ/CHL/FLF/GEN/APR/TET/CIP/DAN/CST/FOS/STR |
| WG20 | E. coli | ND | duck | plasmid | ∼360 | IncI2 | NT | AMP/CTX/CTF/CRO/CAZ/CHL/FLF/GEN/AMK/APR/TET/DOX/CIP/DAN/CST/STR |
| YC33 | S. enterica | Typhimurium | duck | plasmid | ∼190 | IncHI2 | ST19 | AMP/CTX/CTF/CRO/CAZ/GEN/TET/DOX |
| SG2 | S. enterica | Typhimurium | chicken | chromosome | NA | NA | ST19 | AMP/CTX/CTF/CRO/CAZ/CHL/FLF/TET |
| M77 | S. enterica | Indiana | chicken | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY6 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY18 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY23 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY29 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY35 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY36 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
ND, not determined; NT, not typeable; NA, not applicable; AMP, ampicillin; CTX, cefotaxime; CTF, ceftiofur; CRO, ceftriaxone; CAZ, ceftazidime; CHL, chloramphenicol; FLF, florfenicol; GEN, gentamicin; APR, apramycin; TGC, tigecycline; AMK, amikacin; CIP, ciprofloxacin; DAN, danofloxacin; TET, tetracycline; DOX, doxycycline; CST, colistin; FOS, fosfomycin; STR, streptomycin.
The most common resistance phenotype was resistance to AMP, CTX, CTF, CRO, CAZ, FLF, TGC, CIP, DAN and FOS.
blaCTX-M-64 and blaCTX-M-65 co-existed in the same E. coli strain.
Characteristics of blaCTX-M-64-positive E. coli and Salmonella isolates from chickens and ducks throughout Shandong and Guangdong provinces in 2016
| Strain . | Species . | Serotype . | Source . | blaCTX-M-64 location . | Plasmid size (kb) . | Replicon type . | MLST . | Resistance phenotypea . |
|---|---|---|---|---|---|---|---|---|
| SF1b | E. coli | ND | chicken | plasmid | ∼360 | IncI2 | NT | AMP/CTX/CTF/CRO/CAZ/CHL/FLF/GEN/APR/TET/CIP/DAN/CST/FOS/STR |
| SF4b | E. coli | ND | chicken | plasmid | ∼360 | IncI2 | NT | AMP/CTX/CTF/CRO/CAZ/CHL/FLF/GEN/APR/TET/CIP/DAN/CST/FOS/STR |
| WG20 | E. coli | ND | duck | plasmid | ∼360 | IncI2 | NT | AMP/CTX/CTF/CRO/CAZ/CHL/FLF/GEN/AMK/APR/TET/DOX/CIP/DAN/CST/STR |
| YC33 | S. enterica | Typhimurium | duck | plasmid | ∼190 | IncHI2 | ST19 | AMP/CTX/CTF/CRO/CAZ/GEN/TET/DOX |
| SG2 | S. enterica | Typhimurium | chicken | chromosome | NA | NA | ST19 | AMP/CTX/CTF/CRO/CAZ/CHL/FLF/TET |
| M77 | S. enterica | Indiana | chicken | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY6 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY18 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY23 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY29 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY35 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY36 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| Strain . | Species . | Serotype . | Source . | blaCTX-M-64 location . | Plasmid size (kb) . | Replicon type . | MLST . | Resistance phenotypea . |
|---|---|---|---|---|---|---|---|---|
| SF1b | E. coli | ND | chicken | plasmid | ∼360 | IncI2 | NT | AMP/CTX/CTF/CRO/CAZ/CHL/FLF/GEN/APR/TET/CIP/DAN/CST/FOS/STR |
| SF4b | E. coli | ND | chicken | plasmid | ∼360 | IncI2 | NT | AMP/CTX/CTF/CRO/CAZ/CHL/FLF/GEN/APR/TET/CIP/DAN/CST/FOS/STR |
| WG20 | E. coli | ND | duck | plasmid | ∼360 | IncI2 | NT | AMP/CTX/CTF/CRO/CAZ/CHL/FLF/GEN/AMK/APR/TET/DOX/CIP/DAN/CST/STR |
| YC33 | S. enterica | Typhimurium | duck | plasmid | ∼190 | IncHI2 | ST19 | AMP/CTX/CTF/CRO/CAZ/GEN/TET/DOX |
| SG2 | S. enterica | Typhimurium | chicken | chromosome | NA | NA | ST19 | AMP/CTX/CTF/CRO/CAZ/CHL/FLF/TET |
| M77 | S. enterica | Indiana | chicken | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY6 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY18 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY23 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY29 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY35 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
| WY36 | S. enterica | Indiana | duck | chromosome | NA | NA | ST17 | AMP/CTX/CTF/CRO/CAZ/FLF/TGC/CIP/DAN/FOS |
ND, not determined; NT, not typeable; NA, not applicable; AMP, ampicillin; CTX, cefotaxime; CTF, ceftiofur; CRO, ceftriaxone; CAZ, ceftazidime; CHL, chloramphenicol; FLF, florfenicol; GEN, gentamicin; APR, apramycin; TGC, tigecycline; AMK, amikacin; CIP, ciprofloxacin; DAN, danofloxacin; TET, tetracycline; DOX, doxycycline; CST, colistin; FOS, fosfomycin; STR, streptomycin.
The most common resistance phenotype was resistance to AMP, CTX, CTF, CRO, CAZ, FLF, TGC, CIP, DAN and FOS.
blaCTX-M-64 and blaCTX-M-65 co-existed in the same E. coli strain.
Molecular typing
The typing data indicated that the three E. coli isolates were grouped into two PFGE clusters and each cluster had the same novel ST (Figure S1A, available as Supplementary data at JAC Online). The nine Salmonella isolates were grouped into three PFGE clusters designated A, B and C (Figure S1B) and two STs. The STs correlated with specific serovars; ST17 with Salmonella enterica serotype Indiana (n = 7) and ST19 with Salmonella enterica serotype Typhimurium (n = 2). Thus, both horizontal transmission and clonal dissemination were responsible for the distribution of the blaCTX-M-64 gene. One additional Salmonella enterica serotype Enteritidis that harboured CTX-M-64 was also recently identified from a patient.14 This indicated that dissemination of blaCTX-M-64 to Salmonella strains from different hosts had occurred.
Location and genetic context of the blaCTX-M-64 gene
Plasmids were successfully transferred from the three E. coli isolates and blaCTX-M-64 was located on 65 kb IncI2 plasmids (Figure S2a) for all three transconjugants (C-SF1, C-SF4 and C-WG20). The plasmid pWG20 was completely sequenced and the obtained contigs containing blaCTX-M-64 indicated that a 3080 bp ISEcp1-mediated transposition (ISEcp1-blaCTX-M-64-orf477-A/C) event had occurred and this cassette was 100% identical to a region of pCTXM64_C0967 (accession number KP091735) (Figure 1, type II).
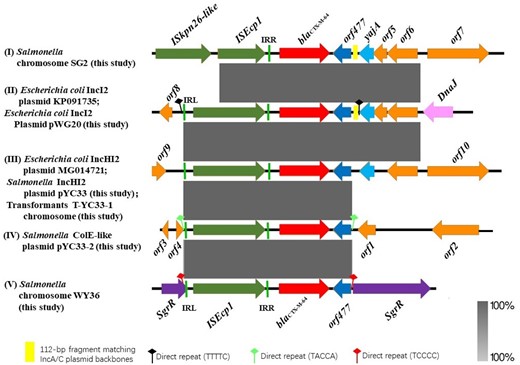
Genomic and molecular analyses for blaCTX-M-64-positive plasmids. Types of genomic environment of the blaCTX-M-64 gene in E. coli and Salmonella isolates: (I) in the chromosome of the Salmonella isolate SG2; (II) in the E. coli IncI2 plasmid pCTXM64_C0967 (KP091735) and the E. coli IncI2 plasmid pWG20; (III) in the E. coli IncHI2 plasmid pFS11Y5CT (MG014721), the Salmonella IncHI2 plasmid pYC33 and the chromosome of the transformant T-YC33-1; (IV) in the Salmonella ColE-like plasmid pYC33-2; and (V) in the chromosome of the Salmonella isolate WY36. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
For the nine blaCTX-M-64-producing Salmonella isolates, only one plasmid was successfully transferred by transformation from one strain (YC33). Interestingly, two different colony characteristics [a small colony type (T-YC33-1) and a large colony type (T-YC33-2)] were observed for the transformants. The MICs of cefotaxime, cefoxitin and ceftriaxone for T-YC33-2 were increased 8- to 16-fold compared with T-YC33-1 (Table S1). PBRT, S1-PFGE and Southern hybridization indicated that the blaCTX-M-64 gene was present on a 190 kb IncHI2 plasmid (pYC33) in YC33 and the context of CTX-M-64 was 100% identical to a region of E. coli IncHI2 plasmid pFS11Y5CT (accession number MG014721) (Figure 1, type III). Unexpectedly, it was located on the chromosome in T-YC33-1 and on a ColE-like plasmid (pYC33-2, 6435 bp) in T-YC33-2 (Figure S2b and S2c). Significantly, the 2968 bp region (ISEcp1-blaCTX-M-64-orf477) was observed in the plasmids pYC33 (Figure 1, type III) and pYC33-2 (Figure 1, type IV) and the chromosome of T-YC33-1, yet was absent from the YC33 endogenous ColE-like plasmid (pYC33-1, 3462 bp) (Figure S3). Additionally, the pYC33 plasmid carrying blaCTX-M-64 was observed partly integrated into the chromosome of T-YC33-1 by PCR mapping (see Table S2 for primers) (Figure S4). This was similar to the results of previous studies in which an IncY and IncA/C2 fusion plasmid harbouring blaCTX-M-15 was partially integrated into the chromosome of Salmonella enterica serotype Concord.15
No transformants were obtained for the remaining eight Salmonella strains despite repeated attempts. The chromosomal location of blaCTX-M-64 in these isolates was confirmed by hybridization (Figure S5). In WY36, the 2968 bp region (ISEcp1-blaCTX-M-64-orf477) was inserted into the SgrR gene (Figure S6) and was similar to the region in YC33 (Figure 1, type V). However, in another chromosomal location in Salmonella isolate SG2, the ISEcp1 was truncated by an IS5 gene and the downstream region of blaCTX-M-64 was similar to that of pWG20 in the E. coli isolate (Figure 1, type I). ISEcp1-mediated transposition seems to be responsible for the integration of the blaCTX-M gene from a plasmid to a chromosomal location.16–19 The high degree of genetic similarity between the blaCTX-M-64 plasmids and chromosomes and the frequent reports of blaCTX-M-64 in E. coli suggest a common mechanism of ISEcp1-mediated transposition in these strains (Figure 1).6,7,20 As ISEcp1-mediated transposition allows horizontal transfer, these elements are likely to further disseminate among Salmonella and other bacterial species.
Conclusions
To the best of our knowledge, this is the first report of chromosomally encoded CTX-M-64 in Salmonella from food animals. These findings indicate that ISEcp1-mediated transposition is likely to be responsible for the spread of blaCTX-M-64 between different plasmids and chromosomes in Enterobacteriaceae, especially E. coli and Salmonella. It is imperative that more attention should be paid to limit the transmission of the blaCTX-M-64 gene alone in regional food chains.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 31772792), and the Special Project for the Cultivation of Major Projects in International Science and Technology Cooperation (grant no. 2019SCAUGH02), and the Graduate Student Oversea Study Program of South China Agriculture University (grant no. 2017LHPY029).
Transparency declarations
None to declare.
Supplementary data
Tables S1 and S2, plus Figures S1 to S6 are available as Supplementary data at JAC Online
References
CLSI. Performance Standards for Antimicrobial Susceptibility Testing—Twenty-Sixth Edition: M100.
CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals—Second Informational Supplement: VET01.
Author notes
Qiu-Yun Zhao and Pin-Xian Chen contributed equally to this work.



