-
PDF
- Split View
-
Views
-
Cite
Cite
Dejun Liu, Weishuai Zhai, Huangwei Song, Yulin Fu, Stefan Schwarz, Tao He, Li Bai, Yang Wang, Timothy R Walsh, Jianzhong Shen, Identification of the novel tigecycline resistance gene tet(X6) and its variants in Myroides, Acinetobacter and Proteus of food animal origin, Journal of Antimicrobial Chemotherapy, Volume 75, Issue 6, June 2020, Pages 1428–1431, https://doi.org/10.1093/jac/dkaa037
Close - Share Icon Share
Abstract
To report a novel tigecycline resistance gene, tet(X6), and its variants in four bacterial species isolated from chickens and pigs in China.
WGS was conducted to identify the suspected resistance genes in the tigecycline-resistant Myroides phaeus 18QD1AZ29W. Functional cloning, homology modelling and molecular docking were performed to compare the function with other Tet(X) variants. Retrospective screening for tet(X6) was conducted for 80 isolates in our WGS data collection, and all genomic environments of tet(X6)-positive isolates were analysed.
The tigecycline-resistant M. phaeus 18QD1AZ29W isolated from a pig farm in Shandong in 2018 was positive for tet(X2) and a novel tet(X) gene, designated tet(X6). Tet(X6) could increase the MICs of all tested tetracyclines/glycylcyclines for Escherichia coli only 2- to 4-fold, which was possibly due to a lower tetracycline binding capacity of Tet(X6) compared with that of other Tet(X) variants. Retrospective screening showed that seven other isolates (7/80, 8.8%), comprising four Proteus spp. and three Acinetobacter spp. from chickens and pigs in Shandong and Guangdong, were positive for three different variants of tet(X6). The analysis of the genomic environment revealed that two tet(X6)-positive isolates from M. phaeus and Proteus cibarius, respectively, contained ISCR2, which may play a role in tet(X6) transmission.
This study identified a novel type of tigecycline resistance gene, tet(X6), in Myroides, Acinetobacter and Proteus from chickens and swine. Tet(X6) conferred lower tetracycline/glycylcycline MICs than other Tet(X) variants, and ISCR2 may play a role in the transmission of tet(X6).
Introduction
Tet(X) is one of the tetracycline-inactivating enzymes that can catalyse the degradation of tetracyclines and was first isolated from the human commensal Bacteroides fragilis.1 It encodes a flavin-dependent monooxygenase that uses flavin adenine dinucleotide (FAD) and exogenous NADPH as bound cofactors to catalyse the oxidation,2 which can enzymatically inactivate most tetracyclines, including tigecycline,3 one of the last-line antibiotics for treating infections due to XDR Gram-negative bacteria.4
To date, five tet(X) variants, tet(X1), tet(X2), tet(X3), tet(X4) and tet(X5), have been identified in various bacterial species. The genes tet(X1) and tet(X2), which have 66% and 99% amino acid identities with the original tet(X), were identified as parts of Bacteroides transposons.5 The variant tet(X1) lacked the FAD-binding domain and has been proven to be unable to degrade tetracyclines/glycylcyclines, while tet(X) and tet(X2) mediate low-level resistance to tigecycline (0.5–2 mg/L, 4-fold increase).3 However, tet(X3) and tet(X4), which were identified in Acinetobacter and Escherichia coli and have 85% and 94% amino acid identities with the original tet(X), confer high-level resistance to tigecycline (0.25–16 mg/L, 64-fold change).6,7 Recently, a novel tet(X) variant, tet(X5), in clinical tigecycline-resistant Acinetobacter baumannii has been characterized.8 It is noteworthy that tet(X3), tet(X4) and tet(X5) were all located in close proximity to ISCR2 elements on horizontally transferable plasmids,6,7 indicating that different tet(X) variants have the opportunity for a wide dissemination and evolution in diverse bacterial species. In the present study, we analysed the sequences of tigecycline-resistant strains and identified a novel tet(X) variant, designated tet(X6).
Materials and methods
Bacterial isolate and functional cloning of tet(X6)
Myroides phaeus 18QD1AZ29W was isolated from a faecal sample from a pig farm in Shandong in 2018. The tigecycline MIC value was 32 mg/L. Genomic DNA was subjected to WGS using a combination of the Illumina HiSeq 2000 system (Sinobiocore, Beijing, China) and the MinION system (Oxford Nanopore Technologies, Shanghai, China). Library preparation and sequence analysis were conducted as described previously.9 To confirm the drug resistance phenotypes conferred by the suspected resistance gene, a 1449 bp DNA fragment, including the putative tigecycline resistance gene and its predicted promoter, was amplified using primers ptet(X6)-F (5′-TTATTAATCAGATAAAATATTTGCAATTTTAGCAATTGACTTTCC-3′) and ptet(X6)-R (5′-GTGATAAACTACCGCATTAAGATGGAGCCGACTTTTAGCAC-3′). The amplicon was ligated into the E. coli–Enterococcus faecalis shuttle vector pAM401 and the recombinant plasmid was introduced into E. coli DH5α by electrotransformation.10 Transformants were selected on LB agar plates containing 30 mg/L chloramphenicol, and the presence of the tet(X6) gene and the MIC values to various tetracyclines were confirmed.
Homology modelling and molecular docking
A structure with the PDB entry 4A6N was chosen as the template for Tet(X6) homology modelling. A homology model of Tet(X6) was then built using Modeller 9.20 software. The FAD cofactor was kept during molecular docking. Tetracyclines were then docked into the substrate-binding site by the Sybyl x-2.1 platform, which performs an exhaustive search of position, orientation and conformation of the ligand in the binding pocket.
Screening for tet(X6) by retrospective analysis
Retrospective screening for tet(X6) was conducted for 80 isolates in our WGS database. All isolates were resistant to tigecycline. They originated from chickens and pigs and samples from meat for consumption (chicken and pork) collected during 2017–18, in Shandong and Guangdong. Whole-genome fragments were assembled using SPAdes 3.9.0, and then analysed using the RAST web annotation server (http://rast.nmpdr.org) and BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed according to EUCAST, 2019.11E. coli strain ATCC 25922 served for quality control purposes.
GenBank accession numbers
The sequences described in this paper have been deposited in the GenBank database under accession number: PRJNA593823.
Results and discussion
In our annual surveillance of tigecycline-resistant bacteria from animals and animal-derived foods, the porcine M. phaeus isolate 18QD1AZ29W was identified. This isolate exhibited resistance to cefepime (MIC = 32 mg/L), florfenicol (MIC = 16 mg/L), kanamycin (MIC = 256 mg/L), colistin (MIC = 128 mg/L) and all tetracyclines/glycylcyclines, including oxytetracycline (MIC = 128 mg/L), tetracycline (MIC = 64 mg/L), chlortetracycline (MIC = 64 mg/L), doxycycline (MIC = 32 mg/L), minocycline (MIC = 32 mg/L), tigecycline (MIC = 32 mg/L), eravacycline (MIC = 32 mg/L) and omadacycline (MIC = 128 mg/L). Genome assembly with the MinION and HiSeq sequencing data revealed that the size of the genome of M. phaeus 18QD1AZ29W was 3 107 673 bp and that this isolate did not harbour plasmids. The gene annotation revealed that this isolate harboured two tet(X2) genes and a tet(X)-like gene.
The tet(X)-like gene was 1137 bp in size and encoded a 378 amino acid protein that exhibited identities of 87.8%, 87.6%, 79.4%, 90.2% and 92.1% to Tet(X), Tet(X2), Tet(X3), Tet(X4) and Tet(X5), respectively (Figure S1, available as Supplementary data at JAC Online). Therefore, this novel tet(X) variant was designated as tet(X6). Genomic mining revealed that similar proteins were found in whole-genome sequences of E. coli, Acinetobacter indicus, Flavobacterium marinum, Pseudomonas aeruginosa and an uncultured bacterium. Among them, the sequences of an uncultured bacterium from a latrine in El Salvador (GenBank accession number: AMP54221.1) and of an E. coli from pig faeces in Denmark (KHO55827.1) showed the highest amino acid identity of 97.1% to Tet(X6) (Figure S1).
Due to the chromosomal location of tet(X6), the functional cloning of this gene was performed. A single intact copy of tet(X6) together with the upstream sequence was amplified and ligated into pAM401 and then transferred to E. coli DH5α by electrotransformation to verify the resistance phenotypes of the novel tet(X6) variant. Only 2- to 4-fold increases in the MICs of all tested tetracyclines/glycylcyclines were observed for the E. coli transformant, designated DH5α-pAM401-tet(X6) (Table 1), indicating that Tet(X6) can elevate the MIC values of tetracyclines/glycylcyclines, but its function was less efficient than that of other Tet(X) proteins, such as Tet(X3) and Tet(X4).6
| Strains . | Description . | MIC (mg/L) of tetracycline antimicrobials . | |||||
|---|---|---|---|---|---|---|---|
| TET . | DOX . | MIN . | TGC . | ERA . | OMA . | ||
| M. phaeus 18QD1AZ29W | the original tet(X6) | 64 | 32 | 32 | 32 | 32 | 128 |
| P. mirabilis 18QD2AZ3W | 4 amino acid substitutions compared with the original tet(X6) | 128 | 128 | 128 | 16 | 16 | 64 |
| A. johnsonii 18QD2AZ57W | 11 amino acid substitutions compared with the original tet(X6) | 128 | 64 | 16 | 32 | 32 | 128 |
| A. lwoffii 18QD2AZ41W | 11 amino acid substitutions compared with the original tet(X6) | 64 | 32 | 16 | 16 | 16 | 64 |
| A. lwoffii 18QD2AZ28W | 11 amino acid substitutions compared with the original tet(X6) | 64 | 32 | 16 | 16 | 16 | 64 |
| P. cibarius 17SZRF8EW | 16 amino acid substitutions compared with the original tet(X6) | 128 | 128 | 128 | 16 | 16 | 64 |
| P. cibarius 17SZRF19EW | 16 amino acid substitutions compared with the original tet(X6) | 128 | 128 | 64 | 16 | 16 | 64 |
| P. cibarius 17SZRZ8EW | 16 amino acid substitutions compared with the original tet(X6) | 128 | 128 | 64 | 16 | 16 | 64 |
| E. coli DH5α+pAM401 | recipient for the tet(X6) gene | 1 | 2 | 2 | 0.125 | 0.25 | 1 |
| E. coli DH5α- pAM401-tet(X6) | transformants of the original tet(X6) | 4 | 4 | 2 | 0.5 | 1 | 2 |
| E. coli DH5α- pAM401-tet(X6)-4 | transformants of the tet(X6) variant with 4 amino acid substitutions | 4 | 4 | 8 | 0.5 | 1 | 4 |
| E. coli DH5α- pAM401-tet(X6)-11 | transformants of the tet(X6) variant with 11 amino acid substitutions | 4 | 8 | 8 | 0.5 | 1 | 4 |
| E. coli DH5α- pAM401-tet(X6)-16 | transformants of the tet(X6) variant with 16 amino acid substitutions | 4 | 8 | 8 | 0.5 | 1 | 4 |
| Strains . | Description . | MIC (mg/L) of tetracycline antimicrobials . | |||||
|---|---|---|---|---|---|---|---|
| TET . | DOX . | MIN . | TGC . | ERA . | OMA . | ||
| M. phaeus 18QD1AZ29W | the original tet(X6) | 64 | 32 | 32 | 32 | 32 | 128 |
| P. mirabilis 18QD2AZ3W | 4 amino acid substitutions compared with the original tet(X6) | 128 | 128 | 128 | 16 | 16 | 64 |
| A. johnsonii 18QD2AZ57W | 11 amino acid substitutions compared with the original tet(X6) | 128 | 64 | 16 | 32 | 32 | 128 |
| A. lwoffii 18QD2AZ41W | 11 amino acid substitutions compared with the original tet(X6) | 64 | 32 | 16 | 16 | 16 | 64 |
| A. lwoffii 18QD2AZ28W | 11 amino acid substitutions compared with the original tet(X6) | 64 | 32 | 16 | 16 | 16 | 64 |
| P. cibarius 17SZRF8EW | 16 amino acid substitutions compared with the original tet(X6) | 128 | 128 | 128 | 16 | 16 | 64 |
| P. cibarius 17SZRF19EW | 16 amino acid substitutions compared with the original tet(X6) | 128 | 128 | 64 | 16 | 16 | 64 |
| P. cibarius 17SZRZ8EW | 16 amino acid substitutions compared with the original tet(X6) | 128 | 128 | 64 | 16 | 16 | 64 |
| E. coli DH5α+pAM401 | recipient for the tet(X6) gene | 1 | 2 | 2 | 0.125 | 0.25 | 1 |
| E. coli DH5α- pAM401-tet(X6) | transformants of the original tet(X6) | 4 | 4 | 2 | 0.5 | 1 | 2 |
| E. coli DH5α- pAM401-tet(X6)-4 | transformants of the tet(X6) variant with 4 amino acid substitutions | 4 | 4 | 8 | 0.5 | 1 | 4 |
| E. coli DH5α- pAM401-tet(X6)-11 | transformants of the tet(X6) variant with 11 amino acid substitutions | 4 | 8 | 8 | 0.5 | 1 | 4 |
| E. coli DH5α- pAM401-tet(X6)-16 | transformants of the tet(X6) variant with 16 amino acid substitutions | 4 | 8 | 8 | 0.5 | 1 | 4 |
TET, tetracycline; DOX, doxycycline; MIN, minocycline; TGC, tigecycline; ERA, eravacycline; OMA, omadacycline.
| Strains . | Description . | MIC (mg/L) of tetracycline antimicrobials . | |||||
|---|---|---|---|---|---|---|---|
| TET . | DOX . | MIN . | TGC . | ERA . | OMA . | ||
| M. phaeus 18QD1AZ29W | the original tet(X6) | 64 | 32 | 32 | 32 | 32 | 128 |
| P. mirabilis 18QD2AZ3W | 4 amino acid substitutions compared with the original tet(X6) | 128 | 128 | 128 | 16 | 16 | 64 |
| A. johnsonii 18QD2AZ57W | 11 amino acid substitutions compared with the original tet(X6) | 128 | 64 | 16 | 32 | 32 | 128 |
| A. lwoffii 18QD2AZ41W | 11 amino acid substitutions compared with the original tet(X6) | 64 | 32 | 16 | 16 | 16 | 64 |
| A. lwoffii 18QD2AZ28W | 11 amino acid substitutions compared with the original tet(X6) | 64 | 32 | 16 | 16 | 16 | 64 |
| P. cibarius 17SZRF8EW | 16 amino acid substitutions compared with the original tet(X6) | 128 | 128 | 128 | 16 | 16 | 64 |
| P. cibarius 17SZRF19EW | 16 amino acid substitutions compared with the original tet(X6) | 128 | 128 | 64 | 16 | 16 | 64 |
| P. cibarius 17SZRZ8EW | 16 amino acid substitutions compared with the original tet(X6) | 128 | 128 | 64 | 16 | 16 | 64 |
| E. coli DH5α+pAM401 | recipient for the tet(X6) gene | 1 | 2 | 2 | 0.125 | 0.25 | 1 |
| E. coli DH5α- pAM401-tet(X6) | transformants of the original tet(X6) | 4 | 4 | 2 | 0.5 | 1 | 2 |
| E. coli DH5α- pAM401-tet(X6)-4 | transformants of the tet(X6) variant with 4 amino acid substitutions | 4 | 4 | 8 | 0.5 | 1 | 4 |
| E. coli DH5α- pAM401-tet(X6)-11 | transformants of the tet(X6) variant with 11 amino acid substitutions | 4 | 8 | 8 | 0.5 | 1 | 4 |
| E. coli DH5α- pAM401-tet(X6)-16 | transformants of the tet(X6) variant with 16 amino acid substitutions | 4 | 8 | 8 | 0.5 | 1 | 4 |
| Strains . | Description . | MIC (mg/L) of tetracycline antimicrobials . | |||||
|---|---|---|---|---|---|---|---|
| TET . | DOX . | MIN . | TGC . | ERA . | OMA . | ||
| M. phaeus 18QD1AZ29W | the original tet(X6) | 64 | 32 | 32 | 32 | 32 | 128 |
| P. mirabilis 18QD2AZ3W | 4 amino acid substitutions compared with the original tet(X6) | 128 | 128 | 128 | 16 | 16 | 64 |
| A. johnsonii 18QD2AZ57W | 11 amino acid substitutions compared with the original tet(X6) | 128 | 64 | 16 | 32 | 32 | 128 |
| A. lwoffii 18QD2AZ41W | 11 amino acid substitutions compared with the original tet(X6) | 64 | 32 | 16 | 16 | 16 | 64 |
| A. lwoffii 18QD2AZ28W | 11 amino acid substitutions compared with the original tet(X6) | 64 | 32 | 16 | 16 | 16 | 64 |
| P. cibarius 17SZRF8EW | 16 amino acid substitutions compared with the original tet(X6) | 128 | 128 | 128 | 16 | 16 | 64 |
| P. cibarius 17SZRF19EW | 16 amino acid substitutions compared with the original tet(X6) | 128 | 128 | 64 | 16 | 16 | 64 |
| P. cibarius 17SZRZ8EW | 16 amino acid substitutions compared with the original tet(X6) | 128 | 128 | 64 | 16 | 16 | 64 |
| E. coli DH5α+pAM401 | recipient for the tet(X6) gene | 1 | 2 | 2 | 0.125 | 0.25 | 1 |
| E. coli DH5α- pAM401-tet(X6) | transformants of the original tet(X6) | 4 | 4 | 2 | 0.5 | 1 | 2 |
| E. coli DH5α- pAM401-tet(X6)-4 | transformants of the tet(X6) variant with 4 amino acid substitutions | 4 | 4 | 8 | 0.5 | 1 | 4 |
| E. coli DH5α- pAM401-tet(X6)-11 | transformants of the tet(X6) variant with 11 amino acid substitutions | 4 | 8 | 8 | 0.5 | 1 | 4 |
| E. coli DH5α- pAM401-tet(X6)-16 | transformants of the tet(X6) variant with 16 amino acid substitutions | 4 | 8 | 8 | 0.5 | 1 | 4 |
TET, tetracycline; DOX, doxycycline; MIN, minocycline; TGC, tigecycline; ERA, eravacycline; OMA, omadacycline.
To illustrate the difference in function between Tet(X6) and other Tet(X) variants, homology modelling and molecular docking assays were performed. Superposing of this model onto the Tet(X2) X-ray structure (PDB no. 4A6N) showed that the tetracycline-binding site of Tet(X6) was essentially identical to that of Tet(X2), with similar results observed also for Tet(X3), Tet(X4) and Tet(X5) (Figure S2a). However, in the model, no hydrogen bond could form between Tet(X6) and FAD, the most important cofactor to catalyse the oxidation reaction, which was in contrast to Tet(X2), Tet(X4) and Tet(X5) (Figure S2b).6 Thus, it showed the lowest total score of pIC50 (Table S1). This could be an explanation for why Tet(X6) functions less efficiently than other Tet(X) variants.
To investigate the dissemination of tet(X6), we conducted a BLAST analysis for the presence of tet(X6) in tigecycline-resistant isolates from our WGS data collection. A total of seven isolates (7/80, 8.8%), three from chickens and four from pigs, were tet(X6) positive, comprising three Proteus cibarius, one Proteus mirabilis, two Acinetobacter lwoffii and one Acinetobacter johnsonii. Sequence comparisons of the deduced Tet(X6) sequences revealed the presence of a further three Tet(X6) variants, which differed from the Tet(X6) protein of M. phaeus isolate 18QD1AZ29W by 4 (n = 1), 11 (n = 3) and 16 (n = 3) amino acids, respectively (Figure S3). Eleven amino acid variations of Tet(X6) in three isolates were identical to that of Tet(X) (KHO55827.1) isolated from a porcine E. coli in Denmark (Figure S1), indicating that Tet(X6) has also emerged in European countries.
The tet(X6) of M. phaeus 18QD1AZ29W was located within a 22 634 bp length MDR genomic island, which was inserted into the housekeeping genes dnaJ and dnaK, which both expressed a chaperone protein. In addition to tet(X6), nine other resistance genes, namely the tetracycline resistance gene tet(X2), the β-lactamase gene blaOXA-347, the lincosamide resistance gene lnu(B), the aminoglycoside resistance gene aadS, the macrolide resistance genes erm(F) and ere(D), the chloramphenicol/florfenicol exporter gene floR as well as a catA and a catB chloramphenicol acetyltransferase gene, were also present (Figure 1). An intact ISCR2 element, the major mobile element associated with tet(X3) and tet(X4),6,7 was located downstream of tet(X2) and upstream of tet(X6) and may play an important role in the transmission of tet(X6). The comparative analysis of the genetic context of the tet(X6) gene variants of M. phaeus 18QD1AZ29W with that of the other seven isolates indicated that four types (types I to IV) of genomic environment of tet(X6) were present (Figure 1). The tet(X6) gene variant in the type III environment was occasionally surrounded by intact or truncated ISCR2 elements, implying that this element may be involved in the transmission of the tet(X6) gene.
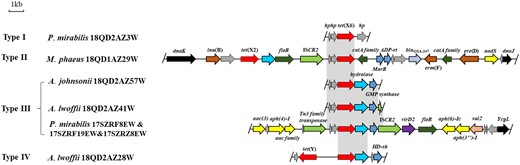
Genetic environments of the tet(X6) genes and comparison of the tet(X6)-carrying regions in this study. The arrows indicate the direction of transcription of the genes. Genes are differentiated by colours. Regions of >97% homology are marked by grey shading.
In summary, this study reported the discovery of four subtypes of the novel tigecycline resistance gene tet(X6) in four bacterial species from chickens and pigs. Tet(X6) conferred lower increases of tetracycline/glycylcyclines MICs than other Tet(X) variants. Although tet(X6) was located on the chromosome in M. phaeus 18QD1AZ29W, the presence of ISCR2 surrounding the tet(X6) gene suggested the possibility of dissemination. Further investigations on both the prevalence and mobility of tet(X6) are needed.
Funding
This work was supported in part by the National Science Foundation of China (31930110) and the National Key R&D Program of China (2018YFD0500300).
Transparency declarations
None to declare.
Supplementary data
Figures S1 to S3 and Table S1 are available as Supplementary data at JAC Online.
References
EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 9.0,



