-
PDF
- Split View
-
Views
-
Cite
Cite
Ruichao Li, Pei Zhang, Xiaorong Yang, Zhiqiang Wang, Séamus Fanning, Juan Wang, Pengcheng Du, Li Bai, Identification of a novel hybrid plasmid coproducing MCR-1 and MCR-3 variant from an Escherichia coli strain, Journal of Antimicrobial Chemotherapy, Volume 74, Issue 6, June 2019, Pages 1517–1520, https://doi.org/10.1093/jac/dkz058
Close - Share Icon Share
Abstract
To characterize the genome of an Escherichia coli harbouring both mcr-1 and mcr-3.19 on a hybrid plasmid and the underlying transmission mechanisms.
Broth microdilution was used to perform antimicrobial susceptibility testing. Conjugation assays and S1-PFGE were used to assess the transferability of mcr genes. Resistance genotypes and genetic contexts were investigated, based on WGS data from the Illumina and MinION platforms. Inverse PCR was performed to test the mcr-3.19-bearing circular intermediate. Bioinformatic tools were used to further characterize the hybrid plasmid.
E. coli CP53 was identified as harbouring both mcr-1 and mcr-3.19 on a 231 859 bp hybrid plasmid pCP53-mcr1_3 containing IncFIA, IncHI1A, IncHI1B and IncN replicons. The genetic structures of mcr-1 and mcr-3.19 were similar to those reported in other mcr-1 and mcr-3.19-bearing plasmids, which suggested that recombination between mcr-bearing plasmids had been mediated by ISs. However, the MDR plasmid pCP53-mcr1_3 cannot transfer via conjugation. Furthermore, another three plasmids were identified in the isolate, two of which encoded resistance genes. In640 duplication between two MDR plasmids was observed. An MDR-region recombination existed in E. coli CP53. A core structure consisting of mcr-3-dgkA existed in mcr-3-bearing plasmids reported, to date. Circular intermediates were observed for mcr-1 and mcr-3.19 regions.
A novel mcr-3.19 was identified along with mcr-1 contained in a hybrid plasmid. This finding suggested that evolution of mcr genes among various plasmids was being driven by mobile elements. Molecular surveillance of mcr gene co-occurrence warrants further investigation to evaluate the public health risk.
Introduction
The emergence of MDR bacteria poses a threat to public health. A mobile colistin resistance gene, mcr-1, conferring resistance to colistin, the drug of last resort used to treat carbapenem-resistant Enterobacteriaceae, was first reported in Escherichia coli mediated by conjugative plasmids in 2015.1 Until now, eight different mcr alleles, from mcr-1 to mcr-8, have been reported from different bacteria.2,3 Studies describing the prevalence of mcr genes in bacteria are increasing and novel mcr alleles are thought to emerge continuously.
The plasmid-mediated gene mcr-3 was first identified in the IncHI2-type plasmid pWJ1 from porcine E. coli.4 Since then, another 18 mcr-3 variants have been identified in Enterobacteriaceae and aeromonads according to the NCBI database. Multiple mcr-1 genes in a single strain have been reported previously.5,6 However, co-location of different mcr genes in a single bacterial isolate is rare.7,8 In this study, we identified a novel mcr-3 allele co-occurring with mcr-1 in a hybrid plasmid found in E. coli. Study of this novel vector may shed light on the coevolution of mcr gene alleles.
Materials and methods
Bacterial strains
Colistin-resistant E. coli strains (n = 52) were isolated from pig faeces in July 2016 in Sichuan, China. Strain identification was confirmed using the VITEK 2 Gram-Negative Identification Card and 16S ribosomal DNA (rDNA)-based sequencing. Antimicrobial susceptibility testing was performed using a panel of antimicrobial compounds (Table S1, available as Supplementary data at JAC Online). Primers targeting mcr-1 and mcr-3 genes were synthesized (Table S2) and then used in PCR assays to screen for the mcr gene-positive isolates.1,4 Inverse primers targeting potential circular intermediates of mcr-1 and mcr-3 were also synthesized (Table S2) and the corresponding inverse PCR products were sequenced. Filter mating assays were conducted to investigate the transferability of mcr genes among bacteria with E. coli J53 (Azir) with 100 mg/L sodium azide and E. coli 26R (Rifr) with 100 mg/L rifampicin as the receipt strains. S1 nuclease PFGE (S1-PFGE) was used to obtain plasmid profiles.
WGS, data analysis and mcr-3-bearing plasmid characterization
WGS combining highly accurate short-read Illumina and long-read Oxford Nanopore Technologies (ONT) MinION platforms was utilized to provide complete genomes via de novo assembly with a hybrid strategy according to a published method.9 The plasmid sequence was confirmed by Sanger sequencing and MinION long-read alignment. Bioinformatic tools including Resfinder, ISFinder and PlasmidFinder were used to analyse these genomes. BRIG and Easyfig were used to generate the genetic comparison figures. To investigate all mcr-3-bearing plasmids previously reported, all mcr-3 plasmid sequences were downloaded from the NCBI database after BLASTn screening with the mcr-3 gene against the non-redundant protein sequence (nr) database. The complete genome sequence of E. coli CP53 was submitted to the NCBI database under the following accession numbers: CP033096 (chromosome), CP033093 (pCP53-38k), CP033094 (pCP53-mcr1_3), CP033095 (pCP53-92k) and CP033097 (pCP53-113k).
Results and discussion
Characterization of strain CP53 coproducing mcr-1 and mcr-3.19
All colistin-resistant E. coli strains (n = 52) isolated from 99 samples of pig faeces were screened with primers targeting mcr-1 and mcr-3 genes. In the end, six isolates were identified as co-harbouring mcr-1 and mcr-3 genes, of which one isolate designated as E. coli CP53 was identified as co-harbouring mcr-1 and mcr-3 genes in the same plasmid. This isolate was found to be resistant to tetracycline, ampicillin, gentamicin, chloramphenicol, trimethoprim/sulfamethoxazole and polymyxin B (8 mg/L), but susceptible to cefotaxime, ceftazidime, ciprofloxacin and imipenem (Table S1). Although conjugation assays were performed with E. coli J53 and E. coli 26R as recipients, no successful conjugants were obtained. This may indicate that the mcr genes were not located on conjugative plasmids. S1-PFGE data demonstrated that E. coli CP53 harboured four plasmids of size 230, 110, 85 and 33 kb (Figure S1). To further explore the genetic structures of these mcr genes and other resistance genotypes, WGS was performed to obtain the complete genome of E. coli CP53.
Genome structures of plasmids
After combing Illumina and MinION sequencing data and performing hybrid assembly, the genome of E. coli CP53 was closed successfully with one chromosome and four plasmids. MLST analysis of E. coli CP53 showed it belonged to ST206 and the chromosome was 4 623 817 bp in length with 50.9% GC content. Previously, ST206 E. coli strains derived from environmental water samples, vegetables and clinical settings were found to harbour mcr and/or blaNDM genes.10,11 This indicated that mcr and/or blaNDM-bearing ST206 E. coli strains have existed along the food production chain. In this study, the plasmids identified were designated as plasmids pCP53-mcr1_3, pCP53-113k, pCP53-92k and pCP53-38k. The distribution of resistance genes among these plasmids is listed in Table S3. Notably, plasmid pCP53-mcr1_3 was found to encode both mcr-1 and mcr-3.19. To the best of our knowledge, this is the first report of a single hybrid plasmid co-harbouring mcr-1 and mcr-3.19.
Online BLASTn of plasmid pCP53-mcr1_3 against the current nr databases showed that most similar plasmids cover 77% of this structure with 99% homology, which indicated that this plasmid was novel in structure. Plasmids pEC2-4, pEQ2, pWJ1 and pKP14812-MCR-1 were most similar to pCP53-mcr1_3 (Figure 1a). Plasmid pCP53-mcr1_3 was 231 859 bp in size, with 47.5% GC content and it encoded 273 hypothetical coding sequences. Multiple replicons including IncFIA, IncHI1A, IncHI1B and IncN were found in plasmid pCP53-mcr1_3, which indicated it is most likely a hybrid plasmid that evolved earlier from recombination events involving several plasmids. A number of antimicrobial resistance loci were identified, including one strAB operon, along with erm(B), mph(A), bleO, mcr-3.19, aac(3)-IId, mcr-1, mer operon, aph(3)-Ia, mef(B), sul3, aadA1, cmlA, aadA2 and dfrA12 located in plasmid pCP53-mcr1_3. Characterization of other plasmids in CP53 is described in the Supplementary data (Table S3 and Figure 2).
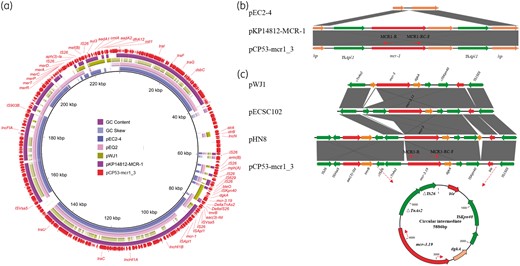
BRIG alignment of plasmids containing mcr-1 and mcr-3 and comparison with plasmid pCP53-mcr1_3. (a) Circular comparison between reference plasmid pCP53-mcr1_3 and most similar plasmids from the NCBI database generated by the BRIG tool. (b) Illustration of insertion event of mcr-1-bearing Tn6330 in pKP14812-MCR-1 and pCP53-mcr1_3 from a conserved region of pEC2-4. Target sequence duplication (AA) was observed. (c) Alignment of mcr-3-bearing genetic regions reported previously and a circular intermediate detected by inverse PCR. It was observed that mcr-3-dgkA-ISKpn40 was conserved in all mcr-3-bearing plasmids. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Characterization of genetic contexts of mcr genes and mcr-3-bearing plasmids
The gene mcr-1 in E. coli CP53 was located within the composite transposon Tn63305 and the genetic environment around Tn6330 was the same as for the plasmid pKP14812-MCR-1 (MH733010) identified previously in Klebsiella pneumoniae (Figure 1b). Sequence alignment indicated the Tn6330 in plasmids pCP53-mcr1_3 and pKP14812-MCR-1 was obtained following the insertion of Tn6330 in the conserved AT-rich region of plasmid pEC2-4 and resulted in a dinucleotide duplication. A circular intermediate was observed with inverse PCR in Tn6330, as reported previously.12 The mcr-3 sequence was accurately identified to be the mcr-3.19 allele reported in Citrobacter freundii (NG_055497) and the genetic environment, IS26-ISVsa5-aac(3)-IId-tmrB-△IS26-△TnAs2-mcr3.19-dgkA-ISKpn40-ble-IS15DI, was also similar to that previously described in the IncR-type plasmid pHN8, also found in E. coli (Figure 1c). Recently, this structure was reported to generate a circular intermediate encoding △IS26-△TnAs2-mcr-3-dgkA-ISKpn40-ble mediated by a truncated IS26 and a complete IS15DI.13 With inverse PCR, a circular intermediate with the structure △IS26-△TnAs2-mcr-3.19-dgkA-ISKpn40-ble was detected (Figure 1c). This mcr-3.19-encoding circular intermediate was similar to that generated by Tn6330 and ISKpn40-mcr-3.11-dgkA-ISKpn40 belonging to composite transposon structures.2,12 These findings suggest that composite transposons appear to play an important role in the translocation of mcr genes.
After screening for mcr-3 genes in the nr database, fourteen sequences were identified (Table S4). Apart from one sequence with an mcr-3 gene cluster (MF455227), the other thirteen completed sequences of mcr-3-bearing plasmids ranged from 50 520 bp to 261 119 bp, belonging to different plasmid replicons including IncR, IncP, IncHI2, IncFII and IncFIB from E. coli, Salmonella enterica and K.pneumoniae found in China, Vietnam, Japan and Canada. Most of these plasmids encoded multiple resistance genes, which indicated that mcr-3 appears to be consistently located in MDR plasmids. One IncP-type plasmid, pECSC102, isolated from E. coli in a chicken farm in Sichuan, China was also positive for mcr-1 and mcr-3.7 The different STs of ECSC102 (ST167) and CP53 (ST206) implied that the two strains may acquire the resistance plasmids respectively. Compared with the hybrid plasmid pCP53-mcr1_3 from E. coli CP53 in a Sichuan pig farm reported in this study, the strains were from different hosts and the genetic contexts of mcr genes were different. This indicated that the two plasmids harbouring both mcr-1 and mcr-3 genes evolved along different pathways. Based on Figure 1(c) and references of the mcr-3 gene,4,7,13–18 the core genetic context of mcr-3 was mcr-3-dgkA surrounded by different ISs including ISKpn40, △TnAs2, IS26 and ISApl1. This was different from the situation for mcr-1, which was always mediated by ISApl1.1,5,19 This implied that the transmission of mcr-3 among plasmids may be more complicated than that of mcr-1.
Conclusions
In this study, we identified a novel hybrid plasmid harbouring both mcr-1 and mcr-3.19 in an E. coli CP53 from animal faeces. High MICs conferred by two mcr alleles constitute a public concern. Although the plasmid was non-conjugative, the MDR region encoding the two mcr alleles may disseminate among different bacteria by conjugative plasmids after translocation. Investigation for co-occurrence of multiple mcr alleles in a single bacterial isolate should be performed to evaluate the risk of colistin resistance among pathogens. Furthermore, systematic research of mcr-3-bearing plasmids or chromosomes should be performed to investigate the transmission ability of this gene.
Acknowledgements
We thank Yan Li, Yangzhou University for organizing information regarding mcr-3-bearing plasmids.
Funding
This work was supported by the National Natural Science Foundation of China (31872523 and 31871899), the Natural Science Foundation of Jiangsu Province (BK20180900) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Transparency declarations
None to declare.



