-
PDF
- Split View
-
Views
-
Cite
Cite
Dan Dan He, Shi Yu Zhao, Hua Wu, Gong Zheng Hu, Jin Feng Zhao, Zhi Yong Zong, Yu Shan Pan, Antimicrobial resistance-encoding plasmid clusters with heterogeneous MDR regions driven by IS26 in a single Escherichia coli isolate, Journal of Antimicrobial Chemotherapy, Volume 74, Issue 6, June 2019, Pages 1511–1516, https://doi.org/10.1093/jac/dkz044
Close - Share Icon Share
Abstract
IS26-flanked transposons played an increasingly important part in the mobilization and development of resistance determinants. Heterogeneous resistance-encoding plasmid clusters with polymorphic MDR regions (MRRs) conferred by IS26 in an individual Escherichia coli isolate have not yet been detected.
To characterize the complete sequence of a novel blaCTX-M-65- and fosA3-carrying IncZ-7 plasmid with dynamic MRRs from an E. coli isolate, and to depict the mechanism underlying the spread of resistance determinants and genetic polymorphisms.
The molecular characterization of a strain carrying blaCTX-M-65 and fosA3 was analysed by antimicrobial susceptibility testing and MLST. The transferability of a plasmid bearing blaCTX-M-65 and fosA3 was determined by conjugation assays, and the complete structure of the plasmid was obtained by Illumina, PacBio and conventional PCR mapping, respectively. The circular forms derived from IS26-flanked transposons were detected by reverse PCR and sequencing.
A novel IncZ-7 plasmid pEC013 (∼118kb) harbouring the blaCTX-M-65 and fosA3 genes was recovered from E. coli isolate EC013 belonging to D-ST117. The plasmid was found to have heterogeneous and dynamic MRRs in an individual strain and the IS26-flanked composite transposon-derived circular intermediates were identified and characterized in pEC013.
The heterogeneous MRRs suggested that a single plasmid may actually be a cluster of plasmids with the same backbone but varied MRRs, reflecting the plasmid’s heterogeneity and the survival benefits of having a response to antimicrobial-related threatening conditions in an individual strain.
Introduction
The increasing prevalence of MDR bacteria in the clinical setting has posed an enormous threat to human and animal health worldwide. Infections with ESBL-producing strains are regarded as major targets for surveillance in the field of antimicrobial drug resistance. These types of infections are of particular concern, because they are often composed of multiple MDR bacteria.1,2 ESBLs, particularly CTX-M-type enzymes, have been widely reported in a number of different bacterial species, isolated from both humans and animals.3 The plasmid-mediated fosfomycin resistance gene, fosA3, has been found in association with other resistance determinants, such as blaCTX-M and rmtB. This association favours the dissemination and maintenance of the fosA3 gene because of co-selection by cephalosporins and/or aminoglycosides.4,5 It has been reported that the fosA3 gene co-localizes with blaCTX-M on several successfully disseminated plasmids, including the IncI1, IncHI2 and IncFII plasmids from Escherichia coli isolates found in commercial livestock and domestic pets in China.5–9 Furthermore, the mobile element IS26, associated with both fosA3 and blaCTX-M, seems to be an important mode of transmission for these resistance determinants, along with horizontal transmission through plasmid conjugation.10–12 Here, a blaCTX-M-65- and fosA3-carrying IncZ-7 plasmid was identified in an E. coli strain. This plasmid had heterogeneous MDR regions (MRRs) containing multiple copies of IS26. Additionally, several IS26-flanked composite transposon-derived circular intermediates were also identified in this work, which may contribute to the presence of the dynamic MRR in an individual strain. This study provides further understanding of the development of MRRs and the rapid dissemination of resistance genes in Enterobacteriaceae.
Materials and methods
Bacterial strain
In September 2012, an E. coli strain, EC013, was identified using the VITEK 32 automated identification system (bioMérieux, Marcy-l'Étoile, France). The strain was recovered from a chicken on a traditional farm in Shangdong province, China. The strain grew on MacConkey agar plates supplemented with 4mg/L cefotaxime to select for cefotaxime-resistant E. coli isolates for further study. The blaCTX-M and fosA3 genotypes were screened using PCR and Sanger sequencing as described previously.7
Antimicrobial susceptibility testing and molecular typing
MICs of antimicrobial agents for the E. coli strain EC013 were determined using a broth microdilution method and interpreted according to the guidelines of the CLSI.13,E. coli strain ATCC 25922 was used as the quality control. E. coli phylogenetic group typing and MLST were performed as described previously.7
Conjugation assay and replicon sequence typing
The conjugation experiment was performed using E. coli EC013 as the donor and E. coli C600 (resistant to rifampicin) as the recipient, and conjugation frequency was calculated as the number of transconjugants per recipient as described previously.7 The transconjugant was screened on MacConkey agar plates supplemented with cefotaxime (4mg/L) and rifampicin (450mg/L). The presence of blaCTX-M and fosA3 in the transconjugant was confirmed with PCR and the MICs of antimicrobial agents for the transconjugant were determined as described above. The replicon sequence typing of the plasmid was confirmed using the PCR-based replicon typing (PBRT) method,14 and then was further classified using primers for repABKI and repAZ.15
Plasmid sequencing and bioinformatics analyses
The plasmid of the transconjugant (TEC013) was extracted using the Qiagen Plasmid Midi Kit (Qiagen, Hilden, Germany), and designated as pEC013. The plasmid was sequenced with the Illumina MiSeq platform (Illumina, San Diego, CA, USA) and the PacBio RSII single-molecule real-time (SMRT) sequencing instrument (Pacific Biosciences, Menlo Park, CA, USA), respectively. For Illumina, the raw sequence data of pEC013 were assembled using Newbler software v2.6,16 generating six contigs. Gaps between contigs were closed by using a PCR-based strategy. Briefly, the primers were designed based on the two orientations of the sequence of each contig and the products were sequenced. For the PacBio RSII SMRT sequencing platform, the average sequencing coverage was ∼135× across the genome. The reads were assembled de novo using the hierarchical genome assembly process with the default settings of the SMRT Analysis v2.3.0 software package (Pacific Biosciences). The plasmid sequence was initially annotated with Rapid Annotation using the Subsystem Technology (v2.0) server (http://rast.nmpdr.org) and curated manually using the BLASTn and BLASTp algorithms (http://blast.ncbi.nlm.nih.gov/blast). The whole plasmid and comparative analysis were generated using EasyFig (http://mjsull.github.io/Easyfig/files.html). Alignment of the nucleotide sequences was generated using SnapGene (www.snapgene.com).
Identification of polymorphic MRRs and circular intermediates
A series of PCR primers, at sites flanking IS26, was used to determine the order of the contigs carrying resistance determinants (Table S1, available as Supplementary data at JAC Online; Figure 1a). In view of the results that the contigs could connect to each other in any direction and array (Figure 2a), and the knowledge that IS26-flanked structures could generate circular DNA intermediates, reverse PCR was performed to detect potential circular forms.
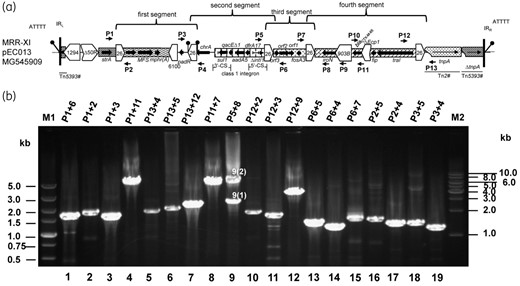
Detection of MRR rearrangements in the plasmid pEC013 by PCR. (a) Positions of primers used for PCR amplification in the plasmid pEC013. (b) Gel electrophoresis of PCR amplicons corresponding to the different IS26-flanked transposons and circular forms of pEC013. The circular intermediate C1 (Figure 2b) corresponds to the combination of amplicon nos 16 and 19, C2 corresponds to amplicon no. 15, and C3 in the circular form corresponds to amplicon no. 12. Corresponding amplicons of different MRRs are as follows: (1) MRR-I: combination of amplicon nos 4 and 7; (2) MRR-II: combination of amplicon nos 4, 11, 17 and 6; (3) MRR-III: combination of amplicon nos 4, 10, 19 and 6; (4) MRR-IV: combination of amplicon nos 4, 10, 18 and 5; (5) MRR-V: combination of amplicon nos 1, 8, 18 and 5; (6) MRR-VI: combination of amplicon nos 1, 8 and 7; (7) MRR-VII: combination of amplicon nos 2, 11, 9(1) and 5; (8) MRR-VIII: combination of amplicon nos 2, 19, 9(1) and 7; (9) MRR-IX: combination of amplicon nos 3, 17, 9(1) and 7; (10) MRR-X: the combination of amplicon nos 3, 17, 9(2), 7 or 3, 17, 13, 8 and 7; (11) MRR-XI: combination of amplicon nos 2, 19, 9(2), 7 or 2, 19, 13, 8 and 7. Amplicon nos 9(1) and 9(2) were generated using the primers P5 and P8.
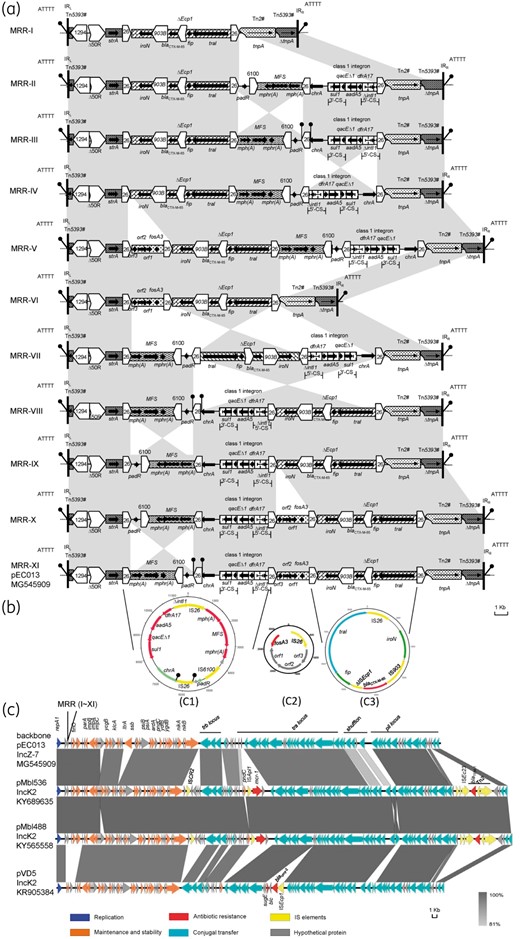
Comparison of pEC013 and IncK2 plasmids, and schematic representations of the circular forms derived from IS26-flanked transposons and different mosaic combinations of MRR from pEC013. (a) Comparison of different combinations (types I–XI) of MRR found in pEC013. ORFs and their direction of transcription are depicted using arrows. IS elements are pointed boxes labelled with the number/name, with Δ representing a truncated gene. Tall bars represent the 38bp inverted repeat (IR) of transposons. Filled circles on stalks are used to indicate direct repeats. #Incomplete transposon. (b) Schematic representation of the circular forms obtained from pEC013 by PCR and sequencing. (c) Sequence comparison between the backbone of pEC013 and other IncK2 plasmids, pMbl536 (KY689635), pMbl488 (KY565558) and pDV45 (KR905384). The degree of homology between different genetic loci is indicated by the degree of solid colour (bottom right corner). ORFs in the circular forms and the backbone of pEC013 are coloured according to their putative functions (bottom). This figure appears in colour in the online version of JAC and in black and white in the printed version of JAC.
Accession number
Because plasmid pEC013 had heterogeneous MRRs, the complete sequence of pEC013 with MRR-XI was submitted to NCBI with the accession number MG545909, and the other heterogeneous structures of the MRRs in pEC013 are illustrated in detail (Figure 2a).
Results and discussion
Genetic characteristics of E. coli strain EC013
E. coli strain EC013, isolated from a chicken on a traditional farm in Shangdong province, China, was found to be resistant to a number of antibiotics, including ampicillin, cefotaxime and fosfomycin, but susceptible to ceftazidime, gentamicin, kanamycin, florfenicol and colistin (Table S2). MLST along with phylogenetic analysis showed that EC013 belonged to the phylogroup D-ST117. The blaCTX-M and fosA3 genes were transferred successfully and the corresponding transconjugant, named TEC013, exhibited resistance to ampicillin, cefotaxime and fosfomycin (Table S2). The conjugation frequency of pEC013 was 7.1×10–5 transconjugants per recipient.
Overall structures of pEC013
Owing to the presence of several repetitive sequences such as IS26, the complete plasmid sequence could not be obtained with Illumina sequencing. Gaps between contigs were closed by a PCR-based strategy. Interestingly, in the present study, the results showed that some contigs could connect to each other in any direction and array, leading to the emergence of heterogeneous MRRs with different rearrangements of genetic components in the same strain (Figure 2). To resolve the problems of the short-read sequences generated by Illumina and to verify the presence of heterogeneous MRRs of plasmids, long-read SMRT sequencing on the PacBio platform was used in an attempt to complete the plasmid sequences, and then a single circular contig of 118.5kb for pEC013 was obtained. The backbone of the plasmid from Illumina was almost identical to the sequence obtained with SMRT sequencing (>99.9%), containing genes responsible for replication, partition, maintenance and conjugation functions. The MRRs were inserted into the plasmid backbone region between repA1 and finO.
Upon analysis of the pEC013 plasmid, a number of interesting features were discovered. Initially, the analysis of plasmid incompatibility groups showed that the IncB/O replicon is present in the plasmid pEC013; however, the IncB/O amplicon of the original PBRT scheme does not distinguish IncB/O and IncZ of the I complex, which share considerable homology.15,17 Further analysis showed that the repAZ gene of IncZ plasmid was positive for pEC013. Additionally, the RNA molecule (RNAI and RNAII) and the complete replicon of pEC013 were respectively 95.8% and 96.2% identical to that of standard Z plasmid (M38523) at the nucleotide sequence level. The difference of 2–12 bases existed in a 117–118bp region between the amplicon of pEC013 and that of the Z plasmids group (Figure S1). Therefore, pEC013 was tentatively designated as an IncZ-7 plasmid. Until recently, the complete sequences of the IncZ-like plasmids were rare. Interestingly, a BLASTn analysis highlighted that the backbone of pEC013 was structurally similar to that of the IncK2 plasmids recovered from poultry retail meat, such as mcr-1-carrying plasmids, pMbl536 (KY689635) and pMbl488 (KY565558), and blaCMY-2-carrying plasmid pDV45 (KR905384).18,19 Previously Praszkier et al.17 showed that there was close correlation among the members (IncK, IncZ, IncB/O and IncI) of the I complex group. Taken together, these results suggest that backbones of IncK2 plasmids and the IncZ-7 plasmid pEC013 may have a common ancestor, originating from poultry, with the potential for dissemination into different strains found along the food chain.
Heterogeneous MRRs of pEC013
Based on contigs from Illumina, various mosaic combinations of MRRs for pEC013 were obtained (n=11, termed types I–XI) using PCR amplification and sequencing. The various mosaic MRRs are four active IS26-flanked composite transposon-mediated rearrangements and deletions (Figure 2a). Subsequently, we retrieved the long-reads from the PacBio platform, harbouring the IS26-flanked transposon fragments, and the BLASTn comparative analysis indicated that there was only one arrangement of MRR for pEC013, corresponding to MRR-X in Figure 2a. The discrepancy between mosaic combinations from Illumina short-reads plus PCR and the single arrangement from PacBio long-reads for the same plasmid was in fact incongruent. It was possible that the single MRR detected through the PacBio platform may be the dominant type of different MRR structures of a plasmid, and occurrence of other MRR structures may be too low to be detected. Previously, two translocatable units (16.3 and 23.7kb in size) in a blaCTX-M-15-carrying ST131 E. coli were detected by SMRT sequencing;12 however, different mosaic combinations of MRRs interspersed with copies of IS26 were not detected. Similarly, variation in the copy number of ISCR1-blaNDM-1 and ISCR1-qnrB6 was detected in a single plasmid molecule by Illumina/Roche 454 and MinION nanopore long-read methods, respectively.20,21 Taken together, we showed that heterogeneous MRRs could be driven by ISs in a single plasmid, suggesting that the DNA sequence of a plasmid may be dynamic in an individual strain.
The MRR bounded by Tn5393 was interspersed with a number of different mobile elements including IS1294, ΔIS50R, IS6100, IS903B, ΔISEcp1 and ΔTn2, and the hypervariable region in this MRR consisted of five IS26 elements flanking four different segments located between strA and ΔTn2. The first segment, IS26-mph(A)-mrx-mphR(A)-IS6100-padR-IS26, and the second segment, IS26-chrA-sul1-qacEΔ1-aadA5-dfrA17-ΔintI1-IS26, could be connected to each other in different directions, resulting in different combinations (Figure 2a). One of these combinations, IS26-ΔintI1-dfrA17-aadA5-qacEΔ1-sul1-chrA-IS26-padR-IS6100-mphR(A)-mrx-mph(A)-IS26, was identical to that of the plasmid pKP1814-3 found in Klebsiella pneumoniae isolated from an outpatient in a secondary hospital in mid-south China,22 except for the simple insertion of IS26 into the usual chrA-padR structure. The third segment was a typical fosA3 context, IS26-fosA3-orf1-orf2-orf3-IS26, with the spacers between the IS26 element and the upstream and downstream fosA3 regions found to be 252 and 1758bp, respectively, and has repeatedly been located in varying regions on a number of plasmids or chromosomes of different bacterial species.10,23,24 The fourth segment, traI-fip-ΔISEcp1-blaCTX-M-65-IS903B-iroN, was found to have 100% nucleotide identity to the corresponding segment in the plasmid pKC396 (HM138653) from an E. coli strain collected in a German university hospital.25
Three corresponding circular forms (termed type ‘C1’ composed of the first two segments, ‘C2’ derived from the third segment and ‘C3’ derived from the fourth segment) of the four IS26-flanked transposons described above were amplified from the pEC013 plasmid by reverse PCR; however, nucleotide sequence analysis showed only one copy of IS26 was detected at the two ends of the transposons for each circular form (Figure 2b). Similarly, Harmer and Hall26 recently described the mobility of IS26 in detail and demonstrated that intermolecular or intramolecular replicative transposition generates circular molecules containing one copy of IS26 with an adjacent DNA segment carrying an antibiotic resistance (or other) gene, which they designated as a ‘translocatable unit’. From these observations, it can be determined how IS26 builds transposons and brings additional transposons into resistance regions. Moreover, the amplicons were generated using the primers P5 and P8 (Figure 1b). The sequence of the larger amplicon (5.8kb) showed that the second segment was located between the first and fourth segments, which exactly coincided with the combination of PCR products 8 and 13 (Figure 1).
Characterization of target site duplication (TSD) flanking the insertion site helped to trace the replicative transposition of IS26 and the form of DNA rearrangements in the plasmids.27 The sequence analysis of all the MRR structures of pEC013 showed that there is only one simple insertion in chrA-padR of the MRR-III, MRR-VIII and MRR-XI structures of pEC013, generating both 8 bp TSDs (ACTAATGC) (Figure 2a, Table S3), which may account for the activity of the first two segments in pEC013 (Figure 2a). Although there were several MRR rearrangements, the 8bp TSDs of each IS26 were very stable (Table S3). Moreover, the absence of direct repeats flanking IS26 except for the chrA-IS26-padR fragment suggested that the tandem arrays of IS26-associated modules on MRR most likely occurred by a homologous recombination event, between a copy of IS26 already present on the plasmid and the IS26 copy of a circular form.
Recently, Li et al.21 reported dynamic MDR structures with various copy numbers of ISCR1-qnrB6 among the plasmidome of Salmonella. Coincidentally, in this study, a blaCTX-M-65- and fosA3-carrying plasmid IncZ-7, identified in an E. coli strain, had polymorphic MRRs containing multiple IS26-flanked composite transposons with different arrangements driven by the insertion and excision of various IS26-derived circular intermediates with a single copy of IS26. These findings were consistent with a previous study, indicating that the movement of IS26-associated resistant genes could occur via a translocatable unit including one copy of IS26.28 Furthermore, the circular DNA intermediates carrying either blaCTX-M or fosA3 in the plasmid exhibited a high degree of flexibility to generate different compositions of MRRs, contributing to the evolution of MRRs and the dissemination of resistance determinants. The different genetic structures of fosA3 and blaCTX-M have been frequently identified on various plasmids or chromosomes from a number of bacterial species, but to our knowledge, the dynamic arrays of fosA3 and blaCTX-M have not yet been reported. Importantly, the different combinations of the MRR identified in a single strain may reflect a dynamic process towards equilibrium involving the insertion, inversion and deletion of a transposable unit.
Conclusions
Novel heterogeneous MRRs with different IS26-flanked transposon arrangements in a single plasmid sample were determined by Illumina plus PCR and PacBio, which were driven by the insertion and excision of various IS26-derived circular intermediates. These findings provide important insights into the development of MRRs and the rapid dissemination of resistance genes in Enterobacteriaceae, and suggest that a plasmid may actually be a cluster of plasmids with the same backbone but with varied MRRs in single strain; further studies should be carried out to investigate the dynamic changes of heterogeneous MRRs mediated by mobile genetic elements.
Funding
This work was supported by grants from the National Natural Science Foundation of China (nos 31702295 and U1504326).
Transparency declarations
None to declare.
References
Clinical and Laboratory Standards Institute.
Author notes
Dan Dan He Shi Yu Zhao authors contributed equally to this work.



