-
PDF
- Split View
-
Views
-
Cite
Cite
A. R. McCullough, S. Parekh, J. Rathbone, C. B. Del Mar, T. C. Hoffmann, A systematic review of the public's knowledge and beliefs about antibiotic resistance, Journal of Antimicrobial Chemotherapy, Volume 71, Issue 1, January 2016, Pages 27–33, https://doi.org/10.1093/jac/dkv310
Close - Share Icon Share
Abstract
The objective of this study was to systematically review quantitative and qualitative studies on the public's knowledge and beliefs about antibiotic resistance.
We searched four databases to July 2014, with no language or study design restrictions. Two reviewers independently extracted data. We calculated the median (IQR) of the proportion of participants who agreed with each statement and synthesized qualitative data by identifying emergent themes.
Of 3537 articles screened, 54 studies (41 quantitative, 3 mixed methods and 10 qualitative) were included (55 225 participants). Most studied adults (50; 93% studies) and were conducted in Europe (23; 43%), Asia (14; 26%) or North America (12; 22%). Some participants [median 70% (IQR 50%–84%); n = 8 studies] had heard of antibiotic resistance, but most [median 88% (IQR 86%–89%); n = 2 studies] believed it referred to changes in the human body. Many believed excessive antibiotic use [median 70% (IQR 59%–77%); n = 11 studies] and not completing antibiotic courses [median 62% (IQR 47%–77%); n = 8 studies] caused resistance. Most participants nominated reducing antibiotic use [median 74% (IQR 72%–85%); n = 4 studies] and discussing antibiotic resistance with their clinician (84%, n = 1 study) as strategies to reduce resistance. Qualitative data supported these findings and additionally identified that: participants believed they were at low risk from antibiotic resistance participants; largely attributed its development to the actions of others; and strategies to minimize resistance should be primarily aimed at clinicians.
The public have an incomplete understanding of antibiotic resistance and misperceptions about it and its causes and do not believe they contribute to its development. These data can be used to inform interventions to change the public's beliefs about how they can contribute to tackling this global issue.
Introduction
Antibiotic resistance is a complex problem that transcends international boundaries. It is predicted to kill 10 million people globally each year and cost the global economy US$100 trillion by 2050.1 Antibiotic use drives resistance2,3 and global rates of antibiotic use4 and resistance5 continue to rise. However, antibiotic resistance is reversible; resistance in the commensals of individuals who are treated with antibiotics reduces exponentially up to 1 year after the end of antibiotic treatment.2 Effective actions by all sectors of society to minimize antibiotic resistance through reduced antibiotic use are urgently required, including global taskforces,1,6 governments,7–9 researchers,10,11 industry,11 clinicians and the public.12,13
More than 70% of people visiting a primary care practitioner in the USA with an acute respiratory infection receive antibiotics.14 Systematic reviews demonstrate that antibiotics can provide only minor benefit for these infections,15–21 yet half of patients who attend their family physician with an acute respiratory tract infection expect an antibiotic.22 Public health campaigns in various countries have sought to dispel the myth that antibiotics are necessary for acute respiratory tract infections23 and several have incorporated messages about antibiotic resistance.24,25 To the best of our knowledge, studies of the public's knowledge and beliefs about antibiotic resistance have not been synthesized across studies or settings and whether people are aware of its reversibility is not known. Analysis of such studies may help to inform future interventions. In this review, we sought to synthesize qualitative and quantitative studies of the public's knowledge and beliefs about antibiotic resistance.
Methods
Protocol and registration
The review protocol was registered on the PROSPERO database (CRD42013005029).
Eligibility criteria
We searched for primary studies of any study design, in any setting and published in any language that measured people's knowledge and beliefs about antibiotic resistance. Studies did not need to meet any predetermined quality criteria to be eligible. Studies that only included clinicians, did not measure knowledge or beliefs about antibiotic resistance or were published in abstract-only form were excluded.
Search and information sources
We searched MEDLINE, EMBASE, PsycINFO and CINAHL from inception until the third week of July 2014. A MEDLINE search strategy was developed and adapted for other databases (Table S1, available as Supplementary data at JAC Online). Search terms also identified studies of clinicians' beliefs about antibiotic resistance; these studies have been reported separately.26 We conducted forward and backward citation searching for all included articles using Web of Science and Scopus. We attempted to identify unpublished studies by contacting authors of articles available in abstract-only form and key researchers in the field.
Study selection
Two reviewers (A. R. M. and J. R. or S. P. and J. R.) independently screened titles and abstracts, followed by full texts of relevant articles. A third reviewer resolved any disagreements (C. B. D. M. or T. C. H.).
Data extraction
Two reviewers (A. R. M. and J. R. or S. P. and J. R.) independently extracted data from included studies. We contacted the author of one (Bulgarian) paper for an English translation.27 We extracted data on study design and participants (Supplementary Data).
To assess survey quality, we extracted the survey method, sampling method, response rate, sample size and description of participants.28 For qualitative articles, we extracted the data collection method, sampling method, type of data analysis, the number of people who analysed the study transcripts and whether findings were validated by participants.29 For knowledge and beliefs about antibiotic resistance outcomes, we extracted data from relevant fixed responses and the percentage of participants who responded affirmatively to the provided statement (yes or strongly agree/agree).
Where appropriate, we collapsed positive Likert responses (e.g. strongly agree and agree) into a single response and calculated the mean percentage of responses across the categories. For intervention studies, we extracted either the pre-intervention data (non-randomized studies) or the post-intervention control group data (randomized studies). One reviewer (A. R. M.) extracted verbatim free-text responses and qualitative outcomes. These included both direct quotes and themes identified by study authors. We treated quotes, themes and free-text data as similar and coded each, line by line, into NVivo®.
Synthesis of results and summary measures
Figure 1 summarizes the methods used for data synthesis. We synthesized quantitative data by grouping similar fixed responses into categories. We calculated the median, IQR and range of participants who agreed with that category. We explored heterogeneity within each category by: publication year (<2011 and ≥2011; 2010 was chosen as the cut-off point because it was the median publication year of included studies); continent (Europe, North America and Asia); and type of participant (parents versus non-parents).
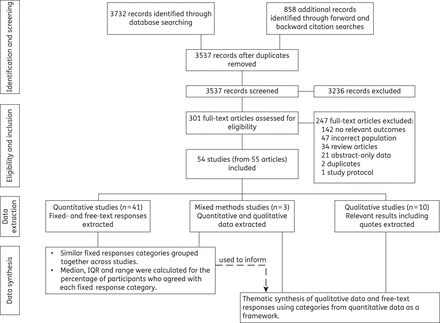
Data were arranged into four overarching categories that emerged from the data: knowledge of antibiotic resistance, beliefs about the importance and causes of antibiotic resistance and strategies to reduce it.
A thematic synthesis was undertaken in four stages as previously reported.26,30 Briefly, data were coded into themes and grouped into the four overarching categories above. Each theme was summarized and given an identifier code (e.g. I1–4 for beliefs about importance themes one to four). Content of qualitative and qualitative themes were cross-referenced to identify common findings.
Results
We identified 54 studies, which involved a total of 55 225 participants (Figure 1). Figure 2 and Supplementary Data show the study characteristics. They were mainly surveys (74%); 54% had high and 34% had moderate response rates. Most studies were conducted in Europe (43%), Asia (26%) or North America (22%) (Figure 2 and Supplementary Data). In nearly all studies (50; 93%) participants were adults, some of which (11) specifically involved parents of children. Half (27; 50%) of included studies were published between 2010 and 2014.
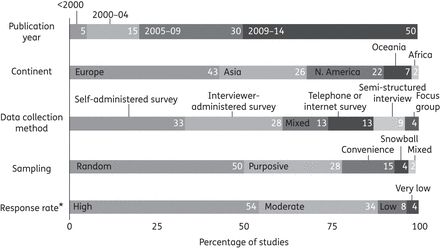
Summary of characteristics of included studies. Numbers denote percentage of studies. *Based on n = 24 studies with data: high = 75%–100% study response rate; moderate = 50%–74% study response rate; low = 25%–49% study response rate; and very low = 0%–24% study response rate.
Knowledge and beliefs about antibiotic resistance
Quantitative data
Across the eight studies that measured awareness of antibiotic resistance, a median of 70% of participants had heard of the term antibiotic resistance. Most conceptualized resistance as some change caused by antibiotics in the person to make the antibiotic ineffective (median 88%, across two studies). A median of 68% of participants, in the seven studies asking about this, believed bacteria were becoming harder to treat with antibiotics and a median of 53% of participants in four studies believed antibiotic resistance was a problem for their country (Figure 3) (full supporting data are in Supplementary Data and five additional statements are in Supplementary Data).
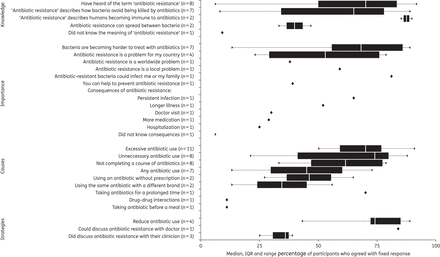
Synthesis of quantitative data on public knowledge and beliefs about antibiotic resistance.
Most believed antibiotic resistance was due to excessive (median 70%, in 11 studies) or unnecessary (median 74%, in 8 studies) antibiotic use and not completing an antibiotic course (median 62%, in 8 studies). Less than half (median 45%, across seven studies) believed that resistance could be caused by the use of any antibiotic.
Of the few (seven) studies asking about strategies to minimize resistance, a median of 74% of participants in four studies nominated reducing antibiotic use and 84% in one study nominated discussing antibiotic resistance with their clinician. However, only a median of 36% of participants in three studies indicated they had discussed resistance with their clinician.
Heterogeneity within each category could not be explained by publication year, continent where the study took place or type of participants questioned.
Qualitative data
Findings from 13 qualitative and mixed methods studies generally supported the quantitative findings (Figure 4 and Supplementary Data). Participants exhibited low awareness of antibiotic resistance (theme K1) and consistently conceived of antibiotic resistance as a change in the human body rather than in bacteria (K2).
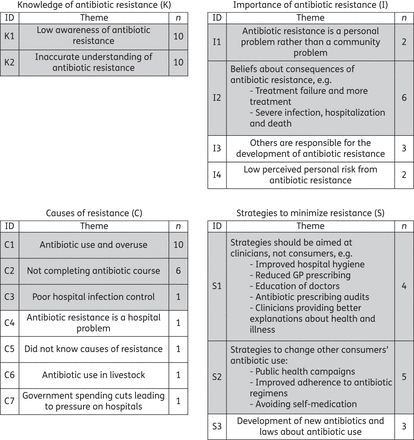
Summary of qualitative synthesis of knowledge and beliefs about antibiotic resistance. ID = theme identifier code; n = number of studies exploring each theme. Grey shading indicates theme was present in both quantitative and qualitative data. GP, general practitioner.
They believed others were largely responsible for the development of antibiotic resistance (I3); perceived they had a low personal risk from resistance (I4); thought their risk increased if they were hospitalized or used prolonged courses of antibiotics (I4); and believed causes of resistance included antibiotic use and overuse (C1) and not completing an antibiotic course (C2). However, they believed that minimizing antibiotic resistance was outside their control and strategies should be aimed at clinicians (S1) or others' antibiotic use (S2).
Discussion
This systematic review of quantitative and qualitative studies demonstrates that the public have an incomplete understanding of and misperceptions about antibiotic resistance. Many participants also believed: they do not contribute to the development of resistance and attributed it to the actions of others; and they are at low risk from antibiotic resistance themselves. They believe the main causes of resistance are using too many antibiotics and not completing a course and that strategies to minimize resistance should largely be aimed at clinicians and other patients.
This review has a number of strengths. It used a robust search strategy and included studies of any design published in any language. Synthesis of qualitative and quantitative studies enabled more in-depth explanations of knowledge and beliefs about antibiotic resistance than would have been achieved using only quantitative data and demonstrated that findings across both qualitative and quantitative studies were similar.
This review also had a number of limitations. Of the studies for which it was possible to report a response rate (n = 24), the majority had a high or moderate response rate. However, many studies did not report a response rate and so the level of non-response bias may be underestimated. Much of the qualitative data were contributed by two key studies,31,32 whose primary aims were to explore beliefs about antibiotic resistance. Exploring knowledge and beliefs about antibiotic resistance were secondary aims in other included qualitative studies. Included studies used different data collection and analysis processes, which may also have affected data quality and subsequent analysis. We did not exclude studies based on quality and this may have affected the findings. Synthesizing the quantitative data by meta-analysis was precluded by heterogeneity in both study design and the questions posed.
We are not aware of any other systematic reviews of the public's knowledge and beliefs about antibiotic resistance. We recently performed a systematic review on clinicians' knowledge and beliefs about antibiotic resistance and found some important similarities.26 These include: clinicians' attribution of responsibility for antibiotic resistance to the actions of others; overusing antibiotics and not completing a course of them as causes of resistance;26 and proposed strategies to reduce resistance focus on clinician behaviour rather than patient behaviour.26 It is not surprising the public feel this way, given that they do not believe the responsibility for tackling antibiotic resistance rests with them. They trust clinicians' decisions to prescribe or withhold an antibiotic.33,34 In contrast, clinicians continue to perceive that most patients expect antibiotics.35 This represents an important dissonance between the two standpoints.
The public believed that they were at low risk from resistance, although research shows that any antibiotic use can lead to resistance in individual patients.2 This finding can be explained in two ways. Firstly, the public tend to underestimate the harms of health interventions.36 Secondly, according to social cognitive theory, the larger the number of people that contribute to the development of a problem, and the more distant its consequences, the lower our perceived personal risk from it.37
The belief that antibiotic overuse and not completing a course of antibiotics causes resistance form part of typical messages distributed during public health campaigns.23 While the former is true,2,3,38 the necessity of completing a course and its potential impact on resistance by not doing so is unclear.39 Better evidence about the causes of antibiotic resistance is needed to resolve uncertainty here, particularly from studies that evaluate the optimal length of antibiotic courses for various indications and the effect of not completing a full course of antibiotics on resistance.39
Some opportunities to intervene and address misperceptions have been identified by this review. The public hold an inaccurate understanding of what antibiotic resistance is and no studies explored if people know that antibiotic resistance is reversible. At a population level, public health campaigns could address these misperceptions by: providing information about how bacteria develop resistance; emphasizing that individual antibiotic use increases individuals' risk from resistance; and also by highlighting that resistance is reversible if antibiotic use is minimized. At the individual level, clinicians could use effective strategies such as shared decision making40 (a process in which patients receive balanced, evidence-based information about benefits and harms of treatment41,42) to alert people to their actual risk of antibiotic resistance following antibiotic use and provide information on how quickly this resistance could be reversed. Current studies have not specifically asked the public about which strategies they would use to minimize resistance, but rather asked more generally what they thought would be effective.32 Input from all stakeholders is needed when designing new interventions to minimize resistance.43
Conclusions
The public have heard of antibiotic resistance, but have an incomplete understanding of and misperceptions about it and its causes and do not believe they contribute to its development. These data can be used to inform the design of interventions that seek to change public beliefs about how they can contribute to tackling this global issue.
Funding
This work was supported by the Centre for Research Excellence in Minimising Antibiotic Resistance from Acute Respiratory Infections funded by the National Health and Medical Research Council (grant number 1044904) and the National Health and Medical Research Council/Primary Health Care Research, Evaluation and Development Career Development Fellowship, with funding provided by the Australian Government Department of Health (grant number 1033038) to T. C. H.
Transparency declarations
T. C. H. received royalties from Elsevier and C. B. D. M. received royalties from BMJ Books and Elsevier, for activities unrelated to the submitted work. All authors: nothing further to declare.
Author contributions
All authors contributed to the study design, drafted the manuscript, gave final approval of the version to be published and are accountable for all aspects of the work. A. R. M., S. P. and J. R. extracted the data. A. R. M. analysed the data and designed figures and tables. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. The corresponding author had final responsibility for the decision to submit for publication.
Data sharing
The full dataset is available from the corresponding author at [email protected].
Acknowledgements
Sarah Thorning, BSc, Grad Dip ILS, Centre for Research in Evidence-Based Practice, Bond University, provided assistance with designing and conducting the searches of the electronic databases and was not compensated beyond her salary for her contribution.
References



