-
PDF
- Split View
-
Views
-
Cite
Cite
Sushmita D. Lahiri, Richard A. Alm, Potential of Staphylococcus aureus isolates carrying different PBP2a alleles to develop resistance to ceftaroline, Journal of Antimicrobial Chemotherapy, Volume 71, Issue 1, January 2016, Pages 34–40, https://doi.org/10.1093/jac/dkv329
Close - Share Icon Share
Abstract
Infections caused by MRSA continue to cause significant morbidity worldwide. Ceftaroline (the active metabolite of the prodrug ceftaroline fosamil) is a cephalosporin that possesses activity against MRSA due to its having high affinity for PBP2a while maintaining activity against the other essential PBPs. PBP2a sequence variations, including some outside of the transpeptidase binding pocket, impact ceftaroline susceptibility. This study evaluated the potential of ceftaroline to select for resistant Staphylococcus aureus clones in isolates containing a variety of PBP2a alleles and with a range of ceftaroline MIC values from different MLST lineages.
Direct resistance selection experiments were performed by plating 20 S. aureus isolates (18 MRSA and 2 MSSA) on agar plates containing increasing concentrations of ceftaroline. Colonies that emerged were tested by standard broth microdilution for changes in ceftaroline susceptibility and genetically characterized.
The frequency of spontaneous resistance to ceftaroline was low for all isolates and, although resistant variants were not obtained on plates containing ≥4-fold the MIC of ceftaroline, six MRSA isolates had a small number of colonies emerge on plates containing 2-fold the MIC of ceftaroline and had a 2- to 8-fold elevation of the ceftaroline MIC, while also impacting the MIC of methicillin compared with the parental isolate. Additional PBP2a mutations located in the ceftaroline-binding pocket, Y446N or A601S, were observed in several of the resistant isolates.
These studies demonstrate that there is a low risk of generating ceftaroline-resistant MRSA isolates, which appears independent of any pre-existing variation in the PBP2a protein sequence or initial ceftaroline MIC.
Introduction
MRSA remains a significant hospital pathogen and can cause a wide range of diseases including skin infections, bacteraemia, wound infections and abscesses as well as pneumonia. Methicillin resistance in Staphylococcus aureus is primarily mediated by the horizontal acquisition of the staphylococcal cassette chromosome (SCC), which encodes an alternative PBP (MecA or PBP2a), which can functionally complement the transpeptidation reaction and allows cell wall biogenesis to proceed in the presence of most β-lactam drugs.1 The distinguishing feature of ceftaroline, the active metabolite of the prodrug ceftaroline fosamil, is the robust activity against MRSA isolates due to its having a high affinity for PBP2a while also maintaining high affinity to other essential S. aureus PBPs.2–4
Several studies have described the characterization of MRSA clinical isolates that are resistant to ceftaroline and have substitutions in PBP2a.5–9 These substitutions are located in the primary ceftaroline-binding pocket in the transpeptidase active site [penicillin-binding domain (PBD)] and give rise to higher-level resistance. Additionally, PBP2a substitutions can be found in a groove in the non-PBD (nPBD) and have been linked to small decreases in ceftaroline susceptibility.5,6,8 This region has been hypothesized to be an important protein–protein interface that interacts with other PBP partners during cell wall biosynthesis,5 and has also been shown to crystallize with cell-wall fragments and a second ceftaroline molecule and to be implicated in the allosteric control of the transpeptidase binding pocket.10 These nPBD variations pre-date the commercial release of ceftaroline and the ceftaroline MIC distribution of MRSA isolates with these nPBD variations overlaps significantly with isolates expressing PBP2a proteins without these substitutions.9 Further, recent molecular analyses suggest that PBP2a sequence-independent factors also may play a role in subtle variations in ceftaroline susceptibility.9
This study was undertaken to evaluate the potential of ceftaroline to select for resistant clones of S. aureus in strains carrying different SCCmec types and having different PBP2a alleles.
Materials and methods
Bacterial strains
The S. aureus isolates (18 MRSA and 2 MSSA) used in this study and the countries of their isolation are shown in Table 1. The S. aureus USA300 isolate was obtained from the Network on Antimicrobial Resistance in Staphylococcus aureus (NRS384) and ATCC 29213 was obtained from the ATCC. The 2010 clinical isolates (ARC prefix) were obtained from JMI Laboratories (North Liberty, IA, USA) whereas the 2012 clinical isolates (TRN prefix) were obtained from International Health Management Associates (Schaumburg, IL, USA). The SA27 isolate was obtained from LSI (Westlake, OH, USA).
Genotypes and frequencies of resistance to ceftaroline for S. aureus isolates
| Strain . | Source . | Year . | Molecular type . | PBP2a protein . | Ceftaroline MIC (mg/L) . | Frequency of resistance . | |||
|---|---|---|---|---|---|---|---|---|---|
| broth . | agar . | 2 × MICa . | 4 × MICa . | 8 × MICa . | |||||
| ATCC 29213b | USA | NA | ST5 | NA | 0.25 | 0.25 | <5.9 × 10−10 | <5.9 × 10−10 | <5.9 × 10−10 |
| USA300 | USA | NA | ST8-MRSA-IV | WTc | 1 | 0.5 | 4.60 × 10−10 | <4.6 × 10−10 | <4.6 × 10−10 |
| ARC3823 | Spain | 2010 | newd-MRSA-IV | WT | 0.5 | 0.5 | <4.2 × 10−10 | <4.2 × 10−10 | <4.2 × 10−10 |
| ARC3824 | Spain | 2010 | ST228-MRSA-I | E239Ke, E447Kf | 8 | 4 | 1.8 × 10−9 | <6.0 × 10−10 | <6.0 × 10−10 |
| ARC3827 | Thailand | 2010 | ST228-MRSA-I | E239Ke | 2 | 1 | 1.1 × 10−8 | <7.6 × 10−10 | <7.6 × 10−10 |
| ARC3828 | Thailand | 2010 | ST228-MRSA-I | E239Ke, E447Kf | 8 | 4 | <6.1 × 10−10 | <6.1 × 10−10 | <6.1 × 10−10 |
| ARC3830 | Thailand | 2010 | ST228-MRSA-I | E239Ke, E447Kf | 8 | 4 | <7.3 × 10−10 | <7.3 × 10−10 | <7.3 × 10−10 |
| ARC3832b | Thailand | 2010 | newg | NA | 0.25 | 0.25 | <7.3 × 10−11 | <7.3 × 10−11 | <7.3 × 10−11 |
| TRN5426 | Portugal | 2012 | ST22-MRSA-IV | WT | 2 | 1 | 7.10 × 10−10 | <2.4 × 10−10 | <2.4 × 10−10 |
| TRN5467 | South Korea | 2012 | ST5-MRSA-II | N146Ke, L357If, I563Tf | 4 | 4 | 2.20 × 10−10 | <2.2 × 10−10 | <2.2 × 10−10 |
| TRN5474 | Taiwan | 2012 | ST228-MRSA-I | N236Ke | 2 | 2 | <5.9 × 10−10 | <5.9 × 10−10 | <5.9 × 10−10 |
| TRN5472 | Italy | 2012 | ST228-MRSA-I | WT | 2 | 2 | <5.3 × 10−10 | <5.3 × 10−10 | <5.3 × 10−10 |
| TRN5427 | Greece | 2012 | ST36-MRSA-II | WT | 2 | 2 | <2.7 × 10−10 | <2.7 × 10−10 | <2.7 × 10−10 |
| TRN5549 | Portugal | 2012 | ST22-MRSA-IV | E150Ke | 2 | 2 | 2.0 × 10−10 | <2.0 × 10−10 | <2.0 × 10−10 |
| TRN5536 | Turkey | 2012 | ST239-MRSA-III | WT | 2 | 2 | <3.3 × 10−10 | <3.3 × 10−10 | <3.3 × 10−10 |
| TRN5545 | Turkey | 2012 | ST239-MRSA-III | N146Ke | 2 | 2 | <2.6 × 10−10 | <2.6 × 10−10 | <2.6 × 10−10 |
| TRN5418 | Chile | 2012 | ST5-MRSA-I | M122Ie, E150Ke | 2 | 2 | <3.5 × 10−10 | <3.5 × 10−10 | <3.5 × 10−10 |
| TRN5572 | Italy | 2012 | ST5-MRSA-II | WT | 2 | 2 | <4.8 × 10−10 | <4.8 × 10−10 | <4.8 × 10−10 |
| TRN5350 | USA | 2009 | ST8-MRSA-II | N236Ke | 2 | 1 | <4.2 × 10−10 | <4.2 × 10−10 | <4.2 × 10−10 |
| TRN5420 | Hungary | 2012 | ST239-MRSA-III | E239Ke | 2 | 2 | <2.5 × 10−10 | <2.5 × 10−10 | <2.5 × 10−10 |
| Strain . | Source . | Year . | Molecular type . | PBP2a protein . | Ceftaroline MIC (mg/L) . | Frequency of resistance . | |||
|---|---|---|---|---|---|---|---|---|---|
| broth . | agar . | 2 × MICa . | 4 × MICa . | 8 × MICa . | |||||
| ATCC 29213b | USA | NA | ST5 | NA | 0.25 | 0.25 | <5.9 × 10−10 | <5.9 × 10−10 | <5.9 × 10−10 |
| USA300 | USA | NA | ST8-MRSA-IV | WTc | 1 | 0.5 | 4.60 × 10−10 | <4.6 × 10−10 | <4.6 × 10−10 |
| ARC3823 | Spain | 2010 | newd-MRSA-IV | WT | 0.5 | 0.5 | <4.2 × 10−10 | <4.2 × 10−10 | <4.2 × 10−10 |
| ARC3824 | Spain | 2010 | ST228-MRSA-I | E239Ke, E447Kf | 8 | 4 | 1.8 × 10−9 | <6.0 × 10−10 | <6.0 × 10−10 |
| ARC3827 | Thailand | 2010 | ST228-MRSA-I | E239Ke | 2 | 1 | 1.1 × 10−8 | <7.6 × 10−10 | <7.6 × 10−10 |
| ARC3828 | Thailand | 2010 | ST228-MRSA-I | E239Ke, E447Kf | 8 | 4 | <6.1 × 10−10 | <6.1 × 10−10 | <6.1 × 10−10 |
| ARC3830 | Thailand | 2010 | ST228-MRSA-I | E239Ke, E447Kf | 8 | 4 | <7.3 × 10−10 | <7.3 × 10−10 | <7.3 × 10−10 |
| ARC3832b | Thailand | 2010 | newg | NA | 0.25 | 0.25 | <7.3 × 10−11 | <7.3 × 10−11 | <7.3 × 10−11 |
| TRN5426 | Portugal | 2012 | ST22-MRSA-IV | WT | 2 | 1 | 7.10 × 10−10 | <2.4 × 10−10 | <2.4 × 10−10 |
| TRN5467 | South Korea | 2012 | ST5-MRSA-II | N146Ke, L357If, I563Tf | 4 | 4 | 2.20 × 10−10 | <2.2 × 10−10 | <2.2 × 10−10 |
| TRN5474 | Taiwan | 2012 | ST228-MRSA-I | N236Ke | 2 | 2 | <5.9 × 10−10 | <5.9 × 10−10 | <5.9 × 10−10 |
| TRN5472 | Italy | 2012 | ST228-MRSA-I | WT | 2 | 2 | <5.3 × 10−10 | <5.3 × 10−10 | <5.3 × 10−10 |
| TRN5427 | Greece | 2012 | ST36-MRSA-II | WT | 2 | 2 | <2.7 × 10−10 | <2.7 × 10−10 | <2.7 × 10−10 |
| TRN5549 | Portugal | 2012 | ST22-MRSA-IV | E150Ke | 2 | 2 | 2.0 × 10−10 | <2.0 × 10−10 | <2.0 × 10−10 |
| TRN5536 | Turkey | 2012 | ST239-MRSA-III | WT | 2 | 2 | <3.3 × 10−10 | <3.3 × 10−10 | <3.3 × 10−10 |
| TRN5545 | Turkey | 2012 | ST239-MRSA-III | N146Ke | 2 | 2 | <2.6 × 10−10 | <2.6 × 10−10 | <2.6 × 10−10 |
| TRN5418 | Chile | 2012 | ST5-MRSA-I | M122Ie, E150Ke | 2 | 2 | <3.5 × 10−10 | <3.5 × 10−10 | <3.5 × 10−10 |
| TRN5572 | Italy | 2012 | ST5-MRSA-II | WT | 2 | 2 | <4.8 × 10−10 | <4.8 × 10−10 | <4.8 × 10−10 |
| TRN5350 | USA | 2009 | ST8-MRSA-II | N236Ke | 2 | 1 | <4.2 × 10−10 | <4.2 × 10−10 | <4.2 × 10−10 |
| TRN5420 | Hungary | 2012 | ST239-MRSA-III | E239Ke | 2 | 2 | <2.5 × 10−10 | <2.5 × 10−10 | <2.5 × 10−10 |
NA, not applicable.
aMIC is the agar-dilution MIC of ceftaroline.
bThese are methicillin-susceptible isolates and do not carry a SCCmec cassette or a PBP2a protein.
cA WT PBP2a sequence is defined as that carried by the vast majority of MRSA isolates as exemplified by strains USA300 and N315 (GenBank identifiers ADV68980 and BAB41256, respectively).9
dThis strain has a novel MLST type due to novel arcC and aroE alleles and the allelic combination is: novel, novel, 1, 1, 12, 19, 3.
eThis substitution is located in the nPBD.
fThis substitution is located in the PBD, although only E447K lies in the transpeptidase binding pocket.9
gThis strain has a novel MLST type due to novel pta allele, but the allelic combination (2, 2, 2, 2, novel, 3, 2) is ST30-like.
Genotypes and frequencies of resistance to ceftaroline for S. aureus isolates
| Strain . | Source . | Year . | Molecular type . | PBP2a protein . | Ceftaroline MIC (mg/L) . | Frequency of resistance . | |||
|---|---|---|---|---|---|---|---|---|---|
| broth . | agar . | 2 × MICa . | 4 × MICa . | 8 × MICa . | |||||
| ATCC 29213b | USA | NA | ST5 | NA | 0.25 | 0.25 | <5.9 × 10−10 | <5.9 × 10−10 | <5.9 × 10−10 |
| USA300 | USA | NA | ST8-MRSA-IV | WTc | 1 | 0.5 | 4.60 × 10−10 | <4.6 × 10−10 | <4.6 × 10−10 |
| ARC3823 | Spain | 2010 | newd-MRSA-IV | WT | 0.5 | 0.5 | <4.2 × 10−10 | <4.2 × 10−10 | <4.2 × 10−10 |
| ARC3824 | Spain | 2010 | ST228-MRSA-I | E239Ke, E447Kf | 8 | 4 | 1.8 × 10−9 | <6.0 × 10−10 | <6.0 × 10−10 |
| ARC3827 | Thailand | 2010 | ST228-MRSA-I | E239Ke | 2 | 1 | 1.1 × 10−8 | <7.6 × 10−10 | <7.6 × 10−10 |
| ARC3828 | Thailand | 2010 | ST228-MRSA-I | E239Ke, E447Kf | 8 | 4 | <6.1 × 10−10 | <6.1 × 10−10 | <6.1 × 10−10 |
| ARC3830 | Thailand | 2010 | ST228-MRSA-I | E239Ke, E447Kf | 8 | 4 | <7.3 × 10−10 | <7.3 × 10−10 | <7.3 × 10−10 |
| ARC3832b | Thailand | 2010 | newg | NA | 0.25 | 0.25 | <7.3 × 10−11 | <7.3 × 10−11 | <7.3 × 10−11 |
| TRN5426 | Portugal | 2012 | ST22-MRSA-IV | WT | 2 | 1 | 7.10 × 10−10 | <2.4 × 10−10 | <2.4 × 10−10 |
| TRN5467 | South Korea | 2012 | ST5-MRSA-II | N146Ke, L357If, I563Tf | 4 | 4 | 2.20 × 10−10 | <2.2 × 10−10 | <2.2 × 10−10 |
| TRN5474 | Taiwan | 2012 | ST228-MRSA-I | N236Ke | 2 | 2 | <5.9 × 10−10 | <5.9 × 10−10 | <5.9 × 10−10 |
| TRN5472 | Italy | 2012 | ST228-MRSA-I | WT | 2 | 2 | <5.3 × 10−10 | <5.3 × 10−10 | <5.3 × 10−10 |
| TRN5427 | Greece | 2012 | ST36-MRSA-II | WT | 2 | 2 | <2.7 × 10−10 | <2.7 × 10−10 | <2.7 × 10−10 |
| TRN5549 | Portugal | 2012 | ST22-MRSA-IV | E150Ke | 2 | 2 | 2.0 × 10−10 | <2.0 × 10−10 | <2.0 × 10−10 |
| TRN5536 | Turkey | 2012 | ST239-MRSA-III | WT | 2 | 2 | <3.3 × 10−10 | <3.3 × 10−10 | <3.3 × 10−10 |
| TRN5545 | Turkey | 2012 | ST239-MRSA-III | N146Ke | 2 | 2 | <2.6 × 10−10 | <2.6 × 10−10 | <2.6 × 10−10 |
| TRN5418 | Chile | 2012 | ST5-MRSA-I | M122Ie, E150Ke | 2 | 2 | <3.5 × 10−10 | <3.5 × 10−10 | <3.5 × 10−10 |
| TRN5572 | Italy | 2012 | ST5-MRSA-II | WT | 2 | 2 | <4.8 × 10−10 | <4.8 × 10−10 | <4.8 × 10−10 |
| TRN5350 | USA | 2009 | ST8-MRSA-II | N236Ke | 2 | 1 | <4.2 × 10−10 | <4.2 × 10−10 | <4.2 × 10−10 |
| TRN5420 | Hungary | 2012 | ST239-MRSA-III | E239Ke | 2 | 2 | <2.5 × 10−10 | <2.5 × 10−10 | <2.5 × 10−10 |
| Strain . | Source . | Year . | Molecular type . | PBP2a protein . | Ceftaroline MIC (mg/L) . | Frequency of resistance . | |||
|---|---|---|---|---|---|---|---|---|---|
| broth . | agar . | 2 × MICa . | 4 × MICa . | 8 × MICa . | |||||
| ATCC 29213b | USA | NA | ST5 | NA | 0.25 | 0.25 | <5.9 × 10−10 | <5.9 × 10−10 | <5.9 × 10−10 |
| USA300 | USA | NA | ST8-MRSA-IV | WTc | 1 | 0.5 | 4.60 × 10−10 | <4.6 × 10−10 | <4.6 × 10−10 |
| ARC3823 | Spain | 2010 | newd-MRSA-IV | WT | 0.5 | 0.5 | <4.2 × 10−10 | <4.2 × 10−10 | <4.2 × 10−10 |
| ARC3824 | Spain | 2010 | ST228-MRSA-I | E239Ke, E447Kf | 8 | 4 | 1.8 × 10−9 | <6.0 × 10−10 | <6.0 × 10−10 |
| ARC3827 | Thailand | 2010 | ST228-MRSA-I | E239Ke | 2 | 1 | 1.1 × 10−8 | <7.6 × 10−10 | <7.6 × 10−10 |
| ARC3828 | Thailand | 2010 | ST228-MRSA-I | E239Ke, E447Kf | 8 | 4 | <6.1 × 10−10 | <6.1 × 10−10 | <6.1 × 10−10 |
| ARC3830 | Thailand | 2010 | ST228-MRSA-I | E239Ke, E447Kf | 8 | 4 | <7.3 × 10−10 | <7.3 × 10−10 | <7.3 × 10−10 |
| ARC3832b | Thailand | 2010 | newg | NA | 0.25 | 0.25 | <7.3 × 10−11 | <7.3 × 10−11 | <7.3 × 10−11 |
| TRN5426 | Portugal | 2012 | ST22-MRSA-IV | WT | 2 | 1 | 7.10 × 10−10 | <2.4 × 10−10 | <2.4 × 10−10 |
| TRN5467 | South Korea | 2012 | ST5-MRSA-II | N146Ke, L357If, I563Tf | 4 | 4 | 2.20 × 10−10 | <2.2 × 10−10 | <2.2 × 10−10 |
| TRN5474 | Taiwan | 2012 | ST228-MRSA-I | N236Ke | 2 | 2 | <5.9 × 10−10 | <5.9 × 10−10 | <5.9 × 10−10 |
| TRN5472 | Italy | 2012 | ST228-MRSA-I | WT | 2 | 2 | <5.3 × 10−10 | <5.3 × 10−10 | <5.3 × 10−10 |
| TRN5427 | Greece | 2012 | ST36-MRSA-II | WT | 2 | 2 | <2.7 × 10−10 | <2.7 × 10−10 | <2.7 × 10−10 |
| TRN5549 | Portugal | 2012 | ST22-MRSA-IV | E150Ke | 2 | 2 | 2.0 × 10−10 | <2.0 × 10−10 | <2.0 × 10−10 |
| TRN5536 | Turkey | 2012 | ST239-MRSA-III | WT | 2 | 2 | <3.3 × 10−10 | <3.3 × 10−10 | <3.3 × 10−10 |
| TRN5545 | Turkey | 2012 | ST239-MRSA-III | N146Ke | 2 | 2 | <2.6 × 10−10 | <2.6 × 10−10 | <2.6 × 10−10 |
| TRN5418 | Chile | 2012 | ST5-MRSA-I | M122Ie, E150Ke | 2 | 2 | <3.5 × 10−10 | <3.5 × 10−10 | <3.5 × 10−10 |
| TRN5572 | Italy | 2012 | ST5-MRSA-II | WT | 2 | 2 | <4.8 × 10−10 | <4.8 × 10−10 | <4.8 × 10−10 |
| TRN5350 | USA | 2009 | ST8-MRSA-II | N236Ke | 2 | 1 | <4.2 × 10−10 | <4.2 × 10−10 | <4.2 × 10−10 |
| TRN5420 | Hungary | 2012 | ST239-MRSA-III | E239Ke | 2 | 2 | <2.5 × 10−10 | <2.5 × 10−10 | <2.5 × 10−10 |
NA, not applicable.
aMIC is the agar-dilution MIC of ceftaroline.
bThese are methicillin-susceptible isolates and do not carry a SCCmec cassette or a PBP2a protein.
cA WT PBP2a sequence is defined as that carried by the vast majority of MRSA isolates as exemplified by strains USA300 and N315 (GenBank identifiers ADV68980 and BAB41256, respectively).9
dThis strain has a novel MLST type due to novel arcC and aroE alleles and the allelic combination is: novel, novel, 1, 1, 12, 19, 3.
eThis substitution is located in the nPBD.
fThis substitution is located in the PBD, although only E447K lies in the transpeptidase binding pocket.9
gThis strain has a novel MLST type due to novel pta allele, but the allelic combination (2, 2, 2, 2, novel, 3, 2) is ST30-like.
Antimicrobial susceptibility testing
The MIC for each isolate was determined using the broth microdilution or agar dilution method following guidelines of document M07-A9 of the CLSI.11 All compounds were tested in accordance with CLSI recommendations. S. aureus ATCC 29213 was used as the quality control isolate. Ceftaroline was obtained from Cerexa, Inc. (Oakland, CA, USA) and all other reference compounds used were obtained from US Pharmacopeial Convention (Rockville, MD, USA).
Frequency of spontaneous resistance emergence
Microorganisms were harvested from 24 h blood agar plates and suspended in Mueller–Hinton 2 broth (MHB2) (Sigma-Aldrich, St Louis, MO, USA) to an OD at 600 nm (OD600) of ∼3.2, which corresponds to a density of ∼109–1010 cfu/mL. A dilution series of this suspension was plated to determine the number of cfu in the original suspension. In addition, 100 μL volumes of the suspension were spread evenly in triplicate onto agar plates containing ceftaroline at 2-fold increasing concentrations relative to the agar dilution MIC value, although the inoculum used is significantly higher than the 104 cfu/spot that is employed in the CLSI-validated agar dilution MIC method. The plates were incubated for 24–48 h at 36°C in ambient air. The numbers of colonies growing on plates at multiples of the agar dilution MIC were counted and the frequency of spontaneous resistance was calculated. If no colonies were observed on the ceftaroline-containing agar plates for a particular concentration, the frequency of mutational resistance was expressed as less than the frequency of obtaining one resistant variant. Representative variants were then passaged on drug-free media and tested for changes in MIC by the broth microdilution method.
WGS and analysis
Genomic DNA purified on the Maxwell 16 platform (Promega, Madison, WI, USA) and quantified using the Qubit fluorometer (Invitrogen Life Technologies, Grand Island, NY, USA) was used as input material for library construction. DNA libraries were prepared using the Nextera library construction protocol (Illumina, San Diego, CA, USA) following the manufacturer's instructions and sequenced on a MiSeq Sequencer (Illumina). For each isolate, ∼2.5 million 150 bp paired-end sequence reads were de novo assembled and analysed using the CLCBio suite of software tools (Cambridge, MA, USA). The SCCmec types of the MRSA isolates were determined by comparison with reference sequences. The MLST profiles of the isolates were determined by comparison of the seven allele sequences with the curated public database available at http://saureus.mlst.net.
Molecular modelling
The structures of S. aureus PBP2a, in the apo form (PDB:1VQQ) and in complex with ceftobiprole (PDB:4DKI), ceftaroline (PDB:3ZG0) or methicillin (PDB:1MWU), and the structures of the nPBD mutant proteins (N146K, E150K and N146K/E150K) (PDB:4BL3, 4BL2 and 4CPK, respectively) were used to support the structural interpretations. The PBP2a mutations identified in this study were mapped onto the known PBP2a structure using Pymol (Schrödinger, www.pymol.org).
Results and discussion
Frequency of spontaneous resistance
Previous studies have identified changes in PBP2a that have been correlated with decreased ceftaroline susceptibility, and these can be located in either the transpeptidase pocket of the PBD or in a region of the nPBD thought to be crucial for the correct regulation and function of PBP2a.5,10 However, a recent analysis of a large number of MRSA isolates with ceftaroline MIC values of 0.5–2 mg/L has revealed that MRSA populations carrying either WT PBP2a proteins or PBP2a proteins with an nPBD variation have overlapping population distributions.9 A set of 20 S. aureus isolates (18 MRSA and 2 MSSA) with a range of SCCmec types, PBP2a variations and ceftaroline MIC values were used to evaluate the potential of ceftaroline to select for variants with reduced susceptibility. The spontaneous frequency of resistance to ceftaroline for all 20 isolates was low, with no variants being isolated at either 4-fold or 8-fold the agar dilution MIC in any strain with calculated frequencies of resistance that ranged from <7.6 × 10−10 to <7.3 × 10−11 (Table 1). Indeed, only six MRSA isolates had any variants emerge at 2-fold the agar dilution MIC (Table 1). These data strongly suggest that strains with an nPBD substitution are not predisposed to higher frequencies of resistance to ceftaroline. Therefore, even if there is an enrichment of PBP2a nPBD substitutions in MRSA isolates with ceftaroline MIC values of 2 mg/L, no greater risk exists for the emergence of a resistant variant from exposure to ceftaroline than with an MRSA strain harbouring a WT PBP2a protein. Further, inspection of the MRSA isolates carrying a WT PBP2a allele suggests that the low frequencies of ceftaroline resistance are independent of the initial MIC value.
Characterization of resistant variants
Representative mutants from the 2-fold MIC plates from the MRSA isolates were selected for characterization. The single mutant from strain USA300 and two mutants from ARC3827 had a ≤2-fold change in their ceftaroline MIC compared with the parental isolate (Table 2), and no genetic changes were identified. Given that the agar dilution MIC for these two isolates tested 2-fold lower than the broth microdilution (Table 1), it is likely that these colonies represented breakthrough growth. The resistant variants from TRN5426 and TRN5549 both had a ceftaroline MIC of 8 mg/L, which represented a 4-fold increase in the ceftaroline MIC when compared with the parent isolate (Table 2), although no changes were observed in the mecA gene. This is consistent with the notion that MecA-sequence-independent factors also can impact ceftaroline susceptibility,9 and these mutants remain under investigation.
| Strain . | PBP2a proteina . | MIC (mg/L) . | |||||
|---|---|---|---|---|---|---|---|
| CPT . | MET . | OXA . | LVX . | VAN . | LZD . | ||
| USA300 | WTb | 1 | 64 | 64 | 1 | 0.5 | 4 |
| USA300-A | WT | 1 | 32 | 32 | 1 | 0.5 | 4 |
| ARC3824 | E239K, E447K | 8 | 1024 | 512 | 16 | 1 | 2 |
| ARC3824-A | E239K, E447K, Y446N | 64 | 1024 | 512 | 16 | 1 | 4 |
| ARC3824-B | E239K, E447K, A601S | 16 | 64 | 128 | 16 | 1 | 2 |
| ARC3824-C | E239K, E447K, A601S | 16 | 64 | 256 | 16 | 1 | 2 |
| ARC3827 | E239K | 2 | 2048 | 1024 | 16 | 1 | 2 |
| ARC3827-A | E239K | 4 | >2048 | 1024 | 16 | 0.5 | 2 |
| ARC3827-B | E239K | 4 | >2048 | 1024 | 16 | 0.5 | 2 |
| TRN5426 | WT | 2 | 2048 | 1024 | >32 | 0.5 | 4 |
| TRN5426-A | WT | 8 | 1024 | 512 | >32 | 0.5 | 2 |
| TRN5467 | N146K, L357I, I563T | 4 | >2048 | >1024 | 32 | 2 | 8 |
| TRN5467-A | N146K, L357I, I563T, Y446N | 32 | >2048 | >1024 | 32 | 1 | 8 |
| TRN5467-B | N146K, L357I, I563T, Y446N | 32 | >2048 | >1024 | 32 | 2 | 4 |
| TRN5549 | E150K | 2 | 2048 | 512 | 16 | 0.5 | 4 |
| TRN5549-A | E150K | 8 | 2048 | 1024 | 16 | 0.5 | 4 |
| Strain . | PBP2a proteina . | MIC (mg/L) . | |||||
|---|---|---|---|---|---|---|---|
| CPT . | MET . | OXA . | LVX . | VAN . | LZD . | ||
| USA300 | WTb | 1 | 64 | 64 | 1 | 0.5 | 4 |
| USA300-A | WT | 1 | 32 | 32 | 1 | 0.5 | 4 |
| ARC3824 | E239K, E447K | 8 | 1024 | 512 | 16 | 1 | 2 |
| ARC3824-A | E239K, E447K, Y446N | 64 | 1024 | 512 | 16 | 1 | 4 |
| ARC3824-B | E239K, E447K, A601S | 16 | 64 | 128 | 16 | 1 | 2 |
| ARC3824-C | E239K, E447K, A601S | 16 | 64 | 256 | 16 | 1 | 2 |
| ARC3827 | E239K | 2 | 2048 | 1024 | 16 | 1 | 2 |
| ARC3827-A | E239K | 4 | >2048 | 1024 | 16 | 0.5 | 2 |
| ARC3827-B | E239K | 4 | >2048 | 1024 | 16 | 0.5 | 2 |
| TRN5426 | WT | 2 | 2048 | 1024 | >32 | 0.5 | 4 |
| TRN5426-A | WT | 8 | 1024 | 512 | >32 | 0.5 | 2 |
| TRN5467 | N146K, L357I, I563T | 4 | >2048 | >1024 | 32 | 2 | 8 |
| TRN5467-A | N146K, L357I, I563T, Y446N | 32 | >2048 | >1024 | 32 | 1 | 8 |
| TRN5467-B | N146K, L357I, I563T, Y446N | 32 | >2048 | >1024 | 32 | 2 | 4 |
| TRN5549 | E150K | 2 | 2048 | 512 | 16 | 0.5 | 4 |
| TRN5549-A | E150K | 8 | 2048 | 1024 | 16 | 0.5 | 4 |
CPT, ceftaroline; MET, methicillin; OXA, oxacillin; LVX, levofloxacin; VAN, vancomycin; LZD, linezolid.
aThe substitutions located in the nPBD are N146K, E150K and E239K. The remaining substitutions are located in the PBD, with Y446N, E447K and A601S being specifically located in the transpeptidase binding pocket.
bA WT PBP2a sequence is defined as that carried by the vast majority of MRSA isolates as exemplified by strains USA300 and N315 (GenBank identifiers ADV68980 and BAB41256, respectively).9
| Strain . | PBP2a proteina . | MIC (mg/L) . | |||||
|---|---|---|---|---|---|---|---|
| CPT . | MET . | OXA . | LVX . | VAN . | LZD . | ||
| USA300 | WTb | 1 | 64 | 64 | 1 | 0.5 | 4 |
| USA300-A | WT | 1 | 32 | 32 | 1 | 0.5 | 4 |
| ARC3824 | E239K, E447K | 8 | 1024 | 512 | 16 | 1 | 2 |
| ARC3824-A | E239K, E447K, Y446N | 64 | 1024 | 512 | 16 | 1 | 4 |
| ARC3824-B | E239K, E447K, A601S | 16 | 64 | 128 | 16 | 1 | 2 |
| ARC3824-C | E239K, E447K, A601S | 16 | 64 | 256 | 16 | 1 | 2 |
| ARC3827 | E239K | 2 | 2048 | 1024 | 16 | 1 | 2 |
| ARC3827-A | E239K | 4 | >2048 | 1024 | 16 | 0.5 | 2 |
| ARC3827-B | E239K | 4 | >2048 | 1024 | 16 | 0.5 | 2 |
| TRN5426 | WT | 2 | 2048 | 1024 | >32 | 0.5 | 4 |
| TRN5426-A | WT | 8 | 1024 | 512 | >32 | 0.5 | 2 |
| TRN5467 | N146K, L357I, I563T | 4 | >2048 | >1024 | 32 | 2 | 8 |
| TRN5467-A | N146K, L357I, I563T, Y446N | 32 | >2048 | >1024 | 32 | 1 | 8 |
| TRN5467-B | N146K, L357I, I563T, Y446N | 32 | >2048 | >1024 | 32 | 2 | 4 |
| TRN5549 | E150K | 2 | 2048 | 512 | 16 | 0.5 | 4 |
| TRN5549-A | E150K | 8 | 2048 | 1024 | 16 | 0.5 | 4 |
| Strain . | PBP2a proteina . | MIC (mg/L) . | |||||
|---|---|---|---|---|---|---|---|
| CPT . | MET . | OXA . | LVX . | VAN . | LZD . | ||
| USA300 | WTb | 1 | 64 | 64 | 1 | 0.5 | 4 |
| USA300-A | WT | 1 | 32 | 32 | 1 | 0.5 | 4 |
| ARC3824 | E239K, E447K | 8 | 1024 | 512 | 16 | 1 | 2 |
| ARC3824-A | E239K, E447K, Y446N | 64 | 1024 | 512 | 16 | 1 | 4 |
| ARC3824-B | E239K, E447K, A601S | 16 | 64 | 128 | 16 | 1 | 2 |
| ARC3824-C | E239K, E447K, A601S | 16 | 64 | 256 | 16 | 1 | 2 |
| ARC3827 | E239K | 2 | 2048 | 1024 | 16 | 1 | 2 |
| ARC3827-A | E239K | 4 | >2048 | 1024 | 16 | 0.5 | 2 |
| ARC3827-B | E239K | 4 | >2048 | 1024 | 16 | 0.5 | 2 |
| TRN5426 | WT | 2 | 2048 | 1024 | >32 | 0.5 | 4 |
| TRN5426-A | WT | 8 | 1024 | 512 | >32 | 0.5 | 2 |
| TRN5467 | N146K, L357I, I563T | 4 | >2048 | >1024 | 32 | 2 | 8 |
| TRN5467-A | N146K, L357I, I563T, Y446N | 32 | >2048 | >1024 | 32 | 1 | 8 |
| TRN5467-B | N146K, L357I, I563T, Y446N | 32 | >2048 | >1024 | 32 | 2 | 4 |
| TRN5549 | E150K | 2 | 2048 | 512 | 16 | 0.5 | 4 |
| TRN5549-A | E150K | 8 | 2048 | 1024 | 16 | 0.5 | 4 |
CPT, ceftaroline; MET, methicillin; OXA, oxacillin; LVX, levofloxacin; VAN, vancomycin; LZD, linezolid.
aThe substitutions located in the nPBD are N146K, E150K and E239K. The remaining substitutions are located in the PBD, with Y446N, E447K and A601S being specifically located in the transpeptidase binding pocket.
bA WT PBP2a sequence is defined as that carried by the vast majority of MRSA isolates as exemplified by strains USA300 and N315 (GenBank identifiers ADV68980 and BAB41256, respectively).9
The mutants recovered from isolate TRN5467, which had a ceftaroline MIC of 4 mg/L and contain three PBP2a substitutions, both generated a ceftaroline MIC that was 8-fold higher than the parent strain (Table 2). These mutant isolates (TRN5467-A and TRN5467-B) were found to carry an additional Y446N substitution in PBP2a. The mutants recovered from isolate ARC3824, which had a ceftaroline MIC of 8 mg/L and contain two PBP2a substitutions, including a Glu447Lys in the transpeptidase binding pocket, had a 2-to 8-fold elevation in MIC values (Table 2). These mutants were found to all carry additional substitutions, either Y446N or A601S in PBP2a. The 8-fold increase in ceftaroline MIC observed in some of these mutants suggests that these mutants could have been obtained directly on plates containing higher concentrations of ceftaroline if more plates had been examined or if slightly higher inoculums had been used. Several studies have reported characterization of MRSA clinical isolates with a ceftaroline MIC of ≥8 mg/L.5,7,9 Of these, the ST228-MRSA-I strains, predominantly isolated from Thailand, contain one nPBD variation (E239K) and one PBD variation (E447K).5,9 Both of these changes were also recently identified in an ST5 strain from the USA,7 which also carried the Y446N mutation and generated a ceftaroline MIC of >32 mg/L, consistent with mutant ARC3824-A (Table 2). Interestingly, in addition to the increase in the ceftaroline MIC seen for these mutants, there was a noticeable change in the MIC of methicillin, which decreased 16-fold in the ARC3824-A601S mutants (ARC3824-B and ARC3824-C; Table 2) concomitant with a smaller (2- to 4-fold) reduction in the oxacillin MIC value. As expected, all mutants exhibited MIC values of levofloxacin, vancomycin and linezolid that were essentially unchanged when compared with the parent isolates (Table 2).
Structural impact of PBP2a changes
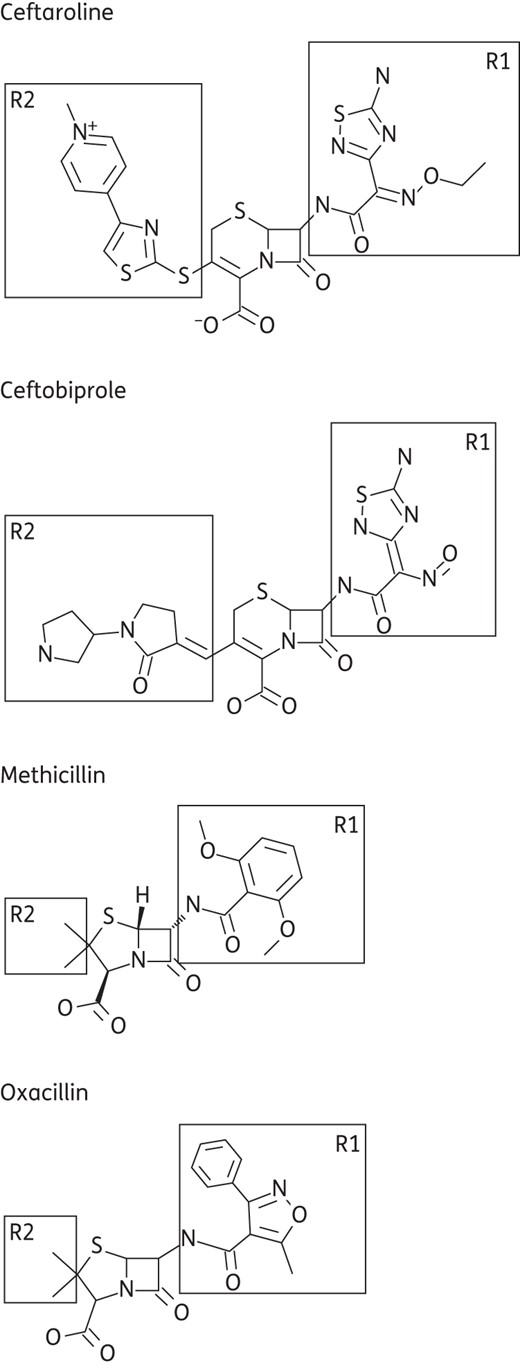
Methicillin resistance mediated by PBP2a is imparted by the formation of a narrow active site opening of its penicillin-binding transpeptidase pocket, due to a Y446–M641 hydrophobic interaction, which limits access to methicillin and the majority of other β-lactams.12 Recent structural data have also shown that Y446 can exist in both an ‘out’ and an ‘in’ conformation in the unliganded binding pocket, which would also impact access to the pocket.10,13 Covalent binding of a β-lactam, and most likely also to a peptidoglycan substrate, necessitates the ‘out’ conformation of Y446 to avoid a steric clash, and hence the dynamics of these two conformations would play a role in PBP2a inhibition. Ceftaroline and ceftobiprole, the two anti-MRSA β-lactams, overcome the ability to access this narrow pocket by their improved potency, which results from interactions with their large R2 groups (Figure 1), in contrast to the smaller R2 groups found in methicillin, oxacillin and other cephalosporins, such as ceftazidime and cefotaxime. Structural data show that the binding modes of ceftaroline and ceftobiprole, while not identical, are very similar with respect to their interaction with Y446 (Figure 2a). In both cases the inhibitors stabilize Y446 in the ‘out’ conformation, but ceftobiprole forms a hydrophobic stacking interaction between its R2 group and Y446, while ceftaroline interacts with Y446 sideways, resulting in the repositioning of Y446 (Figure 2a).10,14 Interestingly, whereas the ‘out’ conformation has also been observed in the methicillin-bound form (Figure 2a), there is no interaction between the inhibitor and this residue, suggesting that while this conformational change is necessary for methicillin to bind, there is no thermodynamic benefit provided by any additional interaction with the ligand.
![Analysis of the transpeptidase binding pocket of PBP2a. Overlays of the unliganded form (grey) with the structures bound with different β-lactam ligands [ceftaroline in cyan (PDB:3ZG0), ceftobiprole in light brown (PDB:4DKI) and methicillin in light green (PDB:1MWU)]. (a) An overlay of the different ligands in the binding pocket is shown relative to the positions of E447 and Y446. The Y446 residue in the unliganded form (grey) is in the ‘in’ conformation. (b) The Y446N change has been modelled onto the ceftaroline (N446 in red) and methicillin (N446 in bright green) structures. The nearest polar interactions are indicated by black dashed lines. (c) The R1 pocket is depicted with respect to A601 and Q521 interactions. (d) The A601S mutation has been modelled, in yellow, on the methicillin structure. The new interaction of the mutated residue with Q521 is indicated by a dashed line.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jac/71/1/10.1093_jac_dkv329/2/m_dkv32902.jpeg?Expires=1747919983&Signature=tearUX6BI7BAog-DEUYlqgEngmXUMTIqeyqmxQdI4jzwWEjsD5r87z9jwVQwyrGgEvLWA6Sv2G5-VA0ufKwqF9-o-YgwLPXTXoerMdSVY1fJ5oxREQIBUuZY4wE7Zn6t4l92umVIM~o~m5FkLTDC3f5lQNLt0K1M0GGKoqmhG06FOAID90Ebshb5lQO2VxiH7egUipc9TTgn~o~l59DJtwfJIG6beKT5SC4bb8u3U-ef8Qc40qmq1RY4G4MmxPJnXAMC7OajvHkXXl7AJB0igrSxZEjf8obyn1~86p6b1LP3TRUx~3aw~x9zfhnYOgHEHVT2MhNQomz6kmBd0V26Gw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Analysis of the transpeptidase binding pocket of PBP2a. Overlays of the unliganded form (grey) with the structures bound with different β-lactam ligands [ceftaroline in cyan (PDB:3ZG0), ceftobiprole in light brown (PDB:4DKI) and methicillin in light green (PDB:1MWU)]. (a) An overlay of the different ligands in the binding pocket is shown relative to the positions of E447 and Y446. The Y446 residue in the unliganded form (grey) is in the ‘in’ conformation. (b) The Y446N change has been modelled onto the ceftaroline (N446 in red) and methicillin (N446 in bright green) structures. The nearest polar interactions are indicated by black dashed lines. (c) The R1 pocket is depicted with respect to A601 and Q521 interactions. (d) The A601S mutation has been modelled, in yellow, on the methicillin structure. The new interaction of the mutated residue with Q521 is indicated by a dashed line.
The difference in the R2 interactions results in a different impact on PBP2a carrying the Y446N mutation. The 8-fold reduced susceptibility to ceftaroline (Table 2) can be explained by the reduced hydrophobic interaction with the R2 rings as well as an unfavourable charge clash between the N446 and the R2 sulphur (Figure 2b). This is evident from the MIC increase observed for mutants with two genetic backgrounds, one that contains a E447K substitution in the transpeptidase binding pocket (ARC3824-A) and one that does not (both TRN5467-A and TRN5467-B). Whereas this clash may impart less impact on ceftobiprole due to the orientation of this residue, the elimination of the hydrophobic stacking is still predicted to reduce susceptibility to ceftobiprole.
The impact of the E447K mutation, adjacent to Y446, previously observed in ceftaroline-resistant clinical isolates5,7 and in laboratory mutants selected with ceftobiprole,15 can be best explained by the introduction of a new salt bridge with neighbouring residues E460 and T582 resulting in the reduced size of the same R2 pocket and reduced susceptibility.5,14 However, it is expected that the Y446N and E447K changes would be independent, as evident from decreased susceptibility in the strains with no E447K change (Table 2). Interestingly, an MRSA isolate in a recent study that only carried the Y446N substitution had a ceftaroline MIC of only 1.5 mg/L, and this observation was supported by binding studies with the recombinant purified PBP2a protein.7 In our study, the acquisition of the Y446N substitution in mutants TRN5467-A and TRN5467-B, which do not carry any other transpeptidase site mutations, resulted in high-level resistance to ceftaroline (MIC = 32 mg/L; Table 2). This suggests that this change, in the presence of at least certain nPBD variations, can significantly hinder inhibition by ceftaroline, although dependence of the Y446N-mediated resistance on either an nPBD change or an E447K change cannot be ruled out. The crystal structure of the N146K variant, although not in the presence of ceftaroline,13 showed that the binding pocket of this variant was very similar to WT. In particular, the dynamic movement of Y446 was similar as this residue was captured in both the ‘out’ and ‘in’ conformations in the two monomers for both structures. This suggests that there is minimal pre-existing change in the transpeptidase pockets due to this nPBD variation.
The A601S mutation is also located in the ceftaroline-binding site, but in the R1 pocket (Figure 2c). Binding of the R1 group results in a conformational change in this part of the pocket that is associated with a significant movement of a loop formed by residues S598 to E602. In the unbound form this loop closes down on the pocket, locked by a salt bridge between Q521 and E602. Upon ceftaroline or ceftobiprole binding, this loop is repositioned and the salt bridge between E602 and Q521 is broken to accommodate the R1 group. The A601 residue (Figure 2c) is very close to Q521 in the unliganded form and also gets repositioned upon ceftaroline binding. However, in the case of binding of methicillin, which contains a smaller R1 group, this conformational change is slightly different (Figure 2c) in that the Q521 side chain remains closer to A601 due to a concomitant side-chain movement of Q521, although there is a shift in the loop (Figure 2c).
The A601S mutation, which is a polar substitution, should result in a newly formed hydrogen bond with Q521 in both the apo and acylated forms of the protein, which would result in stabilization of the methicillin-bound conformation of Q521 (Figure 2d). This is likely to result in a preformed optimal conformation for methicillin binding, which is energetically more favourable for methicillin binding and thus explains the 16-fold potency improvement of methicillin. In contrast, this new hydrogen bond is unfavourable for ceftaroline binding, since it will need to be broken to accommodate the R1 group at this site. In addition, given the close proximity of the R1 group of ceftaroline, a larger serine residue could result in a steric clash, together explaining the reduced susceptibility to ceftaroline resulting from this mutation.
Conclusions
Global surveillance studies have shown that the MIC90 value of ceftaroline for MRSA is 1–2 mg/L depending on geographical location, and characterization of isolates with decreased ceftaroline susceptibility has implicated variations in PBP2a as one of the responsible factors.5–8 Numerous variations have been identified in the nPBD or allosteric domain of PBP2a, and most isolates carrying these alleles have a ceftaroline MIC of 2 mg/L.5,6,8,9 Recent pharmacokinetic/pharmacodynamic and target attainment analyses have suggested that the approved dose of ceftaroline (600 mg every 12 h) could support a susceptible breakpoint of ≤2 mg/L.16,17 In contrast, isolates carrying transpeptidase mutations typically have ceftaroline MIC values of ≥4 mg/L. We evaluated a panel of MRSA isolates with a variety of PBP2a alleles, including those most commonly found in different lineages,9 and determined that the risk of selecting for ceftaroline-resistant clones is low and is independent of the initial ceftaroline MIC and appears to be unrelated to the specific PBP2a allele, although a larger number of isolates would need to be evaluated to confirm this trend. The few mutants that were obtained were characterized and contained either Y446N or A601S mutations that were located in the PBP2a transpeptidase binding pocket. The 2- to 8-fold increase in ceftaroline MIC values for these mutants, as well as the improved potency of methicillin in the A601S mutants, rationalized through interpretation of the co-crystal structure, provides further understanding of the mechanism of action of this anti-MRSA cephalosporin.
Funding
This study was funded by AstraZeneca.
Transparency declarations
Both authors (S. D. L. and R. A. A.) were employees of AstraZeneca at the time of this study and held stock or stock options in the company.
Acknowledgements
We thank Jim Whiteaker and Robert McLaughlin for sequencing and bioinformatics support.
References
Author notes
Present address: Macrolide Pharmaceuticals, 99 Hayden Avenue, Suite 100, Lexington, MA 02421, USA.



