-
PDF
- Split View
-
Views
-
Cite
Cite
Bashar Amer, Louise Juul, Anders Hauer Møller, Hanne Søndergård Møller, Trine Kastrup Dalsgaard, Improved solubility of proteins from white and red clover – inhibition of redox enzymes, International Journal of Food Science and Technology, Volume 56, Issue 1, January 2021, Pages 302–311, https://doi.org/10.1111/ijfs.14632
Close - Share Icon Share
Abstract
New alternative protein sources are needed. Clover grasses as white clover (WC) and red clover (RC) may provide a novel protein source to achieve high-quality food protein. To prevent protein oxidation, endogenous oxidative enzymes as polyphenol oxidase (PPO) and peroxidases were inhibited with sulphite. RC showed higher PPO activity than WC. Low sulphite inhibited the PPO activity in both species, but browning still occurred in RC. Sulphite did not affect the polyphenol (PP) content in WC, rather suggesting peroxidase than PPO activity. In RC juice, the PP content increased in a dose-dependent manner with increasing sulphite. High sulphite impaired browning and increased the content of soluble rubisco in RC, mainly by increasing the content of native rubisco. In vitro digestibility of RC protein increased with increasing sulphite. In conclusion, sulphite inhibited oxidative enzymes and increased the quality of protein extracted from WC and RC.
Introduction
There is an increased demand for protein due to the growing world population and an increase in protein intake. To meet the protein demand, there is a need to increase the efficiency of existing plant-based protein sources, but also to exploit alternative sources of protein for food purposes (Henchion et al., 2017). Most recently, as an example, canola protein has been upgraded to food protein hydrolysates (Alashi et al., 2018). In temperate regions, forage legumes are widely grown but have mostly been used for ruminant feed due to the high fibre content, which monogastrics cannot utilise. Legumes are in general known to have a high content of protein and a composition of essential amino acids, which are typically associated with high-quality protein (Houseman & Connell, 1976; Krawutschke et al., 2012). Hence, white clover (WC) (Trifolium repens L.) and red clover (RC) (Trifolium pratense L.) are among the main species used for grazing and silage making, respectively (Krawutschke et al., 2012). Solati et al. (2017) showed that the crude protein content of WC and RC varies between 15 and 30% of dry matter, depending on season (spring/summer) of harvest. They also showed that the crude protein yield and extracted protein yield decline with plant ageing, whereas dry matter increases from spring to summer. Furthermore, later harvest shifts the compartmentation of proteins towards less soluble protein fractions, probably due to an increase in cell wall-bound proteins (Solati et al., 2017). The soluble protein mainly originates from the photosynthetic enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (rubisco), which is located in the stroma (the soluble fraction of the chloroplast) (Fiorentini & Galoppini, 1983; Salvucci et al., 1986; Barbeau & Kinsella, 1988), and constituted of eight S (small chain) units (13 kDa) and eight L (large chain) units (55 kDa). In C3 plants, up to 42% of the leaf protein is rubisco (Carmo-Silva et al., 2015). The overall content of protein and general chemical composition can further vary upon, for example, growth stage and conditions, and further depend on extraction and processing methods (Houseman & Connell, 1976). The rubisco protein has good functional properties for food applications (Martin et al., 2019) and a good composition of essential amino acids (Barbeau & Kinsella, 1988); why rubisco extracted from WC and RC could be a valuable source of food protein in the future.
Protein from leaves changes colour with ageing, going from yellow to brown (Kiskini et al., 2016). When extracting protein from green plants, the protein yield may be compromised by indigenous redox enzymes due to enzymatic browning reactions. Polyphenol oxidases (PPOs) are a group of redox enzymes that are located in the chloroplasts of plants and widely distributed in forage crops and to a higher extent in RC than WC (Jones et al., 1995; Lee et al., 2019). PPOs are copper metalloenzymes, which carry out o-hydroxylations of monophenols to o-diphenols (cresolase/monophenolase activity) and further the oxidation to o-diquinones (catecholase/diphenolase activity) by using molecular oxygen (Fig. 1). The formed diquinones are coloured and highly reactive electrophiles, which can non-enzymatically react with functional groups of proteins and amino acids, such as amine, amide, sulphydryl, indole and imidazole, or themselves to form brown polymeric pigments. This means that when PPO is active, it leads to enzymatic browning (Sullivan et al., 2004; Bittner, 2006). Even though the physiological role of PPO in plants is not clear, PPO is generally believed to play a role in biotic stress defence, as activity of PPO in plants is increased, for example, upon wounding (Mayer, 2006; Taranto et al., 2017).
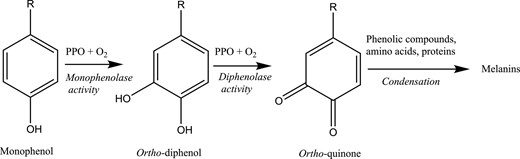
Simplified schematisation of browning process. PPOs: polyphenol oxidases. Figure from Taranto et al. (2017).
Typically, PPO and polyphenols (PPs) are compartmentally separated in living plants. However, in situations such as wounding of leaves, for example, upon harvesting, pressing or ensiling, they might meet and react (Vaughn & Duke, 1984; Bittner, 2006). Thus, upon pressing, a step in the extraction of protein from forage crops, PPO induced enzymatic browning and protein–quinone complex formation in the juice may occur. Along with a change in colour, these reactions can give rise to off-flavours and reduced solubility/functionality and protein digestibility, which reduce the quality of the protein (Kroll et al., 2000; Kroll & Rawel, 2001) and lower the nutritional value of the protein rats and in ruminants (Hurrell et al., 1982; Matheis & Whitaker, 1984; Lee, 2014; Lee et al., 2019).
The enzymatic browning can be avoided by inhibition of PPO, which will decrease the formation of quinone–protein complexes. Sodium sulphite, which is used in this study, and other sulphur-containing compounds act as inhibitors by generating colourless sulpho–polyphenol complexes from quinones by a reduction reaction (Queiroz et al., 2008; Narváez-Cuenca et al., 2011). Hence, this study was performed to investigate the effect of sulphite on the protein solubility in clover juice and the in vitro protein digestibility. Three specific hypotheses were tested: sulphite addition during extraction of protein from WC and RC will (i) inhibit the PPO activity, (ii) increase protein solubility and (iii) improve in vitro protein digestibility of the extracts. To test these hypotheses, the colour, PPO activity, PP content, rubisco content and in vitro protein digestibility were measured after acid precipitation of RC protein.
Materials and methods
Chemicals and reagents
Deionised (18.2 MΩ) filtered water (0.22 μm) came from a Milli-Q system, Millipore SAS (Molsheim, France). L-leucine, methanol, Folin–Ciocalteu’s reagent, sodium carbonate, sodium bicarbonate, sodium sulphite, sodium chloride, sodium hydrogen phosphate, sodium dihydrogen phosphate, D-ribulose 1,5-diphosphate carboxylase (rubisco) from spinach, pyronin Y, 1,4-dithioerythritol (DTE), HCl 37%, L-3,4-dihydroxyphenylalanine, gallic acid, bovine serum albumin, sodium tetraborate, pepsin, pancreatin and fluorescamine were purchased from Sigma-Aldrich (Darmstadt, Germany). Sodium dodecyl sulphate (SDS), trisaminomethane (Trizma Base), coomassie brilliant blue G-250 (Serva, Heidelberg, Germany), glycin (Applichem, Darmstadt, Germany), Spectra Multicolour Board range protein Ladder, Thermo 26634 (Thermo Scientific, Rockford, USA), Criterion Tris-HCl gels (Bio-Rad, Hercules, USA), trichloroacetic acid (TCA) and dried acetone were obtained from Merck, Germany.
Plant material
WC and RC were harvested (1.5 kg of each plant) at 08:00 am on 31 August and 01 September 2017, respectively, by scissors from Aarhus University (AU) planted fields at AU research centre – Foulum, Denmark. Fresh harvested plant material was immediately transferred to a dark and cold room (4 °C) in the laboratory in plastic containers with holes to ensure sufficient airing.
Preparation of plant juices
Fresh plant material (250 g) was washed with 250 mL of treatment solutions, followed by drying using a salad spinner (IKEA, Denmark). Then, washed plant material was juiced into 250 mL of treatment solutions using a double screw juicer (Angel Juicer 8500S, Domotech, Denmark). All processing steps were carried out in a dark room at 4 °C to reduce light oxidation.
Table S1 summarises juiced liquid volume and pulp weight of each sample. Plant juices were centrifuged for 15 min, at 1700 × g, 4 °C (Multifuge 3SR centrifuge, Fisher Scientific) to remove non-soluble fibres. Supernatants were kept in brown glass bottles at −20 °C until analysis.
Removal of chlorophyll by ultracentrifugation
Aliquots (25 mL) of WC and RC juices of different treatments were ultracentrifuged for 30 min at 34940 × g, 4 °C on an Optima L-80XP Ultracentrifuge (Beckman Coulter Inc., Brea, CA) with a titanium fixed-angle 70-Ti Rotor (angle 23°) to remove chlorophyll. Supernatants were transferred to brown glass bottles at −20 °C until analysis.
Protein precipitation and re-dissolving
White clover (WC) and red clover (RC) proteins were precipitated from the supernatants (20 mL) after ultracentrifugation by adjusting pH to 4.5 using 50 and 100 mm hydrochloric acid solutions for control and treated samples, respectively, followed by standing overnight in the dark at 4 °C before centrifugation for 15 min, at 1700 × g, 4 °C (Multifuge 3SR centrifuge, Fisher Scientific). Resulted protein pellets were freeze-dried and kept at −20 °C until analysis. The dried protein pellets were re-dissolved in 20 mL phosphate buffer (100 mM) pH 7.4 by shaking the mixture at 1000 rpm (IKAMAG Reo, Germany) for 120 min at room temperature.
Measurement of colour components
Colour components L* (lightness), a* (green – red) and b* (blue – yellow) of 2 mL of each raw plants juices and supernatants of ultracentrifuged clover juices were measured using a handheld colorimeter (Konica Minolta, Tokyo, Japan) in a 12-well plate.
Determination of polyphenol oxidase (PPO) activity and polyphenol (PP) content
Polyphenol oxidase (PPO) activity was determined using a modified method of Cheng et al. (2007) and Ni Eidhin et al. (2010). After removal of non-soluble fibres, 50 µL of raw plant juices were added to 200 µL of substrate (100 mm solution of L-3,4-dihydroxyphenylalanine (L-DOPA)) in phosphate buffer (pH 6.5) in a microtiter plate, followed by immediate measuring of the change in absorbance at 475 nm every 10 s for 3 min using a microtiter plate spectrophotometer (BioTek Instruments Inc, Winooski, VT 05404, USA). The PPO activity is expressed as units/mL plant juice, where 1 unit causes a change in A475 nm of 0.001/min, when 50 µL plant juice is added to 200 µL substrate solution at RT.
Total polyphenol (PP) content in raw juices was determined using Folin–Ciocalteu spectrophotometric assay as described by Singleton et al. (1999). PPs were extracted into 80% methanol (v/v) by shaking for 60 min (IKAMAG Reo, Germany) followed by centrifugation (Centrifuge 5417 R, F45-30-11, Eppendorf AG, Hamburg, Germany) for 10 min at 4 °C (20817 × g). Extracted PP (15 µL) were mixed with 235 µL of Milli-Q water, 20 µL of 1N Folin–Ciocalteu’s reagent and 30 µL of 0.5 M Na2CO3 solution in a microtiter plate, followed by incubation for 120 min in the dark. Absorbance of samples was measured at 750 nm using a microtiter plate spectrophotometer (BioTek Instruments Inc, Winooski, VT 05404, USA). Pure gallic acid standard was used to prepare calibration solutions in 80% methanol (v/v) followed by same treatment as extracted plants juices. PP content was calculated as mg gallic acid equivalent per mL of plant juice.
Determination of protein content
Bradford (1976) spectrophotometric method was used to determine the total content of soluble protein in juices. In microtiter plates, 10 µL of the standards (aqueous solution of bovine serum albumin, BSA) or samples were mixed with 200 µL of diluted dye (acidic solution of Coomassie Brilliant Blue G-250). Five minutes later, the absorbance was measured at 595 nm using a microtiter plate spectrophotometer (BioTek Instruments Inc, Winooski, VT 05404, USA) at room temperature.
Total protein content in precipitates after freeze-drying was determined using a DUMATHERM instrument (Gerhardt Analytical Systems, Königswinter, Germany) with 1.6 mg O2/mg sample, an O2 flow rate of 200 mL/min and protein factor 6.25.
Quantification of rubisco
Size exclusion was used for quantitative analysis of rubisco using a Yarra 1.8 µm SEC-X150 column (size: 150 × 4.6 mm) (Phenomenex, United States). Two µL of ultracentrifuged plant juice was injected onto a HPLC (Agilent, United States). A 50 mm sodium phosphate buffer with 300 mm NaCl and pH 6.8 was used to elute protein at a flow of 0.3 mL/min. UV absorbance was measured at 280 nm.
SDS-PAGE
SDS-PAGE was carried out on Criterion TGX (8%–16%) precast ready-made gels (Bio-Rad Laboratories Inc., Hercules, CA, USA) using the technique described by Laemmli (1970) under reducing conditions. The protein concentration was determined by using the Bradford assay and adjusted with Milli-Q water to give a final concentration of 2 mg/mL and further diluted 1:1 with SDS-PAGE sample buffer (20 mm Tris, 2% SDS, 20% glycerol, 0.1 mg/mL bromophenol blue, 20 mm dithiothreitol (DTT), pH 6.8) to obtain a final concentration of 1 mg/mL. The samples were heated at 85 °C for 3-5 min prior to electrophoresis, and 20 µL were subsequently applied to each lane. Fixation was obtained in 50% ethanol and 8% phosphoric acid, and the gels were subsequently stained using colloidal CBB according to Kang et al. (2002). Multicolour protein marker (10–260 kDa) (Thermo Scientific, USA) was applied to estimate the protein mass.
Protein digestibility
The digestibility of RC precipitated proteins was evaluated by measuring the free N-terminal level (equivalent to mM leucine) in re-dissolved pellets after digestion with pepsin and pancreatin enzymes at 60 min using a fluorescamine assay as in Pearce (1979) modified by Dalsgaard et al. (2007). In 5-mL Eppendorf tubes, 700 µL of pepsin solution (150 U/mL) in 70 mm NaCl (pH 2) was incubated at 37 °C for 5 min, and then, 300 µL of the protein solutions was added to the tubes and incubated again at 37 °C with shaking (800 r.p.m.) for 15 min. The reaction was terminated by mixing 200 µL of each solution with 200 µL of 20 mm NaHCO3 solution (pH 7). For the second digestion step with pancreatin, 700 µL of pancreatin solution (150 U/mL) was incubated at 37 °C for 5 min in 5-mL Eppendorf tubes, and then, 300 µL of the digested protein solutions with pepsin was added to the tubes and incubated again at 37 °C with shaking (800 rpm). Aliquots of 200 µL of each sample were collected after 0, 15 and 60 min and then heated for 1 min at 90 °C and frozen at −20 °C to stop the digestion.
N-terminal level was determined by mixing 75 µL standard (L-leucine) or sample and 75 µL of 24% trichloroacetic acid in 2-mL Eppendorf tubes according to Dalsgaard et al. (2007), followed by precipitation in ice bath for 30 min. Samples were centrifuged (17949.49 × g) for 20 min at 4 °C. Aliquots of 37.5 µL of the supernatants were mixed with 1130 µL of 100 mm Na-tetraborate buffer and 375 µL of 0.2 mg/mL fluorescamine in dried acetone. Finally, 250 µL of each sample was transferred to a microtiter plate spectrophotometer (BioTek Instruments Inc, Winooski, VT 05404, USA) measurement at 25 °C with shaking 0.5/s, excitation 400/30 nm and emission 485/20 nm. The amount of free N-terminals is calculated as L-leucine equivalents.
Data analysis
A two-way analysis of variance (Anova) statistical method was used to test significance of difference from control and between plant type (RC vs. WC), and treatments (10 mm vs. 25 mm sulphite) using SAS Enterprise Guide version 5.1 (Analytics Software & Solutions).
Results and discussion
The present study investigated the effect of addition of antioxidant (sodium sulphite) on polyphenol oxidation and its effect on protein solubility of proteins from WC and RC, and in vitro digestibility of extracted protein from RC. Sodium sulphite was selected as antioxidant in this study to examine the effect of PPO inactivation on protein solubility and in vitro protein digestibility.
After juicing WC and RC with sulphite, no clear differences in the colour of the raw juices were visible between control and samples with 10 mm sulphite due to the impact of chlorophyll. RC juice with 25 mm sulphite was more green compared to the other RC samples, which appeared more brown (Fig. 2a). RC juice with 25 mm sulphite was less red and exhibited a more yellow, brighter colour after removal of chlorophyll. No visual difference was observed between WC samples (Fig. 2a). The visual appearance was consistent with the colour measurement, showing no difference in lightness factor (L*) or redness factor (a*) of WC juices, while the colour component b* (blue-yellow) was decreased (Δb* = −1.93) in WC juice after treatment with 10 mm sulphite (Fig. 2b). The lightness of RC was reduced with 10 mm sulphite (ΔL* = −1.93, P < 0.0001). However, treating RC with a higher level of sulphite (25 mm) significantly increased the lightness (ΔL* = 9.12, P < 0.0001) of the juice (Fig. 2b). Treatment with 10 mm sulphite increased redness factor (a*) of the RC juice (Δa* = 2.39, P < 0.0001), whereas it was significantly decreased Δa* = −9.81 (P < 0.0001) after treatment with 25 mm sulphite (Fig. 2b). The yellow colour (b*) increased (Δb* = 3.84, p < 0.0001) for the RC juice with 10 mm sulphite but decreased in RC juice (Δb* = −1.54, P < 0.0001) when adding 25 mm sulphite (Fig. 2b).
![Colour of white and red clover juices. (a) White and red clover juices treated in deionised water (control), 10 mm sodium sulphite (red and white clover) and 25 mm sodium sulphite (red clover), before and after removal of chlorophyll by ultracentrifugation (34 940 × g, 30 min, 4 °C), and (b) delta values (sample – control) of colour components (L*, a*, b*) of ultracentrifuged juices. Notations on bars indicate significance of difference (P < 0.05) from control and between treatments testing with a two-way Anova. [Colour figure can be viewed at wileyonlinelibrary.com]](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ijfst/56/1/10.1111_ijfs.14632/1/m_ijfs14632-fig-0002-m.jpeg?Expires=1747956351&Signature=CS14~V0y1Raua8Url-Mrhqx4wvOkpbhdsbVH4JcVR1rhfcHUYrDSYSSfRYOumOdqIspDmSdszRf3frfmTeEj1JmIejpFm1iF3kHM-yNuqsX~mVS3ItnQpcOeGVGNjZ~gGKvj77RVD9QFLQGLUuiqKyP7OlG1yXOsF45N0AYBkWwOaWKPYMJsdYampI~XMsCEOcxwptB1wBfXXeEyAsFVSA3WlrtvIE2L39jdZYeQyvjHQBFqyuu~mAYG~16aAfCbpISgosgOmWnzahZuRfqM9IuV7mnWhWrs4g-XvHxcyqSQxUgB~MF-jwlkyPwD6JtvERhBmxNlIqv~vRvMi5hM4A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Colour of white and red clover juices. (a) White and red clover juices treated in deionised water (control), 10 mm sodium sulphite (red and white clover) and 25 mm sodium sulphite (red clover), before and after removal of chlorophyll by ultracentrifugation (34 940 × g, 30 min, 4 °C), and (b) delta values (sample – control) of colour components (L*, a*, b*) of ultracentrifuged juices. Notations on bars indicate significance of difference (P < 0.05) from control and between treatments testing with a two-way Anova. [Colour figure can be viewed at wileyonlinelibrary.com]
Polyphenol oxidase activity and total PP content were measured (Fig. 3). A higher activity (~8-fold higher) of PPO was observed in RC control juice compared to WC (Fig. 3a). RC has previously shown a higher PPO activity than WC, ryegrass, alfalfa and a number of other legumes (Belanche et al., 2013). Independent on the level of sulphite, the PPO activity was efficiently inhibited in the RC samples (Fig. 3a). Although the addition of 10 mm sulphite deactivated PPO in both WC and RC, it did not change the total level of PP in WC juice, while the addition of sulphite showed a dose-dependent elevation on PP content in RC (~8- and 25-fold for 10 and 25 mm treatments, respectively) (Fig. 3b). Since no significant colour change was observed and the recovery of PP was unaffected by addition of 10 mm sulphite in WC and the PPO was completely inhibited, we did not find a need to treat WC with 25 mm sulphite as for RC, where no colour improvement and PP-recovery increase from 0 to 10 mm sulphite. Sulphur-containing compounds have been reported to decrease the browning of phenolic compounds in plant extracts (Narváez-Cuenca et al., 2011) and model systems containing standard phenolic compounds (Kuijpers et al., 2012). Sulphite can irreversibly inhibit the PPO enzymes by blocking their active copper-B site through covalent binding to one of the copper-coordinating histidines (Kuijpers et al., 2013), and/or by combining with enzymatically produced ortho-quinones and stopping their condensation to melanin (Embs & Markakis, 1965; Sullivan et al., 2004; Bittner, 2006). The inhibitory effect of sulphite on PPO can explain the elevation in total PP content, and an inverse relationship between PPO activity and PP content has earlier been shown (Esmaeili et al., 2017). The increase in lightness (L*) and reduction in red colour component (a*) in RC when adding sulphite (25 mm) may also be explained by less PP oxidation and thus lower formation of quinones and polymeric browning products (Arogundade & Mu, 2012). Treatment of RC with 25 mm sulphite in this study confirmed the effect of the antioxidant on decreasing browning in RC. However, the slight increase in the red colour of RC with low level of sulphite compared to the control is not explainable.
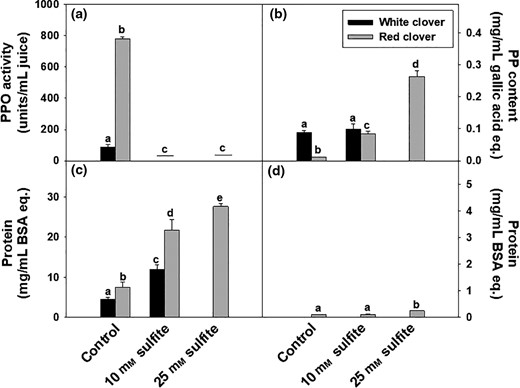
(a) Polyphenol oxidases (PPO) activity with L-DOPA as substrate, (b) polyphenol (PP) content using Folin Ciocalteu’s assay, (c) soluble protein content measured with Bradford, and (d) soluble protein content in supernatant after acid precipitation in samples treated with deionised water (control), 10 mM sodium sulphite (red and white clover) and 25 mM sodium sulphite (red clover). Notations on bars indicate significance of difference between treatments and among plant type, mixed model ANOVA, P < 0.05.
The higher PPO activity in RC when compared to WC (Fig. 3a) might explain why the reduction of PPO activity by 10 mm sulphite showed a significant improvement of PP content in RC compared to the control but not in WC (Fig. 3b). Looking at the increased lightness and reduced redness of sulphite-added juices can provide a quick and easy method for determining the level of sulphite that should be added to sufficiently inhibit PPO and retain PP content. Furthermore, the elevation of non-oxidised PP in RC as a result of sulphite addition might add value if PPs are extracted and utilised as food additives, nutraceuticals and food colourants (Caleja et al., 2017). Phenolic compounds from plant extracts may be efficiently adsorbed by sorpative separation techniques (Silva et al., 2018), and based on our results, it is recommended to study the effect of PP antioxidant, that is sodium sulphite, addition on PP absorption (recovery).
At the same time, the soluble protein content in raw juices was highly affected by addition of sulphite (Fig. 3c). Treatment with 10 mm sulphite increased the protein content 2.6- and 2.9-fold in WC and RC, respectively. The increase in total content of soluble protein in RC juices was dependent on sulphite level; 2.9- and 3.7-fold for 10 and 25 mm sulphite treatments, respectively (Fig 3c). After acid treatment, supernatants contain very little amount of protein (Fig. 3d) indicating almost all soluble proteins were precipitated. SDS-PAGE analysis of proteins in WC and RC juices treated with 10 or 25 mM sulphite showed an intense bands at 50 kDa and 13 kDa corresponding to the large and small rubisco subunits supported by a rubisco analytical standard (Fig. 4). The rubisco bands were more or less absent in the two control samples, and as the same amount of protein were loaded to each lane, we suggest formation of higher polymers/aggregates in the control samples that cannot migrate into the gel. Furthermore, a denser band was observed in the WC, both in the control and the sulphite treated sample even though the total protein content was higher in RC (Fig. 3c). This may reflect higher formation of polymers in the RC than in the white clover samples.
![Reduced SDS-PAGE Criterion TGX (8%–16%) of white and red clover juices after treatment with deionised water (control), 10 mm sodium sulphite (red and white clover) and 25 mm sodium sulphite (red clover). Equal level of protein, 20 µg, was loaded in each lane. [Colour figure can be viewed at wileyonlinelibrary.com]](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ijfst/56/1/10.1111_ijfs.14632/1/m_ijfs14632-fig-0004-m.jpeg?Expires=1747956351&Signature=f9EvFio5Hvu~ND1WGNPWDvBCVbye7JSWVVqpErtNNQfX8o4MgOw9SYqCmTcAmBu5sc-VG6o-HaynXKBsdCHFS7wMQtgn29ak4ozEH2ytWUDTscZAfQChnHw-7zd~uiFeZPLHD0e2AVJG6qOX7AXdEU6zRXBWrR-OSpyoxH1b0h-ofJOqFizm4BXL~1TB8ylsYHaMJt9fBhsX~9xQVm2oNjorAuntEyluiiglJu4ElINbUBxUuPSuEn0ohnDNsTrtw1EUYPXoVVjBwVFLpq-CpzMnpCZ2BBEXrL5IVIDvJ9RzXyQ40DSK-gIiC5ehvrq2FyJ0CrepbPzAw~cc2I3sPw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Reduced SDS-PAGE Criterion TGX (8%–16%) of white and red clover juices after treatment with deionised water (control), 10 mm sodium sulphite (red and white clover) and 25 mm sodium sulphite (red clover). Equal level of protein, 20 µg, was loaded in each lane. [Colour figure can be viewed at wileyonlinelibrary.com]
The rubisco content was quantified using size exclusion chromatography after removal of chlorophyll (Fig. 5). Two dominating peaks, that were both identified as rubisco, were present in the chromatogram (Fig. 5a), and according to an external standard we suggest the first peak to be higher polymers of rubisco and the second peak to be native rubisco, the latter corresponding to a size of approximately 550 kDa. The sum of the two peaks was used to quantify the total rubisco content in the juices. In general, the rubisco content was higher (~7-fold) in RC than in WC (Fig. 5b). For RC, a dose–respond increase of the total rubisco was observed with increased sulphite with a final increase of 4.0-fold for 25 mm sulphite. No significant changes were observed in the content of rubisco for WC control and the sulphite treated sample (Fig. 5b). The latter result was unexpected when compared with the total protein content (Fig. 3c) and SDS-PAGE of WC (Fig. 4) but supported by the PP content, which was unaffected by sulphite addition. This indicated a potential protein–protein cross-linking without any reaction with the polyphenols, suggesting other oxidative enzymes, for example peroxidases, might form phenoxyl radicals resulting in dityrosine formation (Steffensen et al., 2008) but further investigation is needed to understand the underlying mechanism. However, the rubisco band observed in reduced SDS-PAGE indicated that the cross-linking of rubisco was reducible and hence oxidation of cysteine residues and formation of disulphide bridges seemed more likely. Cysteine oxidation by peroxidase activity has previously been reported (Figueroa-Espinoza et al., 1998). Peroxidase activity has previously been identified to be main responsible for the lignification in WC (Lee et al., 2007) and has been proposed to be involved in enzymatic browning in other species as well (Richard-Forget & Gauillard, 1997). Another possibility is autoxidation taking place during processing of WC, although sulphite would also be expected to react with the quinones formed through autoxidation. Oppositely, the dose-dependent increase of PP with increasing sulphite indicated a reaction between PP and proteins in the RC samples (Fig 3b). For the RC juice, the relative ratio between native:polymeric rubisco (peak 1 and 2) changed with sulphite addition where a ratio of 1:1 was observed for 25 mm sulphite and a ratio ~1:3 (native:polymeric rubisco) was observed for control. The higher content of rubisco as well as an increase in relative ratio of native:polymeric rubisco after the addition of sulphite was suggested to be caused by the inhibition of PPO activity.
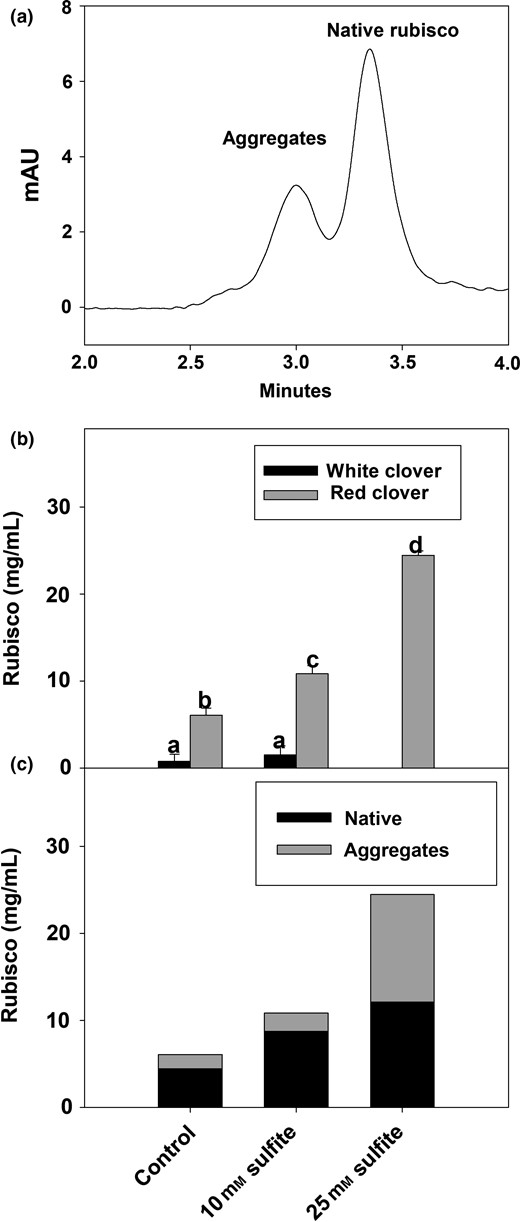
Rubisco content of red and white clover ultracentrifuged juices of samples treated with deionised water (control), 10 mm sodium sulphite (red and white clover) and 25 mm sodium sulphite (red clover), determined by size exclusion. (a) Chromatogram showing the two peaks of rubisco (Rubisco standard), (b) total rubisco content based on size exclusion of supernatants after removal of chlorophyll by ultracentrifugation and (c) distribution of native rubisco peak (peak 2) and higher polymers of rubisco (peak 1) from the size exclusion of red clover juices. Notations on bars indicate significance of difference between treatments and among plant type, two-way Anova, P < 0.05.
After isoelectric precipitation, in vitro digestibility was investigated on protein pellets of RC samples equal in protein content. As the protein recovery of RC was much higher than for WC, protein precipitates from RC were selected the in vitro digestibility study. The three different samples of RC protein (treated with 0, 10 mm and 25 mm sodium sulphite) also allowed for a dose–response effect investigation. When digested with pepsin followed by pancreatin, sulphite improved the protein in vitro digestibility in a dose-dependent manner (Fig. 6). The improved in vitro protein digestibility can be explained by the inhibition of PPO activity, thus inhibition of the formation of protein-quinone and/or protein-protein complexes, which was also observed in models with different PP (Kroll & Rawel, 2001).
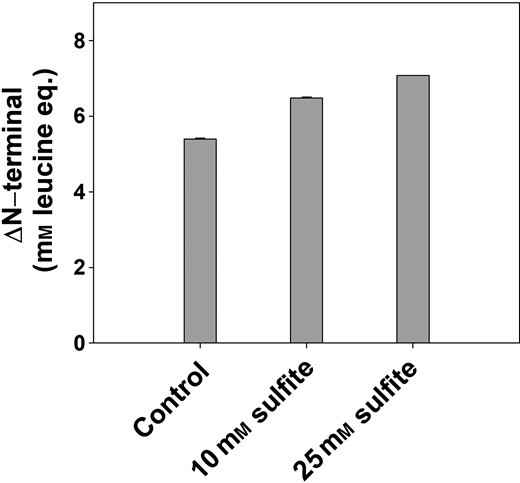
In vitro protein digestibility of precipitated protein from red clover measured as release of peptides (free N-terminal) after 1 h of pepsin digestion followed by 1 h of pancreatic digest. The results are expressed as the level of free N-terminals (equivalent to mm leucine) using a fluorescamine assay. Notations on bars indicate significance of difference between treatments and control, Anova, P < 0.05.
Polyphenol oxidase has previously shown an ability to inhibit protein proteolysis in red clover by catalysing the oxidation of endogenous o-diphenols to quinones, which are subsequence polymerised to cross-linked protein complexes, resulting in inhibition of protein proteolysis (Li et al., 2018). Although this activity of PPO is protecting protein from degradation, our study shows that PPO activity decreases protein solubility and in vitro protein digestibility, potentially by forming protein–quinone complexes. Such complexes have been reported to reduce protein digestibility in the rumen and subsequently increase undegraded dietary protein flow to the small intestine (Lee, 2014). Protein digestibility in rats has previously been investigated with respect to treatment with metabisulphite (Donnelly et al., 1983). The authors suggested improved recovery of lysine and methionine to be important for an improved utilisation of protein from metabisulphite treated lucerne, WC and ryegrass-WC. However, our study indicates that protein solubility may also be an important factor to consider. Recent data have shown that the standardised nitrogen digestibility is 77.4% for RC and 79.3% for WC, respectively. Even though quite low, it may actually be caused by a rather low protein content in the protein concentrate used. The rat study also shows that the sulphur-containing amino acids, methionine and cysteine, are the first limiting amino acids when evaluated for pigs and poultry (Stødkilde et al., 2018). Bittner (2006) suggests cysteine and methionine to be some of the most affected amino acids in the enzymatic browning reaction (Bittner, 2006). We definitely see a potential for these crops, both as feed for monogastrics and for human consumption, but more investigation on inhibition of redox enzymes is needed. Sulphites in general are widely used as preservatives in food, but adverse health effects have been reported after exposure to sulphites (Vally & Misso, 2012). Therefore, the amount of sulphite present in the WC and RC protein after precipitation and drying needs to be further investigated. Although sodium sulphite was selected as antioxidant in this study, it is recommended to test other safer antioxidants such as vitamin C and E for their potential effect on the redox enzymes, and thus, on the quality of extracted plant proteins.
Conclusion
The content of soluble protein in juice from WC and RC and the in vitro protein digestibility in RC protein extract was highly dependent on addition of sulphite. This might be caused by the inactivation of PPO, thus inhibition of cross-linking between PP and soluble protein such as rubisco. Even though there still are much to discover and solve before RC and WC can be counted as food protein, the present study indicates that inhibition of PPO activity improved the protein quality.
Acknowledgment
The study was supported by the Biobase Green Protein project funded by Aarhus University and the Danish Ministry of Food, Agriculture and Fisheries.
Conflict of interest
The authors declare no conflict of interest.
Author contribution
Bashar Amer: Formal analysis (equal); Methodology (equal); Writing-original draft (equal); Writing-review & editing (equal). Louise Juul: Formal analysis (equal); Methodology (equal); Writing-original draft (equal). Anders Hauer Møller: Writing-original draft (equal); Writing-review & editing (equal). Hanne Søndergård Møller: Formal analysis (equal); Methodology (equal). Trine Kastrup Dalsgaard: Conceptualization (lead); Funding acquisition (lead); Methodology (equal); Project administration (lead); Writing-original draft (equal); Writing-review & editing (equal).
References
The review article by Bittner presents a thorough description of the reaction between quinones (products of polyphenol oxidation) and proteins to form quinone-protein complexes, the knowledge provided by this review has enabled the authors of this study to make the hypothesis that the inhibition of PP oxidation might increase protein solubility, and improve in vitro protein digestibility by preventing the formation of quinone-protein complexes.
Although, a non-plant-based protein (myoglobin) was used in Kroll et al., 2001 to study the reaction between phenols and protein, the authors have cited this article as it provides in-depth description of the structural changes and in vitro digestibility of myoglobin after the reaction with phenols and quinones. The results from this study can explain the data obtained in our current study.
Mixing red clover with alfalfa in this study led to decreased proteolysis, which was attributed to the formation of quinone-protein complexes by presence of polyphenols oxidase (PPO) in red clover. This result might explain the higher in vitro digestibility of red clover protein as a result of the inhibition of PPO enzyme in our study.
This comprehensive comparison of the functional properties of RuBisCO with proteins used for food applications (e.g., whey and soy proteins) is recommending the utilization of RuBisCO as a potential nutritious and functional food ingredient to face global food security and protein supply. Results from this study goes well with our research to improve recovery of RuBisCO from other new resources.
This study presents sulphite, which was also used in our study, as an anti-browning agent. Furthermore, it also reveals the mechanism by which sulphite inhibits the browning by producing sulfo-O-diphenol products instead of forming O-quinones that are responsible for browning.
Author notes
The peer review history for this article is available at https://publons.com/publon/10.1111/ijfs.14632



