-
PDF
- Split View
-
Views
-
Cite
Cite
Changwan Cui, Yiping Lu, Yuanyi Yue, Si Wu, Shuang Wang, Miao Yu, Zhengrong Sun, Camel milk regulates T-cell proliferation to alleviate dextran sodium sulphate-induced colitis in mice, International Journal of Food Science and Technology, Volume 55, Issue 4, April 2020, Pages 1648–1660, https://doi.org/10.1111/ijfs.14434
Close - Share Icon Share
Abstract
The incidence of inflammatory bowel disease (IBD) is increasing in China. Current clinical methods of treatment for IBD are accompanied by side effects, and thus, the search for effective therapeutic drugs with minimal adverse effects is necessary. In the present study, the effects of camel milk (CM) on dextran sodium sulphate (DSS)-induced acute and chronic colitis in a mouse model were investigated. The results showed CM effectively alleviated the injury induced by DSS to the colon mucosa and imbalance of immune cells in mice. However, treatment with CM significantly increased the body weight of mice and decreased the disease activity index (DAI), histopathological score, proliferation of Th17 cells and concentration of inflammatory cytokine IL-17. The results from the present study indicate CM possesses intestinal protective effects.
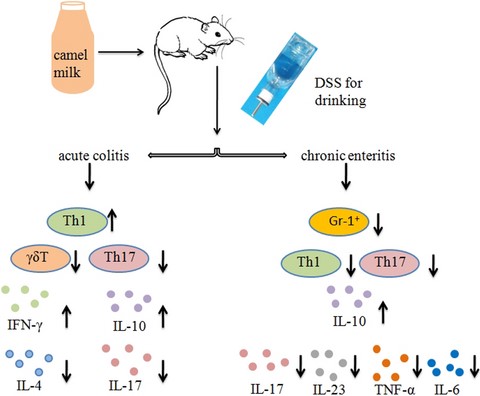
Introduction
Inflammatory bowel disease (IBD) is a relapsing, chronic gastrointestinal inflammation mediated by a variety of factors that seriously distress the patient quality of life and includes Crohn's disease (CD) and ulcerative colitis (UC) (Maloy & Powrie, 2011). The aetiology and chronicity processes of IBD are associated with destroyed intestinal epithelial barrier and uncontrolled immune proliferation of pro-inflammatory and anti-inflammatory cells as the key event, followed by derangement of intestinal flora (Fiocchi, 1998).
Th1 cells secreting IL-12, IFN-γ, TNF-α and other inflammatory factors participate in the pathogenesis of CD. Th2 immune response is the main mucosal inflammation response in patients with UC (Geremia et al., 2014). The number of Th17 cells in the peripheral blood of IBD patients is higher than normal and associated with disease activity. The increase of IL-17 secreted by Th17 cells in the intestinal mucosa is more evident in CD patients (Yang et al., 2018). Rectifying the imbalance of mucosal immunity may have preventive or adjuvant therapeutic effects on IBD (Dieleman et al., 1998; Maloy & Powrie, 2011). Th17 cells secreting IL-17 and IL-22 promote a variety of cells including epithelial cells, macrophages and fibroblasts producing TNF-α, IL-6, chemotactic cytokines and GM-CSF to mediate the occurrence and development of inflammation (Yang et al., 2018).
Current IBD therapeutic medicine, including sulfasalazine, corticosteroids and immunosuppressive agents, causes secondary effects as organism immune response down-regulated (Murthy et al., 2015; Tursi et al., 2015; Lenzen et al., 2018). The scientific basis of the application of camel milk as a natural health production primarily comes from its marked antioxidant, immunomodulatory function, anti-inflammatory properties, insulin-like and anti-apoptotic attributes. CM has unique composition that differs from other ruminants’ milk, containing more calcium, lactoferrin, immunoglobulins and less fat. CM is also advocated as an alternative to cow milk for those who are allergic or intolerant to cow's milk (Ahamad et al., 2017; Khalesi et al., 2017). CM contains several secretory antibodies including IgA and IgM. It has higher antibacterial and antiviral activities than milk. CM contains numerous bioactive proteins with immunoregulatory effect including lysozymes, lactoperoxidase and N-acetyl glucosaminidase (Arab et al., 2014; Mihic et al., 2016; Zhu et al., 2016). The oligosaccharides contained in CM inhibit pathogenic microorganisms attaching to colon mucosa and favour the multiplication of intestinal tract bifidobacteria. Drinking CM down-regulated the expression of TNF-α mRNA and improve disorders of glucose and lipid metabolism in diabetic patients (Soliman et al., 2015; Ayyash et al., 2018). Furthermore, CM has been reported to exhibit protective and alleviating properties against diabetic nephropathy and alcohol-induced hepatic injury, virus hepatitis (Mohamed et al., 2015). Thus, we aimed to investigate the potential alleviating effects of CM, a natural product with anti-inflammatory features, in DSS-induced mice colitis.
In the current study, colon inflammation was evaluated by change in body weight, colon length and histopathological score. Additionally, the immune status was monitored by assessing the levels of pro- and anti-inflammatory cell proliferation and cytokine concentration. This study offers a new demonstration that CM can regulate the internal homeostasis to relieve colitis symptoms in mice. To the best of our knowledge, this may be the first report describing the ameliorating effect of CM on DSS-induced colitis via its anti-inflammatory features.
Methods
Mice and materials
Male C57BL/6 mice, 6–8 weeks of age, (Beijing Vital River Laboratory Animal Technology Co. Ltd. Beijing, China) were used in the experiments. The mice were kept at China Medical University SPF animal experiment centre under constant temperature (21 ± 1 °C). Mice were acclimatised for 1 week before experiments and given standard chow and water. Experimentation on animals was approved by the Animal Care and Use Committee of China Medical University. CM used in this study was purchased from Wang Yuan Camel Milk Company (Xinjiang, China). The milk was obtained from Bactrian camels living in the desert area of Altai in Xinjiang Uyghur Autonomous Region of China and pasteurised. The main components are shown in Fig. S1.
Antibodies and reagents
Antibodies for flow cytometry analysis, including anti-CD3, anti-Gr-1, anti-CD4, anti-CD44, anti-IL-17A, anti-IL-10, anti-IFN-γ, anti-F4/80, anti-B220, anti-αβTCR, anti-γδTCR, anti-CD8, anti-NK1.1, anti-CD25 and anti-CD28, were purchased from BD Biosciences (San Diego, CA, USA). To measure spontaneous cytokine production by LPL, ELISA kits (BD Biosciences) for IL-23, TNF-α, IL-1β, IL-6, IL-4, IL-10, IL-17 and IFN-γ were used in the experiments.
Experimental design and treatment protocol
No colitis
Twenty-four rats were randomly divided into two groups (n = 12 per group). Group I (Control group) received DDW with oral gavage for 14 days. Group II (CM experimental group) received CM (2 g kg−1 body weight in 200 μL DDW/mouse/day) by oral gavage for 14 days (Fig. S2).
Acute colitis
Forty-four rats were randomly divided into two groups (n = 22 per group). Mice were treated with oral administration of DDW (200 μL mouse/day, as control) or CM (2 g kg−1 body weight in 200 μL DDW/mouse/day) for 19 days. For induction of acute colitis, mice were administered 3% DSS (MP Biomedicals, LLC, Irvine, CA, USA) on days 14–19 (Fig. S2).
Chronic colitis
Forty-four rats were randomly divided into two groups (n = 22 per group). Mice were orally administrated with DDW (200 μL mouse/day, as control) or CM (2 g kg−1 body weight in 200 μL DDW/mouse/day) for 39 days. To induce chronic colitis, mice were given alternating administration of 1.5% DSS on day 14, and 5 days of DSS followed by 5 days of DDW. The body weight and the disease activity index (DAI) were recorded daily. DAI scoring process is given as previous articles (O'Sullivan et al., 2017) (Fig. S2).
Tissue collection and preparation
Mice intestines were cut into 2-mm pieces, treated two times for 10 min in PBS solution with 3 mm EDTA at 37 °C and 20 min in RPMI-1640 with 1% FBS (all from Gibco, Grand Island, NY, USA). To obtain LP cells, intestine pieces were mixed at 37 °C for 90 min in RPMI-1640 with 20% FBS, 100 U mL−1 collagenase and 5 U mL−1 DNase I (both from Sigma-Aldrich, St Louis, MO, USA). The suspension was centrifuged through a 45%/66.6% discontinuous Percoll® (Pharmacia, Uppsala, Sweden) gradient at 600× g for 20 min. The living cells were stained with trypan blue.
Flow cytometry
Isolated LPL from mice intestines were stained with the following monoclonal antibodies: anti-F4/80, anti-Gr-1, anti-CD3, anti-B220, anti-NK1.1, anti-γδTCR, anti-CD4, anti-CD8, anti-CD25 or anti-CD44. For intracellular cytokine staining, LPL were stimulated with PMA (25 ng mL−1) and ionomycin (1 μg mL−1) for 5 h in the cell incubator; then, BFA (10 μg mL−1; Sigma-Aldrich) was added for the last 4 h of incubation. The cells were harvested, washed and stained with the following monoclonal antibodies: anti-IL-10, anti-IL-4, anti-IL-17A or anti-IFN-γ for 30 min at 4 °C. Flow cytometry data were acquired on an LSRII flow cytometer and analysed using Cell Quest software (BD Biosciences).
ELISA for cytokines
Extracted cells were cultured at 37 °C for 24 h under 5% CO2 in 96-well flat-bottomed plates in a volume of 0.2 mL RPMI 1640 containing 10% FBS (all from Gibco) to detect the culture supernatant cytokines, IL-23, TNF-α, IL-1β and IL-6. To measure cytokines IL-4, IL-10, IL-17 and IFN-γ, 2 × 105 cells were cultured at 37 °C for 48 h in 96-well flat-bottomed plates coated with anti-CD3 (10 μg mL−1) and soluble anti-CD28 monoclonal antibodies (1 μg mL−1). Cytokines secreted into culture supernatants were detected using ELISA kit (BD Biosciences) according to the manufacturer's protocol. The absorbance was measured using an Infinite M200 microplate reader (Tecan Group Ltd., Männedorf, Switzerland). Optical densities were detected at 420 nm. Protein contents were normalised to per milligram of tissue.
Histopathological analysis
The colons were removed and immersed in 10% neutral formalin for 24 h. The specimens were washed, dehydrated with alcohol, cleared in xylene and embedded in paraffin for another 24 h. Full-thickness colon biopsy specimens (6 μm in thickness) were stained with H&E and examined under a light microscope (Leica Microsystems, Wetzlar, Germany). All histopathological processing and assessments of specimens were performed by an experienced technician/observer blinded to the identity of the sample being examined to avoid any bias. Damage to the intestinal mucosa was scored as follows: epithelium (0, intact epithelium; 1, globlet cell deficiency; 2, loss of globlet cells in large areas; 3, crypt damage; 4, loss of crypts in large areas) and infiltration (0, no infiltration; 1, infiltration around the crypt base; 2, moderate infiltration around the muscularis; 3, extensive infiltration reaching the muscularis mucosae and thickening of the mucosa with abundant oedema; 4, infiltration of the submucosa) (Sun et al., 2008; Chen et al., 2017).
Statistical analysis
Parametric data were expressed as means ± standard deviation (Sd). Differences between two groups were determined using Student's t-test for normally distributed data or Wilcoxon two-sample otherwise. Statistical analyses were conducted using SPSS program, version 17. A two-tailed P-value < 0.05 was considered statistically significant.
Results
Populations of LPL and their cytokines in colons of CM-treated mice
To assess the impact of CM on the immune system of mice, changes in immune cells and immune factors were detected in mice administered CM or DDW. Compared with mice treated with DDW, the percentage of CD4+ T and CD44+CD4+ cells increased, and CD8+ T cells decreased in colons of mice administered CM (Fig. 1c,e). The absolute number of CD4+ T-LPL increased in mice treated with CM (Fig. 1g). Significant differences were not observed in the H&E staining results, percentage or absolute number of macrophages (CD11b+F4/80+), neutrophils (CD11b+Gr-1+), NK cells (NK1.1+CD3−), NKT cells (NK1.1+CD3+), T cells (CD3+B220−), B cells (CD3−B220+), γδT (CD3+γδTCR+) cells and T-reg (CD4+CD25+) cells between CM- and DDW-treated mice (Fig. 1).
![Changes in histopathological activity and LP cells after CM treatment. a. Histological score (original magnification, ×400). b-g. Proliferation of CD11b+Gr-1+, CD11b+F4/80+, CD3+, B220+, CD4+,CD8+, CD4+CD25+, CD4+CD44+, CD8+CD44+, γδ T, NK and NKT cells in LP. h. Absolute number of LP cells. Data are expressed as means ± Sd (*P < 0.05). Experiments were performed twice. [Colour figure can be viewed at wileyonlinelibrary.com]](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ijfst/55/4/10.1111_ijfs.14434/1/m_ijfs14434-fig-0001-m.jpeg?Expires=1750215716&Signature=yT8JKBDQlcSj3BhyQMl87-jG2DPFWQn3kTYSMDNisweLm9aiHD30VnPXs9AIBZnxpD0FbasMA0M9NeQUJVkF1SKRy2lTUFlpQKIpDjoXXK7-8iB0Xje3u3yDe5rXL~SXu632w6yE-qbFzE4UffIBUSKwM26XaEiSshYj7icwQ~YL8Q37AZNL0J4zu0A4eV0GeqGCey9MvzgvMY16f-NlY3mamEqeU3quATIdSla7WLtIq9GQkBSqvMxMdRPDght0WApjpbjT1INUpJBJ3741LJ8-G1AYzRcrDkOaZ2KBlZHM-qlLWL~sEByT3dbmFtpxbYq~3m3wIadRXdc1xHjFKQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Changes in histopathological activity and LP cells after CM treatment. a. Histological score (original magnification, ×400). b-g. Proliferation of CD11b+Gr-1+, CD11b+F4/80+, CD3+, B220+, CD4+,CD8+, CD4+CD25+, CD4+CD44+, CD8+CD44+, γδ T, NK and NKT cells in LP. h. Absolute number of LP cells. Data are expressed as means ± Sd (*P < 0.05). Experiments were performed twice. [Colour figure can be viewed at wileyonlinelibrary.com]
The number and frequency of IFN-γ-producing CD4+ cells in LPL were noticeably higher in the colons of mice treated with CM than mice treated with DDW (Fig. 2a,b). The number and frequency of IL-17-producing CD4+ cells showed no significant changes. The IFN-γ and IL-10 concentrations were higher and the IL-4 concentration was lower in the colons of mice treated with CM (Fig. 2). The results indicated CM can promote the proliferation and secretion of Th1 cells.
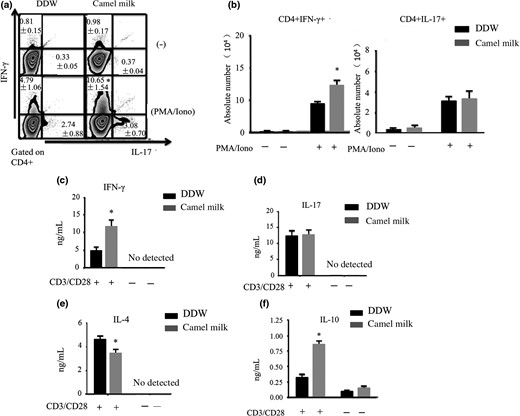
Proliferation of Th1 and Th17 cells and cytokine secretion. a, b. Proportion and absolute number of CD4+IFN-γ+, and CD4+IL-17A+ T-LPL. c–f. Concentrations of cytokines IL-4, IL-10, IL-17 and IFN-γ were measured using ELISA. The data are expressed as means ± Sd (*P < 0.05). Experiments were performed twice.
Effects of CM on acute colitis symptoms in mice
Th1 and Th2 cells are strongly associated with the pathogenesis of colitis induced by DSS. The influence of feeding CM on the acute colitis induced by DSS in mice was measured. Compared with the DDW-treated control group, the weight and survival rate of CM-treated mice were significantly increased. The histological score and DAI were decreased (Fig. 3).
![Observed changes in the acute colitis mice model. a–e. Body weight, survival rate, DAI, histological score and colon length (original magnification, ×400). Data are expressed as means ± Sd (*P < 0.05, **P < 0.01). Experiments were performed twice. [Colour figure can be viewed at wileyonlinelibrary.com]](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ijfst/55/4/10.1111_ijfs.14434/1/m_ijfs14434-fig-0003-m.jpeg?Expires=1750215716&Signature=0CRHfiB-gjggn~142VTaYAzEJ3PpZPR06LykgNonbt7eoq1z7RMAxF6cbVh5Cvo7035GpFCV0BLUbnmuvEC61d7A0FxdLx-tP5--RWg1GTMHtQ8qT0VCxVZt1BxfUqMtN0TnRsp9A3Yh73piN8Hl1zi~ndvIeUwwh6ALFV-YCtZ5XORZabz8JHroyysCYbZ~LyxsFjRu2RjQCsDZuvtFTZ2wADjyEV0ynHvnHdDW~NaDplL9hn8iCblr8~EglIZ8BVyGAg8HvraQzEethzg4rWENLCRqZCJ5Oo1vYmBqooUhVPvC-FzxiJnYKPlASeknqpniFVG2Rq8LjWt~v27zZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Observed changes in the acute colitis mice model. a–e. Body weight, survival rate, DAI, histological score and colon length (original magnification, ×400). Data are expressed as means ± Sd (*P < 0.05, **P < 0.01). Experiments were performed twice. [Colour figure can be viewed at wileyonlinelibrary.com]
The frequency of IFN-γ-producing CD4+ LP cells was increased (Fig. 4e). In contrast, the proportion of γδT (CD3+γδTCR+) cells was decreased, and the frequency and number of IL-17-producing CD4+ LPL were lower in CM-treated than in DDW-treated mice (Fig. 4c,f). Statistical differences were not observed in percentage or absolute number of macrophages (CD11b+F4/80+), neutrophils (CD11b+Gr-1+), T cells (CD3+B220−), B cells (CD3−B220+) or CD4+CD44+ T cells between CM- and DDW-treated mice (Fig. 4). Subsequently, the concentration of cytokines secreted by LPL was measured. The LPL-secreted IFN-γ and IL-10 concentrations were higher, and the IL-4 and IL-17 concentrations were lower in CM-treated than in DDW-treated mice. Differences in IL-1β, IL-23, IL-6 or TNF-α were not found (Fig. 5). These results indicated the proliferation and secretion of Th1 cells increased and the proliferation and secretion of Th17 decreased with the administration of CM.
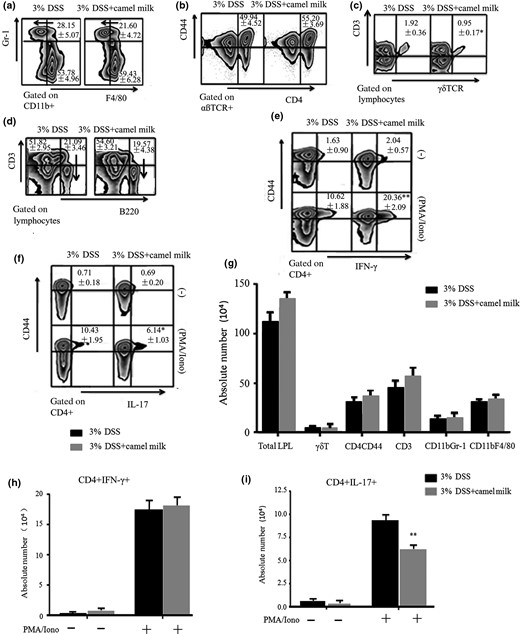
Changes of LP cells in the acute colitis mice model. a–f. The proportions of CD11b+Gr-1+, CD11b+F4/80+, CD3+, B220+, CD4+CD44+, γδ T cells, CD4+IFN-γ+ and CD4+IL-17A+ T-LPL g–i. The absolute number of LPL. Data are expressed as means ± Sd (*P < 0.05, **P < 0.01). Experiments were performed twice.
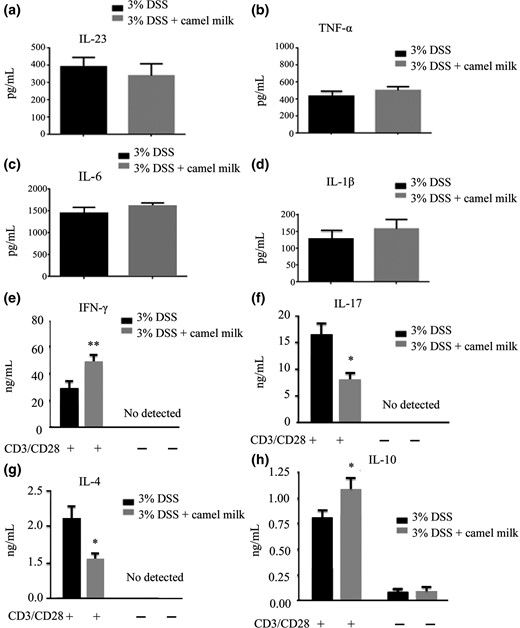
Concentration of cytokines secreted by LP cells. a–h. LPLs were isolated from the colons of mice and cultured in 96-well flat-bottomed plates. Concentration of cytokines IL-23, IL-6, IL-1β, TNF-α, IL-4, IL-10, IL-17 and IFN-γ was measured using ELISA. Data are expressed as means ± Sd (*P < 0.05, **P < 0.01). Experiments were performed twice.
Effects of CM on symptoms of chronic colitis in mice
Pro-inflammatory cells and cytokines are increased in the pathogenesis of DSS-induced chronic colitis. To determine whether CM has a protective effect on chronic colitis in mice, the change in immune cells and cytokines were examined in the colon LP. Weight and disease activity indices of CM-fed mice significantly improved. Noticeable differences were found in histological scores between CM-treated and control group mice (Fig. 6).
![Symptoms in the DSS-induced chronic colitis model. a–d. Colon length, histological score (original magnification, ×400), weight loss and DAI. Data are expressed as means ± Sd (*P < 0.05). Experiments were performed twice. [Colour figure can be viewed at wileyonlinelibrary.com]](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ijfst/55/4/10.1111_ijfs.14434/1/m_ijfs14434-fig-0006-m.jpeg?Expires=1750215716&Signature=5NhPZfWeQGoDFCJOJMS-OtEBzwT9O60UAp7e9~LGt0xz3Psih04oxt2z0rrvD3jGRxUzeAgyTbrtJV7iQg-tkfwSLH6PBDr1BlvesmGL-6r~hrxbyrn5Td11kFjX5JuEvmOBTmqT3sZUV7G7rz7KcIEAQuGIKjJ7RSFYF9xJl6pDEiHrpCzkQ7xIzh5r9TjrPSOO6za8kB7UeOdke6zJhLjU5Ak-SRo1mEWnfPFwf5DUajAy8x~Q6FSRAzxtNrTw-Sv47FDUNDOZB~-e~RCpsPjndZrmvpMPjmj5S8aSCE~gihXTUP9AhiafoczAKuhVnjkC3o~V49bb7qNeFZux3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Symptoms in the DSS-induced chronic colitis model. a–d. Colon length, histological score (original magnification, ×400), weight loss and DAI. Data are expressed as means ± Sd (*P < 0.05). Experiments were performed twice. [Colour figure can be viewed at wileyonlinelibrary.com]
The effects of CM in mice during chronic colitis induced by DSS were further characterised. The frequency of CD11b+Gr-1+ T cells decreased in the LP of CM-fed mice (Fig. 7a). The frequency and number of CD4+ LPL producing IFN-γ and IL-17 was lower in CM-treated than in DDW-treated mice (Fig. 7e,f,g). Distinctive differences were not found in percentage or absolute number of macrophages (CD11b+F4/80+), γδT (CD3+γδTCR+), CD4+ or CD8+T cells between CM- and DDW-treated mice (Fig. 7). In addition, the concentration of LP-secreted cytokines in the intestines of mice was measured. The IL-23, IL-6, TNF-α and IL-17 concentrations were lower; conversely, the IL-10 concentration was significantly higher in CM-treated mice than in DDW-treated mice (Fig. 8). Differences in IL-1β, IL-4 and IFN-γ concentrations were not found. The results indicated the proliferation and secretion of Th1 and Th17 were inhibited with CM treatment.
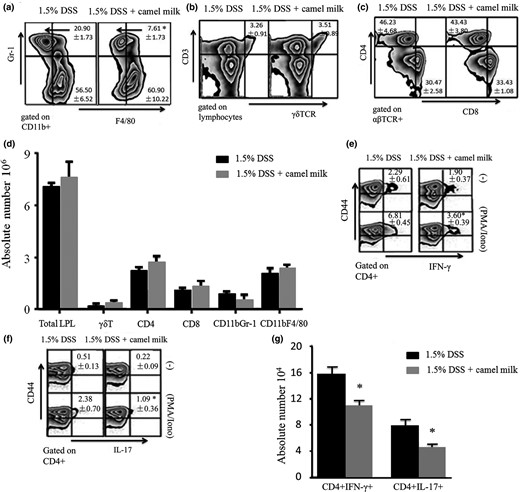
Analysis of the frequency of LP cells in mice treated with CM. a–g. The proportion and the absolute number of CD11b+Gr-1+, CD11b+F4/80+, CD4+, CD8+, γδT cells and IFN-γ- or IL-17-producing CD4+CD44+ LPL cells. Data are expressed as means ± Sd of 10 mice from each group (*P < 0.05). Experiments were performed twice.
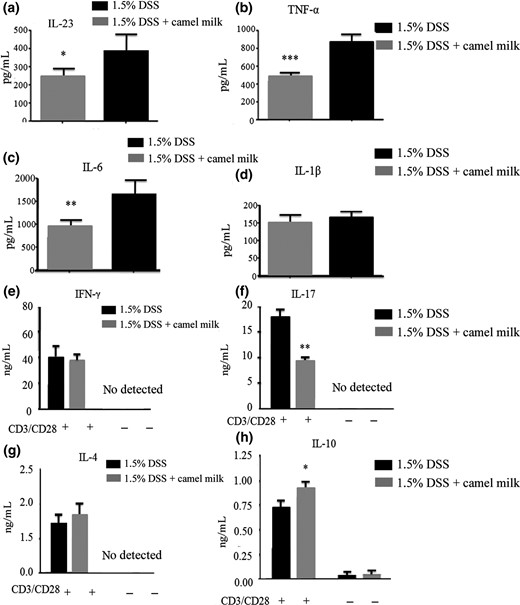
Concentration of cytokines secreted by LP cells in mice administered CM. a–d. LPL cells were isolated from the colons of mice after administration of 1.5% DSS and then cultured for 24 h. Concentration of pro-inflammatory factors IL-23, IL-6, IL-1β and TNF-α was detected using ELISA. e–h. LPL cells were cultured for 48 h with anti-CD3 and anti-CD28 monoclonal antibodies. Concentration of cytokines IL-4, IL-10, IL-17 and IFN-γ was measured using ELISA. Data are expressed as means ± Sd (*P < 0.05, **P < 0.01, ***P < 0.001). Experiments were performed twice.
Discussion and conclusion
Inflammatory bowel disease is characterised by a chronic relapsing intestinal inflammation with epithelial damage and immune system imbalance. Gut inflammation may develop from the augmentation of pro-inflammatory cells and their secreted cytokines in the intestinal mucosa (Maloy & Powrie, 2011). In the present study, whether CM relieves the process of colitis in mice was evaluated. As expected, CM alleviated symptoms in the mouse model of DSS-induced colitis. Noticeably, CM treatment reduced pro-inflammatory cell proliferation and differentiation and up-regulated anti-inflammatory cells in the colons of mice. The current study may be the first in which the beneficial effects of CM were demonstrated in a mouse model of DSS-induced colitis as an experimental model of human IBD.
Throughout centuries, CM as an important diet supplement has been consumed as fresh milk or a milk product for the management of various diseases. Goat milk and cow milk have high-protein homology and cross-reactivity and induce allergic reactions in people. However, CM is distinct from the milk of other animals in the Bovidae family and causes less allergic reactions (Soliman et al., 2015; Ayyash et al., 2018). CM restores the immune system imbalance and improves symptoms in a variety of immune diseases, such as allergies, asthma and rashes. The relieving effects of CM on colitis were associated with alleviating oxidative stress by restraining lipid peroxides and restoring glutathione (GSH) in the gut LP. In addition, consuming CM inhibits cell apoptosis as indicated by caspase-3 activity (Alhaider et al., 2014; Uversky et al., 2017). To confirm the role of CM in the prevention of IBD, animal studies are needed. In the present study, macroscopic changes, proliferation and differentiation of immunological cells, and cytokine secretion, were evaluated using a mouse model. The effective attenuation of the pathological injury in the colon induced by DSS demonstrated that CM relieves IBD symptoms. In addition, the nutritional value of CM cannot be ignored.
Gut inflammation is affected by the release and composition of immune cells as well as the interaction of immune factors in the gut mucosa. In the present study, CM up-regulated Th1 cells in the intestinal mucosa of normal and acute colitis mice. Macrophages secrete a large number of pro-inflammatory cytokines to enhance phagocytosis and cytotoxicity, promoting the production of TNF and damage the structure and function of tight junctions. Finally, the intestinal epithelial barrier is destroyed (Lissner et al., 2015). In the early stage of IBD inflammatory response, neutrophils gather in the mucosa, generating neutrophil extracellular traps (NETs) to induce extensive vascular lesions and inflammation (Leshner et al., 2012). In the present study, the changes in innate immune cells, such as neutrophils and macrophages, were not as significant as in Th cells. The adaptive immune response was proven to contribute to the disease relief process. Th1 cells mainly secrete IL-2 and IFN-γ and differentiate from Th0 cells in the presence of IL-12 and other cytokines. The Th1 cell response was an important component of the DSS-induced acute colitis process (Badr et al., 2017; Broggi et al., 2017). ELISA results demonstrated the secretion of IFN-γ was increased; however, the IL-4 concentration was decreased in the LP of the intestinal mucosa in the acute colitis model treated with CM. These results are in agreement with recent research showing similar effects of CM on hepatitis (Mohamed et al., 2015). In patients with hepatitis, immune dysfunction is common and Th2 cells are increased while Th1 cells are decreased. After drinking CM, serum cytokine IFN-γ was increased and the Th1 immune response was enhanced to purge the virus. Lactoferrin and lysozymes contained in CM can directly inhibit infection by invading pathogens and strengthen the local Th1 response, thereby enhancing the immune regulatory function, an important host defence approach against bacterial infections (Ebaid et al., 2013, 2015; Badr et al., 2017).
The Th17 pathway was shown associated with the pathogenesis of colitis (O'Connor et al., 2009; Aguiar et al., 2017). Th17 cells exacerbated the immunopathogenesis of IBD. The results from the present study showed CM modulates the proliferation and differentiation of Th17 cells and cytokine secretion in the DSS-induced intestinal colitis. Th17 response was mutually modulated by STAT3 (Atarashi et al., 2015). Retinoid-related orphan receptor (ROR)γ, an orphan nuclear receptor, induces Th17 and Th1 differentiation (Chen et al., 2003; Kim et al., 2017; Nakanishi et al., 2018). In the chronic colitis model, cytokines including IL-17, TNF-α, IL-6 and IL-23, as well as the percentage and absolute number of Th1 and Th17 cells, were significantly decreased in LP of mice treated with CM compared with mice treated with DDW. TNF-α produced by LPL in the intestinal mucosa is an important inflammatory factor relevant to the pathogenesis of IBD, including the recruitment of inflammatory cells into the LP, the promotion of local inflammation of the intestinal mucosa, neutrophil/granulocyte aggregation and macrophage differentiation (Feagan et al., 2008; Schiering et al., 2014; Chamoun et al., 2018; Lenzen et al., 2018). A previous study result showed CM can reduce TNF-α concentration in serum, thus decreasing the arthritis index and inflammatory cell migration into the intestinal tract, which is similar to the observations in the current study (Arab et al., 2017). Furthermore, Treg cells producing IL-10 influence homeostasis in the colon.
In recent microbiological studies, probiotics contained in CM regulated mucosal immunological cells and cytokines by depressing pathogenic infection (Ebaid et al., 2011; Lennon et al., 2014; Tozer et al., 2015). Clostridia spp. and Lactobacilli spp. affect the proliferation and polarisation of Th cells (Atarashi et al., 2015). Probiotics such as Bifidobacteria spp. promote Th1 responses (Mazmanian et al., 2005), and Clostridia spp. and Bacteriodes fragilis (Lee & Mazmanian, 2010; Farkas et al., 2015) induce the proliferation of Tregs in the LP. Virtually, the observed CM lowering of pro-inflammatory factors may be implicated in the colonic microflora.
In conclusion, CM is a regulator with a two-way effect on the immune system, which is associated with adaptive immune response. Hence, CM can be beneficial in maintaining intestinal homeostasis in IBD patients. Administration of CM corrected the imbalance of pro-inflammatory cells and pro-inflammatory cytokines in the acute and chronic mouse model of IBD. The beneficial bacteria contained in CM may be involved in the regulation of the intestinal microflora and alter the intestinal mucosal immune system. In a broader perspective, further studies are needed to determine if microflora in CM play a role in alleviating inflammation.
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Author contributions
CC prepared and analysed the data and edited the manuscript. YL, YY, SW, SW, YM established the animal model. ZS helped edit the manuscript. All authors have read and approved the final version of the manuscript.
Funding
This study was funded by NSFC grant 81171581.



